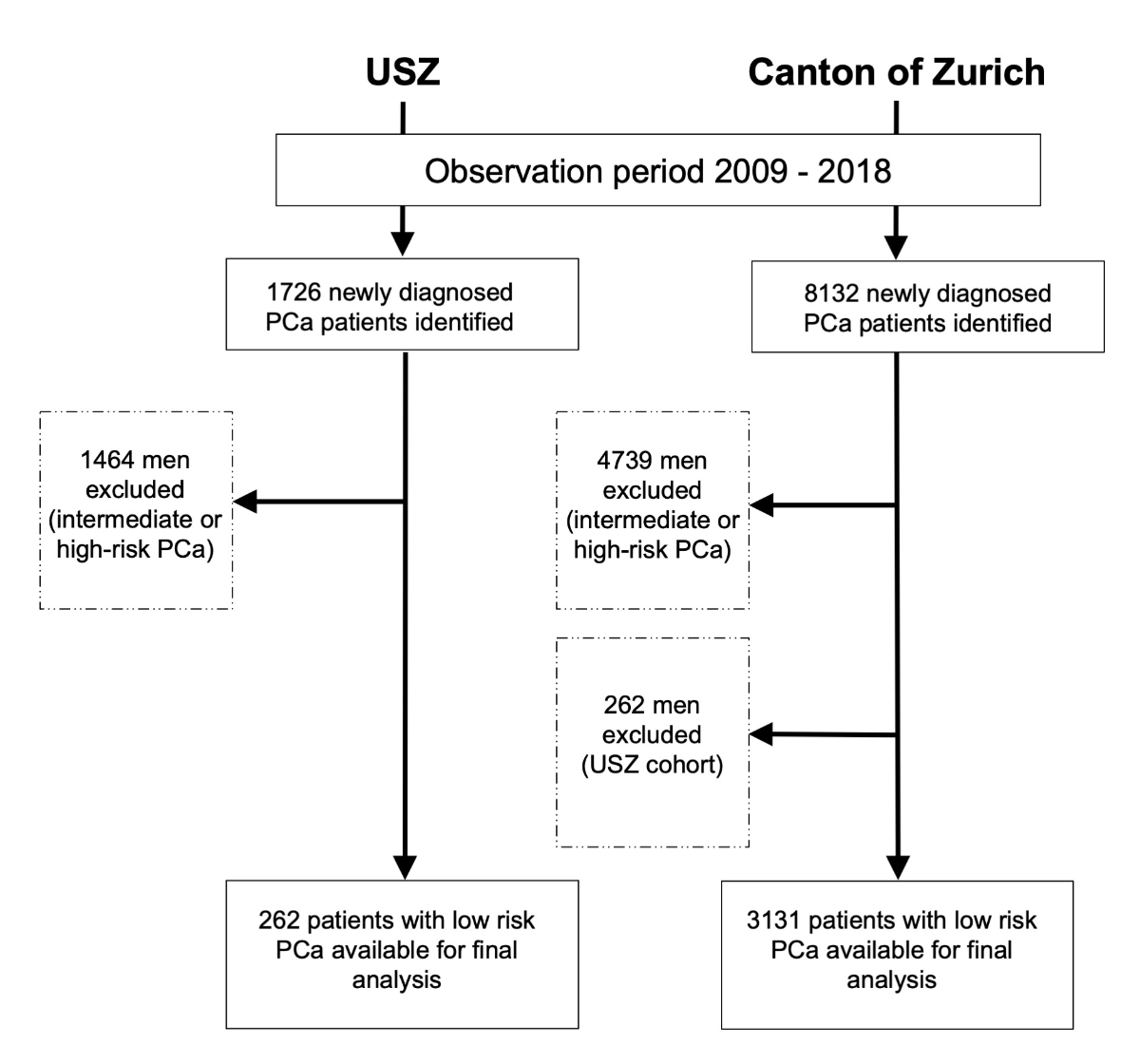
Figure 1Study flow chart. USZ: University Hospital of Zurich
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40103
German Oncologic Society
European Association of Urology
interquartile range
International Society of Urological Pathology
prostate specific antigen
In the last 10 years, active surveillance has emerged as the preferred management option for low-risk prostate cancer. In comparison with watchful waiting, which is only symptom-based surveillance of patients, active surveillance is seen as a curative strategy. Patients in active surveillance undergo regular follow-ups with periodical measurement of the prostate-specific antigen (PSA) level and re-biopsy. Active surveillance is widely recommended by clinical guidelines based on the excellent oncological long-term outcomes comparable to those after immediate active treatment [1–3]. When active treatment is selected, prostate cancer is either surgically removed (radical prostatectomy) or treated with radiotherapy. Despite the clear benefits of active surveillance, its uptake has been shown to vary among different centres and regions [4]. The decision to pursue active surveillance is not only influenced by patients’ and physicians’ preferences but also varies in different institutions according to their internal policies. Furthermore, regional differences have also been identified [5–7].
In the early 2000s, the German Oncologic Society (Deutsche Krebsgesellschaft, DKG) started to certify cancer centres in German-speaking countries to standardize patient care according to clinical guidelines to improve patient outcomes. In these certified centres, standardized pathways for different clinical scenarios are mandatory(standard operating procedures). All newly diagnosed prostate cancer cases must be discussed in a multidisciplinary tumour board before any treatment decision is made. Our tertiary academic centre (University Hospital of Zurich) has been a DKG-certified prostate cancer centre since 2009.
The primary aim of this study was to analyze the uptake of active surveillance in our tertiary academic centre. The secondary aim was to compare our results with those retrieved from the population-based cancer registry of our region (canton of Zurich).
All patients diagnosed with low-risk prostate cancer from 01/2009 to 12/2018 in our tertiary academic centre (University Hospital of Zurich, Zurich, Switzerland) were retrospectively, manually screened in our patient data management system. Low-risk prostate cancer was defined as prostate cancer with a Gleason score of ≤3 + 3 = 6 [8]. Men who had a higher Gleason score were excluded. Men who underwent a confirmatory biopsy and were diagnosed with a higher Gleason score within three months after the initial diagnosis were also excluded.
Age, date of diagnosis, PSA level at diagnosis, biopsy results, clinical T stage and initial selected treatment for each patient were recorded. Possible treatments were as follows: active surveillance, watchful waiting, radical prostatectomy, radiotherapy (i.e. brachytherapy or external beam radiation) and systemic treatment (i.e. androgen deprivation therapy). The overall numbers and proportions of the different treatments were analyzed separately for each year and for the entire observation period.
To compare the selected treatments for low-risk prostate cancer in our certified prostate cancer centre with those in other urological centres in our region, we analyzed the data from the cancer registry of the canton of Zurich during the same period (from 01/2009 to 12/2018). Identically to the University Hospital of Zurich cohort, only men newly diagnosed with low-risk prostate cancer were included in the cancer registry cohort. The same inclusion and exclusion criteria were applied. The clinical data and treatment details were retrieved from the cancer registry database and were analyzed the same way. To avoid inclusion of the same patient in both cohorts, we excluded University Hospital of Zurich patients from the cancer registry patients.
The results of the University Hospital of Zurich and cancer registry cohorts were compared for the entire observation period and for each year separately. In the cancer registry cohort, active surveillance and watchful waiting were not coded separately but coded collectively as “observation”. Thus, it was not possible to distinguish between active surveillance and watchful waiting in the cancer registry cohort.
With the aim of comparing observation against direct treatment within the two cohorts, both active surveillance and watchful waiting were also summarized as observation in the University Hospital of Zurich cohort.
Finally, a subgroup analysis of very low-risk prostate cancer (defined as prostate cancer with a Gleason score of ≤6 and additionally PSA levels at diagnosis of <10 ng/ml) was performed for both cohorts and compared with each other.
Data entry, evaluation and visualization were all conducted using Microsoft Excel (Version 2016, Microsoft Corporation, Redmond, United States of America). The clinical data were evaluated using descriptive statistics.
The internal data analysis of the University Hospital of Zurich cohort was reviewed and approved by the local ethics committee (KEK Nr. 2021-02041). Generally, all cancer cases in the canton of Zurich are registered with presumed consent, and registration is based on the decision by the Zurich Government Council from 1980 and the Cantonal Cancer Registration Law of 2016. No additional approval from the ethical committee of the canton of Zurich was necessary for this monitoring project.
From 2009 to 2018, a total of 1726 men were diagnosed with prostate cancer at the University Hospital of Zurich. Of them, 262 men (15.2%) met the criteria for low-risk prostate cancer and the inclusion criteria for this study and were included in the final analysis (figure 1).

Figure 1Study flow chart. USZ: University Hospital of Zurich
After exclusion of the University Hospital of Zurich patients from the cancer registry cohort, the final cancer registry cohort consisted of 8132 patients with prostate cancer, of whom 3393 (41.7%) had low-risk prostate cancer. Ultimately, the final cancer registry cohort from the canton of Zurich consisted of 3131 patients (38.5%) (figure 1).
The clinical characteristics of both cohorts for the entire observation period and for each year are illustrated in table 1. The University Hospital of Zurich cohort was diagnosed at a median age of 63.5 years [interquartile range (IQR): 58–68 years], with a median PSA level of 5.7 ng/ml (IQR: 3.8–8.7 ng/ml). Conversely, the cancer registry cohort was diagnosed at a median age of 67 years (IQR: 62–73 years), with a median PSA level of 6.3 ng/ml (IQR: 4.7–9.0 ng/ml). The PSA levels were available for all men in the University Hospital of Zurich cohort and were missing for 1075 men (34.3%) in the cancer registry cohort.
Table 1Clinical characteristics of the men with low-risk prostate cancer, including the number of men in each observed year and overall selected treatment.
| Patient characteristics | No. (%) | ||
| University Hospital of Zurich, n = 262 | Cancer registry, Zurich n = 3131 | ||
| Age, year | Median | 63.5 | 67 |
| IQR | 58−68 | 62–73 | |
| PSA level, ng/ml | Median | 5.7 | 6.3 |
| IQR | 3.8–8.7 | 4.7–9 | |
| <10 ng/ml | 216 (82.4) | 1637 (52.3) | |
| ≥10 ng/ml | 46 (17.6) | 419 (13.4) | |
| Unknown | 0 (0) | 1075 (34.3) | |
| Clinical T category | cT1a–cT2a | 251 (95.8) | 2027 (64.7) |
| cT2b–cT4 | 10 (3.8) | 100 (3.2) | |
| Unknown | 1 (0.4) | 1004 (32.1) | |
| Year of diagnosis | 2009 | 31 (11.8) | 378 (12.1) |
| 2010 | 58 (22.1) | 331 (10.6) | |
| 2011 | 42 (15.0) | 395 (12.6) | |
| 2012 | 28 (10.7) | 302 (9.7) | |
| 2013 | 24 (9.2) | 293 (9.4) | |
| 2014 | 23 (8.8) | 255 (8.1) | |
| 2015 | 20 (7.6) | 267 (8.5) | |
| 2016 | 10 (3.8) | 304 (9.7) | |
| 2017 | 10 (3.8) | 271 (8.7) | |
| 2018 | 16 (6.1) | 335 (10.7) | |
| Treatment | Observation (active surveillance/watchful waiting) | 146 (55.7) | 502 (16.0) |
| Active treatment | 115 (43.9) | 2220 (70.9) | |
| Unknown | 1 (0.4) | 409 (13.1) | |
Abbreviations: IQR: Interquartile range, PSA: Prostate-specific antigen
In the University Hospital of Zurich cohort, 146 men (55.7%) opted for observation, while 115 men (43.9%) underwent active treatment [radical prostatectomy: n = 88 (33.6%), radiation: n = 27 (10.3%), systemic therapy: n = 0 (0%)]. In one patient, the selected treatment was unknown (0.4%).
In the cancer registry cohort, 502 men (16.0%) opted for observation, while 2220 men (70.9%) underwent active treatment [radical prostatectomy: n = 2087 (66.7%), radiation: n = 91 (2.9%), systemic treatment: n = 42 (1.3%)]. In 409 men (13.1%), the selected treatment was unknown. Assuming all unknown cases in the cancer registry cohort received the same proportion of active treatment to observation as the dedicated cases or all unknown cases had received an observational strategy, 18.9% and 29.1% of the cancer registry cohort patients, respectively, could have been allocated to the observation group.
Figure 2 shows the proportion of the different treatments selected per year in the University Hospital of Zurich cohort and cancer registry cohort. In the University Hospital of Zurich cohort, observation was performed in 35.4% of the patients in 2009. Subsequently, the proportion of men who underwent observation increased continuously over the years up to 88.2% in 2018. In the cancer registry cohort, 12.2% of men underwent observation in 2009. No relevant increase was observed in the subsequent years, with only 16.2% of men undergoing observation in 2018.
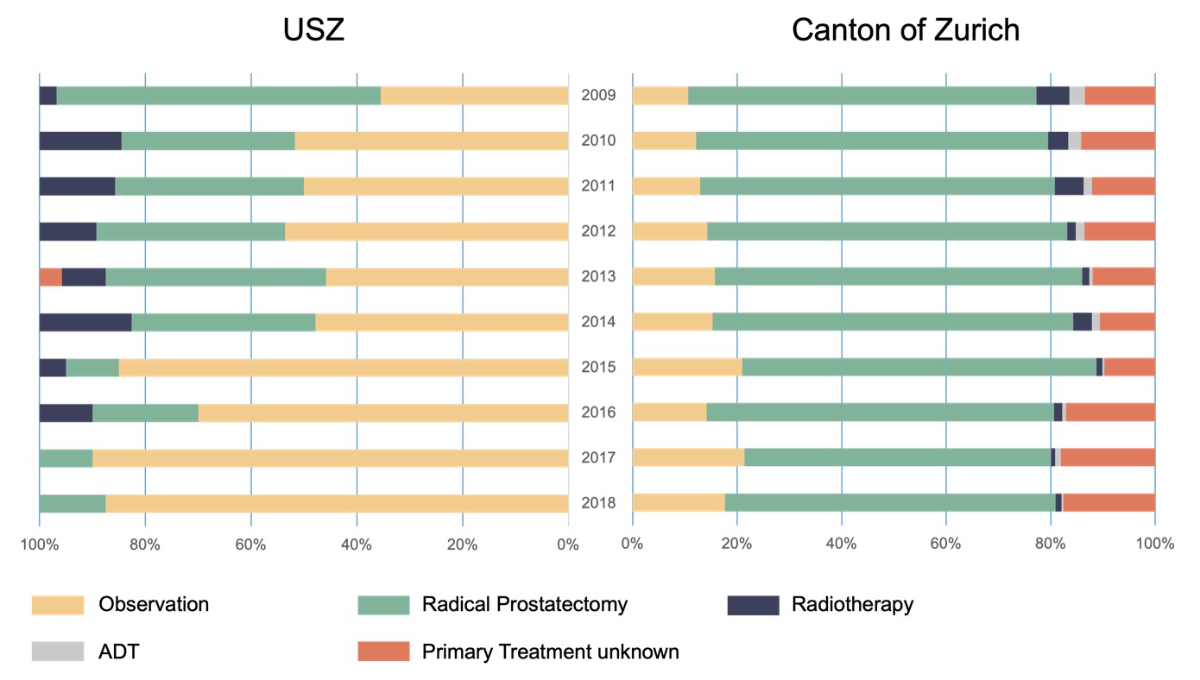
Figure 2Proportion of each treatment in men with low-risk prostate cancer from 2009 to 2018 in the University Hospital of Zurich and the canton of Zurich. Abbreviations: ADT: Androgen deprivation therapy, USZ: University Hospital of Zurich
The results of the subgroup analysis of the men with very low-risk prostate cancer compared with the entire group of men with low-risk prostate cancer are illustrated in figure 3 for both cohorts. In the University Hospital of Zurich cohort, no relevant differences in the proportion of men undergoing observation for low-risk or very low-risk prostate cancer were detected (55.7% vs 58.8%, figure 3). In the cancer registry cohort, the proportion of the use of observation was slightly higher in the patients with very low-risk prostate cancer than in those with low-risk prostate cancer (22.6% vs 16.0%). Over the entire observation period, an increase in the proportion of the use of observation was detected among the patients with very low-risk prostate cancer in the cancer registry cohort (13.9% in 2009 vs 25.9% in 2018).
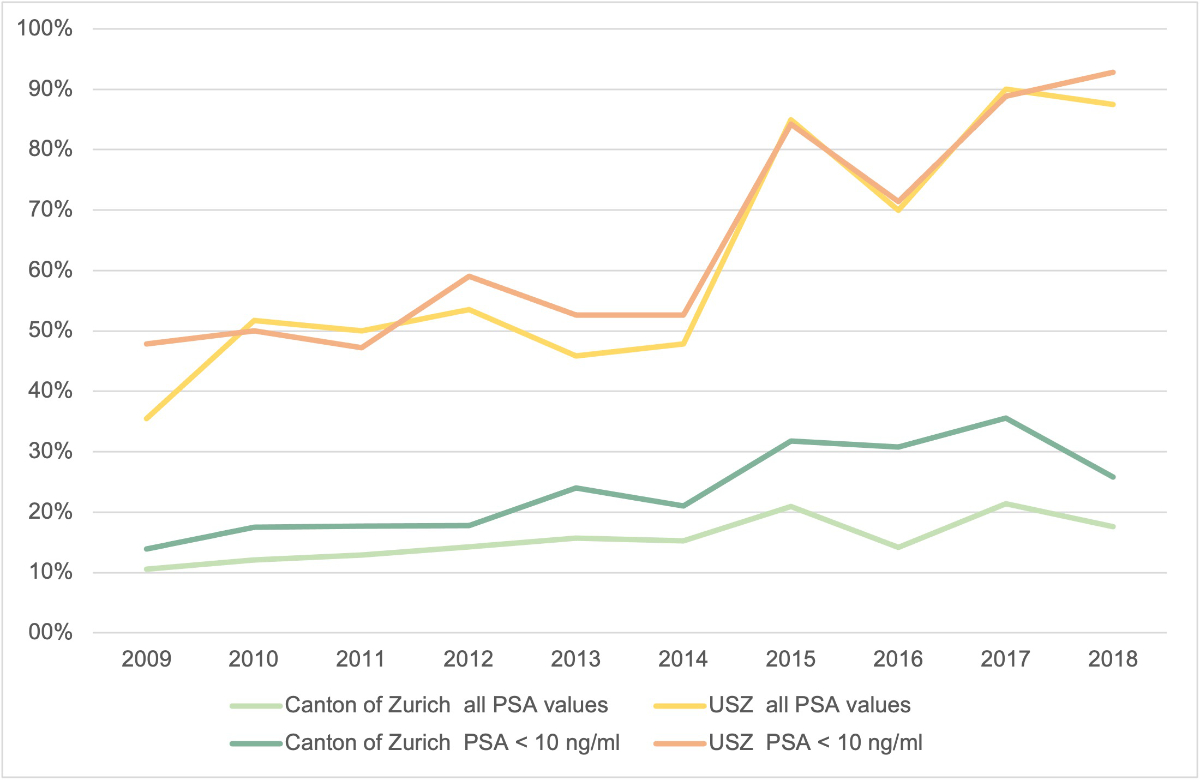
Figure 3Proportion of the use of observation in men with low-risk prostate cancer and very low-risk prostate cancer (PSA level of ≤10 ng/ml) from 2009 to 2018 in the University Hospital of Zurich and the canton of Zurich. Abbreviations: PSA: Prostate-specific antigen, USZ: University Hospital of Zurich
Active surveillance for low-risk prostate cancer is an oncologically safe management option, which significantly reduces overtreatment and the risk of morbidity associated with treatment [9]. In the prostate cancer guidelines of the European Association of Urology, active surveillance has been recommended as the primary management option for patients with low-risk prostate cancer since 2009 [10]. Despite this recommendation, the use of active surveillance varies significantly among different regions and centres. In our certified prostate cancer centre, the use of active surveillance was already considerably high in 2009 and constantly increased to almost 90% in 2018. In contrast, active surveillance was rarely used in the canton of Zurich in 2009, and, more importantly, the proportion of its use did not relevantly increase during the 10-year observation period.
Large variations in the use of active surveillance in different settings have previously been shown [6, 11]. The reasons for the differences in the uptake of active surveillance are diverse. Adherence to clinical guidelines has been shown to be generally lower in non-academic settings [12, 13]. Treatment-associated financial benefits for institutions and physicians (e.g. private vs public service) might be another explanation for such differences [14]. Furthermore, it has been shown that a higher socioeconomic status of a patient increases the probability to undergo active surveillance [15].
Country-specific differences in healthcare systems can also influence the uptake of active surveillance. A non-guideline-conforming treatment is less likely to be reimbursed, and thus, active surveillance is more commonly performed in countries with more centralized systems (e.g. Great Britain, Scandinavia, Canada) than in countries without these regulations. In Sweden, 74% of men with low-risk prostate cancer and 91% of men with very low-risk prostate cancer were treated with active surveillance in 2014 [7]. In Canada and Australia, 85% and 73% of patients with low-risk prostate cancer underwent active surveillance, respectively [16, 17]. Low uptake of active surveillance since the beginning of its recommendation is unfortunately common and has been reported in some studies. For example, in the United States of America, active surveillance was conducted in less than 25% of cases between 2010 and 2015, with some regions pursuing active surveillance only in 5% of cases [5]. In the Swiss healthcare system, there are no regulations regarding the treatment option for prostate cancer. Thus, patients are free to decide whether they want to undergo active surveillance or active treatment.
During the observation period of our study, our academic centre was the only DKG-certified prostate cancer centre in the canton of Zurich. In the early 2000s, the DKG started to certify cancer centres, with the goal of standardizing care for patients with cancer and with a strong focus on well-defined clinical pathways (standard operating procedures) and mandatory interdisciplinary (e.g. urology, radio-oncology, oncology, pathology) case discussions in tumour boards. The tumour board provides a management recommendation for every newly diagnosed prostate cancer case. Pretreatment interdisciplinary case discussions are known to increase guideline adherence, particularly for patients with low-risk prostate cancer [18]. Furthermore, certified centres are committed to participate in regular audits and disclose their numbers of specific cancer cases (including treatment option and treatment expertise) [19]. It has been shown that patients with prostate, breast or colon cancer treated in certified cancer centres have better treatment outcomes after surgery than patients treated in non-certified centres [20–22].
Given that the use of active surveillance is not centrally regulated in Switzerland and that our centre was the only certified prostate cancer centre in the canton of Zurich during the observation period, it seems reasonable to assume that the certification process with standardized pathways had a significant impact on the uptake of active surveillance. The use of active surveillance in our region is comparable to that in countries with centralized healthcare systems. In contrast, the remaining (non-academic) centres in the canton of Zurich did not adopt active surveillance as standard management for low-risk prostate cancer, and the overall use of active surveillance remained substantially low throughout the observation period.
The aspect of overtreatment, despite clinical evidence and guidelines, is restricted not only to prostate cancer or the field of urology. For example, it is well known that women with low-risk breast cancer often undergo mastectomy instead of breast-preserving treatment, which is recommended in many clinical guidelines [23]. Primary care physicians and patients should be encouraged to ask for second opinions in certified cancer centres to avoid unnecessary treatment and reduce morbidity and healthcare costs. In men with low-risk prostate cancer, the decision to undergo active treatment might be influenced by many individual aspects of the treating physician or of the patient himself or his relatives. Therefore, it can be helpful to ask for a recommendation from a certified interdisciplinary tumour board that follows well-defined and evidence-based guidelines. Men with low-risk prostate cancer should aim for a second opinion before making a final treatment decision [5].
Our study has some limitations. The PSA levels were missing for a number of men in the cancer registry cohort, and therefore, correct classification into low- or very low-risk prostate cancer was not possible. Furthermore, the treatment option in the cancer registry cohort was missing in 13% of the cases. However, even if all men with unknown information on treatment had selected surveillance (active surveillance or watchful waiting), the proportion of men undergoing surveillance would have still been considerably lower in the cancer registry cohort than in the University Hospital of Zurich cohort. In the cancer registry cohort, data on specific institutions or hospitals (public vs private) and patients’ insurance status, socioeconomic status, race or access to an interdisciplinary tumour board were not available for analysis. Given that it was not possible to identify individual centres from the cancer registry, it is possible that certain centres had much higher active surveillance rates than the reported overall rate.
Despite clear guideline recommendations, active surveillance for low-risk prostate cancer is still widely underused. Our analysis showed that access to a certified interdisciplinary tumour board significantly increases the use of active surveillance. Continuous education of physicians and patients as well as low-threshold access to a second opinion from an interdisciplinary prostate cancer centre can help to promote guideline-conforming management of low-risk prostate cancer to reduce unnecessary treatment.
The data that support the findings of this study are available from the first author, CP, upon reasonable request.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Mottet N, van den Bergh RC, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2020:1–20.
2. Schaeffer E, Srinivas S, Antonarakis ES, Armstrong AJ, Bekelman JE, Cheng H, et al. Prostate cancer, version 1.2021: featured updates to the nccn guidelines. JNCCN Journal of the National Comprehensive Cancer Network. 2021;19:134–43.
3. Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J Urol. 2018 Apr;199(4):990–7.
4. Al Hussein Al Awamlh B, Patel N, Ma X, Calaway A, Ponsky L, Hu JC, et al. Variation in the Use of Active Surveillance for Low-Risk Prostate Cancer Across US Census Regions. Front Oncol. 2021 May;11:644885.
5. Washington SL 3rd, Jeong CW, Lonergan PE, Herlemann A, Gomez SL, Carroll PR, et al. Regional Variation in Active Surveillance for Low-Risk Prostate Cancer in the US. JAMA Netw Open. 2020 Dec;3(12):e2031349.
6. Löppenberg B, Friedlander DF, Krasnova A, Tam A, Leow JJ, Nguyen PL, et al. Variation in the use of active surveillance for low-risk prostate cancer. Cancer. 2018 Jan;124(1):55–64.
7. Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, Stattin P. Uptake of active surveillance for very-low-risk prostate cancer in Sweden. JAMA Oncol. 2017 Oct;3(10):1393–8.
8. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA; Grading Committee. The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016 Feb;40(2):244–52.
9. Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015 Jan;33(3):272–7.
10. Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al.; European Association of Urology. [EAU guidelines on prostate cancer]. Actas Urol Esp. 2009 Feb;33(2):113–26. 10.1016/S0210-4806(09)74110-5
11. Werntz RP, Eggener SE. Re: Use of Active Surveillance or Watchful Waiting for Low-risk Prostate Cancer and Management Trends Across Risk Groups in the United States 2010-2015. Eur Urol. 2019 Aug;76(2):252.
12. Iskandar H, Yan Y, Elwing J, Early D, Colditz GA, Wang JS. Predictors of Poor Adherence of US Gastroenterologists with Colonoscopy Screening and Surveillance Guidelines. Dig Dis Sci. 2015 Apr;60(4):971–8.
13. Cacciamani G, Artibani W, Briganti A, N’Dow J. Adherence to the European Association of Urology Guidelines: A National Survey among Italian Urologists. Urol Int. 2018;100(2):139–45.
14. Zhang Z, Modi PK, Shahinian V, Herrel LA, Dupree JM, Yan P, et al. Active Surveillance vs Immediate Treatment-Which Has a Greater Financial Incentive for Urologists? Urol Pract. 2020 May;7(3):182–7. 10.1097/UPJ.0000000000000081
15. Butler SS, Loeb S, Cole AP, Zaslowe-Dude C, Muralidhar V, Kim DW, et al. United States trends in active surveillance or watchful waiting across patient socioeconomic status from 2010 to 2015. Prostate Cancer Prostatic Dis. 2020 Mar;23(1):179–83.
16. Timilshina N, Ouellet V, Alibhai SM, Mes-Masson AM, Delvoye N, Drachenberg D, et al. Analysis of active surveillance uptake for low-risk localized prostate cancer in Canada: a Canadian multi-institutional study. World J Urol. 2017 Apr;35(4):595–603.
17. Ong WL, Evans SM, Evans M, Tacey M, Dodds L, Kearns P, et al. Trends in Conservative Management for Low-risk Prostate Cancer in a Population-based Cohort of Australian Men Diagnosed Between 2009 and 2016. Eur Urol Oncol. 2021 Apr;4(2):319–22.
18. Aizer AA, Paly JJ, Zietman AL, Nguyen PL, Beard CJ, Rao SK, et al. Models of care and NCCN guideline adherence in very-low-risk prostate cancer. J Natl Compr Canc Netw. 2013 Nov;11(11):1364–72.
19. Kowalski C, Graeven U, von Kalle C, Lang H, Beckmann MW, Blohmer JU, et al. Shifting cancer care towards Multidisciplinarity: the cancer center certification program of the German cancer society. BMC Cancer. 2017 Dec;17(1):850.
20. Butea-Bocu MC, Müller G, Pucheril D, Kröger E, Otto U. Is there a clinical benefit from prostate cancer center certification? An evaluation of functional and oncologic outcomes from 22,649 radical prostatectomy patients. World J Urol. 2021 Jan;39(1):5–10.
21. Kobayashi H, Yamamoto H, Miyata H, Gotoh M, Kotake K, Sugihara K, et al. Impact of adherence to board-certified surgeon systems and clinical practice guidelines on colon cancer surgical outcomes in Japan: A questionnaire survey of the National Clinical Database. Ann Gastroenterol Surg. 2020 Apr;4(3):283–93.
22. Kreienberg R, Wöckel A, Wischnewsky M. Highly significant improvement in guideline adherence, relapse-free and overall survival in breast cancer patients when treated at certified breast cancer centres: an evaluation of 8323 patients. Breast. 2018 Aug;40:54–9.
23. Harding C, Pompei F, Burmistrov D, Wilson R. Use of mastectomy for overdiagnosed breast cancer in the United States: analysis of the seer 9 cancer registries. J Cancer Epidemiol. 2019 Jan;2019:5072506.