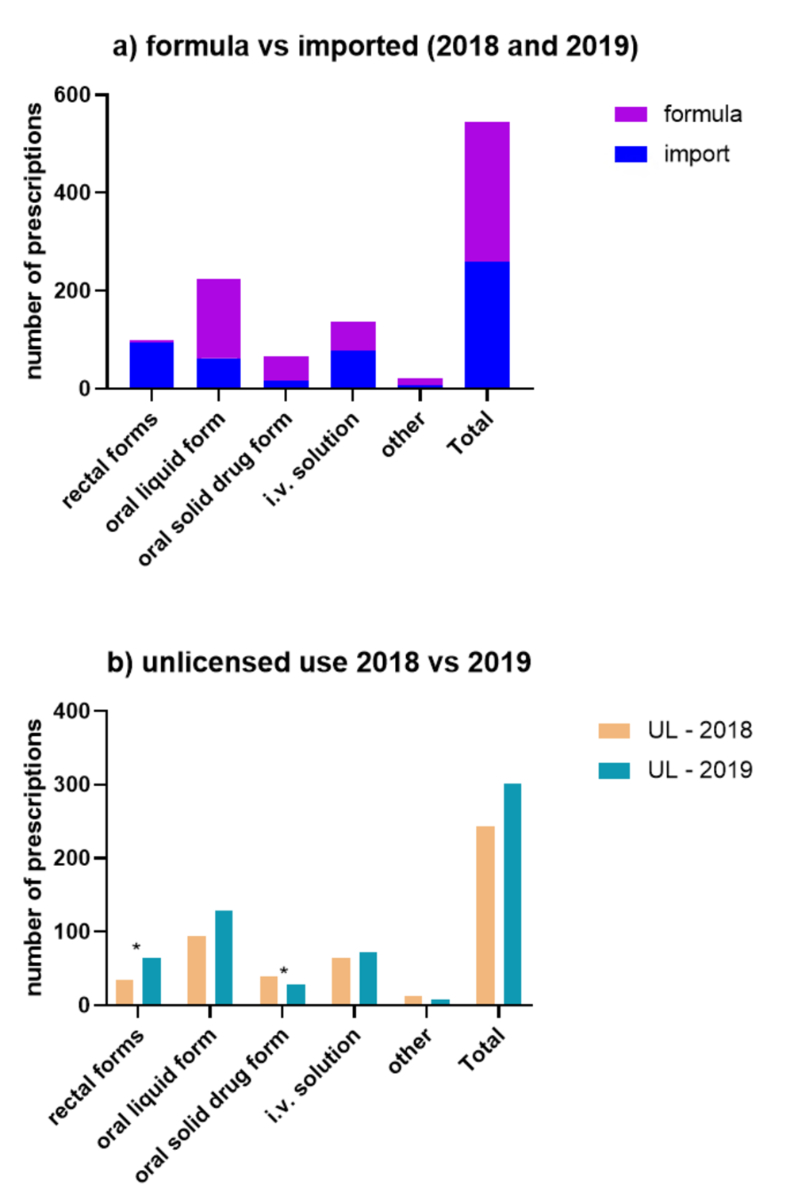
Figure 1Drug forms. (A) Formula vs imported (2018 and 2019). (B) Unlicensed use (UL) 2018 vs 2019.
DOI: https://doi.org/https://doi.org/10.57187/s.3369
Computerized physician order entry (electronic prescription in the hospital)
National Coordinating Council for Medication Error Reporting
Pharmaceutical Care Network Europe
Unlicensed drugs are medicines that have no marketing authorisation in the country in which they are used [1–3]. In Switzerland, this applies to drugs without market authorisation by Swissmedic. These may be medicines imported from foreign countries, henceforth referred to as “imported drugs”, or those prepared by a hospital pharmacy or another licensed manufacturer, henceforth referred to as “formula drugs”. In Switzerland, drugs can be imported by health care professionals, if a valid market authorisation exists in a country with a comparable regulatory system and no alternative drug authorised for the same indication is available [1]. Formula drugs do not have to be authorised by the licensing agency but must be manufactured by authorised manufacturers [1].
Worldwide, the proportion of unlicensed prescriptions in paediatrics differs vastly across regions, ranging between 0.1% and 74.4% [4, 5]. Recent studies from Europe have shown rates between 3.2% and 30% [6–8]. To our knowledge, only one 2006 study from Switzerland has been published, in which Di Paolo et al. [9] described the extent of paediatric off-label and unlicensed use in a university hospital in the French-speaking part of the country. They found that 24% of all prescriptions were unlicensed drugs.
The most important reasons for the use of unlicensed drugs in paediatrics are a lack of a suitable galenic formulation, dosage or specific substance on the national market for paediatric patients [4, 5, 10]. Another increasingly important reason for drugs to be imported or manufactured is drug shortages [11, 12].
Unlicensed drugs carry a higher risk of being prescribed erroneously [4, 13–15] because they have neither proper labelling (undesirable effects, cautions and contraindications) nor dosing instructions. This applies especially to formula drugs, whereas imported drugs do have a summary of product characteristics but often in a foreign language [9]. Unlicensed use is furthermore associated with a higher rate of adverse events [16] and underreporting of adverse events [4]. Extemporaneously prepared formula drugs additionally carry the risks of compounding errors, non-validated stability, and possible reactions to ingredients and excipients [4].
In studies of unlicensed drugs, “off-label” use is also often included [5, 8]. Off-label use describes the use of a licensed drug outside of the summary of product characteristics in terms of age, indication, route of administration or other deviations from registered use. [3]. Off-label use is also frequent in the paediatric population [4]. In Europe, the proportion of off-label use of drugs is estimated to represent between 13% and 69% of all drug use in the hospital setting [17].
As Bonati et al. [18] argued, clinical evidence is the most important reason for the use of medicine but not necessarily the official license. In recent years, efforts have been underway to provide evidence for drug use in case of no license.
The evidence for commonly used drugs in paediatrics in Switzerland has been collected in the databases SwissPedDose [19] and PedEDose [20]. Hence, many drugs at the University Children’s Hospital Zurich may be prescribed off-label, but their use is still evidence-based. Therefore, we decided to focus on unlicensed medicines because these drugs are completely lacking in the Swiss market for the paediatric population. Furthermore, to what extent imported and formula drugs are currently being prescribed in hospital care in Switzerland is unclear.
The study of Di Paolo et al. [9] was conducted several years ago. Since then, no additional data from the German-speaking part of Switzerland has been available. The prevalence of unlicensed drug prescriptions differs from country to country. The costs of unlicensed drugs are often not covered by insurance, or only after bureaucratic approval, which is relevant for patients who leave the hospital with prescriptions for unlicensed drugs. Furthermore, because unlicensed drugs are associated with a higher risk for patients [4, 13–16], we aimed to qualify unlicensed drug use in the University Children’s Hospital Zurich and explore whether unlicensed drugs are more prone to prescribing errors than licensed drugs. This will help in understanding the current situation in paediatric general wards in Switzerland.
We conducted a sub-analysis of a retrospective observational single-centre study, which previously investigated the influence of a computerised physician order entry (CPOE) on prescribing errors in paediatrics [21].
The database for this study comprised 1,000 patients, randomly selected among all patients who stayed at six general wards of the University Children’s Hospital Zurich during the study periods. Each 500 patients were selected from all patients hospitalised in two timeframes (1,688 patients in Oct–Dec 2018 and 1,608 patients in Oct–Dec 2019), which allowed a comparison of the two periods on the development of the rate of unlicensed, imported and formula drugs over time. Only patients with at least one prescribed medication were eligible. All medications prescribed within the first 24 hours after admission were included except for parenteral nutrition; lipids; blood cell transfusions; insulin; solutions for dialysis; solutions for fluid management, such as NS, NS-D5W and D5W; and acetated Ringers.
All drugs were assigned licensed or unlicensed. The unlicensed drugs were further divided into imported or formula drugs. The galenic drug formulations were categorised into five main classes, comprising several similar drug forms: rectal forms (suppositories and other rectal forms), oral liquid forms (e.g. suspensions, solutions, syrups), oral solid forms (e.g. tablets, capsules, soft capsules), i.v. solutions (e.g. i.v. concentrated solutions, powder for the preparation of an i.v. solution, i.v. infusion solutions) and others (e.g. nasal sprays, topical ointments, solutions for intravesical instillation).
Patients were divided into four age groups according to the EMA classification [22]: newborn (term newborn infants: 0–27 days), infants (infants and toddlers: 28 days to 23 months), children (2 to 11 years) and adolescents (12 to 18 years).
A medication review was performed for all patients to assess prescribing errors. Errors were categorised according to the Pharmaceutical Care Network Europe (PCNE) classification [23], and their severity was ranked according to the National Coordinating Council for Medication Error Reporting (NCC MERP) index as adapted by Forrey et al. [24]. To validate prescribing error assessment and severity classification, interrater reliability was calculated. All patients underwent a medication review by the first rater, a clinical pharmacist, and a random sample of 5% of all included patients underwent an additional review by the second rater, another clinical pharmacist. Consequently, interrater reliability could be assessed. As described in our previous article, the overall error rate was lower in 2019 after the implementation of the CPOE than in 2018 [21]. Because the error rates differed significantly between the two years, we decided to analyse only error rates related to prescriptions in 2019 to exclude the influence of the CPOE.
The study database was built with Microsoft SQL Server 2019 Master Data Services. Data were collected by the first rater. The evaluation and visualisation of the anonymised data used Microsoft Power BI Desktop, and statistical analyses were conducted with RStudio 2022.02.1 and IBM® SPSS® Statistics Version 27. The rates of unlicensed, imported and formula drugs versus licensed drugs, as well as the rates of prescribing errors, were compared by t-test or chi-square test where appropriate. A significance level of 0.05 was defined.
This study was performed following the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Zurich (PB_2019-00030, project related to subsequent use of non-genetic personal health data), and informed consent for further use of their data was obtained from all patients or their parents.
The total of 1,000 patients from both periods were prescribed 5,022 medicines, of which 544 (10.8%) were unlicensed drugs; 5.1% were imported drugs and 5.7% were formula drugs. A total of 340 patients (34%) received at least one unlicensed drug. Of the imported drugs, 244 (95%) came from Germany, with the remaining 14 (5%) from countries such as the USA, Great Britain, Italy, Sweden and the Netherlands.
In 2018, 243 (10.6%) of the total 2,299 drug prescriptions were unlicensed drugs, and in 2019, 301 (11.1%) of the total 2,723 prescriptions were unlicensed (table 1). This increase was not statistically significant. The proportion of formula drugs did not differ between the two years, but the rate of imported drugs increased statistically significantly from 4.3% to 5.8% (p = 0.019). On the patient level, 151 (30.2%) were prescribed at least one unlicensed drug in 2018, along with 189 (37.5%) in 2019. The proportion of patients who were prescribed an imported drug also increased statistically significantly from 17.8% (2018) to 26.8% (2019).
Table 1Rate of unlicensed, formula and import drugs in 2018 and 2019.
| Category | Prescriptions 2018 | Prescriptions 2019 | p-value | Patients 2018 | Patients 2019 | p-value |
| n = 2,299 | n = 2,723 | n = 500 | n = 500 | |||
| Unlicensed | 243 (10.6%) | 301 (11.1%) | 0.582 | 151 (30.2%) | 189 (37.5%) | 0.011* |
| Formula | 143 (6.2%) | 143 (5.3%) | 0.143 | 84 (16.8%) | 91 (18.2%) | 0.560 |
| Import | 100 (4.3%) | 158 (5.8%) | 0.019* | 89 (17.8%) | 134 (26.8%) | 0.001* |
* indicates significant value
As table 2 shows, newborns had the highest proportion of unlicensed drugs and adolescents the lowest. Unlicensed drug use in adolescents increased statistically significantly from 2018 to 2019, whereas no difference occurred in other age groups over time.
Table 2Unlicensed prescriptions in the four age groups.
| Age group | Prescriptions 2018 | Prescriptions 2019 | Prescriptions total | p-value |
| Newborns | 11 (16.9%) | 4 (13.3%) | 15 (15.8%) | 0.660 |
| Infants | 82 (12.0%) | 114 (14.6%) | 196 (13.4%) | 0.139 |
| Children | 129 (11.3%) | 133 (10.1%) | 262 (10.6%) | 0.324 |
| Adolescents | 21 (5.2%) | 50 (8.5%) | 71 (7.1%) | 0.037* |
* indicates significant value
Table 3 shows the 10 most frequently imported drugs. All drugs in the top 10 list were imported from Germany.
Table 3Top 10 imported and formula drugs.
| Imported drug product name | Substance | Prescriptions (% of unlicensed prescr.) | Patients | 2018 prescr. | 2019 prescr. | Reason for import |
| Nurofen Junior suppositories 60 mg | Ibuprofen | 59 (10.8%) | 54 | 19 | 40 | No suppository in paediatric dosage on the CH market |
| Midazolam ratiopharm oral solution 2 mg/ml | Midazolam | 36 (6.6%) | 36 | 1 | 35 | Formulation not on the CH market |
| Gentamicin-Ratiopharm SF 40 mg/ml i.v. solution | Gentamicin | 35 (6.4%) | 35 | 20 | 15 | Formulation not on the CH market |
| Nurofen Junior suppositories 125 mg | Ibuprofen | 28 (5.1%) | 27 | 10 | 18 | No suppository in paediatric dosage on the CH market |
| Prednisolut 50 mg powder w solv for i.v. use | Prednisolone | 21 (3.9%) | 21 | 3 | 18 | Formulation not on the CH market |
| Lasix liquidum oral solution 10 mg/ml | Furosemide | 12 (2.2%) | 11 | 6 | 6 | Formulation not on the CH market |
| Prednisolut Trockensub 25 mg powder w solv for i.v. use | Prednisolone | 11 (2.0%) | 11 | 4 | 7 | Formulation not on the CH market |
| Gentamicin-Ratiopharm SF 80 mg/2 ml i.v. solution | Gentamicin | 8 (1.5%) | 7 | 7 | 1 | Formulation not on the CH market |
| Petnidan oral suspension 50 mg/ml | Ethosuximide | 6 (1.1%) | 6 | 3 | 3 | Formulation not on the CH market |
| Novalgin suppositories 300 mg | Metamizole | 6 (1.1%) | 4 | 2 | 4 | No suppository in paediatric dosage on the CH market |
| Formula drug product name | Substance | Prescriptions (% of unlicensed prescr.) | Patients | 2018 prescr. | 2019 prescr. | Reason for formula manufacture |
| Macrogol 4000 Plv | Macrogol | 69 (12.7%) | 56 | 36 | 33 | Clinical reasons |
| Lisinopril Susp 1 mg/ml | Lisinopril | 21 (3.9%) | 21 | 10 | 11 | Formulation not on the CH market |
| Kaliumchlorid Inf Lös 15% | Potassium chloride | 21 (3.9%) | 19 | 13 | 8 | Safety concerns |
| Adrenalin Inj Lös 10 mg/10 ml | Epinephrine | 19 (3.5%) | 19 | 2 | 17 | Size of the ampule |
| Spironolacton Susp 5 mg/ml Ora-Blend SF | Spironolactone | 19 (3.5%) | 19 | 7 | 12 | Formulation not on the CH market |
| NaCl 25% Inf Konz 5 g/20 ml | Sodium chloride | 14 (2.6%) | 13 | 8 | 6 | Vial instead of glass ampule and size |
| Hydrochlorothiazid Susp 5 mg/ml OraBlend SF | Hydrochlorothiazide | 11 (2.0%) | 11 | 2 | 9 | Formulation not on the CH market |
| Spironolacton Kaps 6 mg | Spironolactone | 6 (1.1%) | 6 | 6 | 0 | Dosage not on the CH market |
| Tacrolimus Susp 0.5 mg/ml Ora-Blend SF | Tacrolimus | 6 (1.1%) | 5 | 3 | 3 | Dosage not on the CH market and safety concerns |
| Captopril Lös 1 mg/ml | Captopril | 5 (0.9%) | 5 | 4 | 1 | Substance not on the CH market |
| Hydrochlorothiazid Kaps 6 mg | Hydrochlorothiazide | 5 (0.9%) | 5 | 5 | 0 | Dosage not on the CH market |
All formula drugs were produced by authorised manufacturers (other hospital pharmacies, community pharmacies or drug manufacturers) but not by the hospital pharmacy of the University Children’s Hospital due to a lack of suitable premises. The 10 most frequently prescribed formula drugs are displayed in table 3.
The most frequently prescribed forms of unlicensed drugs were oral liquid forms, followed by i.v. solutions, rectal forms and oral solid forms (figure 1).

Figure 1Drug forms. (A) Formula vs imported (2018 and 2019). (B) Unlicensed use (UL) 2018 vs 2019.
The rate of rectal forms increased significantly in 2019 (p = 0.028), whereas the rate of oral solid drug forms decreased significantly (p = 0.017). The other drug forms had no significant difference in their rate.
The use of unlicensed drugs was associated with statistically significantly more prescribing errors than the use of licensed drugs: 31.6 errors per 100 prescriptions (95% CI: 26.1–37.0) versus 24.3 errors per 100 prescriptions (95% CI: 22.3–26.2; p = 0.024). In particular, formula drugs were prone to errors, with 36.4 errors per 100 prescriptions (95% CI: 28.4–44.2) vs 24.5 errors per 100 prescriptions (95% CI: 22.6–26.3) in non-formula drugs (licensed in Switzerland or another country; p = 0.012). Imported drugs were not associated with an increased error rate: 27.2 errors per 100 prescriptions (95% CI: 19.6–34.8) vs 25.0 errors per 100 prescriptions (95% CI: 23.1–26.8) in non-imported drugs (licensed in Switzerland or formula drug; p = 0.570). Most of the errors were of minor severity. To estimate whether errors were clinically relevant, we more closely examined those that could potentially lead to harm (NCC MERP severity E–I). We found a rate of 14.6 such errors per 100 prescriptions (95% CI: 10.9–18.3) in unlicensed drugs versus 10.0 errors per 100 prescriptions (95% CI: 8.7–11.3) in licensed drugs (p = 0.060). This difference just missed the significant level. The use of formula drugs led to a rate of 11.9 potentially harmful errors per 100 prescriptions (95% CI: 6.5–17.3) versus non-formula drugs: 10.5 per 100 prescriptions (95% CI: 9.2–11.7; p = 0.616). The use of imported drugs was associated with a rate of 17.1 potentially harmful errors per 100 prescriptions (95% CI: 11.9–22.2) vs non-imported drugs with 10.1 potentially harmful errors per 100 prescriptions (95% CI: 8.9–11.4; p = 0.045).
In total, 95 errors were detected in the 301 prescriptions of unlicensed drugs in 2019, and 52 errors occurred in the 143 formula drug prescriptions. The most frequently observed error overall was the PCNE type 5.2 error: “necessary information not provided”. This type of error comprised the majority of cases of minor formal errors, such as missing drug form, missing route of administration or missing concentration of the solution but could also include errors of potentially harmful severity (NCC MERP E–I), such as missing the number of maximum doses that may be administered in cases of on-demand analgesics. This error type 5.2 occurred in 32 cases (62%) of formula drugs, whereas the rate was 46% for licensed drugs and 28% for imported drugs. Dosing errors (PCNE 3.1–3.5) were found in 29 unlicensed prescriptions. Most frequently affected by dosing errors were prednisolone i.v. solution, epinephrine i.v. solution, ethosuximide oral solution, furosemide oral solution, ibuprofen suppositories, metamizole suppositories and midazolam nasal spray.
The overall proportion of unlicensed drugs at 10.8% was lower than the 24% reported by Di Paolo et al. [9]. Notably, Di Paolo et al. investigated unlicensed drugs in different kinds of wards, including paediatric intensive care units and neonatal wards, where the use of unlicensed drugs is higher [5]. The proportion of such use on medicine wards in Di Paolo’s study was 16%, which is closer to our rate. In relation to the results reported by Gore et al. [4] and Shuib et al. [5] in their review articles (0.1%–74.4%), our rate is in the lower range. Kaisto et al. [25] recently reported 8% unlicensed drug prescriptions in Finland, also describing a reduction of unlicensed use from 2011 to 2021. Therefore, the reduction in the proportion of unlicensed use compared to the finding of 2006 by Di Paolo et al. seems plausible.
Although the prescription rate of unlicensed drugs did not differ significantly between 2018 and 2019, the proportion of patients prescribed an unlicensed drug increased significantly. This finding may be explained by the increase in drug shortages that took place in recent years [11, 12]. A look at the drug stock at the hospital pharmacy of the University Children’s Hospital Zurich shows that in 2019, 15.7% of the drugs were unlicensed (8.0% imported drugs, 7.7% formula drugs). In 2022, 16.8% of the drugs in stock were unlicensed, with 9.2% being imported drugs and 7.6% being formula drugs. Therefore, unlicensed drugs remain an important pillar in the treatment of paediatric patients.
The significant increases in prescriptions of imported drugs and patients receiving an unlicensed drug in 2019 can be explained by the fact that, if no licensed option is available in Switzerland, the hospital pharmacy usually tries to favour drugs that are at least licensed in other countries over formula drugs. Therefore, there may have been adaptions of the hospital formulary, leading to an increased proportion of imported drugs and a reduction of formula drugs. Imported drugs are licensed in other countries where they have undergone an authorisation process, whereas formula drugs are not subject to regulatory review.
Most of the imported drugs were purchased in Germany. This brought the advantage that the summary of product characteristics was also in German.
The distribution of unlicensed use among the four age groups showed that the use of unlicensed drugs was higher the younger the patients were. This complies with the findings of others [9, 25] and could be another factor explaining the lower use of unlicensed drugs in our study compared to Di Paolo et al. [9]. The patients in our population were older, with a median age of 4.3 years (range 0–18.8 years), whereas the patients in the Di Paolo et al. study had a median age of 1.6 years. This finding is also explicable by the fact that younger paediatric patients cannot swallow tablets and therefore need other galenic formulations.
All imported drugs in the top 10 were imported because no identical galenic form exists on the Swiss market or the available galenic form is not on the market in an appropriate dosage for paediatric use (e.g. ibuprofen and metamizole) but appropriate forms are licensed in other countries. Three substances are listed twice in the top 10 list (different dosages): ibuprofen suppositories, gentamicin i.v. solution and prednisolone i.v. solution. Ibuprofen suppositories are helpful for not only paediatric patients in hospital care but also for ambulatory patients in primary or pharmacy care.
A reason that these drugs are licensed in other countries but not Switzerland could be that the Swiss market is small compared to other markets. Therefore, pharmaceutical companies are not interested in licensing a drug in all available formulations in Switzerland.
Formula drugs are manufactured for several reasons. A lack of appropriate dosage and galenic formulation (especially oral liquid formulations) on the Swiss market was the main reason that a drug was manufactured as a formula drug (table 3). Other reasons for the production of formula drugs were safety concerns, especially with potassium chloride ampules. The concentrated drug is rated to be of high risk [26]; therefore, it is favourable if the manufactured drug label has features that make it well-distinguishable from other drugs. The potassium chloride formula drug comes with an orange label, whereas the licensed products do not have special labels to mark the high-alert drug.
Other reasons to use formula drugs instead of licensed drugs were lack of appropriate ampule sizes (adrenaline, sodium chloride 25%), clinical reasons (macrogol), or that the substance was not at all available in Switzerland as a licensed drug (captopril). Overall, the variety of reasons that a drug was produced as a formula drug was greater than the reasons to import a drug. The advantage of formula drugs is that they can be manufactured exactly the way users need them, i.e. in any given ampule size required in clinical practice and many dosages and concentrations, given the data on product stability. Formula drugs may even be necessary in case licensed products do not fulfil the safety requirements of a hospital, like the example of potassium chloride ampules in our study.
The comparison of the two years (figure 1b) shows that the number of oral solid drugs decreased, whereas the number of oral liquid formulations increased. This can be explained by the fact that, wherever possible, the hospital pharmacy tries to find an oral liquid formulation instead of capsules because this is easier to adapt the dosage to the weight of a patient. Capsules are produced only when no established liquid formulation is available. Unfortunately, liquid oral forms do not seem to be profitable for companies and thus are often not on the market.
A disadvantage of oral liquid formulations can be their taste; paediatric patients often do not accept or like the taste of the liquid drug, even when masked with syrup or other flavoured liquids. In contrast, the capsule content can be dissolved in liquid with a flavour of the patient’s choice. Rectal drug forms also increased significantly, though we could not find a direct reason for this and interpret it as a random finding.
Our finding that prescriptions of unlicensed drugs were more prone to errors aligns with previous findings [4, 13-15]. Notably, formula drugs were at especially high risk of being prescribed inappropriately. They showed a strikingly high rate of PNCE error 5.2 (“necessary information not provided”). This is a plausible finding because formula drugs do not have a summary of product characteristics or a leaflet, in which prescribers could find additional information on the drug, dosage, administration, potential adverse drug reactions and so on. Therefore, it is unsurprising that prescriptions of these drugs were not comprehensive enough and often lacked information. This finding implies that imported drugs are in many cases the better option for patient safety than formula drugs.
Dosing errors occurred often in reserve drugs for anaphylactic reactions (prednisolone i.v. and epinephrine i.v.), which can be explained by the fact that routine anaphylaxis treatment is used in these cases, where fixed dosages are more likely to result in doses outside of the range recommended by the literature. Fixed dosages were also the reason that dosing errors occurred frequently in suppositories (ibuprofen, metamizole). The other types of prescribing errors did not display any special pattern of errors that could be attributed to the licensing status of the drugs.
The strengths of this study are the considerable size of the sample and that the findings were validated through review by two independent raters. All patient data were accessible for the rater to evaluate prescribing errors, leading to a comprehensive medication review.
A limitation is that our findings may not be generalisable to the wider population in Switzerland or worldwide because we investigated only general paediatric wards, whereas neonatal and paediatric intensive care patients and oncologic patients are known to have especially high rates of unlicensed prescriptions. We also did not include primary care or multiple centres. Furthermore, the retrospective nature of our study imposes a limitation on error rating. In studies where the authors included drugs that must be manipulated as unlicensed drugs, the proportion of unlicensed drugs was accordingly higher than in our study. We decided not to include such cases, and extracting them from the database was not possible. Therefore, our results may not be directly comparable to these studies.
To our knowledge, this study is the first to describe the prevalence of unlicensed drug use in paediatric general wards in the German-speaking part of Switzerland. Unlicensed drugs are frequently prescribed in paediatric hospital care. Around every tenth drug prescription on general wards in the University Children’s Hospital Zurich is unlicensed. Imported and formula drugs each account for around half of the unlicensed prescriptions. Oral liquid solutions were the most frequently prescribed unlicensed drug form. Prescribing errors occurred significantly more often with unlicensed drugs than with licensed drugs, and formula drugs had the highest rate of prescribing errors compared to imported licensed drugs. Given increasing drug shortages, leading to the fact that more licensed drugs must be replaced by unlicensed drugs for short- or long-term treatment, our findings highlight the risk that unlicensed drugs carry. Future efforts by politicians and pharmaceutical companies should be made to ensure that more drugs suitable for children are licensed in Switzerland.
We thank Beat Bangerter from the University Children’s Hospital Zurich for his technical support in the creation and use of the database.
Author’s contributions: Principal investigator and supervision of the thesis of AS: AC – Conception, design, and methods: AC, AS – Data collection: AS – Medication review: AS (first rater), MP (second rater) – Data analysis and initial manuscript: AS – Review, editing and final approval of the manuscript: AC, AS, MP, CM.
This study was not funded, but the main study [21] was funded by the GSASA (Swiss Association of Public Health Administration and Hospital Pharmacists) grant for the scientific project of national reach 2014.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Federal Act on Medicinal Products and Medicinal Devices, 812.21 (2000).
2. Aronson JK, Ferner RE. Unlicensed and off-label uses of medicines: definitions and clarification of terminology. Br J Clin Pharmacol. 2017;83(12):2615-25. Epub 20170926. doi: . PubMed PMID: 28779556; PubMed Central PMCID: PMC5698582.
3. Neubert A, Wong IC, Bonifazi A, Catapano M, Felisi M, Baiardi P, et al. Defining off-label and unlicensed use of medicines for children: results of a Delphi survey. Pharmacol Res. 2008;58(5-6):316-22. Epub 20080918. doi: . PubMed PMID: 18852048.
4. Gore R, Chugh PK, Tripathi CD, Lhamo Y, Gautam S. Pediatric Off-Label and Unlicensed Drug Use and Its Implications. Curr Clin Pharmacol. 2017;12(1):18–25.
5. Shuib W, Wu XY, Xiao F. Extent, reasons and consequences of off-labeled and unlicensed drug prescription in hospitalized children: a narrative review. World journal of pediatrics : WJP. 2021;17(4):341-54. Epub 20210602. doi: . PubMed PMID: 34080130.
6. Camacho Arroyo MT, Rivas Paterna AB, Meneses Monroy A, Cabrera García L, Blázquez González P, Mancebo Salas N, et al. Off-label and unlicensed drug use in a pediatric intensive care unit of a tertiary care Spanish hospital. A descriptive study. Arch Argent Pediatr. 2023;121(1):e202102550. Epub 20221006. doi: . PubMed PMID: 36194689.
7. Moulis F, Durrieu G, Lapeyre-Mestre M. Off-label and unlicensed drug use in children population. Therapie. 2018;73(2):135-49. Epub 20180216. doi: . PubMed PMID: 29580614.
8. Teigen A, Wang S, Truong BT, Bjerknes K. Off-label and unlicensed medicines to hospitalised children in Norway. J Pharm Pharmacol. 2017;69(4):432-8. Epub 20160623. doi: . PubMed PMID: 27334565; PubMed Central PMCID: PMC5396330.
9. Di Paolo ER, Stoetter H, Cotting J, Frey P, Gehri M, Beck-Popovic M, et al. Unlicensed and off-label drug use in a Swiss paediatric university hospital. Swiss Med Wkly. 2006 Apr;136(13-14):218–22.
10. Vieira I, Sousa JJ, Vitorino C. Paediatric Medicines - Regulatory Drivers, Restraints, Opportunities and Challenges. J Pharm Sci. 2021 Apr;110(4):1545–56.
11. Miljković N, Batista A, Polidori P, Kohl S, Horák P. Results of EAHP's 2019 Medicines Shortages Survey. Eur J Hosp Pharm. 2020;27(4):202-8. Epub 20200529. doi: . PubMed PMID: 32471816; PubMed Central PMCID: PMC7335625.
12. Blankart KE, Felder S. Do Medicine Shortages Reduce Access and Increase Pharmaceutical Expenditure? A Retrospective Analysis of Switzerland 2015-2020. Value Health. 2022 Jul;25(7):1124–32.
13. Agency EM. Evidence of harm from off-label or unlicensed medicines in children. London: EMA; 2004.
14. Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999 Sep;88(9):965–8. 10.1111/j.1651-2227.1999.tb00191.x
15. Conroy S. Association between licence status and medication errors. Arch Dis Child. 2011;96(3):305-6. Epub 20101203. doi: . PubMed PMID: 21131639.
16. Bellis JR, Kirkham JJ, Thiesen S, Conroy EJ, Bracken LE, Mannix HL, et al. Adverse drug reactions and off-label and unlicensed medicines in children: a nested case-control study of inpatients in a pediatric hospital. BMC Med. 2013;11:238. Epub 20131107. doi: . PubMed PMID: 24229060; PubMed Central PMCID: PMC4231613.
17. Schrier L, Hadjipanayis A, Stiris T, Ross-Russell RI, Valiulis A, Turner MA, et al. Off-label use of medicines in neonates, infants, children, and adolescents: a joint policy statement by the European Academy of Paediatrics and the European society for Developmental Perinatal and Pediatric Pharmacology. Eur J Pediatr. 2020;179(5):839-47. Epub 20200103. doi: . PubMed PMID: 31897842.
18. Bonati M, Jacqz-Aigrain E, Choonara I. Licensed medicines, off-label use or evidence-based. Which is most important? Arch Dis Child. 2017;102(1):53-4. Epub 20160817. doi: . PubMed PMID: 27535476.
19. Tilen R, Panis D, Aeschbacher S, Sabine T, Meyer Zu Schwabedissen HE, Berger C. Development of the Swiss Database for dosing medicinal products in pediatrics. Eur J Pediatr. 2022;181(3):1221-31. Epub 20211105. doi: . PubMed PMID: 34739591; PubMed Central PMCID: PMC8897330.
20. Higi L, Käser K, Wälti M, Grotzer M, Vonbach P. Description of a clinical decision support tool with integrated dose calculator for paediatrics. Eur J Pediatr. 2021;•••:
21. Satir AN, Pfiffner M, Meier CR, Caduff Good A. Prescribing errors in children: what is the impact of a computerized physician order entry? Eur J Pediatr. 2023 Jun;182(6):2567–75.
22. EMA. ICH E11 (R1) guideline on clinical investigation of medicinal products in the paediatric population 2017 [cited 2022 25.11.2022]. international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-1.pdf]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e11r1-guideline-clinical-investigation-medicinal-products-pediatric-population-revision-1_en.pdf
23. Association PCNE. The PCNE CLassification V 9.1: Pharmaceutical Care Network Europe; 2020 [cited 2022 20.06.2022]. Classification for Drug related problems].
24. Forrey RA, Pedersen CA, Schneider PJ. Interrater agreement with a standard scheme for classifying medication errors. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2007;64(2):8. doi: . PubMed PMID: 17215468.
25. Kaisto H, Sepponen K, Lindell-Osuagwu L, Sankilampi U. Off-label and unlicensed medicine prescribing in university hospital paediatric wards in Finland: A prospective study. Br J Clin Pharmacol. 2023;n/a(n/a). Epub 20230330. doi: . PubMed PMID: 36998116.
26. Organization WH. Medication safety in high-risk situations. World Health Organization; 2019.