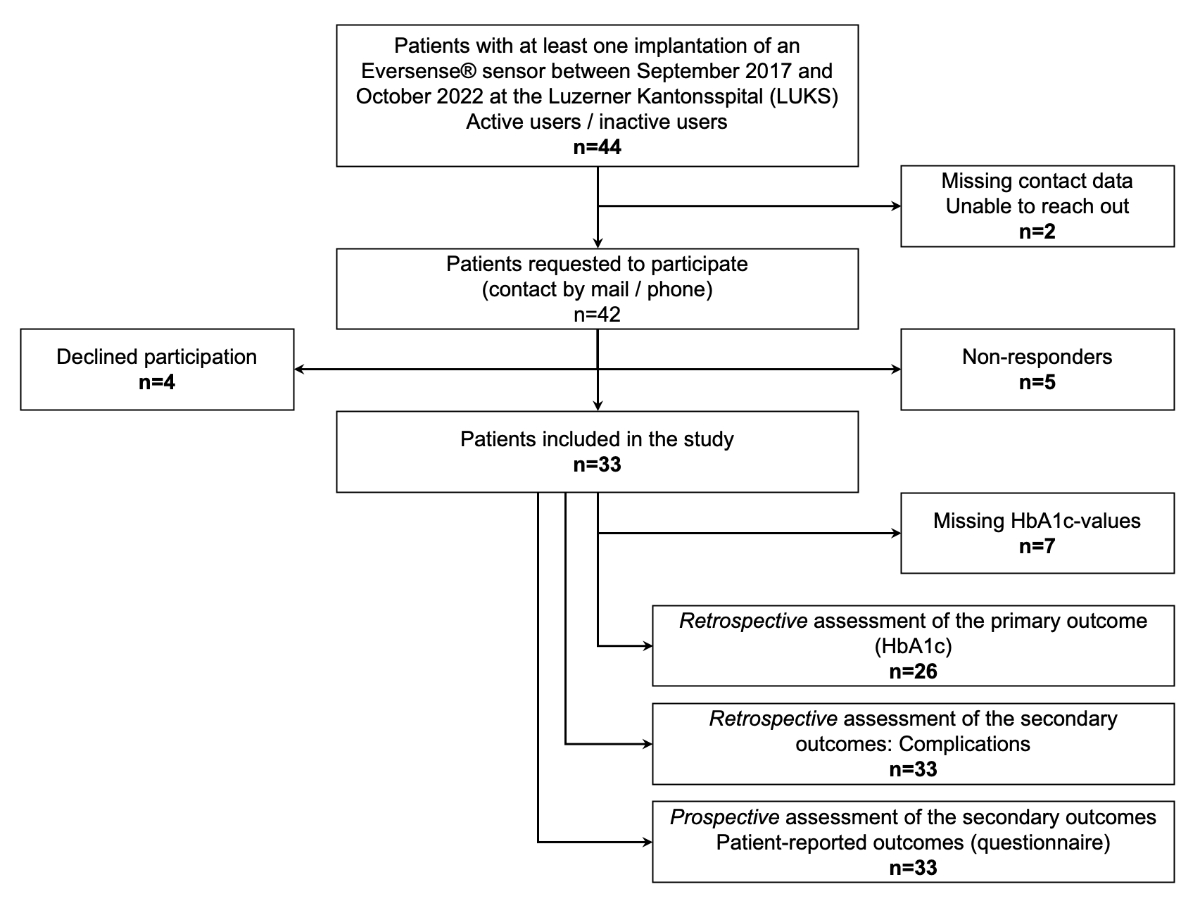
Figure 1Study flow chart.
DOI: https://doi.org/https://doi.org/10.57187/s.3366
To avoid and reduce diabetic complications and glucose fluctuations (hypo- and hyperglycaemia) persons with diabetes mellitus type 1 depend on close monitoring of their glucose values. One of the main goals of diabetes treatment is to keep glucose values in a defined range throughout the day (often defined as “time in range”; TIR; 3.9–10.0 mmol/l). Glycaemic control of a patient is assessed by HbA1c, which provides information about the average blood glucose concentration for the past 2–3 months and correlates closely with the risk of developing diabetic complications [1, 2]. However, HbA1c levels are influenced by high and low glucose values, and glucose excursions throughout the day are insufficiently reflected by HbA1c measurements only (e.g. false-low HbA1c in case of recurrent hypoglycaemia) [3]. Continuous glucose monitoring systems (CGMSs) and flash glucose monitoring systems (FGMSs) have become a mainstay in the monitoring of glucose levels in type-1 diabetics. They allow dynamic monitoring of glucose values and are more and more integrated into automated insulin delivery systems (hybrid closed loop [HCL] systems). Studies have shown that CGMS and FGMS devices can improve glycaemic control and reduce hypoglycaemia in patients with diabetes mellitus [4–8].
The Eversense® CGM System is the first and only continuous glucose monitoring system that uses a small sensor that is fully implanted in the subcutaneous tissue. The system was approved in Europe in 2017 and consists of several generations with different sensor systems: the Eversense® system (lasting 90 days), the Eversense XL® system (lasting 180 days) and the newest Eversense E3® system (lasting 180 days, with reduced frequency of calibration and approved for treatment decisions). The glucose sensor measures glucose levels in the interstitial fluid, which are then transferred to a transmitter attached to the skin. The transmitter calculates the glucose data, sending them to an app on a mobile device. Several pivotal studies have shown the safety and accuracy of the Eversense® system and its effectiveness in improving glycaemic control [9–11].
This study aimed to investigate the effectiveness and safety of the Eversense® CGM System at a large diabetes centre (Luzerner Kantonsspital; LUKS) in terms of HbA1c change and safety issues in a clinical setting with a prolonged follow-up. Compared to other CGMSs and FGMSs, the Eversense® system initially requires more effort to become operational (a minor surgical procedure for implantation of the sensor every 6 months). Therefore, a special interest in this study relied on patient-reported outcomes assessed with a study-specific questionnaire.
This was a prospective and retrospective observational study. It was conducted from January 2022 to October 2022 at the LUKS. The participants were patients with type 1 diabetes mellitus for whom the Eversense® System was prescribed and inserted by physicians at the LUKS.
Since the start of the Eversense® System at our institution in 2017, every patient who underwent a sensor implantation was registered on a list that provided the basic data to define the project population. This list recorded the start date (first sensor implantation), stop date (if applicable), the precise date of every sensor change and the interval of change for each patient. This allowed for identifying patients who had premature sensor dysfunction (cf. below). We included all patients who had at least one sensor implantation between September 2017 and October 2022, irrespective of the status “active user” (still using the system) or “inactive user” (stopped using the system). All these patients were contacted by mail and, if necessary, by phone. The informed consent and the questionnaire were sent by mail with an attached reply envelope. If patients consented to participate, they could send back the informed consent form and completed questionnaire. Only patients who refused to participate in the study were excluded from the project.
The exact study data flow is depicted in figure 1.

Figure 1Study flow chart.
The Eversense® CGM System consists of a glucose sensor that is fully implanted in the subcutaneous tissue and measures glucose levels in the interstitial fluid. The measurement is performed by a fluorescent hydrogel-based copolymer matrix that is grafted to the outside of the encasement and increases its fluorescence intensity by binding glucose. Changes in glucose concentrations result in changes in fluorescence intensity, which can be detected by the sensor’s optical system [12]. This light signal then is captured by a removable transmitter, which is worn externally on the skin directly over the sensor and provides on-body vibration alerts. Within the transmitter, the signal is converted into a glucose reading and transmitted to a specific app (Eversense® Mobile App) on a compatible mobile device (smartphone) every 5 minutes. The app allows patients to check their glucose levels, including the trend (rising or falling), and receive notifications or alerts based on the glucose settings [13]. The system needs regular calibration with capillary blood glucose measurement (usually once per day).
When the sensor is implanted, the desired sensor location is first drawn on the patient’s upper arm. The area is anaesthetised with lidocaine, and a skin incision of 5 mm is made, followed by tunneling with a dissector. The prepared and pre-hydrated sensor is then inserted with the appropriate instrument 3–5 mm below the skin surface into the subcutaneous fat tissue. Minor bleeding is stopped by compression and then the wound is closed with skin closure strips (Steri-Strips®). The transmitter is positioned using the placement aid and connected to the sensor. For the explantation or change procedure, the sensor position is palpated or sonographically displayed. After local anaesthesia and skin incision, the sensor is first bluntly exposed with a clamp and then sharply dissected with a surgical scissor. The sensor is subsequently removed. The new sensor is inserted on the ipsi- or contralateral arm as described above. In case of a reimplanted ipsilateral side, a new incision is made, which must be at least 2–3 cm from the older incision.
Initially, sensor changes were necessary every 90 days. After the approval of the Eversense® XL and Eversense® E3 systems, re-implantation was due every 180 days. To guarantee quality and reduce operator variability, sensor implantation and removal were performed exclusively by two company-certified physicians at the LUKS (LB and SF).
Primary outcome: change in HbA1c levels
The primary endpoint was the change in HbA1c levels from the baseline (before implantation of the sensor) to 6 ± 2 and 12 ± 2 months and the last follow-up (newest available value) after implantation. This data was collected retrospectively from the electronic health records information system of the LUKS. Missing data records were obtained from the patients’ diabetologists and GPs. For the analysis of the HbA1c values, we included only patients who had at least three different HbA1c measurements (including the mandatory baseline value).
HbA1c was measured by turbidimetric inhibition immunoassay (TINIA; Roche cobas®, Roche Diagnostics, Rotkreuz, Switzerland). HbA1c was also measured using a DCA Vantage® POCT analysis system (Siemens Healthineers, Erlangen, Germany) and a Roche cobas b 101® POCT analysis system (Roche Diagnostics, Rotkreuz, Switzerland). External HbA1c (performed at some GP offices, different systems) values were also used for this study. All values were standardised to the National Glycohemoglobin Standardization Program and Diabetes Control and Complications Trial, which allowed for a comparison between the different measurements.
Secondary outcomes: complications
We assessed the following secondary outcomes retrospectively: number of premature sensor breakdowns and adverse events related to the implantation procedure: infection, major bleeding, and major difficulties with implantation or explantation.
Premature sensor breakdown was defined as a sensor dysfunction before the predicted expiry date (<90 days with the Eversense® system and <180 days with the Eversense® XL/E3 system). Apart from the incidence, the time interval between implantation and breakdown (in months) and the year of implantation of the dysfunctional device was recorded.
Infection was defined by clinical symptoms and signs (pain, purulent secretion at the implantation site) and the need for sensor removal and drainage. Major bleeding was defined as an event necessitating surgical intervention to stop haemorrhage. Major difficulties with implantation or explantation were respectively defined as events where the sensor could not be placed in the tissue or the extraction procedure was complicated by sensor dislocation and the need to widen the primary incision.
Secondary outcomes: patient-reported outcomes
A specific questionnaire was designed to prospectively assess patient-related outcomes with the Eversense® System. It contained a total of 12 questions about general handling, satisfaction and possible complications with the system. It also included three specific questions about the effects of wearing the Eversense® sensor on the patient’s frequency and awareness of hypoglycaemia.
This study used descriptive statistics. To compare HbA1c values (baseline vs 6 or 12 months after insertion or last follow-up), a Wilcoxon matched-pairs signed-rank test was used. A p-value of ≤0.05 was considered statistically significant. All tests were performed using GraphPad Prism (Version 10.0.3, GraphPad Software, Boston, MA, USA).
The study was performed following the Declaration of Helsinki and was approved by the ethics committee Ethikkommision Nordwest- und Zentralschweiz (Project ID: 2021-02514). Informed consent was obtained from all study subjects.
A total of 33 patients gave informed consent and completed the questionnaire (return rate 79%). Seven patients had missing HbA1c values. The median age of participants was 47 years (IQR 34.5–54.5), the median diabetes duration was 18.8 years (12.2–23.8), and the median time between the baseline (before first sensor implantation) and last follow-up HbA1c was 50 months (22.3–58.5). Other baseline characteristics are given in table 1.
Table 1Baseline characteristics (n = 33 patients). For categorical variables: n (%), for continuous variables: median (IQR).
| Variable | Value | |
| Age (years) | 47 (34.5–54.5) | |
| Sex | Male | 29 (88) |
| Female | 4 (12) | |
| Diabetes duration (years) | 18.8 (12.2–23.8) | |
| Body Mass Index (kg/m2) | 27.6 (24.0–28.6) | |
| Insulin delivery | MDI | 23 (70) |
| CSII | 10 (30) | |
| Measurement of blood glucose before | CGMS (i.e, Dexcom®, Medtronic Guardian®) | 6 (18) |
| FGMS (i.e., FreeStyle® libre 1/2) | 6 (18) | |
| Capillary | 20 (60) | |
| Unknown | 1 (3) | |
| Diabetes complications | Cardiovascular* | 2 (6) |
| Retinopathy** | 5 (15) | |
| Nephropathy | 2 (6) | |
| Neuropathy*** | 2 (6) | |
CGMS: continuous glucose monitoring system; FGMS: flash glucose monitoring system; CSII: continuous subcutaneous insulin infusion; MDI: multiple daily injections.
* Macroangiopathy (i.e., coronary artery disease)
** Retinopathy: non-proliferative or proliferative defined by fundoscopy
*** Neuropathy: defined by clinical signs (e.g. pallhypaesthesia)
The time course of the individual HbA1c values is displayed in table 2. With a median HbA1c of 7.55%, the patients presented at the baseline with good diabetes control but considerable interindividual variation. Compared to the baseline, HbA1c values changed by –0.25%, –0.45 and –0.2 after 6 and 12 months and at last-follow-up, respectively (p = 0.278, 0.308 and 0.296). Considering the questionnaire responses, 23/33 (70%) patients rated their HbA1c value as improved by using the device, whereas 10/33 (30%) patients reported no change in HbA1c. No patient reported worsening of the HbA1c value.
Table 2Primary outcome: course of HbA1c values (n = 26 patients).
| Baseline (before sensor implantation) | After 6 ± 2 months | After 12 ± 2 months | At last follow-up | |
| HbA1c (%) (IQR) | 7.55 (7.00–8.40) | 7.30 (6.80–8.10) | 7.10 (6.70–7.90) | 7.35 (6.98–7.73) |
| p-value* | – | 0.278 | 0.308 | 0.296 |
| Median of differences* (95% CI) | – | –0.100 (–0.600–0.300) | –0.200 (–0.600–0.200) | –0.050 (–0.600–0.300) |
| Missing data | 0 | 1 | 3 | 0 |
* Compared to baseline.
In total, 178 sensor implantations were performed. Insertion and replacement of the sensors were associated with a low rate of complications (table 3). We recorded 16 (9%) premature sensor breakdowns; dysfunction happened after a median of 4 months (3.0–4.75) and was restricted to the years 2019 and 2020. No sensor dysfunctions were registered after 2020. One case of late-onset sensor infection occurred 5 months after implantation, which required surgical excision and drainage. No major bleeding events occurred, even in patients with inhibitors of platelet aggregation or anticoagulation. The most common problems were difficulties in sensor location and removal due to adhesions and sometimes sensor dislocation. In some cases, this led to long explantation procedures (>60 min.). Major difficulties with sensor removal necessitating extension of the primary incision and wound closure with stitches were recorded in four cases.
Table 3Secondary outcomes: complications (in total: 178 sensor removals).
| Type of complication | n (%) |
| Premature sensor breakdown | 16 (9) |
| Infection | 1 (0.6) |
| Major bleeding | 0 |
| Complicated removal | 4 (2) |
However, with the implementation of adapted dissectors, the use of ultrasound for localisation and the increasing experience of the operators, the rate of complicated removals was minimised. The use of the newest generation of sensors (E3) was also associated with a clear reduction in the frequency of sensor malfunction.
The following figures represent the other results of the questionnaire. A total of 33 patients returned a questionnaire, with not all questions always answered.
General aspects of diabetes management (stability of glucose values), general satisfaction with the device, hypoglycaemia management
Most patients found that their diabetes management had improved by using the Eversense® System and estimated that their glucose values were more stable. More than half of the patients (17/33, 52%) were generally satisfied with the device (figure 2).
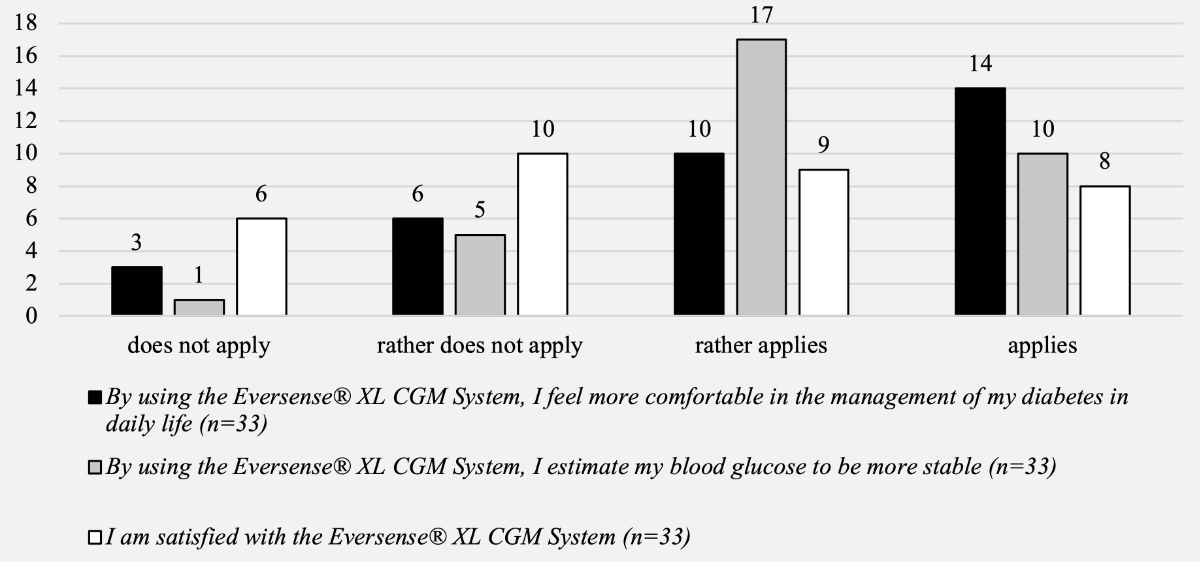
Figure 2Patient-reported outcomes – general aspects of diabetes management and satisfaction with the system.
Furthermore, most patients reported feeling more confident about the management of hypoglycaemia, having a better perception of hypoglycaemia, and reduced occurrence of hypoglycaemia (figures 3 and 4).
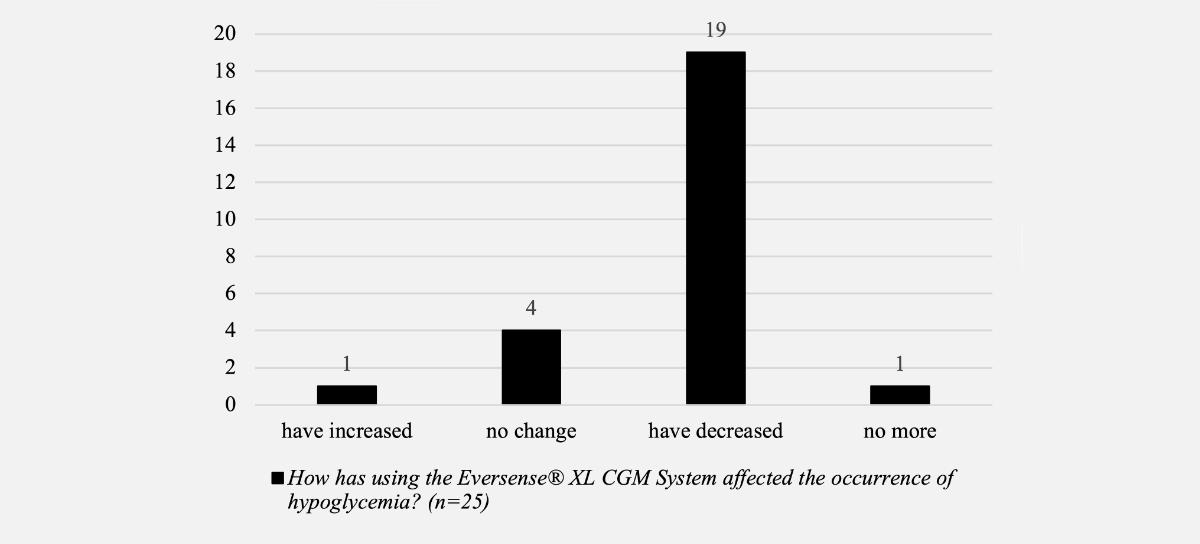
Figure 3Patient-reported outcomes – occurrence of hypoglycaemia.
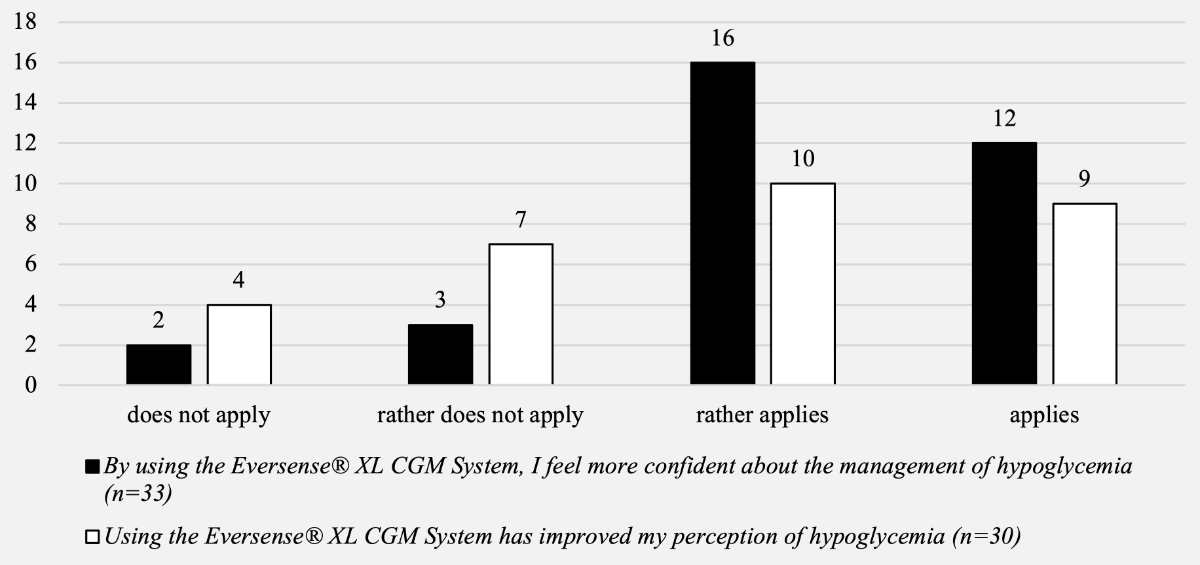
Figure 4Patient-reported outcomes – questions about confidence in hypoglycaemia management and perception of hypoglycaemia.
Aspects of handling the device (transmitter, mobile app, wearing the transmitter), insertion and removal
Questions focused on the handling of the device showed that on average, patients only had slight to no difficulties with the Eversense®Smart Transmitter (84%) and the Eversense® CGM Mobile App (94%; figure 5). Of the patients, 16/31 (52%) found wearing the sensor a slight disturbance, whereas 7/31 patients (23%) found it not disturbing at all (figure 6).
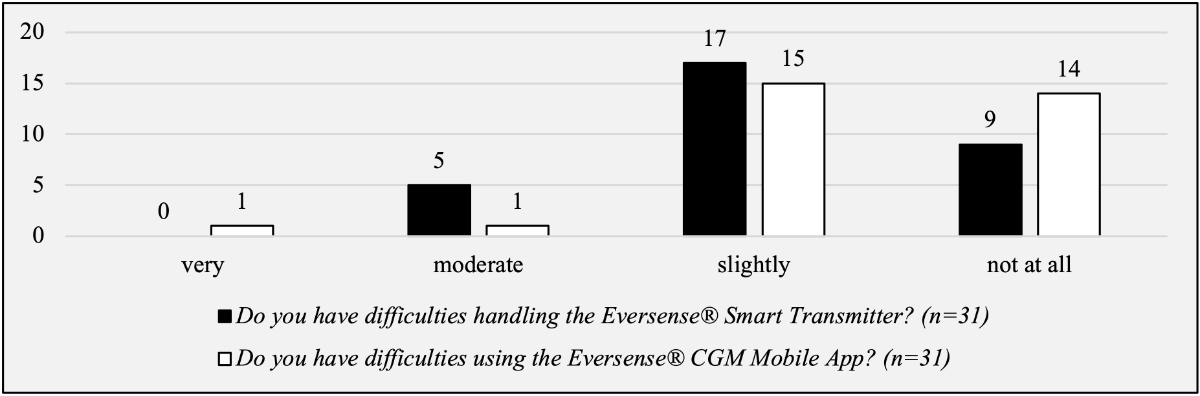
Figure 5Patient-reported outcomes – questions about the handling of the device.
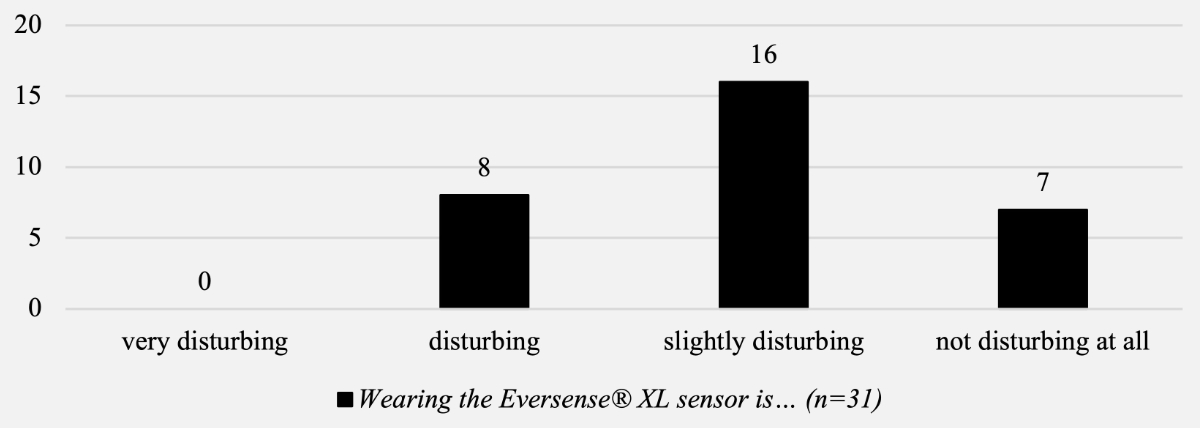
Figure 6Patient-reported outcomes – question regarding the wearing of the device.
Most patients felt that the replacement procedure for the Eversense®sensor was a burden for them. Eight (26%) felt that the change was a great burden for them, and only six (19%) felt that the change was not a burden for them at all (figure 7).
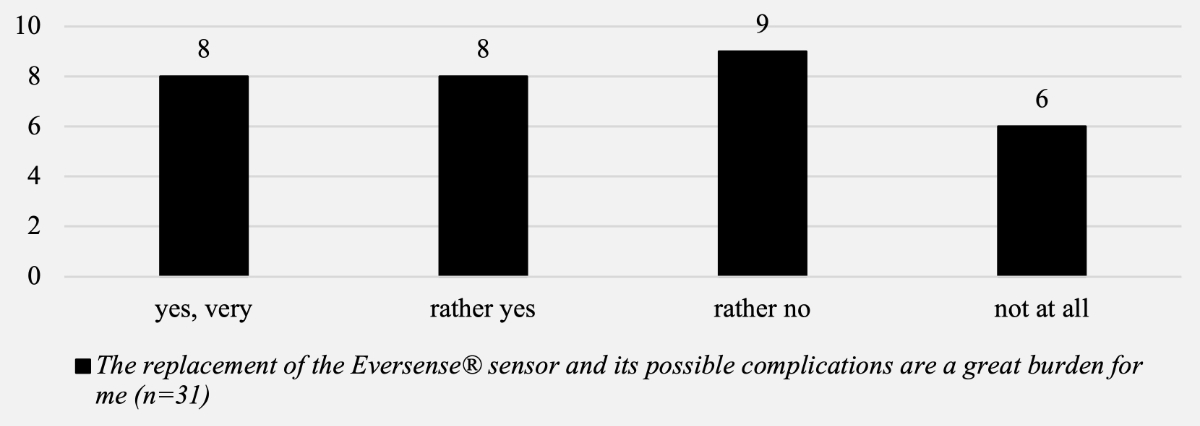
Figure 7Patient-reported outcomes – question about sensor replacement.
Table 4 shows the complications, difficulties and negative aspects reported by the patients in the questionnaire. The most frequent issues were technical problems with the sensor (30%), problems with the incision or scar (30%), and the removal procedure (24%).
Table 4Complications and negative aspects of using the Eversense® XL CGM System (n = 33).
| Complication | n (%) |
| None | 6 (18.2) |
| Problems with the sensor (early expired, repeated loss of contact with the transmitter) | 10 (30.3) |
| Problems with the incision/scar (pain, cosmetic aspects) | 10 (30.3) |
| Sensor removal is difficult/a burden | 8 (24.2) |
| Discrepancy between capillary glucose measurement and Eversense® measurement | 6 (18.2) |
| Vibrations disturb during the day/night | 5 (15.2) |
| The transmitter does not stick well to the skin (while sweating/swimming) | 2 (6.1) |
| Skin reactions (redness/rash) | 2 (6.1) |
| Long-lasting pain | 2 (6.1) |
| Loss of perception of the vibration alerts | 2 (6.1) |
| Hypoglycaemia was not detected | 2 (6.1) |
| Sensor impacted in the tissue | 2 (6.1) |
| Sensor dislocation after implantation | 1 (3.0) |
| Too expensive | 1 (3.0) |
| No sharing with other apps | 1 (3.0) |
| Need for abstention from physical activity after implantation | 1 (3.0) |
| Effort is a burden (daily charging/replacement, constant calibration and finger pricks every 12 h) | 1 (3.0) |
This single-centre study performed at a Swiss diabetes outpatient clinic evaluated the effectiveness of a newer implantable CGMS in terms of HbA1c changes, complications and patient-reported outcomes. To the best of our knowledge, this is the first and only single-centre study presenting data in a realistic clinical setting with a follow-up over several years. In this sample of well-controlled patients with type 1 diabetes mellitus, implantation of the Eversense® XL CGM sensor was associated with an HbA1c reduction of around –0.2% at the last follow-up, albeit this was statistically not significant.
The impact of CGMSs on diabetes control is well-established. Previous studies have shown that they significantly reduce HbA1c levels, reduce the time spent in hyper- and hypoglycaemia, reduce the frequency of hypoglycaemia and improve quality of life [14]. Two newer metanalyses on the influence of CGMSs on diabetes reported HbA1c reductions of –0.24% [15] and –0.23% [16]. Several studies have already been conducted on the Eversense® CGM System and its effect on the HbA1c value, showing that it can reduce HbA1c levels by –0.35% [11] and –0.43% [17] in patients with type 1 diabetes mellitus.
Our results are not completely in line with this data. This could be due to various factors. Our sample size was small, and data was collected and analysed retrospectively. Our study also relied on a real-world setting, lacking controlled study modalities but evaluating long-term outcomes (>1 year). HbA1c reduction is more pronounced in patients with HbA1c levels of >8%, as shown in the metanalysis of Teo et al. [16]. Our patients showed a baseline level below 8%, which could be a contributing factor to the weaker effect of implanting an Eversense® sensor.
However, a recently published randomised and prospective study [18] with the Eversense® XL CGM System in nearly 150 patients with an HbA1c of >8% compared the effect of the system between patients with implanted and activated sensors (enabled group) and implanted but inactivated sensors (control group). After 6 months, the HbA1c was 0.1% lower in the enabled group, supporting our findings.
The second part of the study investigated patient-reported outcomes with a questionnaire specifically developed for this investigation. The questionnaire showed that the majority of patients reported subjectively better HbA1c levels, and more stable glucose values throughout the day and that the Eversense® CGM System led to a better management of the disease. Additionally, patients had a better perception of hypoglycaemia, had fewer hypoglycaemic events and felt more confident in managing hypoglycaemia when using the CGMS. This agrees with other data regarding the quality of life of CGMS users [14].
From a technical viewpoint, most patients stated that they had only slight to no difficulties using the Eversense® Smart Transmitter or the Eversense® CGM Mobile App, which indicates the convenience and usability of the system. Frequently mentioned negative aspects related to technical errors (premature sensor dysfunction) and issues with the procedure (difficult removal). In nearly 200 implantation procedures, we have experienced a low rate of complications over the past 5 years. As reported by the patients and in our experience, most problems with the systems were related to a reduced lifespan of the sensor and difficulties with the explantation of the sensors. However, importantly, those technical problems and difficulties with the sensor removal decreased dramatically over the past 2 years due to several factors (training of operators, new and adapted implantation procedures, use of ultrasound for localisation, implementation of the newest generation of sensors). Our experiences are consistent with the newest published data [19]. Today, the insertion and removal of Eversense® sensors represents a simple and rapid procedure, and a lifespan of 180 days is achieved in all cases.
In our cohort, 19 of the 33 participants (57.6%) have currently stopped wearing the Eversense® CGM System. The reasons for stopping or changing the CGM system included the availability of newer and smaller sensors, pre-calibrated sensors and the wish for an HCL insulin delivery system (which is not possible with the Eversense® system).
However, this research has several limitations. The study population was small compared to similar studies, which could undermine the validity of the study, and the data were assessed retrospectively. Another limitation is the lack of an adequate control group, which would give us more certainty that the changes in HbA1c were caused by using the Eversense® XL CGM System. The follow-up in this project covers several years, in which the system underwent several adaptions (e.g. introduction of new sensor generations); this could lead to bias regarding the outcomes.
The questionnaire we created contained only a few questions and showed only a rough outline of patient satisfaction. Therefore, a more detailed discussion of satisfaction is difficult. However, this information can serve as an indication of what aspects patients could be questioned about in more detail.
Finally, no objective conclusion can be drawn on the impact of the Eversense® CGM System on absolute hypoglycaemia rate, TIR, time-above-range or time-below-range since these parameters were not assessed due to a low rate of CGMS and FGMS users at the baseline.
The results of this study can be helpful as assistance in counselling patients with type 1 diabetes mellitus. They can support patients in their decisions about their optimal therapy. The results of the questionnaire can be used to educate patients about the strengths and weaknesses of the Eversense® CGM System and their frequency.
This observational study demonstrated a numerical but non-significant reduction in HbA1c by using the Eversense® XL CGM System in patients with type 1 diabetes mellitus. Patient-reported outcomes with the Eversense®CGM System showed a subjective positive impact on hypoglycaemia rates, greater confidence in managing hypoglycaemia and diabetes in general, and easy handling of the transmitter and mobile app. Technical issues (early sensor breakdown and difficult sensor removal) must be considered but are now very rare, as were major complications following implantation.
We thank all patients that participated in our study and the team of diabetes nurses at the Luzerner Kantonsspital that assisted all implantations and removals in the past five years with great commitment.
There was no funding of this study.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. The institution of SF received in the past speakers’ honoraria from Roche Diagnostics and Ascensia Diabetes Care. No other potential conflict of interest related to the content of this manuscript was disclosed.
1 Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000 Feb;342(6):381–9. 10.1056/NEJM200002103420603
2 Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec;353(25):2643–53. 10.1056/NEJMoa052187
3 Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A View Beyond HbA1c: Role of Continuous Glucose Monitoring. Diabetes Ther. 2019 Jun;10(3):853–63. 10.1007/s13300-019-0619-1
4 Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, et al.; DIAMOND Study Group. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann Intern Med. 2017 Sep;167(6):365–74. 10.7326/M16-2855
5 Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA. 2017 Jan;317(4):379–87. 10.1001/jama.2016.19976
6 Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al.; DIAMOND Study Group. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017 Jan;317(4):371–8. 10.1001/jama.2016.19975
7 Benkhadra K, Alahdab F, Tamhane S, Wang Z, Prokop LJ, Hirsch IB, et al. Real-time continuous glucose monitoring in type 1 diabetes: a systematic review and individual patient data meta-analysis. Clin Endocrinol (Oxf). 2017 Mar;86(3):354–60. 10.1111/cen.13290
8 Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, et al.; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012 Dec;55(12):3155–62. 10.1007/s00125-012-2708-9
9 Christiansen MP, Klaff LJ, Brazg R, Chang AR, Levy CJ, Lam D, et al. A Prospective Multicenter Evaluation of the Accuracy of a Novel Implanted Continuous Glucose Sensor: PRECISE II. Diabetes Technol Ther. 2018 Mar;20(3):197–206. 10.1089/dia.2017.0142
10 Christiansen MP, Klaff LJ, Bailey TS, Brazg R, Carlson G, Tweden KS. A Prospective Multicenter Evaluation of the Accuracy and Safety of an Implanted Continuous Glucose Sensor: the PRECISION Study. Diabetes Technol Ther. 2019 May;21(5):231–7. 10.1089/dia.2019.0020
11 Kropff J, Choudhary P, Neupane S, Barnard K, Bain SC, Kapitza C, et al. Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter, Pivotal Trial. Diabetes Care. 2017 Jan;40(1):63–8. 10.2337/dc16-1525
12 EV-XL-Broschure-HCP_Deutsch_20220207.pdf [Internet]. [cited 2022 Oct 19]. Available from: https://www.ascensia-diabetes.ch/siteassets/eversense/asset-library/EV-XL-Broschure-HCP_Deutsch_20220207.pdf
13 LBL-1402-50-101_Rev_D_Eversense_User_Guide_mmol_DE-1.pdf [Internet]. [cited 2022 Oct 19]. Available from: https://global.eversensediabetes.com/wp-content/uploads/LBL-1402-50-101_Rev_D_Eversense_User_Guide_mmol_DE-1.pdf
14 Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017 Dec;40(12):1631–40. 10.2337/dc17-1600
15 Dicembrini I, Cosentino C, Monami M, Mannucci E, Pala L. Effects of real-time continuous glucose monitoring in type 1 diabetes: a meta-analysis of randomized controlled trials. Acta Diabetol. 2021 Apr;58(4):401–10. 10.1007/s00592-020-01589-3
16 Teo E, Hassan N, Tam W, Koh S. Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: a systematic review of randomised controlled trials and meta-analysis. Diabetologia. 2022 Apr;65(4):604–19. 10.1007/s00125-021-05648-4
17 Irace C, Cutruzzolà A, Nuzzi A, Assaloni R, Brunato B, Pitocco D, et al. Clinical use of a 180-day implantable glucose sensor improves glycated haemoglobin and time in range in patients with type 1 diabetes. Diabetes Obes Metab. 2020 Jul;22(7):1056–61. 10.1111/dom.13993
18 Renard E, Riveline JP, Hanaire H, Guerci B; on behalf of the investigators of France Adoption Clinical Trial. Reduction of clinically important low glucose excursions with a long-term implantable continuous glucose monitoring system in adults with type 1 diabetes prone to hypoglycaemia: the France Adoption Randomized Clinical Trial. Diabetes Obes Metab. 2022 May;24(5):859–67. 10.1111/dom.14644
19 Garg SK, Liljenquist D, Bode B, Christiansen MP, Bailey TS, Brazg RL, et al. Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: the PROMISE Study. Diabetes Technol Ther. 2022 Feb;24(2):84–92. 10.1089/dia.2021.0182