Exploring the feasibility and safety of laparoscopic
anti-reflux surgery with the new RefluxStop™ device: a retrospective
cohort study of 40 patients
DOI: https://doi.org/https://doi.org/10.57187/s.3365
Yannick Fringelia,
Ioannis Linasb,
Ulf Kesslera,
Joerg Zehetnera
a Department of Visceral Surgery,
Hirslanden Klinik Beau-Site, Bern, Switzerland
b Department of Gastroenterology,
Hirslanden Klinik Beau-Site, Bern, Switzerland
Summary
AIMS OF
THE STUDY: Anti-reflux surgery aims to restore the
anti-reflux barrier and reduce the retrograde flow of stomach contents.
However, traditional surgical techniques generally involve some degree of encircling
of the oesophagus, which can result in adverse effects such as dysphagia and the
inability to belch or vomit. Based on the first published results, a novel surgical
technique – with the RefluxStop™ device – appears promising
for treating gastroesophageal reflux disease (GERD) with minimal postoperative
dysphagia. This study describes the initial clinical experience with this
procedure in a cohort of patients with chronic gastroesophageal reflux disease to
evaluate its feasibility and safety in clinical practice.
METHODS: This
retrospective cohort study examined the first 40
patients who underwent laparoscopic anti-reflux surgery with the RefluxStop™
device at a private hospital in Switzerland. The procedure involves implanting a
nonactive device on the outside of the gastric fundus to stabilise a narrow oesophagogastric
plication. Feasibility was assessed based on the proportion of patients in whom
the device could be successfully implanted, with a discussion of the operative details.
Intraoperative and postoperative complications, adverse effects, and changes in
gastroesophageal reflux disease-related quality of life (GERD-HRQL
questionnaire) are also reported.
RESULTS: Between May 2020 and April 2022, 40 patients underwent elective
surgery for laparoscopic hiatal hernia repair and RefluxStop™ device
implantation. All patients had typical symptoms of gastroesophageal reflux
disease, such as heartburn and regurgitation; 20 (50%) had preoperative
dysphagia. Laparoscopic surgery was feasible in all patients except one who
required laparotomy due to adhesions and associated bleeding when accessing the
abdomen. The median operating time was 57.5 minutes (interquartile range = 51.75–64.25
minutes) with no device-related intraoperative or postoperative complications.
All patients were imaged one day and three months postoperative, confirming the
correct placement of the device. Reflux symptoms (heartburn and acid
regurgitation) were significantly improved in all patients at three months (p <0.0001).
CONCLUSION: These preliminary results support the feasibility and safety of introducing
this novel laparoscopic anti-reflux surgical treatment option in clinical
practice.
Introduction
Gastroesophageal reflux disease (GERD) is a
highly prevalent condition, representing a massive disease burden. Globally,
around one billion individuals suffer from GERD [1], and a 77.5% increase in
prevalence has been reported over recent decades, from 442 million in 1990 to
784 million in 2019 [2], translating to substantial healthcare costs. A recent population-based
survey in the USA found that one in three respondents reported GERD symptoms within
the past week [3].
The retrograde flow of acidic stomach
contents into the oesophagus, and in some cases into the pharynx and
respiratory tract, causes inflammation and damage that results in symptoms.
However, medical treatment does not treat the fundamental underlying
abnormality. Furthermore, proton pump inhibitors, which form the mainstay of
medical treatment, fail to adequately control symptoms in up to half of those
taking them [3].
Anti-reflux surgery, most commonly in the
form of laparoscopic fundoplication, is recommended for chronic gastroesophageal
reflux disease [4] and is often performed when pharmacological approaches such
as proton pump inhibitors are unsuitable or provide incomplete symptom relief [5].
Patients may also prefer the option of a “definitive” treatment over lifelong
medication, although some degree of continued medical treatment may be required.
Surgical adverse effects, primarily dysphagia, bloating, and flatulence, are highly
relevant [6], and efforts to innovate and refine surgical techniques to reduce
their incidence have resulted in methods such as partial fundoplication and magnetic
sphincter augmentation [7]. While early trials showed that magnetic sphincter augmentation
led
to improved outcomes in terms of bloating, postoperative dysphagia remains a
common complaint [8–10].
Therefore, efforts to reduce surgical adverse
effects, particularly postoperative dysphagia, continue. Consequently, a new surgical
implant – the RefluxStop™ device
– was designed for use in laparoscopic anti-reflux
surgery. The initial Conformité
Européenne (CE)-mark study involved 50
patients and reported favourable outcomes at one year, as determined by gastroesophageal
reflux disease health-related quality of life (GERD-HRQL) scores
(86% improvement) and 24 h pH outcomes (normalised in 98% of patients) [11].
Reports following longer follow-up in this ongoing study are awaited.
In 2020, we began to offer this procedure at
our institution in addition to other existing anti-reflux surgical options. We present
our initial experience with this procedure, seeking to answer two key
questions: Would performing this procedure be feasible in all cases? Would the
outcomes support the continued use of this technique in clinical practice?
Methods
Study design
This retrospective cohort study examined the
first 40 patients with documented typical gastroesophageal reflux disease
symptoms who underwent laparoscopic anti-reflux surgery with the RefluxStop™ device
(Implantica, Zug, Switzerland) in a private hospital setting in Switzerland. The
data was collected from their medical records.
Surgery was performed by a single surgeon
(JZ) between May 2020 and April 2022. RefluxStop surgery was offered to
patients whom the surgeon judged suitable candidates based on the investigations
detailed below and accounting for the patient’s situation (figure 1). The patients
were informed about the procedure and availability of limited data [11] before
obtaining consent. This study was ethically approved by the Institutional Review
Board of the University of Bern, Switzerland (approval no. 2018-01827) and conducted
according to the Declaration of Helsinki. The costs of the surgery, including
the device, were covered by the patient’s insurance.
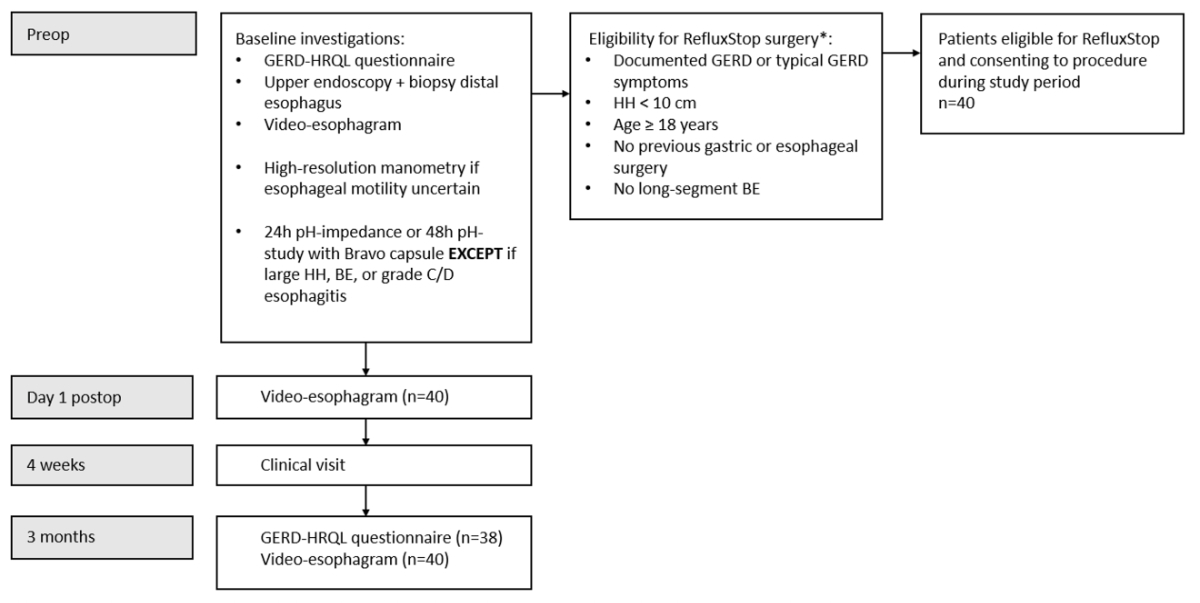
Figure 1Flowchart of the eligibility criteria for
surgery and follow-up.
* Other surgical options available at this institution
include complete or partial fundoplication and magnetic sphincter augmentation.
GERD: gastroesophageal reflux disease; HH: hiatal hernia; BE: Barrett’s oesophagus.
Patient selection
This analysis included all patients who underwent
surgery with the RefluxStop™ device at our institution between the above dates.
To be eligible for surgery, patients must have documented gastroesophageal
reflux disease or typical symptoms of gastroesophageal reflux disease and be
aged >18 years. Patients with hiatal hernia >10 cm, long-segment
Barrett’s oesophagus, or a history of oesophageal or gastric surgery were ineligible
for this surgery (figure 1).
Preoperative assessments
The preoperative work-up consisted of an upper
endoscopy with biopsies of the distal oesophagus, a standardised history and
physical examination, and a standardised questionnaire for reflux disease
(GERD-HRQL, 0–75 points plus an additional quality-of-life question [12]); see figure
S1 in the Appendix for the original questionnaire used. Oesophageal
motility was assessed by video oesophagram under fluoroscopy, with the contrast-enhanced
liquid medium swallowed in upright and supine positions according to a standardised
protocol [13]. In selected patients where the assessment of oesophageal
motility was inconclusive, high-resolution manometry was performed at a specialised
reflux centre. All patients, except those with a large hiatal hernia, Barrett’s oesophagus,
or
reflux oesophagitis Grade C or D according to the Los Angeles classification [14],
underwent either a 24 h pH-impedance study or a 48 h pH-study with the Bravo
capsule for confirmation and to assess the severity of reflux disease.
Surgical procedure and RefluxStop™ device
implantation
The main principles of the RefluxStop
technique are that it maintains the lower oesophageal sphincter (LES) in an
intra-abdominal position, recreates an acute angle of His (often flattened due
to a hiatal hernia), and avoids encircling the oesophagus; a 90–110° attachment
of the stomach to the oesophagus is formed instead of a 360° or
270° wrap. The device is sutured into a pocket created on the gastric
fundus to stabilise the fundus and avoid reherniation of the lower oesophageal
sphincter. Additional technical details on implanting the RefluxStop™
device, including the importance of its positioning relative to the lower oesophageal
sphincter, have been previously described [11].
The operating room
set-up for RefluxStop surgery is similar to other laparoscopic anti-reflux
surgery. After creating a pneumoperitoneum in the left
upper quadrant, trocars are placed in the typical laparoscopic anti-reflux
surgery positions. With an Optiview trocar, the camera is introduced about 5–7 cm
above the umbilicus, paramedian to the left. A 10-mm trocar is placed in the
left upper quadrant, and a 5-mm trocar in the right upper quadrant and left
flank. An epigastric access is created for the Nathanson liver retractor, held
by the iron intern, to elevate the left lobe of the liver.
After opening the pars flaccida with a
harmonic scalpel, the right crus is identified, and the oesophagus is visualised.
The anterior aspect of the oesophagus is dissected with caution to preserve the
anterior vagus nerve, and the top of the left crus is identified. The short
gastric vessels are taken down, and the complete left crus is visualised and freed
from adhesions. An 18 cm long easy-flow
drainage tube is placed around the distal oesophagus for retraction. Mediastinal
dissection of the distal oesophagus is performed, preserving the vagal nerves, and
if present, the hiatal hernia is reduced, and the hernia sac is resected. With
sufficient dissection, an intra-abdominal length of at least 4.5 cm should be
achieved with only slight traction on the oesophagus, providing a maximum 1.5 cm
downward movement of the angle of His. A hiatal hernia closure is
performed with 2 to 3 figure-of-eight sutures with Gore Sutures (Gore Inc.,
Sedona, AZ, USA), avoiding compression of the oesophagus. In cases with
excessive fat pad at the angle of His, further resection of the fat pad is
performed.
Then, the angle of His is recreated using two
rows of sutures with V-loc (Medtronic Inc., Dublin, Ireland) non-resorbable 3–0,
creating an oesophagogastric plication. The first row of sutures is placed to approximate
the oesophagus and the gastric fundus, starting at the angle of His and working
caudocranially until about 4 cm of the distal oesophagus and the fundus are
joined. A slight tension on the oesophagus during this plication moves the angle
of His downward by no more than 1.5 cm to allow the surgeon to reach higher up the
oesophagus. The second row of sutures is placed 1–1.5 cm anteriorly to the
first suture row, taking care to avoid creating folds or kinks. Then, a single
Gore suture is placed to secure the fundus at the top end between the two
suture lines.
After switching out the 5-mm port in the
left flank with a 22-mm reusable port, the prepared RefluxStop™ device is introduced
with a dedicated deployment tool (Implantica, Zug, Switzerland). Next to the
suture line and parallel to the oesophagus, the device is gently placed at the
top of the fundus without tension into a fundic pocket. With one suture row
from cranial to caudal and a second suture row from caudal to cranial, taking
care to avoid narrowing or kinking of the oesophagus, the RefluxStop™ device is
secured in position at the top of the fundus next to the oesophagogastric
suture line. A safe intra-abdominal position of the RefluxStop™ device is thus
achieved without tension on the easy-flow drainage tube. The deployment tool and the
easy-flow drainage tube
are then removed.
Postoperative follow-up
The follow-up of the patients on
postoperative day 1 included a video oesophagram. The patients were on a
blended soft diet for the first week postoperative, followed by a soft diet for
3–4 weeks. Hospital visits with history and physical examination were conducted
four weeks and three months after the procedure. The patients completed a standardised
reflux questionnaire (GERD-HRQL) preoperative and three months postoperative. A
second video oesophagram was performed three months postoperative. Reflux
symptoms and dysphagia were recorded at each hospital visit. Surgical
complications were documented according to the Dindo-Clavien classification [15].
Study outcomes
The primary outcome, relating to
feasibility, was the proportion of patients who could undergo the surgery with
the device positioned correctly. The device position was determined in line
with the manufacturer’s instructions for use, which consider the position of its
top relative to the upper edge of the lower oesophageal sphincter. The device’s
position was considered optimal if its top was >1 times its size
above the upper edge of the lower oesophageal sphincter, acceptable if 0.5–1
times above the upper edge of the LES, failure risk if 0–0.5 times above
the upper edge of the LES and unacceptable if its position was entirely below
the upper edge of the LES (see figure S2 in the Appendix).
The secondary outcomes included intraoperative
complications and postoperative complications within 90 days. These included
complications related to the device directly (e.g. migration or penetration of
the implant) or the overall procedure (e.g. trocar hernia, infectious
complications [wound infection or abscess], and reoperations).
Additional secondary outcomes were the incidence
of postoperative dysphagia requiring endoscopic balloon dilatation, improvement
in gastroesophageal reflux disease symptoms (defined using the GERD-HRQL
score), and improvement in quality of life related to gastroesophageal reflux
disease (defined as improvement from “dissatisfied” or “neutral” response to “satisfied”
or “neutral” [if initially “dissatisfied”]).
Statistical analyses
The data were collected and stored using
Microsoft Excel (version 16.76; Microsoft, Seattle, WA, USA) under licence
(Microsoft 365). Statistical analyses were performed using GraphPad Prism (version
9; GraphPad Software, San Diego, CA, USA). Continuous variables are expressed
as mean (standard deviation) or median (interquartile range [IQR]), and
categorical variables are expressed as frequencies (percentages). Continuous
paired preoperative and postoperative observations were compared using the
Wilcoxon signed-rank test. The significance level was
set at 0.05. The sample size was based on the available data at the time of
writing rather than a sample size calculation.
Results
The patients’ demographic characteristics,
baseline clinical parameters, and operative and postoperative characteristics
are summarised in table 1. Twenty-nine patients (72.5%) had a large hiatal
hernia, defined as ≥4 cm, at the time of surgery. In addition,
31 patients had ineffective oesophageal motility (IEM). Moreover, 20 patients
(50%) had preoperative dysphagia, of whom 19 (95%) had IEM. All 40 patients (100%)
achieved complete follow-up,
defined as a video oesophagram three months postoperative
and completed clinical visits. The GERD-HRQL questionnaire was completed by all
40 patients preoperatively (100%) and 38 (95.0%) three months
postoperative.
Table 1Patients’ demographic
characteristics, baseline clinical parameters, and operative and postoperative
characteristics. Continuous values are expressed as the median [interquartile
range].
|
Characteristic |
n = 40 |
| Demographics |
Sex (female), n (%) |
16 (40) |
| Age (years) |
60 [51–71] |
| BMI (kg/m2) |
26.3 [24.6–28.9] |
| ASA classification (1–6) |
2 [2–3] |
| Reflux-related
clinical parameters |
Size
of hiatal hernia (cm) |
4 [3–5] |
| Barrett’s oesophagus, n (%) |
16 (40) |
| Ineffective oesophageal motility, n (%) |
31 (77.5) |
| Preoperative dysphagia, n (%) |
20 (50) |
| Operative characteristics |
Device implanted
in the correct position |
40
(100) |
| Operative time (minutes) |
57.5 [51.75–64.25] |
| Conversion to laparotomy, n (%) |
1 (2.5) |
| Intraoperative complication, n (%) |
1 (2.5) |
| Postoperative characteristics |
Hospital stay (days) |
4 [3–5] |
| Complications within 90 days*, n (%) |
6 (15) |
|
Grade I |
0 |
| Grade II |
1 (2.5) |
| Grade IIIa |
3 (7.5) |
| Grade IIIb |
2 (5) |
| Grade IVa |
0 |
| Grade IVb |
0 |
| Grade V |
0 |
| Dysphagia requiring dilatations, n (%) |
3 (7.5) |
|
Number of dilatations performed |
4 [4–5] |
Surgery and postsurgical assessments
In all patients, the intended RefluxStop
procedure was feasible (i.e. the RefluxStop™ device was successfully implanted
in the correct position). The surgery was performed laparoscopically on 39/40 (97.5%)
patients. In one patient who had undergone previous open surgery, the procedure
was converted to an open procedure due to bleeding adhesions while establishing
laparoscopic access to the abdomen. After conversion and correction of the
vascular injury, the intended procedure was safely performed as described in
the Methods section. The median operating time was 57.5 minutes (IQR = 51.75–64.25
minutes).
Three patients experienced postoperative
complications. One underwent an urgent laparoscopic reoperation the same day
due to a postoperative haemorrhage caused by dissection of the short gastric
vessels at the fundus. One reported persistent fatigue four weeks postoperative
due to pericardial effusion. The pericardial effusion, which might have been
related to mediastinal dissection or due to cardiac failure, was treated
successfully with non-steroidal anti-inflammatory drugs and did not require
pericardiocentesis. One developed a trocar hernia in the epigastric area
(camera trocar), discovered on postoperative day 24 with acute incarcerated
omentum, and underwent direct open closure of the trocar hernia defect.
All patients underwent imaging (video
fluoroscopy) on postoperative day one, and the correct positioning of the RefluxStop™
device was confirmed (figure 2). The patients’ median hospital stay was four
days (IQR = 3–5 days). All patients tolerated the prescribed diet as described
in the Methods section.
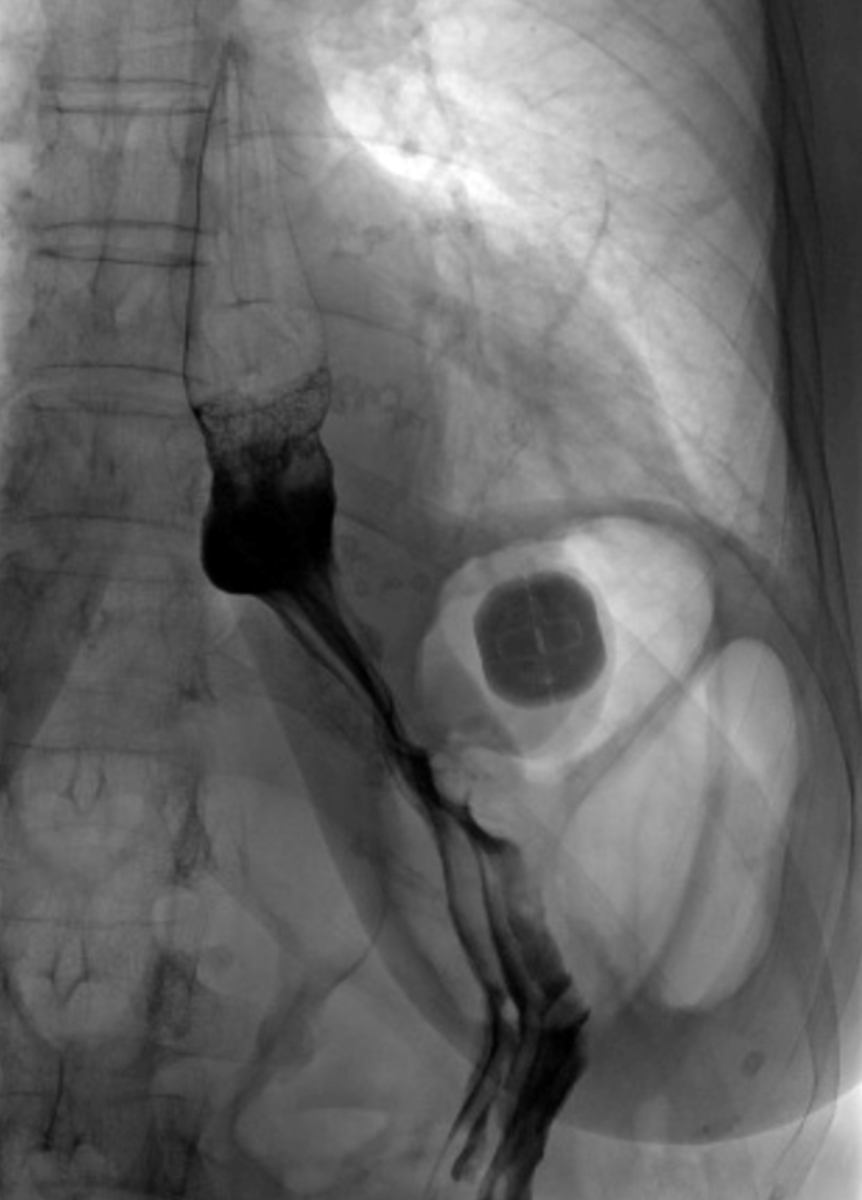
Figure 2Video oesophagram
on postoperative day one depicting the correct positioning of the RefluxStop™
device, leaving the food passageway unaffected.
Clinical outcomes at four weeks and three months
Based on the results of the GERD-HRQL
questionnaire, gastroesophageal reflux disease symptoms (heartburn and
regurgitation) improved in all 38 patients who completed the questionnaire preoperatively
and postoperatively (figure 3). The median (IQR) GERD-HRQL score decreased
significantly from 35 (28.5–49.0) preoperatively to 2 (0–3)
at three months postoperative (p <0.0001). Sub-scores (0–30 points)
for heartburn and regurgitation also showed significant improvement in all
patients three months postoperative. Among the 38 patients who answered the question
about their quality of life related to gastroesophageal reflux disease preoperatively
and postoperatively, 28 (73.7%) initially reported they felt dissatisfied or
neutral, and all (100%) reported an improvement three months postoperative (figure
4).
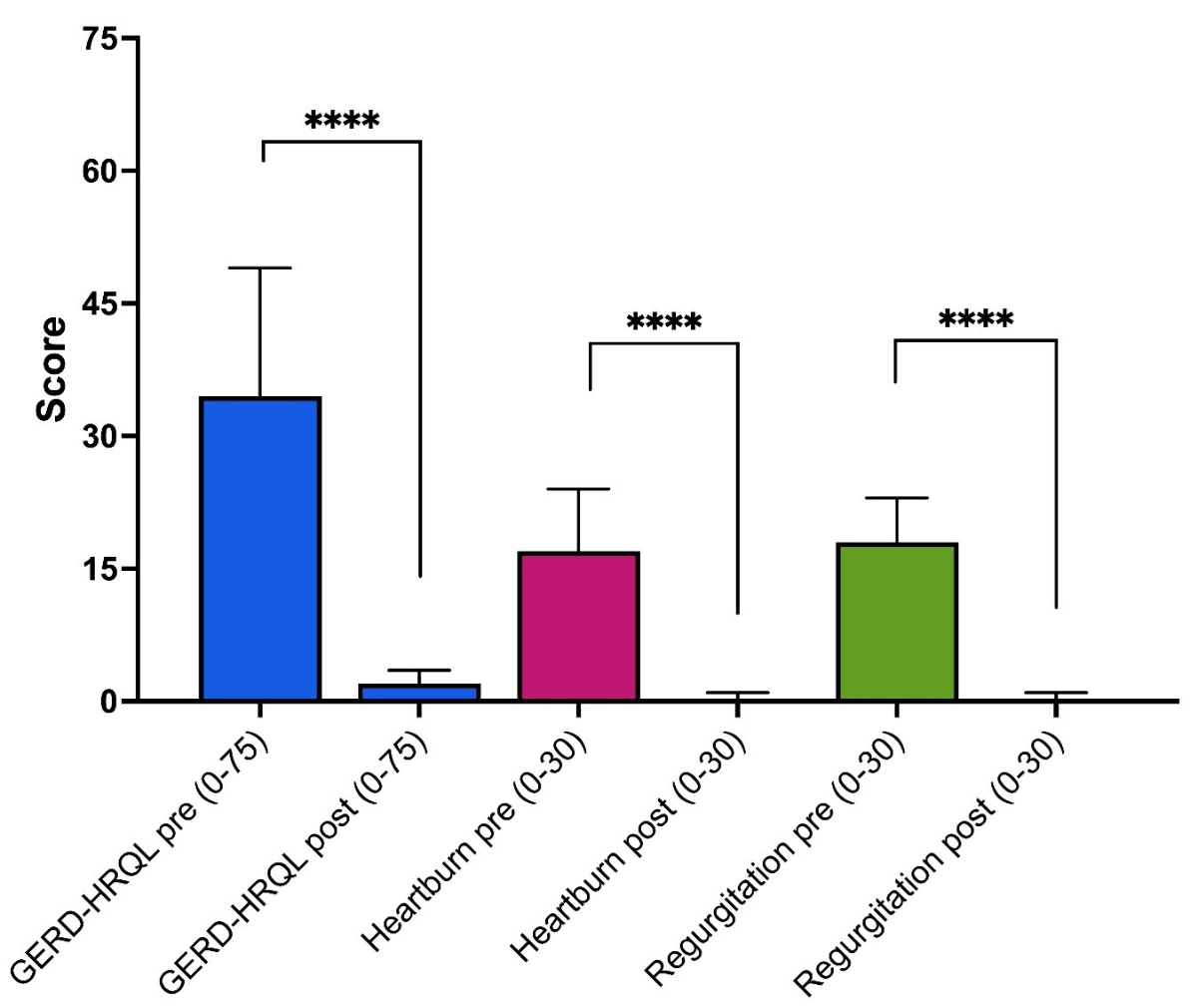
Figure 3Preoperative
and postoperative GERD-HRQL scores (without proton pump inhibitors) for all
patients (n = 38) who completed the questionnaire before and three
months after surgery, with detailed analysis for heartburn and regurgitation. The
scores are presented as the median (box) and interquartile range (whiskers).
Paired comparisons had p-values <0.0001 (****). GERD-HRQL: gastroesophageal
reflux disease health-related quality of life.
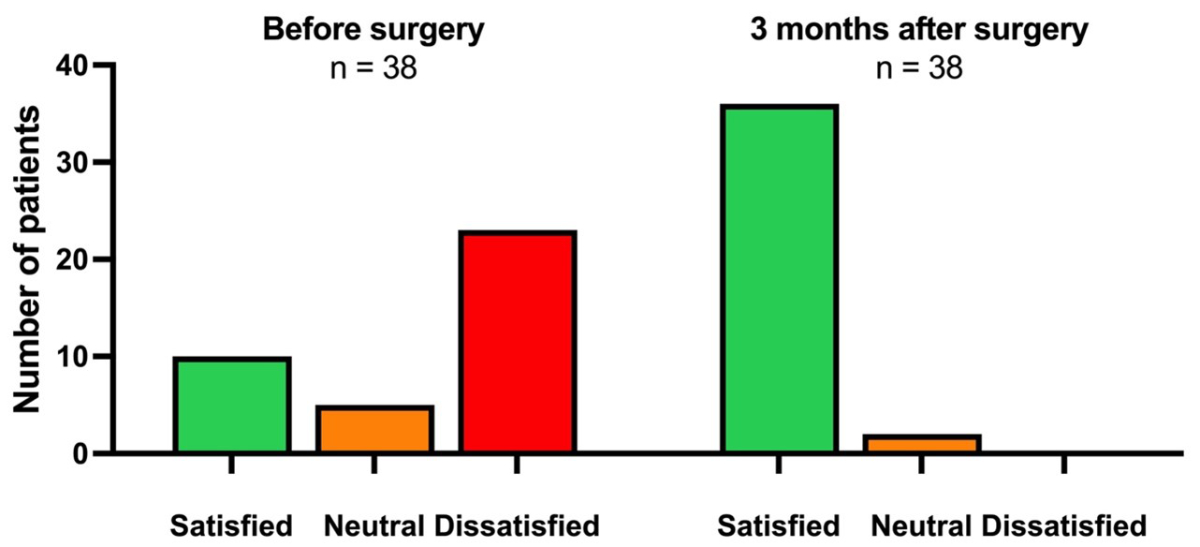
Figure 4Preoperative
and postoperative (three months) quality of life
related to gastroesophageal reflux disease. Only patients (n = 38) who
completed the questionnaire before and three months after surgery were
included.
Three patients reported postoperative
dysphagia with frequent vomiting and inability to eat a normal diet. However,
these patients all had IEM before surgery, and two had preoperative dysphagia.
Due to the severity of symptoms in these three patients, early dilations were
performed 3–4 weeks postoperative. Following the initial phase and subsequent
implementation of a normal diet, additional dilations were required to provide
satisfactory results. In one patient, dilations with 18- to 20-mm balloons were
successful; in the other two patients, further endoscopic dilations had to be
performed after initial dilation with 18- and 20-mm balloons, with an EndoFLIP
balloon to 25 mm, to resolve their dysphagia.
At the three-month clinical follow-up, 20 patients
who had previously suffered from dysphagia showed a reduction in severity or complete
resolution of symptoms (figure 5). No new onset of dysphagia was recorded at the
three-month follow-up.
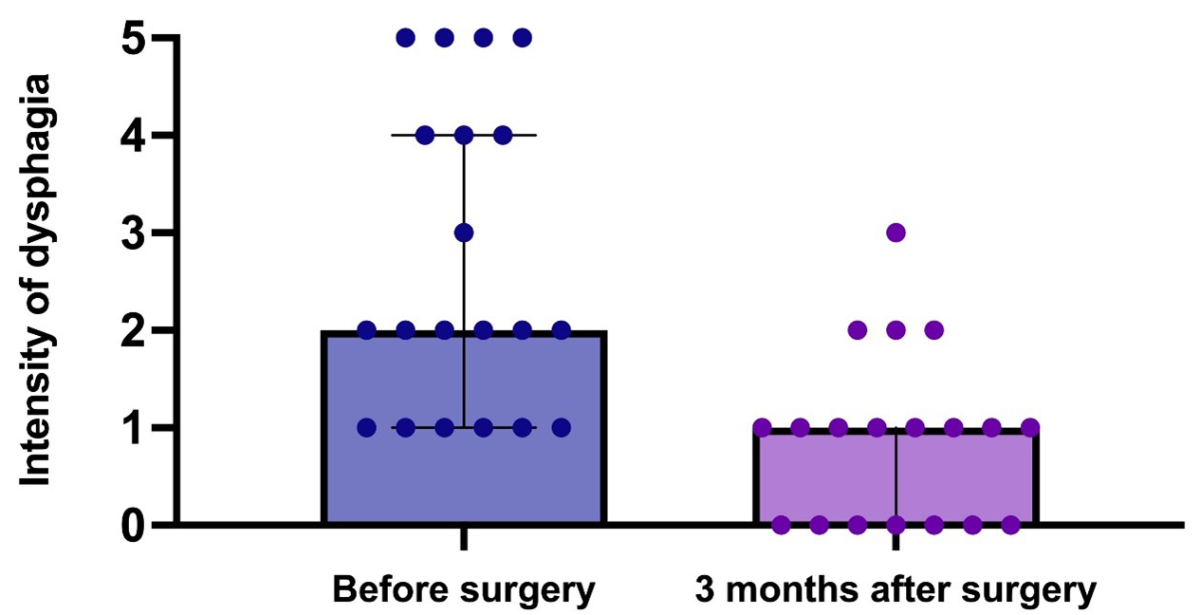
Figure 5Preoperative and postoperative (three months) dysphagia
intensity (0–5) in patients who had preoperative dysphagia (n = 20). The
scatter plots show the median (box) and
interquartile range (whiskers).
Radiologic outcomes at three months
The video oesophagrams three months
postoperatively confirmed the correct positioning of the RefluxStop™ device in
all patients, and no device-related complications were observed.
Device-related complications
Overall, there were no device-related
complications at surgery or the four-week and three-month clinical follow-ups. No
device-related reoperations occurred in this study cohort during the follow-up period.
Discussion
Following the initial study by Bjelovic et
al. for the Conformité
Européenne (CE)-mark [11], this is the second study on laparoscopic anti-reflux
surgery with the RefluxStop™ device.
In this initial experience with a cohort of
40 patients undergoing laparoscopic anti-reflux surgery with the RefluxStop™ device
in a private hospital setting, the device was successfully implanted in all
patients who were considered appropriate candidates preoperatively and who consented
to undergo the procedure. The device was positioned correctly, and all 40
patients showed improved or resolved reflux symptoms, confirmed by a
significant improvement in GERD-HRQL scores and low rates of dysphagia despite many
in the cohort having IEM preoperatively. We consider the complication rates at 90
days – with two patients requiring reoperation (acute bleeding and trocar
hernia), one developing pericardial effusion, and three patients requiring
endoscopic dilations – to be comparable to other laparoscopic anti-reflux
surgery techniques. Richter’s review of side effects and complications from
fundoplication reported open conversion in 0–24% (though <2.4% in high
volume centres), bleeding and splenic injury in <1%, severe postoperative
nausea and vomiting in 2–5%, and early dysphagia in 10–50% of adults hospitalised
for anti-reflux surgery, with 4.7–8.3% having at least one complication [16].
There were no procedure-related
complications during surgery, which is indicative of the safety of the implant and
its delivery system (deployment tool). The trocar hernia reported by one patient in
this series was from the epigastric camera port, not from the port used for the deployment
tool.
The goal of the RefluxStop™ device is to fill
the existing gap in modern laparoscopic anti-reflux surgery techniques,
enabling a non-wrap variation of surgical repair, thereby mitigating the adverse
effects seen with current procedures such as laparoscopic Nissen [17] and
Toupet [18] fundoplication. Among the most frequently recognised adverse
effects of a 360° wrap (Nissen) is the inability to belch and vomit, leading to bloating
and flatulence [18]. While such effects are thought to be less common with
partial than complete fundoplication [4], at least in the short term [19], they
are still reported and deter many patients from undergoing anti-reflux surgery
[19]. Therefore, a gap exists for a definitive surgical treatment for gastroesophageal
reflux disease that both repairs the underlying dysfunction of the lower oesophageal
sphincter and reconstructs the angle of His while avoiding adverse effects such
as dysphagia, which is thought to be caused by encircling and putting pressure
on the oesophagus.
Recent minimally invasive techniques
attempt to mimic or replace fundoplication, often in combination with
laparoscopic hiatal hernia repair. Laparoscopic magnetic sphincter augmentation
with the LINX™ reflux management system (Johnson & Johnson, Newark, NJ,
USA), comprised of a dynamic band of magnetic beads, has been available for over
a decade since being approved by the US Food and Drug Administration in 2012. This
technique has been shown to have fewer adverse effects (bloating and flatulence)
than the Nissen technique and preserved the ability to vomit and belch in most
patients [9, 20, 21]. However, the circular placement around the distal oesophagus
limits its use to patients with normal oesophageal motility [22–24], and it is
not indicated for those with moderate preoperative dysphagia [25]. One postulate advantage
of the RefluxStop™
device is less persistent postoperative dysphagia since the oesophagus is not
encircled, unlike in Nissen fundoplication [18] and magnetic sphincter
augmentation [22]. In this study, three patients with postoperative
dysphagia (one of whom had new onset dysphagia) were effectively treated by
balloon dilation without recurrence of reflux symptoms. We consider this rate
to be relatively low, particularly because 50% (20/40) of the patients had
dysphagia preoperatively, of whom 19 had IEM, and 77.5% (31/40) had IEM based on the
preoperative video oesophagrams.
These preoperative rates of dysphagia and IEM may well have influenced outcomes
by leading to a higher rate of dysphagia than would occur in a more general
population. This observation is particularly interesting given the limitations
of existing treatments for those with IEM and supports the suitability of
using the RefluxStop™ device as an alternative to a wrap.
One crucial step of the procedure to ensure
a tension-free reconstruction during the oesophagogastric plication is establishing
sufficient oesophageal length during the mediastinal dissection. Similarly, the
fundic pocket that contains the RefluxStop™ device close to the oesophagogastric
suture must be closed without creating tension around the implant since a taut
pocket may increase the likelihood of gastric migration. In this study, video oesophagrams
at one day and three months postoperative confirmed no cases of device migration.
It should be noted that, as a safety measure, the RefluxStop™ device is made of
five silicon components, held together with a Vicryl suture (assembled on the
back table during surgery). This five-part design, rather than a larger single
component, aims to prevent a gastric or intestinal obstruction if migration occurs.
This study had some limitations. Firstly, its
follow-up was limited to 3 months, and symptoms and/or hiatal hernia could recur
after that time. Secondly, it was limited by its retrospective study design. There
were no data on postoperative pH studies or manometry since these are not
performed routinely in our clinical practice. However, Bjelovic et al. provided
data on postoperative pH [11]. Thirdly, it did not include a control group since
the focus was to describe the initial experience with this procedure in
clinical practice to assess its feasibility and safety. Such features could be
incorporated into future study designs.
This patient population had a high rate of
preoperative dysphagia and dysmotility, which may have affected the final
results regarding postoperative dysphagia [16], although, as reported above, our
findings show a low rate of new-onset or persistent dysphagia. Postoperative
dysphagia rates are likely to be lower in a larger cohort with fewer patients
with IEM. The high proportion of patients with IEM in this study was influenced
by the fact that, at our institution, we also offer other procedures besides the
RefluxStop™ device, such as magnetic sphincter augmentation (LINX™). While some
patients will have undergone magnetic sphincter augmentation, it is unsuitable
for those with IEM; therefore, there will have been a selection bias toward
those with IEM undergoing surgery with the RefluxStop™ device. In addition,
72.5% of our patients had a large hiatal hernia. Such patients are often deemed unsuitable
for current standard-of-care procedures that encircle the food passageway; this
technique appears to offer a solution that can be used successfully in these cases.
Using a video oesophagram to evaluate for IEM
before surgery limited the accuracy of the IEM diagnosis in this cohort since
it is not a standardised test for evaluating IEM. We did not systematically collect
information about anti-reflux
medication use at three months. Nonetheless, as the responses to the GERD-HRQL
questionnaire are intended to describe symptoms in the absence of medication,
the comparison of the GERD-HRQL scores before and three months after surgery
remains valid, and the statistically significant improvement in the score at three
months is not confounded by ongoing intake of proton pump inhibitors or other
medical anti-reflux therapy.
Assessing or comparing the length of stay
in this study with other anti-reflux surgery procedures was not thought to be
helpful since, in Switzerland, all patients are kept at least two nights for
optimal hospital reimbursement.
Future research should focus on clinical
trials comparing outcomes against traditional surgical techniques and look
in-depth at the physiology of how this device and procedure exert their effect.
Conclusions
In this explorative study, our preliminary
observations indicate that the RefluxStop™ device is a feasible treatment option
for gastroesophageal reflux disease to introduce in similar healthcare settings
since the device was safely and correctly implanted in all 40 patients with a
low complication rate. Furthermore, it appears suitable for a broader patient
population that includes IEM since short-term clinical outcomes were favourable
in this patient cohort. Further studies with larger patient cohorts and longer
follow-ups are required.
Joerg Zehetner, MD, MMM, FACS
Hirslanden Klinik Beau-Site Bern
Schaenzlihalde 1
CH-3013 Bern
joerg.zehetner[at]hirslanden.ch
References
1. Nirwan JS, Hasan SS, Babar ZU, Conway BR, Ghori MU. Global Prevalence and Risk Factors
of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis.
Sci Rep. 2020 Apr;10(1):5814. 10.1038/s41598-020-62795-1
2. Zhang D, Liu S, Li Z, Wang R. Global, regional and national burden of gastroesophageal
reflux disease, 1990-2019: update from the GBD 2019 study. Ann Med. 2022 Dec;54(1):1372–84.
10.1080/07853890.2022.2074535
3. Delshad SD, Almario CV, Chey WD, Spiegel BM. Prevalence of Gastroesophageal Reflux
Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology. 2020 Apr;158(5):1250–1261.e2.
10.1053/j.gastro.2019.12.014
4. Slater BJ, Dirks RC, McKinley SK, Ansari MT, Kohn GP, Thosani N, et al. SAGES guidelines
for the surgical treatment of gastroesophageal reflux (GERD). Surg Endosc. 2021 Sep;35(9):4903–17.
10.1007/s00464-021-08625-5
5. Savarino V, Marabotto E, Zentilin P, Demarzo MG, de Bortoli N, Savarino E. Pharmacological
Management of Gastro-Esophageal Reflux Disease: An Update of the State-of-the-Art.
Drug Des Devel Ther. 2021 Apr;15:1609–21. 10.2147/dddt.S306371 10.2147/DDDT.S306371
6. Greenberg JA, Stefanova DI, Reyes FV, Edelmuth RC, Harik L, Thiesmeyer JW, et al. Evaluation
of post-operative dysphagia following anti-reflux surgery. Surg Endosc. 2022 Jul;36(7):5456–66.
10.1007/s00464-021-08888-y
7. Dunn C, Bildzukewicz N, Lipham J. Magnetic Sphincter Augmentation for Gastroesophageal
Reflux Disease. Gastrointest Endosc Clin N Am. 2020 Apr;30(2):325–42. 10.1016/j.giec.2019.12.010
8. Sheu EG, Nau P, Nath B, Kuo B, Rattner DW. A comparative trial of laparoscopic magnetic
sphincter augmentation and Nissen fundoplication. Surg Endosc. 2015 Mar;29(3):505–9.
10.1007/s00464-014-3704-6
9. Reynolds JL, Zehetner J, Wu P, Shah S, Bildzukewicz N, Lipham JC. Laparoscopic Magnetic
Sphincter Augmentation vs Laparoscopic Nissen Fundoplication: A Matched-Pair Analysis
of 100 Patients. J Am Coll Surg. 2015 Jul;221(1):123–8. 10.1016/j.jamcollsurg.2015.02.025
10. Ayazi S, Chowdhury N, Zaidi AH, Chovanec K, Komatsu Y, Omstead AN, et al. Magnetic
sphincter augmentation (MSA) in patients with hiatal hernia: clinical outcome and
patterns of recurrence. Surg Endosc. 2020 Apr;34(4):1835–46. 10.1007/s00464-019-06950-4
11. Bjelović M, Harsányi L, Altorjay Á, Kincses Z, Forsell P, Gunjić D, et al.; Investigators
of the RefluxStop™ Clinical Investigation Study Group. Non-active implantable device
treating acid reflux with a new dynamic treatment approach: 1-year results : RefluxStop™
device; a new method in acid reflux surgery obtaining CE mark. BMC Surg. 2020 Jul;20(1):159.
10.1186/s12893-020-00794-9
12. Velanovich V. 25 Years of the GERD-HRQL symptom severity instrument: an assessment
of published applications. Surg Endosc. 2023 Jan;37(1):255–65. 10.1007/s00464-022-09463-9
13. Allen JE, White C, Leonard R, Belafsky PC. Comparison of esophageal screen findings
on videofluoroscopy with full esophagram results. Head Neck. 2012 Feb;34(2):264–9.
10.1002/hed.21727
14. SS Sami KR. The Los Angeles Classification of Gastroesophageal Reflux Disease. Video
Journal and Encyclopedia of GI Endoscopy. 2013;1(1):103-4 DOI:10.1016/S2212-0971(13)70046-3.
15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new
proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann
Surg. 2004 Aug;240(2):205–13. 10.1097/01.sla.0000133083.54934.ae
16. Richter JE. Gastroesophageal reflux disease treatment: side effects and complications
of fundoplication. Clin Gastroenterol Hepatol. 2013 May;11(5):465–71. 10.1016/j.cgh.2012.12.006
17. Ayazi S, DeMeester SR, Hagen JA, Zehetner J, Bremner RM, Lipham JC, et al. Clinical
Significance of Esophageal Outflow Resistance Imposed by a Nissen Fundoplication.
J Am Coll Surg. 2019 Aug;229(2):210–6. 10.1016/j.jamcollsurg.2019.03.024
18. Schwameis K, Zehetner J, Rona K, Crookes P, Bildzukewicz N, Oh DS, et al. Post-Nissen
Dysphagia and Bloating Syndrome: Outcomes After Conversion to Toupet Fundoplication.
J Gastrointest Surg. 2017 Mar;21(3):441–5. 10.1007/s11605-016-3320-y
19. Analatos A, Håkanson BS, Ansorge C, Lindblad M, Lundell L, Thorell A. Clinical Outcomes
of a Laparoscopic Total vs a 270° Posterior Partial Fundoplication in Chronic Gastroesophageal
Reflux Disease: A Randomized Clinical Trial. JAMA Surg. 2022 Jun;157(6):473–80. 10.1001/jamasurg.2022.0805
20. Rona KA, Reynolds J, Schwameis K, Zehetner J, Samakar K, Oh P, et al. Efficacy of
magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc.
2017 May;31(5):2096–102. 10.1007/s00464-016-5204-3
21. Warren HF, Reynolds JL, Lipham JC, Zehetner J, Bildzukewicz NA, Taiganides PA, et
al. Multi-institutional outcomes using magnetic sphincter augmentation versus Nissen
fundoplication for chronic gastroesophageal reflux disease. Surg Endosc. 2016 Aug;30(8):3289–96.
10.1007/s00464-015-4659-y
22. Tsai C, Steffen R, Kessler U, Merki H, Lipham J, Zehetner J. Postoperative Dysphagia
Following Magnetic Sphincter Augmentation for Gastroesophageal Reflux Disease. Surg
Laparosc Endosc Percutan Tech. 2020 Aug;30(4):322–6. 10.1097/sle.0000000000000785 10.1097/SLE.0000000000000785
23. Rona KA, Tatum JM, Zehetner J, Schwameis K, Chow C, Samakar K, et al. Hiatal hernia
recurrence following magnetic sphincter augmentation and posterior cruroplasty: intermediate-term
outcomes. Surg Endosc. 2018 Jul;32(7):3374–9. 10.1007/s00464-018-6059-6
24. Baison GN, Jackson AS, Wilshire CL, Bell RC, Lazzari V, Bonavina L, et al. The Impact
of Ineffective Esophageal Motility on Patients Undergoing Magnetic Sphincter Augmentation.
Ann Surg. 2023 Apr;277(4):e793–800. 10.1097/sla.0000000000005369 10.1097/SLA.0000000000005369
25. Bonavina L, DeMeester TR, Ganz RA. LINX(™) Reflux Management System: magnetic sphincter
augmentation in the treatment of gastroesophageal reflux disease. Expert Rev Gastroenterol
Hepatol. 2012 Dec;6(6):667–74. 10.1586/egh.12.47
Appendix

Figure S1The GERD-HRQL
questionnaire used to assess patients before and three months after surgery.

Figure S2Device position categorisation according to
the manufacturer’s instructions for use, reproduced with the kind permission of
Implantica (Zug, Switzerland).






