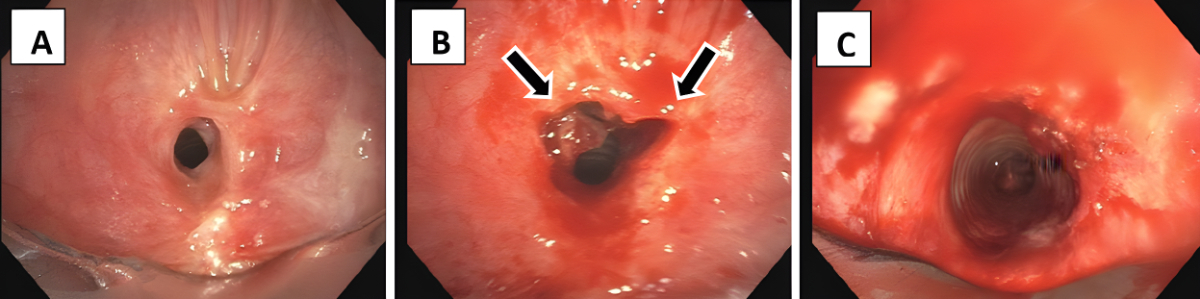
Figure 1The web-like stenosis before endoscopic intervention (A), when it was cut radially at two defined locations at 10 and 2 o’clock(arrows) (B) and after dilation using rigid tracheoscopes with gradually increased diameter (C).
DOI: https://doi.org/https://doi.org/10.57187/s.3363
Benign tracheal stenosis is a relatively rare yet impactful chronic condition characterised by symptoms such as dyspnoea, inspiratory stridor and poor quality of life. Most commonly, benign tracheal stenosis is observed in young to middle-aged women, which may lead to misdiagnosis of bronchial asthma and therefore delayed diagnosis of benign tracheal stenosis [1–3]. There are a few potential causes, including damage from a prior intubation [1], damage from inhaling chemicals or heat, an autoimmune disease, gastro-oesophageal reflux disease (GERD) or an unknown cause, in which case it is known as idiopathic subglottic stenosis [4, 5]. The most common causes of benign tracheal stenosis are post-intubation tracheal stenosis (PITS) and post-tracheostomy tracheal stenosis (PTTS), accounting for 19% and 65% of cases, respectively [6, 7]. Nowadays, the incidence of post-intubation and post-tracheostomy tracheal stenosis is estimated to be approximately 4.9 cases per million per year [8, 9]. Interestingly, the presence of oestrogen receptors in tracheal mucosa could play a pivotal role in the pathogenesis and increased incidence in females [10, 11]. Basically, two treatment strategies have been proposed. Surgical resection of the diseased tracheal segment was first described by Pearson and Andrews in the 1970s, with resection of the stenotic area and subsequent end-to-end anastomosis [12, 13]. This has been the procedure of choice in benign tracheal stenosis measuring <2 cm and located in the proximal part of the trachea. However, this method is associated with procedure-related 30-day mortality of 2.6% and a procedure-related morbidity rate of up to 45% [12, 14–20]. Furthermore, restenosis after months to years is an issue. According to Macchiarini et al., restenosis can even occur one month post-operatively [14, 19]. The reported recurrence rate after surgery ranges from 4% to 36%, depending on the experience of the surgeon and other medical issues such as excessive tension, devascularisation and comorbidities such as diabetes, connective tissue disorders and poor nutritional status [21–24]. On the other hand, endoscopic tracheoplasty is a minimally invasive alternative. This technique was first described in 1987 by Stanley Shapshay who combined laser resection with rigid bronchoscopy in tracheal and subglottic stenosis [25]. Since then, the technique has been modified repeatedly with dilation using rigid bronchoscopy or balloon catheter after cutting with electrocautery, electric knife or laser [25]. Ultimately, there is no generally recognised gold standard for the therapy of benign tracheal stenosis. Neither is there evidence comparing the surgical and endoscopic therapies. In this retrospective single-centre study, we aimed to evaluate the safety and outcome of endoscopic tracheoplasty in patients with benign tracheal stenosis, using rigid tracheoscopy, radial incision, dilation and local glucocorticoid administration.
All patients who underwent endoscopic tracheoplasty due to benign tracheal stenosis from 2013 to 2022 at the University Hospital Zurich, Switzerland, were included in this study. Patients with tracheal stenosis due to malignancy or goitre were excluded. Medical history, pulmonary function test, details on the intervention including post-interventional course, histology and follow-up data were drawn from patients’records. The first follow-up visit was scheduled at six to eight weeks post-intervention, in accordance with internal Standard Operating Procedures (SOPs), as wound healing typically concludes after approximately six weeks. Thereafter,the patients were invited to report symptoms suggestive of restenosis. When symptoms suggestive of restenosis recurred, a repeat spirometry along with a comprehensive medical history, physical examination and tracheoscopy were performed. The primary outcome of our study was procedure-related safety of endoscopic tracheoplasty in patients with benign tracheal stenosis. Secondary outcomes were success rate and recurrence rate, especially following introduction of local triamcinolone acetonide administration and post-interventional budesonide inhalation.
All patients included in the study signed a general consent to retrospectively obtain anonymised patient data. The study was approved by the ethics committee of Zurich (BASEC-Nr. 2020-01573).
Endoscopic tracheoplasty was performed under general anaesthesia as an inpatient procedure. After the induction of total intravenous anaesthesia and muscle relaxation, the patient’s trachea was intubated with a rigid tracheoscope (Karl Storz SE & Co. KG, Tuttlingen, Germany),whereas the tip of the scope was placed between the vocal cords and stenosis using 4.5 mm, 0° Hopkins®optics (Karl Storz SE & Co. KG). After initiation of high-frequency jet ventilation, a flexible videobronchoscope (190 series,Olympus,Tokyo, Japan) was introduced into the tracheoscope and the web-like stenosis was cut radially at two defined locations at 10 and 2 o’clock (figure 1) with an electric papillotomy needle (MTW Endoskopie, Wesel, Germany), which was inserted over the working channel of the bronchoskope, and Endo-cut (VIO® 200D, APC 2, Erbe, Tübingen, Germany; effect 4, 40–50 W). Then the stenosis was dilated using rigid tracheoscopes with gradually increased diameter. Since 2016, 20–40 mg triamcinolone acetonide (Kenacort®, Dermapharm AG, Hünenberg, Switzerland) has been administered topically into the former stenotic area to prevent early recurrence due to scarring. In addition, from 2020, inhaled budesonide 200 µg bid has been initiated after hospital discharge to prevent recurrence (Pulmicort® Turbuhaler®, AstraZeneca, Baar, Switzerland). Since this is an uncontrolled single-arm study, the recurrence rate after sole tracheoplasty was historically compared to tracheoplasty including local triamcinolone administration without and with subsequent inhaled budenoside.

Figure 1The web-like stenosis before endoscopic intervention (A), when it was cut radially at two defined locations at 10 and 2 o’clock(arrows) (B) and after dilation using rigid tracheoscopes with gradually increased diameter (C).
Continuous data are reported using means (±standard deviation [SD]).SPSS(IBM, Armonk, NY, USA) version 27 was used for descriptive data analysis.
In total, 22 patients (mean age 57.3 [±SD 17.3] years, 86.4% female) were included and observed during a mean follow-up time of 4.5 years. Details concerning comorbidities, risk factors and symptoms are shown in table 1. Mean time from symptom onset to diagnosis of benign tracheal stenosis was 14.2 (±SD 27.6) months. At histology, none of the included patients had findings suggestive of malignancy, amyloidosis or granulomatous disease. All patients experienced immediate improvement of their symptoms. A follow-up was conducted after 7.8 (±SD 4.3) weeks including a spirometry as shown in figure 2. Numeric pulmonary function values improved only a little (table 2), but the flow-volume curve regained its normal shape, while inspiratory and expiratory flattening had disappeared. At follow-up, none of the patients reported dyspnoea on exertion or at rest, nor did they have any stenosis-related breathing difficulties.
Table 1Demographic and clinical characteristics.
| Frequency n (%) / mean(±SD) | |||
| No. of participating patients | 22 (100.0%) | ||
| Demographics | Female | 19 (86.4%) | |
| Age | 57.3 (±SD 17.3) | ||
| Smoking | Never smoker | 17 (77.3%) | |
| Active smoker | 3 (13.6%) | ||
| Former smoker | 2 (9.1%) | ||
| Body Mass Index(kg/m2) | 18.5–24.9 | 12 (54.6%) | |
| 25–29.9 | 4 (18.2%) | ||
| 30–34.9 | 3 (13.6%) | ||
| 35–39.9 | 2 (9.1%) | ||
| ≥40 | 1 (4.5%) | ||
| Previous misdiagnosis of bronchial asthma | 3 (13.6%) | ||
| Other comorbidities | Gastro-oesophageal reflux disease and/or proton pump inhibitor use | 8 (36.4%) | |
| Rheumatological disease | 3 (13.6%) | ||
| Thyroid disease(excl.goitre) | 6 (27.3%) | ||
| Type 2 diabetes mellitus | 5 (22.7%) | ||
| Hypertension | 9 (40.9%) | ||
| Symptoms | Cough | 12 (54.5%) | |
| Stridor | 21 (95.5%) | ||
| Voice abnormality | 2 (9.1%) | ||
| Dysphagia | 2 (9.1%) | ||
| Dyspnoea | mMRC Score 3 | 16 (72.7%) | |
| mMRC Score 4 | 6 (27.3%) | ||
| Previous interventions to trachea | Orotracheal intubation | 8 (36.4%) | |
| Tracheostomy | 6 (27.3%) | ||
| Tracheal surgery to treat benign tracheal stenosis | 3 (13.6%) | ||
mMRC: modified Medical Research Council.
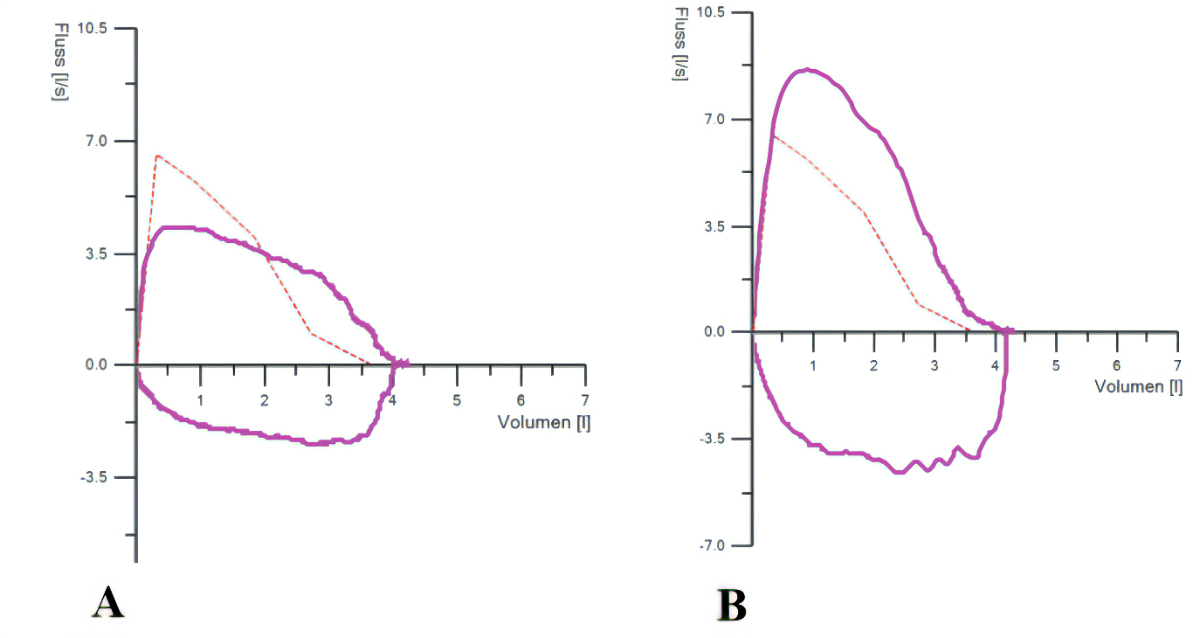
Figure 2Spirometry before (A) and 8 weeks after endoscopic tracheoplasty (B).
Table 2Pre- and post-intervention pulmonary function test.
| Pre-intervention, mean (±SD) | Post-intervention, mean (±SD) | |
| FEV1 (l) | 2.47 (±SD 0.61) | 2.62 (±SD 0.83) |
| FEV1 predicted % | 92.5% (±SD 17.73%) | 94.38% (±SD 16.61%) |
| FVC (l) | 3.32 (±SD 1.01) | 3.34 (±SD 0.98) |
| FVC predicted % | 100.65% (±SD 16.73%) | 101.38% (±SD 16.23%) |
FEV1: forced expiratory volume per second in litres and predicted value; FVC: forced vital capacity in litres and predicted value.
The procedure was performed as described in the Methods section. Average procedure time was 42 (±SD 15.6) minutes and length of hospital stay was 2 (±SD 1) days. The stenoses in all patients were located subglottically at a distance of 19.5 (±SD 9.1) mm from the vocal cord. On average, the stenosis length was 8.0 (±SD 10.7) mm, with a percentage luminal stenosis of 57.2% (±SD 13.6%). In 1994, a study by Myer et al. was published, in which subglottic stenoses were classified based on their degree of stenosis using various sizes of endotracheal tubes: grade I ≤50%, grade II 51–70%, grade III 71–99% and grade IV with no detectable lumen. This grading, known as the “Myer-Cotton classification”, continues to be used for subglottic stenoses to this day [26]. Applied to our patient cohort, 9 patients had grade I stenosis before the first endoscopic therapy, 9 had grade II and 4 had grade III stenosis. None of the patients had Grade IV stenosis or showed any cartilage involvement. There were no adverse events during the procedure.
Post-interventional complications were rare with transient hoarseness after one intervention (2.6%). In total, ten (45.5%) patients had 16 recurrent tracheal stenoses after an average of 21.1 (±SD 18.0) months following endoscopic tracheoplasty. Two patients had three recurrences each (one with idiopathic origin and one with status after cricotracheal resection after a previous intubation), two had two recurrences each (one with status after tracheostomy and one with status after cricotracheal resection and anastomosis after a previous intubation) while six patients had one recurrence each (two of idiopathic origin; two with status after intubation; one with status after tracheotomy during thyroidectomy in Graves’ disease; and one with status after cricotracheal resection and anastomosis after a previous intubation and also hypothyroidism). In our patient cohort, only one patient needed stent placement due to inability to manage the stenosis using the rigid tracheoscope. We opted to insert a self-expanding Ultraflex stent (12 × 30 mm). Upon subsequent follow-up assessments, the expansion of the stent revealed a well-healed tracheal lining. Currently, the patient remains free of symptoms. The presentation of patients, along with whether they received a triamcinolone injection after the incision and dilation during all treatments, as well as additional budesonide inhalation, and peri-interventional complications, is listed in table 3. Also included are the relative percentage values within each of the various recurrence groups. Ultimately, 38 procedures were performed in 22 patients. Topical triamcinolone was administered during 27 procedures, from which 10 (37.0%) experienced recurrence. In the remaining 11 cases, which were treated before 2016, triamcinolone was not used. Of these, 6 had recurrent tracheal stenosis (54.5%). From 2020 onwards, 8 patients received inhaled budesonide as part of the new post-intervention regime. Of these, only 1 has experienced a recurrence to date (12.5%).
Table 3Procedure-related complications and recurrence after endoscopic tracheoplasty.
| Intervention | Number of patients* | Procedure-related complications, n (%) | Recurrence, n(%) | Mean time to recurrence, months (SD) |
| Tracheoplasty without triamcinolone | 11 | 1 (9.1%)** | 6 (54.5%) | 15 (±SD 14.1) |
| Tracheoplasty with triamcinolone | 19 | 0 | 9 (47.4%) | 18.4 (±SD 18.5 ) |
| Tracheoplasty with triamcinolone and budesonide | 8 | 0 | 1 (12.5%) | 5 (±SD 0) |
* Patients with multiple tracheoplasties were counted for each intervention.
** Patient with transient hoarseness.
In this retrospective study, we aimed to share our single-centre experience on endoscopic treatment of benign tracheal stenosis, challenging the traditional way of surgical repair. Over the course of the observed period, a total of 22 patients were enrolled in this study. The sample size is contingent upon incidence, reflecting the rarity of this condition. Consequently, an extended observation period was accepted. In uncomplicated benign tracheal stenosis (i.e. web-like stenosis), endoscopic tracheoplasty is possibly a considerable alternative to surgical repair regarding safety and success rate. However, the recurrence rate of 42.1% after endoscopic tracheoplasty in our study is an issue, since the reported recurrence rate after surgical repair is 4–36% [21–23]. Yet, after topical administration of triamcinolone during endoscopic tracheoplasty, the risk of recurrence was only 37.0% compared to 54.5% without triamcinolone. According to the treatment of keloids of the skin, the administration of triamcinolone in the mucosa seems to be crucial to avoid relapse and lower the amount of inflammation of mucosal tissue [27, 28]. Similar results have been achieved using mitomycin C as adjuvant treatment in tracheal stenosis to lower the recurrence rate [29–31]. Mitomycin C has been shown to impair fibroblast reproduction and proliferation, and thus refibrosis rate was temporarily reduced by induction of the cytokine TGF-β, which inhibits collagen production and therefore fibrosis [29].
In a study by Wierzbicka et al., the corticosteroid injection following non-invasive bronchoscopic dilation was investigated according to the cause of benign tracheal stenosis: (1) autoimmune disease (granulomatosis with polyangiitis), (2) traumatic or (3) idiopathic. All patients received the same therapy with endoscopic dilation and intramucosal corticosteroid injection with granulomatosis with polyangiitis showing the highest success rate of 75%, compared to 71% and 56% with traumatic and idiopathic aetiology, respectively [32]. In the end, several studies emphasised that, regardless of the cause, additional adjuvant corticosteroid injection is superior compared to endoscopic dilation alone [5, 24, 32]. In 2016, we introduced triamcinolone to be administered locally into the lesion at the end of the procedure. However, since the effect of triamcinolone vanishes over time, we additionally introduced inhaled budesonide from 2020 to standard post-interventional care after tracheoplasty. As historically compared to the group without corticosteroid administration, we found that risk of recurrence was considerably lower after triamcinolone injection, and even lower after additional budenoside inhalation. Interestingly, inhaled budesonide and the inhaled use of the antifibrotic drug nintedanib have recently been shown to reduce the risk of recurrent tracheal stenosis in an animal model [33]. The promising effect of nintedanib was able to be reproduced in a rabbit model showing that it effectively prevented tracheal stenosis by inhibiting fibrosis and inflammation. The antifibrotic effect of nintedanib may be achieved by inhibiting fibroblast proliferation, migration and differentiation, and suppressing the TGF-β1/Smad2/3 and ERK1/2 signalling pathways [34].
Although in many studies surgical resection is considered to be the only definitive treatment option for benign tracheal stenosis, more recent studies have shown that web-like or simple stenosis can also be safely and successfully treated by endoscopic procedures such as balloon dilation or stent insertion [4, 30, 35]. In the study conducted by Ozdemir et al., two categories were set up. Simple stenoses that measured less than 1 cm in length and complex stenoses that were longer than 1 cm and showed signs of inflammation, malignancy and cartilage involvement. They concluded that simple stenoses can be successfully treated with balloon dilation and laser, while complex stenoses require surgical resection or stenting with regular follow-ups, since stents have a wall-stabilising effect and prevent cartilage loss [35, 36]. However, treatment options varied for tracheal stenosis of benign and malignant origins. While benign tracheal stenosis is easily treatable using endoscopic procedures, primary malignant tracheal stenosis requires surgical resection [27]. Although endoscopic interventions carry the risk of recurrence of benign tracheal stenosis, safety seems to be superior as compared to uncontrolled data from surgical repair [16, 37, 38]. While surgical resection used to be the treatment of choice of benign tracheal stenosis, it is associated with several complications including restenosis, necrosis and anastomotic dehiscence in 4% of cases [16, 38]. The closer the stenosis is to the larynx, the greater the risk of vocal cord dysfunction and difficulty in swallowing. According to Bibas et al., voice changes or difficulty swallowing are reported in 2–4% of patients after surgery but have not been reported after endoscopic treatment [22, 24, 39]. The reported post-operative mortality after surgery ranges from 1.8% to 5%, whereas no fatalities have been reported after endoscopic interventions [38]. Restenosis after surgery can occur in up to 36%, necessitating repeat treatment. However, revision surgery of a restenosis is often no longer possible after surgical resection. According to Donahue et al., revision surgery is associated with a high complication rate of 39% [40]. Therefore, endoscopic treatment even after a surgical resection might be a safe treatment option for restenosis [27].
Our collaborative and multidisciplinary approach incorporates a team of healthcare professionals, encompassing ear-nose-throat specialists, anaesthetists and interventional pulmonologists, alongside other healthcare experts. Specifically addressing tracheal stenosis linked to intubation, we acknowledge the absence of a universally agreed-upon optimal treatment algorithm. This highlights the ongoing necessity for research to develop effective treatment strategies tailored to this particular cause of tracheal stenosis [5, 35].
There are several limitations to our study, which are mainly due to the small sample size, its retrospective and uncontrolled nature and to the relatively short follow-up period after endoscopic tracheoplasty. However, given the rarity of benign tracheal stenosis and the single-centre approach, it is ambitious to gather a sufficiently high sample size for a randomised trial. Furthermore, assessment of recurrence was based on self-reported symptoms rather than scheduled follow-ups, resulting in a considerable risk of reporting bias.
According to our retrospective, real-life data, endoscopic tracheoplasty was a successful treatment for benign tracheal stenosis with few complications and zero peri-interventional mortality. The high risk of recurrence must be considered and can possibly be lowered by means of local triamcinolone injection and inhaled budesonide.
Author contributions: Study design (DF, CS), data collection (DK), data analysis (DK), drafting of the manuscript(all authors). Approval of the final version of the manuscript (all authors).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Lorenz RR. Adult laryngotracheal stenosis: etiology and surgical management. Curr Opin Otolaryngol Head Neck Surg. 2003 Dec;11(6):467–72.
2. Zubairi AB, Dildar B, Husain SJ, Khan MF. Tracheal stenosis mimicking severe acute asthma. BMJ Case Rep. 2010 Oct 12:2010:bcr1220092517. Epub 2010/01/01. doi: . PubMed PMID: 22789696; PubMed Central PMCID: PMCPMC3029208.
3. Nagappan R, Parkin G, Wright CA, Walker CS, Vallance N, Buchanan D, et al. Adult long-segment tracheal stenosis attributable to complete tracheal rings masquerading as asthma. Crit Care Med. 2002 Jan;30(1):238–40.
4. Marchioni A, Andrisani D, Tonelli R, Andreani A, Cappiello GF, Ori M, et al. Stenting versus balloon dilatation in patients with tracheal benign stenosis: The STROBE trial. Laryngoscope Investig Otolaryngol. 2022;7(2):395-403. Epub 2022/04/19. doi: . PubMed PMID: 35434321; PubMed Central PMCID: PMCPMC9008152.
5. Ravikumar N, Ho E, Wagh A, Murgu S. The role of bronchoscopy in the multidisciplinary approach to benign tracheal stenosis. J Thorac Dis. 2023;15(7):3998-4015. Epub 2023/08/10. doi: . PubMed PMID: 37559626; PubMed Central PMCID: PMCPMC10407490.
6. Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy. A prospective study of 150 critically ill adult patients. Am J Med. 1981 Jan;70(1):65–76.
7. D'Andrilli A, Venuta F, Rendina EA. Subglottic tracheal stenosis. J Thorac Dis. 2016;8(Suppl 2):S140-7. Epub 2016/03/17. doi: . PubMed PMID: 26981264; PubMed Central PMCID: PMCPMC4775266.
8. Nouraei SA, Ma E, Patel A, Howard DJ, Sandhu GS. Estimating the population incidence of adult post-intubation laryngotracheal stenosis. Clin Otolaryngol. 2007 Oct;32(5):411–2.
9. Zias N, Chroneou A, Tabba MK, Gonzalez AV, Gray AW, Lamb CR, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med. 2008;8:18. Epub 2008/09/23. doi: . PubMed PMID: 18803874; PubMed Central PMCID: PMCPMC2556644.
10. Valdez TA, Shapshay SM. Idiopathic subglottic stenosis revisited. Ann Otol Rhinol Laryngol. 2002 Aug;111(8):690–5.
11. Benjamin B, Jacobson I, Eckstein R. Idiopathic subglottic stenosis: diagnosis and endoscopic laser treatment. Ann Otol Rhinol Laryngol. 1997 Sep;106(9):770–4.
12. Pearson FG, Andrews MJ. Detection and management of tracheal stenosis following cuffed tube tracheostomy. Ann Thorac Surg. 1971 Oct;12(4):359–74. 10.1016/S0003-4975(10)65137-5
13. Grillo HC, Donahue DM, Mathisen DJ, Wain JC, Wright CD. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg. 1995 Mar;109(3):486–92. 10.1016/S0022-5223(95)70279-2
14. Amat B, Esselmann A, Reichle G, Rohde HJ, Westhoff M, Freitag L. The electrosurgical knife in an optimized intermittent cutting mode for the endoscopic treatment of benign web-like tracheobronchial stenosis. Arch Bronconeumol. 2012 Jan;48(1):14–21. 10.1016/j.arbr.2011.07.007
15. Grillo HC, Mathisen DJ. Surgical management of tracheal strictures. Surg Clin North Am. 1988 Jun;68(3):511–24. 10.1016/S0039-6109(16)44531-7
16. Wright CD, Li S, Geller AD, Lanuti M, Gaissert HA, Muniappan A, et al. Postintubation Tracheal Stenosis: management and Results 1993 to 2017. Ann Thorac Surg. 2019 Nov;108(5):1471–7.
17. Puri HV, Asaf BB, Mundale VV, Pulle MV, Bishnoi S, Munjal M, et al. Predictors of Anastomotic Complications After Resection and Anastomosis for Tracheal Stenosis. Indian J Otolaryngol Head Neck Surg. 2021;73(4):447-54. Epub 2021/10/26. doi: . PubMed PMID: 34692457; PubMed Central PMCID: PMCPMC8520546.
18. Segura-Salguero JC, Díaz-Bohada L, Ruiz Á J. Perioperative management of patients undergoing tracheal resection and reconstruction: a retrospective observational study. Braz J Anesthesiol. 2022;72(3):331-7. Epub 2022/02/21. doi: . PubMed PMID: 35183604; PubMed Central PMCID: PMCPMC9373245.
19. Macchiarini P, Verhoye JP, Chapelier A, Fadel E, Dartevelle P. Partial cricoidectomy with primary thyrotracheal anastomosis for postintubation subglottic stenosis. J Thorac Cardiovasc Surg. 2001 Jan;121(1):68–76.
20. Marulli G, Rizzardi G, Bortolotti L, Loy M, Breda C, Hamad AM, et al. Single-staged laryngotracheal resection and reconstruction for benign strictures in adults. Interact Cardiovasc Thorac Surg. 2008 Apr;7(2):227–30.
21. Menapace DC, Modest MC, Ekbom DC, Moore EJ, Edell ES, Kasperbauer JL. Idiopathic Subglottic Stenosis: Long-Term Outcomes of Open Surgical Techniques. Otolaryngol Head Neck Surg. 2017 May;156(5):906–11.
22. Bibas BJ, Terra RM, Oliveira Junior AL, Tamagno MF, Minamoto H, Cardoso PF, et al. Predictors for postoperative complications after tracheal resection. Ann Thorac Surg. 2014 Jul;98(1):277–82.
23. Madariaga ML, Gaissert HA. Reresection for recurrent stenosis after primary tracheal repair. J Thorac Dis. 2016;8(Suppl 2):S153-9. Epub 2016/03/17. doi: . PubMed PMID: 26981266; PubMed Central PMCID: PMCPMC4775269.
24. Gelbard A, Anderson C, Berry LD, Amin MR, Benninger MS, Blumin JH, et al. Comparative Treatment Outcomes for Patients With Idiopathic Subglottic Stenosis. JAMA Otolaryngol Head Neck Surg. 2020;146(1):20-9. Epub 2019/11/02. doi: . PubMed PMID: 31670805; PubMed Central PMCID: PMCPMC6824232
25. Shapshay SM, Beamis JF Jr, Hybels RL, Bohigian RK. Endoscopic treatment of subglottic and tracheal stenosis by radial laser incision and dilation. Ann Otol Rhinol Laryngol. 1987;96(6):661–4.
26. Myer CM 3rd, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994 Apr;103(4 Pt 1):319–23.
27. Ferreirinha J, Caviezel C, Weder W, Opitz I, Inci I. Postoperative outcome of tracheal resection in benign and malignant tracheal stenosis. Swiss Med Wkly. 2020 Dec;150:w20383.
28. Griffith BH. The treatment of keloids with triamcinolone acetonide. Plast Reconstr Surg. 1966 Sep;38(3):202–8.
29. Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope. 2009 Feb;119(2):272–83.
30. Schweinfurth JM. Endoscopic treatment of severe tracheal stenosis. Ann Otol Rhinol Laryngol. 2006 Jan;115(1):30–4.
31. Cataneo DC, Ximenes AM, Cataneo AJ. Mitomycin C in the endoscopic treatment of tracheal stenosis: a prospective cohort study. J Bras Pneumol. 2018;44(6):486-90. Epub 2019/02/07. doi: . PubMed PMID: 30726324; PubMed Central PMCID: PMCPMC6459742.
32. Wierzbicka M, Tokarski M, Puszczewicz M, Szyfter W. The efficacy of submucosal corticosteroid injection and dilatation in subglottic stenosis of different aetiology. J Laryngol Otol. 2016 Jul;130(7):674–9. 10.1017/S0022215116001122
33. Wei P, Huang Z, Gan L, Li Y, Qin C, Liu G. Nintedanib ameliorates tracheal stenosis by activating HDAC2 and suppressing IL-8 and VEGF in rabbit. Am J Transl Res. 2020;12(8):4739-48. Epub 2020/09/12. PubMed PMID: 32913546; PubMed Central PMCID: PMCPMC7476127.
34. Fan Y, Li X, Fang X, Liu Y, Zhao S, Yu Z, et al. Antifibrotic Role of Nintedanib in Tracheal Stenosis After a Tracheal Wound. Laryngoscope. 2021 Sep;131(9):E2496–505.
35. Özdemir C, Kocatürk CI, Sökücü SN, Sezen BC, Kutluk AC, Bilen S, et al. Endoscopic and Surgical Treatment of Benign Tracheal Stenosis: A Multidisciplinary Team Approach. Ann Thorac Cardiovasc Surg. 2018;24(6):288-95. Epub 2018/06/08. doi: . PubMed PMID: 29877219; PubMed Central PMCID: PMCPMC6300420.
36. Gaissert HA, Grillo HC, Mathisen DJ, Wain JC. Temporary and permanent restoration of airway continuity with the tracheal T-tube. J Thorac Cardiovasc Surg. 1994 Feb;107(2):600–6. 10.1016/S0022-5223(94)70109-1
37. Cui PC, Zhao DQ, Guo ZH, Liang LP, Wang W. [Effect of partial cricotracheal resection and extended cricotracheal resection for severe laryngotracheal stenosis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020 Feb;55(2):94–7.
38. Brichet A, Verkindre C, Dupont J, Carlier ML, Darras J, Wurtz A, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J. 1999 Apr;13(4):888–93.
39. Auchincloss HG, Wright CD. Complications after tracheal resection and reconstruction: prevention and treatment. J Thorac Dis. 2016;8(Suppl 2):S160-7. Epub 2016/03/17. doi: . PubMed PMID: 26981267; PubMed Central PMCID: PMCPMC4775259.
40. Donahue DM, Grillo HC, Wain JC, Wright CD, Mathisen DJ. Reoperative tracheal resection and reconstruction for unsuccessful repair of postintubation stenosis. J Thorac Cardiovasc Surg. 1997 Dec;114(6):934–8. 10.1016/S0022-5223(97)70007-2