
Figure 1Flow chart of patient inclusion. Out of 1069 potential patients, 227 patients were excluded, while 842 patients were included in the analysis.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40091
The quality of care and performance is a central aspect of healthcare policies, and quality control is required by the law [1]. For many years, there have been calls for a uniform quality assessment applicable to different domains, for example, by the United States Institute of Medicine [2]. Focusing on cancer care, the National Comprehensive Cancer Network in the United States has elaborated a list of quality measures for endorsement [3]. Many of these goals are certainly part of daily management by medical oncology providers in Switzerland, but there is a lack of universally accepted definitions, methods, and ways to measure and report them to the public [4, 5].
Cancer centre certification programs aim to ensure quality by focusing on procedures and timely assessment of the outcomes of mostly surgical interventions. Certification programs in Switzerland are almost all voluntary, and data from individual certified centres are not publicly available [6, 7].
Outcome parameters such as survival data as well as factors that impact outcomes, including guideline adherence, availability of specialized tumour boards, and treatments in a study setting, are of utmost importance [8–12]. Additionally, patient-reported outcome measures and experiences are promising tools but are not widely implemented in daily practice. Other benchmarks published by the American Society of Clinical Oncology focus on surrogate parameters such as personnel, productivity, revenues, expenses, and salaries but not on outcomes [13]. Apart from the almost exclusively voluntary certification programs and the mandatory regulations for a few, mainly surgical, procedures in cancer care, the Swiss healthcare system does not consider them binding benchmarks, and they are not systematically implemented, collected, and reported [14].
The main aim of this study was to investigate the overall survival (OS) outcomes of patients receiving care from medical oncologists at our institution in Switzerland, a medium-sized in-house medical oncology service (MOS) at Olten Cantonal Hospital. For logistical reasons, we evaluated only patients with at least one outpatient visit at the medical oncology service during the study period. We examined the overall survival of patients with six types of cancer from 2008 to 2017 and compared it with survival data provided by the National Agency for Cancer Registration (NACR), which we set as national benchmarks. We hypothesized that for the six cancer types, the overall survival at the Olten medical oncology service is comparable to the national data.
Several studies have demonstrated improved survival for cancer types if treatment adheres to guidelines and treatment protocols [8, 9]. We also therefore investigated adherence to internationally recognized guidelines at the Olten medical oncology service and hypothesized that treatments follow the guidelines in at least 80% of cases.
Olten Cantonal Hospital, a teaching hospital of the medical faculties of the Universities of Bern and Basel, is a 200-bed hospital that provides primary care and extended primary care for the eastern part of the Canton of Solothurn, serving a population of approximately 100,000 inhabitants. Since 2008, Olten Cantonal Hospital has had an in-house medical oncology service with three board-certified medical oncologists (up to 2017: 180% full time equivalent), one board-certified haematologist (80% full time equivalent), and two residents (200% full time equivalent). Starting 2013, the medical oncology service has had institutionalized cooperation in oncology training with the Centre for Oncology, Haematology, and Transfusion Medicine at Aarau Cantonal Hospital, an approximately 600-bed hospital. Together, the two oncology services have operated a breast care unit certified by the European Society of Breast Cancer Specialists since 2013. In 2014, the medical oncology service was certified as a cancer care institution by the Swiss Society of Medical Oncology. On average, 280–300 new patients with cancer are seen each year at the Oltenmedical oncology service. From 2008 to 2020, an average of 6848 outpatient consultations per year took place.Tumour board case discussions are held once a week, involving all different diagnostic and therapeutic specialists. As a general internal rule, all new cancer cases treated at the medical oncology service must be presented to the tumour board. The number of tumour board discussions increased over time from 349 in 2008 to 814 in 2020. Cooperation with other institutions allows for comprehensive decision-making.
We used January 1, 2008, the founding date of the medical oncology service, as the starting point for this study. All patients with cancer with at least one outpatient visit at the medical oncology service up to December 31, 2017, were included. To possibly allow for a broad statement on the performance of the medical oncology service, we focused the investigation on six frequent or highly curable types of cancer (mean 36.3% of newly diagnosed patients per year): breast cancer, testicular cancer, colon cancer, non-small-cell lung cancer, Hodgkin lymphoma, and diffuse large B-cell lymphoma (DLBCL). Figure 1 shows the inclusion and exclusion criteria. Patients with a corresponding disease were selected from the chronologically updated database of new patients at the medical oncology service. Patient and cancer characteristics, comorbidities at the first consultation, and date of the last follow-up visit were extracted from the medical records. The date of death was determined by reviewing the medical records or by contacting the local municipalities when this datum was missing from our files.

Figure 1Flow chart of patient inclusion. Out of 1069 potential patients, 227 patients were excluded, while 842 patients were included in the analysis.
The Swiss Cancer Registries collect vital status data by linkage with federal mortality data and verification of vital status with the cantonal registration offices. Only primary tumours per incidence year were included, and all cases of relapse were excluded from this study. Follow-up information was available until December 31, 2017.
As the primary endpoint, the outcomes of the patients with cancer at the medical oncology service were evaluated. To examine the impact of the medical oncology service, we calculated and defined the overall survival as the time from the first consultation at the medical oncology service to the date of death. Patients who were still alive or lost to follow-up at the time of the analysis were censored at the last confirmable date of being alive. Where subgroups were too small to analyse or where the median time to death was not yet reached, the data were classified as not applicable.
The outcomes of the patients at the medical oncology service were compared with national outcome data, calculated and provided by the NACR for each of the six cancer types, including all primary cancers from 2008 to 2017. In 2008, 15 out of 26 cantons kept a cancer registry, covering 62.5% of the Swiss population. In 2017, this number increased to 23 cantons and 94.0% population coverage. Completeness of case ascertainment is generally high in Switzerland [15], with proportions of death certificate-only cases ranging below 1.5% for all incidence years included in the study. Notably, the NACR data up to 2020 do not include any data from the Canton of Solothurn, to which Olten belongs.
The overall survival of the patients was estimated using the Kaplan–Meier method. As is customary for registry data, the NACR used the time of histological diagnosis as the start date to calculate the overall survival, whereas the medical oncology service used the date of the first consultation in the outpatient service.
Guideline conformity, the secondary descriptive endpoint, was assessed by comparing the treatment decision with stage-specific guidelines that were valid at the respective time period: National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, the European Society for Medical Oncology Guidelines, and/or the American Society of Clinical Oncology Clinical Practice Guidelines [16–18]. These guidelines are recognized by the voluntary certification program of the Swiss Society of Medical Oncology [6]. Adherence to these guidelines was assessed via chart review by the oncology fellow at the medical oncology service, and cases of uncertainty were discussed and decided with the senior oncologist at the medical oncology service. Details of the respective therapies such as dose, dose intensity, treatment duration, application of granulocyte colony-stimulating factor (G-CSF), antibiotic prophylaxis, and characteristics of surgery and radiotherapy were not analysed. Patients who were not treated according to the guidelines were classified into two groups: patients for whom there was no medical explanation recorded for this decision, and patients who received individually adjusted treatment. Individual adjustment means, for example, that patients declined an adjuvant treatment or that bleomycin was omitted for an elderly patient owing to an increased risk for pneumonitis.
All data from the hospital’s internal medical records were given a neutral number and were transferred to a table. Data that contributed to the identification of the test participants were kept separately from the study data. The median follow-up period at the medical oncology service was estimated using the reverse Kaplan–Meier method. For the time-to-event endpoint (overall survival), the Kaplan–Meier method was used to estimate the median 1- and 5-year overall survival probabilities. The analyses were not adjusted for stage, age, or any other possible influencing factor. Therefore, all analyses were considered exploratory. Log–log transformation was used for calculating the 95% confidence interval (CI) concerning survival function. To compare the medical oncology service data with the NACR data, we used the approximation of comparing the 95% CI. Therein, a non-overlapping 95% CI was considered to indicate a statistically significant difference with a p value of <0.05.
Treatment according to the guidelines was considered as a categorical variable, and the exact log-rank test based on a permutation test was used. To investigate whether a correlation exists between patient treatment following the guidelines and overall survival, we calculated Harrell’s C index for each subgroup [19]. Harrell’s C index is a parameter between 0 and 1 that is used to determine the correlation of concordant pairs. A Harrell’s C index of 0.5 indicates a 50/50 chance and therefore represents a lack of influence of the analysed variable on the outcome. The reason for not using hazard ratios was that there were partly too few events in the patient subgroups. Statistical tests were performed at a 5% level, and the significance level was not adjusted for multiple testing. For the analysis, we used the software for statistical computing R version 4.1, package survival version 3.2. The analysis methods were standard, and no self-implemented methods were used. The code can be shared upon request.
The study was approved by the regional ethics committee (EKNZ 2018-02230), and the requirement of informed consent was waived based on Article 34 of the Swiss Federal Human Research Act (non-genetic health-related personal data; it is impossible or disproportionately difficult to obtain consent or to provide information on the right to dissent, or this would impose an undue burden on the person concerned; no documented refusal is available).
Of the initially included 1069 patients at the medical oncology service, 227 (21.2%) had to be excluded for various reasons; of them, 47 patients were excluded owing to missing data (41 owing to lack of data and 6 owing to absence of histological samples) (figure 1). The data on the remaining 842 patients with cancer for all analysed outcomes were complete and analysed up to September 8, 2020. The baseline patient characteristics are shown in table 1.
Table 1Baseline characteristics of the patients at the medical oncology service at Olten Cantonal Hospital from incidence year 2008 to 2017.
| Total | n | % | Age (average), year | Female sex (%) | Male sex (%) | Stage 1 (%) | Stage 2 (%) | Stage 3 (%) | Stage 4 (%) |
| 842 | 100 | 63.1 | 514 (61.0) | 328 (39.0) | 261 (31.0) | 118 (14.0) | 180 (21.4) | 283 (33.6) | |
| Breast cancer | 343 | 40.7 | 63.4 | 333 (97.1) | 10 (2.9) | 186 (54.2) | 53 (15.5) | 44 (12.8) | 60 (17.5) |
| Testicular cancer | 50 | 5.9 | 37.7 | 0 | 50 (100) | 39 (78.0) | 8 (16.0) | 3 (6.0) | 0 (0.0) |
| Colon cancer | 143 | 17 | 66.4 | 60 (42.0) | 83 (58.0) | 3 (2.1) | 29 (20.3) | 50 (35.0) | 61 (42.7) |
| Non-small-cell lung cancer | 237 | 28.1 | 67.5 | 88 (37.1) | 149 (62.9) | 18 (7.6) | 12 (5.1) | 69 (29.1) | 138 (58.2) |
| Hodgkin lymphoma | 23 | 2.7 | 44.8 | 9 (39.1) | 14 (60.9) | 2 (8.7) | 9 (39.1) | 7 (30.4) | 5 (21.7) |
| Diffuse large B-cell lymphoma | 46 | 5.5 | 64.7 | 24 (52.2) | 22 (47.8) | 13 (28.3) | 7 (15.2) | 7 (15.2) | 19 (41.3) |
The median follow-up period of the 842 patients at the medical oncology service was 69.7 months (95%CI = 64.7, 72.0). Figure 2 shows the Kaplan–Meier curve for the overall survival stratified by cancer type. The median overall survival for colon cancer was 62.6 months (95% CI = 45.6, 112.9), while that for non-small-cell lung cancer was 14.0 months (95% CI = 11.5, 16.8). For breast cancer, testicular cancer, Hodgkin lymphoma, and diffuse large B-cell lymphoma, the median overall survival was not reached.
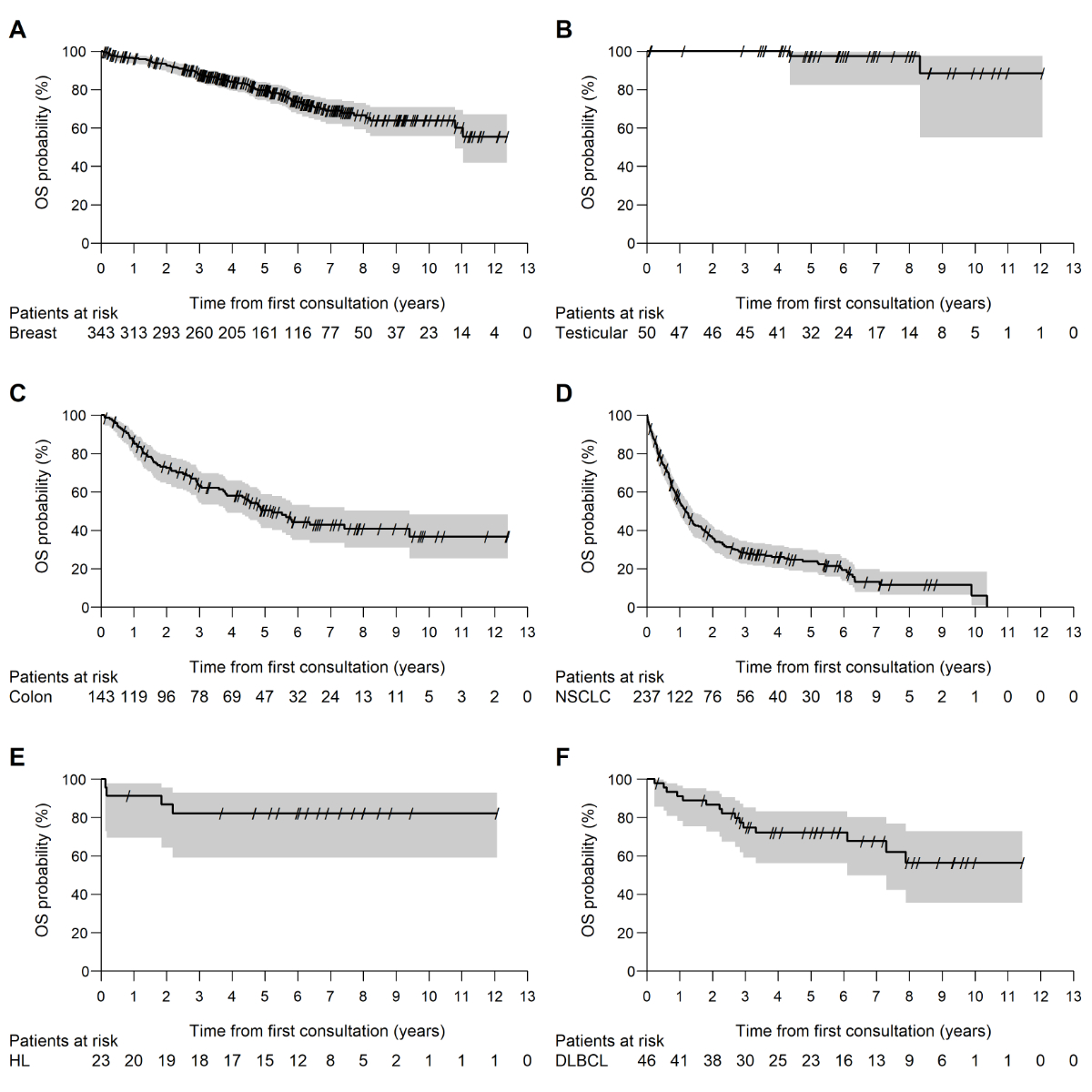
Figure 2Kaplan–Meier curve for overall survival at the medical oncology service stratified by cancer type. Breast cancer (A), testicular cancer (B), colon cancer (C), non-small cell lung cancer (D), Hodgkin lymphoma (E), diffuse large B-cell lymphoma (F).OS: overall survival; NSCLC: non-small-cell lung cancer; HL: Hodgkin lymphoma; DLBCL: diffuse large B-cell lymphoma.
The outcomes for patients at the medical oncology service and in the NACR data regarding the 1- and 5-year overall survival (OS) showed, with an overlapping 95% CI, no statistically significant difference for colon cancer (1-year OS: MOS = 86.4%, NACR = 81.9%; 5-year OS: MOS = 50.4%, NACR = 58.8%), non-small-cell lung cancer (1-year OS: MOS = 55.2%, NACR = 56.3%; 5-year OS: MOS = 23.8%, NACR = 25.5%), Hodgkin lymphoma (1-year OS: MOS = 91.3%, NACR = 94.7%; 5-year OS: MOS = 82.2%, NACR = 89.2%), and diffuse large B-cell lymphoma (1-year OS: MOS = 91.2%, NACR = 77.8%; 5-year OS: MOS = 72.2%, NACR = 62.3%). The 1-year overall survival of the patients with testicular cancer could not be compared statistically because no death occurred during the first year of follow-up at the medical oncology service and thereby, no 95% CI could be calculated on the basis of the 50 cases included in the analysis. The NACR data showed a 1-year overall survival of 99%. The 5-year overall survival for testicular cancer was not significantly different: 97.3% in both the medical oncology service and NACR collectives. A significant difference with a non-overlapping 95% CI was observed in the 5-year overall survival among the patients with breast cancer (MOS = 78.9%, NACR = 84.5%), with the medical oncology service data being inferior to the NACR data. In the medical oncology service collective, there were approximately 2.5 times (17.5% vs. 6.9%) as many patients with stage 4 breast cancer as in the NACR collective. In the NACR data set for breast cancer, which was stratified by stage, 3.6% of the patients were excluded owing to missing stage information. In the comparison of the 5-year overall survival for each stage, no significant difference between the two data sets was found. Additionally, the time interval from histological diagnosis to the first consultation at the medical oncology service among the patients with breast cancer was, on average, about 1 week (stage 1 = 7.1 days, stage 2 = 7.7 days, stage 3 = 5.7 days, and stage 4 = 7.6 days). This time period was not part of the medical oncology service data on the overall survival. The comparisons are shown in tables 2–4.
Table 2One-year overall survival of the patients treated at the medical oncology service at Olten Cantonal Hospital compared with NACR data from incidence year 2008 to 2017.
| Cancer type | Medical oncology service at Olten Cantonal Hospital | NACR | ||||||
| n (%) | Mean age | Overall survival | 95% CI | n (%) | Mean age | Overall survival | 95% CI | |
| 843 (100) | Year | % | 96,370 (100) | Year | % | |||
| Breast cancer | 343 (40.7) | 63.4 | 96.1 | (93.4, 97.7) | 46,074 (47.8) | 62.6 | 96.6 | (96.4, 96.8) |
| Testicular cancer | 50 (5.9) | 37.7 | 100.0 | (NA, NA) | 3412 (3.5) | 38.0 | 99.0 | (98.6, 99.3) |
| Colon cancer | 143 (17.0) | 66.4 | 86.4 | (79.6, 91.1) | 19,762 (20.5) | 71.3 | 81.9 | (81.4, 82.5) |
| Non-small-cell lung cancer | 237 (28.1) | 67.5 | 55.2 | (48.5, 61.3) | 20,940 (21.7) | 68.2 | 56.3 | (55.6, 57.0) |
| Hodgkin lymphoma | 23 (2.7) | 44.8 | 91.3 | (69.5, 97.8) | 1972 (2.0) | 42.4 | 94.7 | (93.6, 95.6) |
| Diffuse large B-cell lymphoma | 46 (5.5) | 64.7 | 91.2 | (78.1, 96.6) | 4210 (4.4) | 67.7 | 77.8 | (76.4, 79.0) |
NACR: National Agency for Cancer Registration operated by the National Institute for Cancer Epidemiology and Registration, 2008–2017; NA: not applicable; CI: confidence interval
Table 3Five-year overall survival of the patients treated at the medical oncology service at Olten Cantonal Hospital compared with NACR data from incidence year 2008 to 2017.
| Cancer type | Medical oncology service at Olten Cantonal Hospital | NACR | ||||||
| n (%) | Mean age | Overall survival | 95% CI | n (%) | Mean age | Overall survival | 95% CI | |
| 843 (100) | Year | % | 963,270 (100) | Year | % | |||
| Breast cancer | 343 (40.7) | 63.4 | 80.0 | (74.4, 83.9) | 46,074 (47.8) | 62.6 | 84.5 | (84.1, 84.9) |
| Testicular cancer | 50 (5.9) | 37.7 | 97.3 | (82.3, 99.6) | 3412 (3.5) | 38.0 | 97.3 | (96.5, 97.9) |
| Colon cancer | 143 (17.0) | 66.4 | 50.4 | (41.22, 58.8) | 19,762 (20.5) | 71.3 | 58.8 | (58.0, 59.7) |
| Non-small-cell lung cancer | 237 (28.1) | 67.5 | 23.8 | (18.1, 29.9) | 20,940 (21.7) | 68.2 | 25.5 | (24.8, 26.3) |
| Hodgkin lymphoma | 23 (2.7) | 44.8 | 82.2 | (59.2, 92.9) | 1972 (2.0) | 42.4 | 89.2 | (87.4, 90.7) |
| Diffuse large B-cell lymphoma | 46 (5.5) | 64.7 | 72.2 | (56.1, 83.2) | 4210 (4.4) | 67.7 | 62.3 | (60.5, 64.0) |
NACR: National Agency for Cancer Registration operated by the National Institute for Cancer Epidemiology and Registration; CI: confidence interval
Table 4Breast cancer outcomes at the medical oncology service at Olten Cantonal Hospital compared with NACR data from incidence year 2008 to 2017. For the NACR data, 1669 cases (3.6%) were excluded owing to missing stage information.
| Stage | Medical oncology service at Olten Cantonal Hospital | NACR | ||||
| 5-year overall survival in % | 95% CI | n (%) | 5-year overall survival in % | 95% CI | n (%) | |
| 1 | 92.9 | (86.9, 96.1) | 186 (54.2) | 95.0 | (94.6, 95.4) | 18,460 (40.1) |
| 2 | 80.9 | (66.4, 89.6) | 53 (15.5) | 88.5 | (87.9, 89.1) | 17,024 (37.0) |
| 3 | 71.0 | (50.8, 84.1) | 44 (12.8) | 74.3 | (72.9, 75.7) | 5748 (12.5) |
| 4 | 40.0 | (26.7, 52.9) | 60 (17.5) | 36.2 | (33.9, 38.6) | 3173 (6.9) |
NACR: National Agency for Cancer Registration operated by the National Institute for Cancer Epidemiology and Registration, 2008–2017; CI: confidence interval
Approximately 92.4% of the patients in the curative setting and 85.8% of the patients in the palliative setting received first-line treatment according to the guidelines [16–18]. Among6.9% of the patients in the curative setting, treatment was individually adjusted: 36.8% (14 cases) owing to comorbidities, 57.9% (22 cases) owing to patients’ wishes, and 5.3% (2 cases) owing to individual medical decisions. Among 12.2% of the patients in the palliative setting, treatment was individually adjusted: 58.1% (18 cases) owing to comorbidities, 38.7% (12 cases) owing to patients’ wishes, and 3.2% (1 case) owing to technical limitations. The groups of individually adjusted treatments were included in the number of patients not treated according to the guidelines. Guideline adherence by cancer type is shown in Table 5. A better descriptive outcome for the patients treated according to the guidelines could be seen only in some subgroups, with Harrell’s C index closer to 1 than to 0 for breast cancer stage 2 (0.59), colon cancer stage 3 (0.58), non-small-cell lung cancer stages 1 and 2 (0.61 and 0.62, respectively), Hodgkin lymphoma stage 3 (1.0), and diffuse large B-cell lymphoma stages 1 and 4 (0.86 and 0.60, respectively). Owing to the small number of events, the calculation of the CIs for Harrell's C index for the examination of statistical significance was dispensed with.
Table 5Guideline adherence at the medical oncology service at Olten Cantonal Hospital by cancer type.
| Curative setting (n, %) | Palliative setting (n, %) | |||||
| According to the guidelines | Individually adjusted | Not according to the guidelines | According to the guidelines | Individually adjusted | Not according to the guidelines | |
| Breast cancer | 261 (93.2) | 16 (5.7) | 3 (1.1) | 55 (87.3) | 6 (9.5) | 2 (3.2) |
| Testicular cancer | 50 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Colon cancer | 71 (87.7) | 10 (12.3) | 0 (0.0) | 57 (91.9) | 5 (8.1) | 0 (0.0) |
| Non-small-cell lung cancer | 70 (87.5) | 10 (12.5) | 0 (0.0) | 131 (83.4) | 22 (14.0) | 4 (2.5) |
| Hodgkin lymphoma | 20 (95.2) | 1 (4.8) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 0 (0.0) |
| Diffuse large B-cell lymphoma | 40 (95.2) | 1 (2.4) | 1 (2.4) | 2 (50.0) | 2 (50.0) | 0 (0.0) |
Prolongation of life is an important goal of medicine [20]. Herein, we set the aggregated data from the National Agency for Cancer Registration (NACR) as a benchmark because to the best of our knowledge, no comparable survival outcome data have been published by any single medical oncology service in Switzerland. It is reassuring that the Olten medical oncology service data on the overall survival are in line with the national survival estimates.
Guideline conformity of the therapeutic decision for first-line treatment at the medical oncology service is at least as high as or higher than that reported in previous studies on colon cancer (35.7–96.4%) and pancreatic cancer (34.5%) in the United States [8, 9]. These two studies investigated whether patients received stage-appropriate therapy without analysing the details of applied dose intensity and supportive treatments, as we did in our study. Ess et al. showed a large variability in the treatment of patients with breast cancer in Switzerland throughout different steps. This variability was discussed as a possible reason for the geographical disparities in the survival of patients with breast cancer in Switzerland [12]. Further, European studies show a wide variation of adherence to treatment between cancer types and between stages [21–23]. To the best of our knowledge, there are also no published data on this topic from other Swiss institutions of medical oncology.
The aim of compliance with certification program requirements is to meet quality standards and thereby improve results. A recent study in Germany based on registry and health insurance data compared the outcome of patients treated at certified centres with the outcome of patients treated at non-certified centres and found an overall survival advantage for almost all cancer types treated at certified centres [24]. Hodgkin lymphoma is one of the highly curable cancers wherein medical oncology is the cornerstone of treatment. A large-scale German study found no difference in treatment efficacy between specialized treatment centres and small practices for patients in clinical studies for Hodgkin lymphoma [25]. This finding raises the question of the extent to which, as with surgical procedures, a medical oncology centre’s size and patient volume affect outcomes and whether other factors, such as adherence to protocols and guidelines, and networking with other centres, have the greatest impact on outcomes [12, 26–29].
The quality of medicine is certainly not limited to a comparable overall survival. As already proposed by Hippocrates, medicine must strive to achieve goals such as “… to do away with the sufferings of the sick; to lessen the violence of their disease …” [30]. Contemporary philosophers and ethicists have defined four core goals of medicine, articulated by the Hastings Centre in 1996 as follows: “the prevention of disease and injury and promotion and maintenance of health; the relief of pain and suffering caused by maladies; the care and cure of those with a malady and the care of those who cannot be cured; the avoidance of premature death and the pursuit of a peaceful death” [31, Executive Summary]. Although it is beyond the objectives of our study, except for “avoidance of premature death,” the Swiss healthcare system has no systematically collected data on how well institutions perform relative to many patient needs. Patient-reported outcome measures and experiences (PROMs and PREMs) are promising tools for assessing patients’ perspectives on these goals [10, 32, 33]. However, to date, they are not widely used in the clinical practice of oncology in Switzerland.
Our study has several limitations. Apart from the retrospective nature of the paper-based chart review, the present data were not adjusted for potential confounders such as distinct subtypes of a particular cancer, age, or comorbidities. While death is an unambiguous event, the classification of adherence to the guidelines is subject to potential bias.
After presentation by the surgical specialists and discussion at the tumour board, many patients with early stages of breast cancer, colon cancer, and non-small-cell lung cancer with no need of adjuvant treatment or patients needing only a simple endocrine therapy for breast cancer were not invited for a consultation at the medical oncology service. These patients were informed by their respective surgical specialist regarding the recommendation of the tumour board. Thereby, their data were not included in this study but were included in the NACR data set. For breast cancer, this led to an imbalance in the stage distribution, with around 2.5 times as many patients under stage 4 in the medical oncology service data set as in the NACR data set as well as in comparison with international registry data [34]. In the NACR data set, 3.6% of the patients with breast cancer were excluded owing to missing stage information. A few patients may have died shortly after diagnosis or may have been transferred to an institution without a visit to the medical oncology service. These patients were not part of our data but were included in the NACR data. A further limitation of the study was the small subgroups at the medical oncology service, which increases the possibility that genuine effects might have been missed owing to low power. We recognize the missing granularity for specific subtypes (e.g., triple-negative breast cancer or non-small-cell lung cancer with a driver mutation), which have substantially distinct prognoses [35, 36]. Nevertheless, the high adherence to the guidelines and the comparable outcome do not support the assumption of significant differences in the overall survival for the majority of the subtypes.
In Switzerland, the uniform and nationwide collection of data on the progression and recurrence of cancer and data on comorbidities in a limited number of malignancies started only recently in 2020 [37]. We assessed the progression-free survival (PFS) as well as the Charlson comorbidity index [38] of our patients, but only data from clinical trials are publicly available for comparison. However, there are inherent impediments and limitations when comparing clinical trial data with real-world data. For this reason, we omitted the collected PFS as well as the analysis of outcome according to the Charlson comorbidity index from this report.
Except for a few malignancies, the management of cancer is characterized by the interdisciplinary nature of the treatment, which involves other specialties in addition to medical oncology. Thus, in general, it is difficult to determine the relative contribution of medical oncology to the overall survival, which might limit the application of this benchmark for medical oncology services.
With the goal of improving the quality of cancer care, a continuous re-evaluation of patient outcomes using uniformly structured data on performed procedures, comorbidities, clinical outcome parameters, and metrics based on patients’ perspectives is needed. However, reliable comparisons across institutions may be affected by small case numbers, hampering the accuracy of outcome estimates.
In Switzerland, the evaluation of the quality of care, diagnosis, and treatment is explicitly anchored in the law [37] as one of the goals. The data compiled by the NACR and hopefully also data provided by certification programs might offer the opportunity to develop risk-adjusted outcome indicators and to set benchmarks on outcomes in the future.
We would like to thank L. Holer and S.Hayoz, SAKK Coordinating Center, Bern, Switzerland, for their support with subgroup analysis and calculating the 95% CI of the data from medical oncology service.
This research received no specific grant from any funding agency.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Art. 59a (Daten der Leistungserbringer) Bundesgesetz as of 18 March 1994 über die Krankenversicherung (KVG; SR 832.10).
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington (DC). US: National Academies Press; 2001.
3. D’Amico TA, Bandini LA, Balch A, Benson AB, Edge SB, Fitzgerald CL, et al. Quality measurement in cancer care: a review and endorsement of high-impact measures and concepts. J Natl Compr Canc Netw. 2020 Mar;18(3):250–9. 10.6004/jnccn.2020.7536
4. Colosia AD, Peltz G, Pohl G, Liu E, Copley-Merriman K, Khan S, et al. A review and characterization of the various perceptions of quality cancer care. Cancer. 2011 Mar;117(5):884–96. 10.1002/cncr.25644
5. Ramsdale EE, Csik V, Chapman AE, Naeim A, Canin B. Improving quality and value of cancer care for older adults. Am Soc Clin Oncol Educ Book. 2017;37(37):383–93. 10.1200/EDBK_175442
6. Swiss Society of Medical Oncology SSMO [Internet]. Basel: Swiss Society of Medical Oncology; c2019 [cited 2022 June 18]. Zertifikat Beantragen; [about 2 screens]. Available from: https://www.sgmo.ch/qualitaetssicherung/zertifikat-beantragen/#
7. German Cancer Society [Internet]. Berlin: Deutsche Krebsgesellschaft e. V., c2021 [cited 2022 September 22]. Certification; [about 2 screens]. Available from: https://www.krebsgesellschaft.de/gcs/german-cancer-society/certification.html
8. Boland GM, Chang GJ, Haynes AB, Chiang YJ, Chagpar R, Xing Y, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013 Apr;119(8):1593–601. 10.1002/cncr.27935
9. Visser BC, Ma Y, Zak Y, Poultsides GA, Norton JA, Rhoads KF. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB (Oxford). 2012 Aug;14(8):539–47. 10.1111/j.1477-2574.2012.00496.x
10. Smith TG, Castro KM, Troeschel AN, Arora NK, Lipscomb J, Jones SM, et al. The rationale for patient-reported outcomes surveillance in cancer and a reproducible method for achieving it. Cancer. 2016 Feb;122(3):344–51. 10.1002/cncr.29767
11. Berardi R, Morgese F, Rinaldi S, Torniai M, Mentrasti G, Scortichini L, et al. Benefits and limitations of a multidisciplinary approach in cancer patient management. Cancer Manag Res. 2020 Sep;12:9363–74. 10.2147/CMAR.S220976
12. Ess S, Savidan A, Frick H, Rageth C, Vlastos G, Lütolf U, et al. Geographic variation in breast cancer care in Switzerland. Cancer Epidemiol. 2010 Apr;34(2):116–21. 10.1016/j.canep.2010.01.008
13. Bourbeau B, Harter D, Towle E. Results From the ASCO 2019 Survey of Oncology Practice Operations. JCO Oncol Pract. 2020 May;16(5):253–62. 10.1200/OP.20.00009
14. Konferenz der kantonalen Gesundheitsdirektorinnen und –direktoren [Internet] Bern: GDK; c2022 [cited 2022 September 22]. HSM-Planungsverfahren; [about 2 screens]. Available from: https://www.gdk-cds.ch/de/hochspezialisierte-medizin/hsm-planungsverfahren
15. Lorez M, Bordoni A, Bouchardy C, Bulliard JL, Camey B, Dehler S, et al. Evaluation of completeness of case ascertainment in Swiss cancer registration. Eur J Cancer Prev. 2017 Sep;26 Joining forces for better cancer registration in Europe:139-46. 10.1097/CEJ.0000000000000380
16. National Comprehensive Cancer Network [Internet]. Plymouth Meeting (PA): National Comprehensive Cancer Network; c2022. [cited 2022 Sep 28]. NCCN Guidelines Treatment by Cancer Type. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx
17. European Society for Medical Oncology [Internet]. Lugano: European Society for Medical Oncology; c2022. [cited 2022 Sep 28]. ESMO Clinical Practice Guidelines. Available from: https://www.esmo.org/guidelines
18. American Society of Clinical Oncology [Internet]. Alexandria (VA): American Society of Clinical Oncology (ASCO); c2022. [cited 2022 Sep 28]. Guidelines, tools, resources. Available from: https://www.asco.org/research-guidelines/quality-guidelines/guidelines
19. Koziol JA, Jia Z. The concordance index C and the Mann-Whitney parameter Pr(X>Y) with randomly censored data. Biom J. 2009 Jun;51(3):467–74. 10.1002/bimj.200800228
20. Garrett TM, Baillie HM, Garrett RM. The ethics of distribution. In: Garrett TM, Baillie HM, Garrett RM, editors. Health care ethics: principles and problems. 5th ed. Upper Saddle River (NJ): Pearson; 2010. pp. 83–7.
21. Heins MJ, de Jong JD, Spronk I, Ho VK, Brink M, Korevaar JC. Adherence to cancer treatment guidelines: influence of general and cancer-specific guideline characteristics. Eur J Public Health. 2017 Aug;27(4):616–20.
22. van de Water W, Bastiaannet E, Dekkers OM, de Craen AJ, Westendorp RG, Voogd AC, et al. Adherence to treatment guidelines and survival in patients with early-stage breast cancer by age at diagnosis. Br J Surg. 2012 Jun;99(6):813–20. 10.1002/bjs.8743
23. Jochum F, De Rozario T, Lecointre L, Faller E, Boisrame T, Dabi Y, et al. Adherence to European ovarian cancer guidelines and impact on survival: a French multicenter study (FRANCOGYN). Int J Gynecol Cancer. 2021 Nov;31(11):1443–52. 10.1136/ijgc-2021-002934
24. Aerzteblatt.de. [Internet]. Berlin: Deutscher Ärzteverlag GmbH; c2022 [cited 2022 Sep 28]. Geringere Sterblichkeit bei Therapie in zertifizierten Krebszentren; [about 3 screens]. Available from: https://www.aerzteblatt.de/nachrichten/133643/Geringere-Sterblichkeit-bei-Therapie-in-zertifizierten-Krebszentren
25. Klimm B, Brillant C, Skoetz N, Müller H, Engert A, Borchmann P. The effect of specialized cancer treatment centers on treatment efficacy in Hodgkin’s lymphoma. Dtsch Arztebl Int. 2012 Dec;109(51-52):893–9. 10.3238/arztebl.2012.0893
26. Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002 Apr;346(15):1128–37. 10.1056/NEJMsa012337
27. Huo YR, Phan K, Morris DL, Liauw W. Systematic review and a meta-analysis of hospital and surgeon volume/outcome relationships in colorectal cancer surgery. J Gastrointest Oncol. 2017 Jun;8(3):534–46. 10.21037/jgo.2017.01.25
28. Gooiker GA, van Gijn W, Post PN, van de Velde CJ, Tollenaar RA, Wouters MW. A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer. Are breast cancer patients better of with a high volume provider? Eur J Surg Oncol. 2010 Sep;36 Suppl 1:S27–35. 10.1016/j.ejso.2010.06.024
29. Stahel RA, Curioni-Fontecedro A, Rohrmann S, Dafni U, Sandner U, Opitz I, et al. Survival outcome of non-small cell lung cancer patients: Comparing results between the database of the Comprehensive Cancer Center Zürich and the Epidemiological Cancer Registry Zurich and Zug. Lung Cancer. 2020 Aug;146:217–23. 10.1016/j.lungcan.2020.05.037
30. Baker R. History of medical ethics. In: Bynum WF, Porter R, editors. Companion encyclopedia of the history of medicine. 1st ed. London: Routledge; 1993. p. 860.
31. Hastings Center. The goals of medicine. Setting new priorities. Hastings Cent Rep. 1996;26(6):S1–27. 10.2307/3528765
32. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013 Jan;346 jan28 1:f167. 10.1136/bmj.f167
33. Cook O, Daiyan Y, Yeganeh L, Davies AG, Kwok A, Webber K, et al. Exploration of the use and impact of patient-reported experience measures (PREMs) in oncology settings: a systematic review. J Clin Oncol. 2020 Oct;38(29_suppl Suppl 29):166. 10.1200/JCO.2020.38.29_suppl.166
34. American Cancer Society [Internet]. Atlanta: American Cancer Society, Inc; c2019 [cited 2021 August 20]. Breast cancer facts & figures 2019-2020; [about 44 pages]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf
35. Fallahpour S, Navaneelan T, De P, Borgo A. Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open. 2017 Sep;5(3):E734–9. 10.9778/cmajo.20170030
36. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020 Aug;383(7):640–9. 10.1056/NEJMoa1916623
37. Bundesgesetz as of 18 March 2016 über die Registrierung von Krebserkrankungen (Krebsregistrierungsgesetz [Cancer Registration Act] KRG; SR 818.33).
38. Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013 Nov;5 Suppl 1:3–29. 10.2147/CLEP.S47150