Tackling alert fatigue with a semi-automated
clinical decision support system: quantitative evaluation and end-user survey
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40082
Hendrike Dahmkeab,
Rico Fiumefreddoc,
Philipp Schuetzc,
Remo De Iacod,
Claudia Zaugga
a Hospital Pharmacy, Kantonsspital Aarau, Aarau,
Switzerland
b Department of Pharmaceutical Sciences, University of
Basel, Basel, Switzerland
c Department of General Internal and Emergency Medicine,
Kantonsspital Aarau, Aarau, Switzerland
d CISTEC AG, Zürich, Switzerland
Summary
STUDY AIMS: Clinical decision support systems (CDSS) embedded in hospital
electronic health records efficiently reduce medication errors, but there is a
risk of low physician adherence due to alert fatigue. At the Cantonal Hospital
Aarau, a CDSS is being developed that allows the highly accurate detection and
correction of medication errors. The semi-automated CDSS sends its alerts
either directly to the physician or to a clinical pharmacist for review first. Our
aim was to evaluate the performance of the recently implemented CDSS in terms
of acceptance rate and alert burden, as well as physicians’ satisfaction with
the CDSS.
METHODS: All alerts generated by the clinical decision support systems
between January and December 2021 were included in a retrospective quantitative
evaluation. A team of clinical pharmacists performed a follow-up to determine whether
the recommendation made by the CDSS was implemented by the physician. The
acceptance rate was calculated including all alerts for which it was possible
to determine an outcome. A web-based survey was conducted amongst physicians to
assess their attitude towards the CDSS. The survey questions included overall
satisfaction, helpfulness of individual algorithms, and perceived alert burden.
RESULTS: In 2021, a total of 10,556 alerts were generated, of which 619 triggered
a direct notification to the physician and 2,231 notifications were send to the
physician after evaluation by a clinical pharmacist. The acceptance rates were 89.8%
and 68.4%, respectively, which translates as an overall acceptance rate of 72.4%.
On average, clinical pharmacists received 17.2 alerts per day, while all of the
hospital physicians together received 7.8 notifications per day. In the survey,
94.5% of physicians reported being satisfied or very satisfied with the CDSS.
Algorithms addressing potential medication errors concerning anticoagulants received
the highest usefulness ratings.
CONCLUSION: The development of this semi-automated clinical decision
support system with context-based algorithms resulted in alerts with a high
acceptance rate. Involving clinical pharmacists proved a promising approach to
limit the alert burden of physicians and thus tackle alert fatigue. The CDSS is
well accepted by our
physicians.
Introduction
A
medication error is any preventable event that can cause or lead to either
inappropriate medication use or harm to the patient during the process of
medication [1]. Studies suggest that approximately 5% of all medication involves
a medication error; the estimate varies depending on the definition of medication
error and the collective of patients observed in the study [2–4]. Medication
errors are not only a risk to patient safety, but they also contribute to
health costs [5–7].
Clinical
decision support systems (CDSSs) embedded in hospital electronic health records
(EHR) can result in significant reduction of medication errors [8, 9]. However,
the most frequently mentioned problem of a CDSS is the phenomenon of alert
fatigue, which describes the ignoring or overriding of alerts [10]. Common
reasons for alert fatigue are the reception of high numbers of irrelevant
alerts and desensitisation from repeated exposure to the same alert,
particularly in a demanding and complex working environment [11]. Especially
interruptive pop-up notifications seem to trigger alert fatigue, with override rates
between 50% and >90% [12, 13]. There is a trade-off between interruption of
workflow and visibility of the alerts for interruptive and non-interruptive
CDSSs [14].
A CDSS can only achieve its potential if its target users actually
use it [15]. Therefore, the focus has changed from sensitive alerts towards the
increase of CDSS alert efficiency and usability [16, 17]. While the number of
CDSSs is increasing, information concerning physicians’ perception of such CDSSs
is still scarce. Recent surveys reveal that some physicians seem to be
completely dissatisfied, complaining of high numbers of irrelevant alerts [18],
while others appear to appreciate the added value despite the system's
shortcomings [19].
In our hospital,
we designed and introduced a semi-automated CDSS
(KPharm) into the hospital's EHR. The semi-automated approach involves role
tailoring, meaning that some alerts are automatically send to the prescribing
physicians, while others are first assessed by a clinical pharmacist. With 20
different context-based algorithms currently running, we evaluated the CDSS’s performance
in terms of alert burden and acceptance rate. Furthermore, we captured the physicians’
satisfaction with our CDSS with an end-user survey.
Methods
Setting
KPharm is a
clinical decision support system that was developed and implemented in a
tertiary care hospital with 669 beds in the north-western part of Switzerland. It
was initiated as a collaboration between the department of internal medicine
and the clinical pharmacy with the aim of increasing drug safety. The overall
goal of KPharm is the early detection of medication errors or situations with
an increased risk of an adverse drug event (ADE). A team of clinical
pharmacists and physicians developed drug safety algorithms, each covering a
variety of potential high-risk situations. The algorithms had been in use in an
external system since 2018. Starting from 2020, they were continuously
implemented in the hospital’s EHR (KISIM™ by CISTEC).
The
algorithms were designed taking the following principles into consideration:
- High
specificity: To fight alert fatigue, we aim to achieve a high specificity while
maintaining a tolerable sensitivity.
- Timing:
Considering the daily workflow of ward rounds, we introduced a time lag of one
hour. This allows physicians to complete the task of prescribing and to work
autonomously from the CDSS. The CDSS acts as a
safety net. Many alerts have shown to be self-limiting in the first few hours.
- Non-interruptive
notifications: Each interruption in the workflow increases the probability of medication
errors [20]. Unlike common CDSSs, we use
non-interruptive notifications that are displayed in the patient’s EHR. Here,
each health care professional working with the patient can see them, which
decreases the risk that they are being missed.
- Parametric:
The sensitivity of the alerts can be controlled with parametric thresholds. Further,
the CDSS can be easily accustomed to new drugs of
hospital specific guidelines.
- Semi-automation:
For each individual alert, we can define if it automatically produces a notification
in the patient's EHR or if it is first evaluated by a clinical pharmacist.
The drug
safety algorithms continuously check the medication of inpatients while taking
into account various aspects, such as prescribed dose, already administered
doses, patient characteristics, and laboratory values. Since diagnoses are not
stored in a coded manner, the algorithms cannot consider them. When a potential
drug-related problem is detected, an alert is triggered. The CDSS is
semi-automated: alerts addressing critical errors with high specificity (e.g.,
duplication of anticoagulants or digoxin overdoses) are automatically displayed
in the patient's EHR. For some alerts, the automatisation is only temporarily
(on weekends, holidays). The remaining alerts are evaluated by the clinical
pharmacist on day duty. In our hospital, clinical pharmacists’ day duty mainly
consists of responding to the telephone and assessing the alerts, which permits
the timely evaluation of these during office hours. To assure consistency, the
alerts are processed according to an internal guidebook. The guidebook takes
further aspects into account that cannot be used by the algorithm, e.g.,
because the information is not available in a structured form. If the alert is
considered relevant, a notification is generated which is displayed in the “messages”
line of the EHR. In critical cases,
the prescribing physician is contacted directly by telephone. The algorithms
will cancel the alerts and notifications autonomously as soon as the conditions
that triggered them no longer apply. The workflow is illustrated in figure 1.
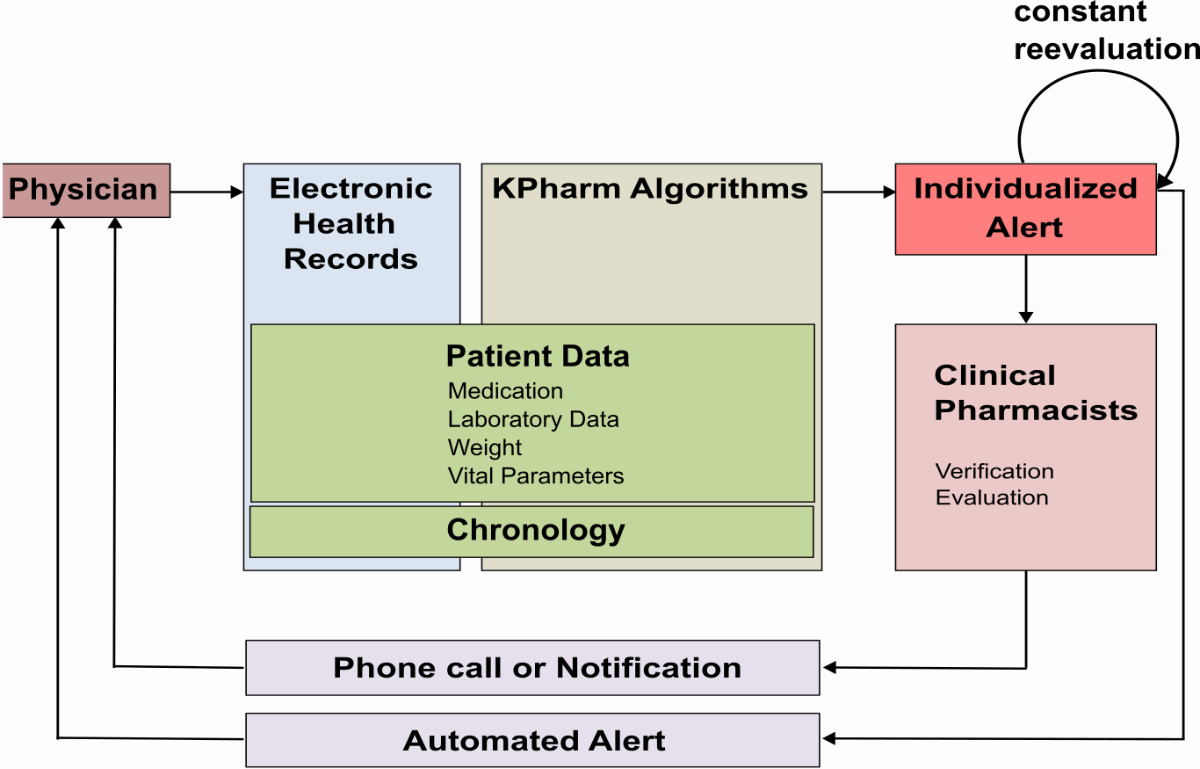
Figure 1Workflow
of the KPharm clinical decision support system: After the physician prescribes
a medication, the context-based KPharm algorithms access the information in the
electronic health records, as well as the chronology of events, to trigger
individualised alerts. Alerts are forwarded either directly to the physician or
first to a clinical pharmacist for review. An automatic constant re-evaluation
assures that only relevant alerts are displayed. KPharm: semi-automated clinical decision
support system.
The text of
the notification itself contains, in addition to the triggering factors, a brief
explanation and a specific recommendation for action (figure 2). If desired,
the physician can respond to the notification for clarification, cancel it, or
leave it in the EHR as a reminder.
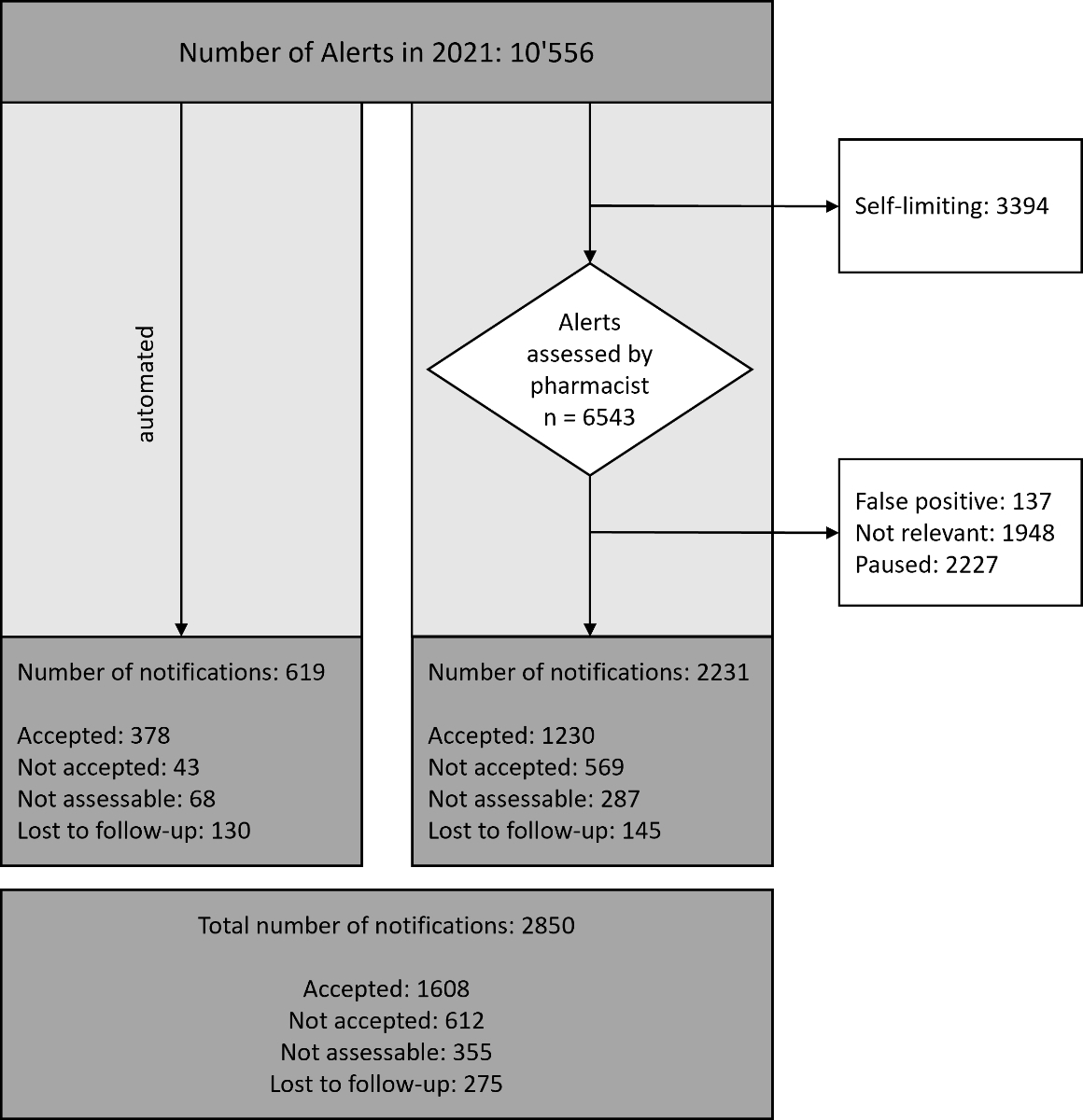
Figure 2Processing of all alerts in 2021: The left column shows the alerts that were automatically
sent to physicians. The right column shows the alerts that were first sent to clinical
pharmacists. All alerts that reached physicians through both ways are summarised in
the box at the bottom.
By the end
of 2021, we had integrated 19 algorithms that can trigger 194 different alerts,
including error messages. The decision to automate individual alerts was taken
as a group (CZ, RF, HD). We considered the frequency of alerts and how often they
were correct and accepted by the physician, as well as the potential patient
harm if they were not processed immediately. A list of the alerts and their
automation status can be found in the appendix 1.
Data
The clinical
decision support system is integrated into the hospitals’ EHR. The clinical pharmacists
process the alerts in an interface for checking, where the status of each alert
is documented. Alerts that are technically wrong (false positive) were
identified during this step. Alerts that were no longer valid before being
reviewed by a clinical pharmacist were automatically flagged as “self-limiting”. Alerts
that were technically
correct but not relevant for the individual patient were either paused or marked
as “not relevant” and were not forwarded to the
physician. The CDSS does not automatically capture whether
the notifications are read and accepted by physicians. Thus, when the alert
resulted in a notification to the physician, the clinical pharmacists performed
a retrospective follow-up to assess whether the notification had been accepted
or dismissed. We considered an intervention as accepted if it led to an
adjustment in patient management within a reasonable period. The period
considered reasonable depended on the recommendation of action in the
notification and the time of day in which the notification was send. If
discontinuation or dose adjustment of a drug was suggested, we expected a
change in medication on the same day, or if the notification was sent late in
the afternoon, the following morning. In case of uncertainty, e.g., if the notification
was sent shortly before the patient was discharged from the hospital or if the
medication was discontinued for a reason not related to the notification, the
follow-up was deemed not assessable.
Web-based survey
The questionnaire
was designed with key stakeholders of the development team in German language (appendix
2). The questionnaire is composed of three categories: demographic information,
opinions specifically concerning KPharm, and opinions concerning CDSSs in general.
The a priori outcome measures were overall
satisfaction and the helpfulness of the individual algorithms. We collected the
following demographic information: gender, age, professional title, clinic,
percentage of employment, and time at workplace. Concerning KPharm, we asked about
overall satisfaction, perceived frequency of alerts, and a rating of the
individual algorithms. We proposed several statements covering relevant aspects
of CDSSs to capture physicians’ attitudes towards KPharm.
Subsequently, physicians rated how important those aspects are for CDSS in
general. Additional questions targeted the preferred mode of receiving
notifications, further drug related problems to be solved in the future, and
experience with other CDSSs. We used an even Likert scale for items that
required rating. At the time of the survey, 16 algorithms were fully operating,
allowing for 193 individual alerts. For the survey, the algorithms for direct
oral anticoagulants (DOACs) were treated as one.
The survey
was distributed to physicians working on wards in our hospital via email using electronic
software (SurveyMonkey™, Momentive Inc., San Mateo, California, USA). Physicians
from wards that do not work with the EHR were not contacted. The survey period
was 16 days, with two reminders being sent on days 7 and 14. We promoted the
survey in the hospitals intranet and raffled three vouchers for a lunch at the
hospital’s canteen amongst the participants as an incentive. By participating,
the physicians agreed to their anonymised data being published.
Ethics approval
The study
was conducted according to the guidelines of the Declaration of Helsinki and
approved by the north-western and central Switzerland ethics committee
(Project-ID: 2021-01379)
The online
survey did not fall within the scope of the Swiss Human Research Act.
Therefore, authorisation from the ethics committee was not required. All
participants of the online survey agreed to their answers being published in an
anonymised form.
Statistical
analysis
Information
on all alerts in 2021 was imported from the EHR. Quantitative data were summarised
using counts and proportions. We calculated the acceptance rates as the
fraction of accepted notifications out of all notifications for which the
outcome was known. Alert burden was defined as the mean number of alerts
pharmacists and physicians must process in a day.
The results
of the online survey were received via export from the SurveyMonkey™ homepage. Demographic
characteristics were aggregated to conduct descriptive analysis using counts
and proportions and median and range, where appropriate. Responses to the
question concerning overall satisfaction and alert burden were stratified by
profession. Due to the small number of participants, attending physicians and
chiefs of service were analysed together. We analysed Likert scales using
counts and proportions and omitted non-assessable responses. For the statements
covering relevant aspects of CDSSs, Cronbach’s
alpha was calculated. With a value of 0.85, it showed good validity.
All analyses
were carried out using Jupyter® Notebook (version 6.1.5) with Python™ (version 3.9.2)
and the additional packages numpy (version 1.23.5), pandas (version 1.5.2), and
matplotlib (version 3.6.3) [21–23]. The code is available from github upon
request.
Results
Quantitative
evaluation
In the year
2021, the CDSS analysed 22,195 patient charts and generated
10,556 alerts for 5,204 individual patients. The highest number of alerts
received for an individual patient was 27. Only a small fraction (5.9%, n = 619)
of the alerts was automated and directly generated a notification to the
prescribing physician. The other alerts were directed to a clinical pharmacist
for assessment. About a third of the alerts (32.2%, n = 3,394) were
self-limiting, meaning that the alert had already ended itself before assessment.
The remaining 6,543 alerts were assessed by the respective clinical pharmacist
on duty, which corresponds to a workload of 17.2 alerts per day. In the course
of 2021, clinical pharmacists performed 2,231 (34.1% of all assessed alerts)
interventions by either phone call or notification. Other alerts were
considered correct but not relevant for the specific clinical situation and
were either ended (n = 1,948, 29.8%) or paused (n = 2,227, 34.0%). A small
number of alerts (n = 135, 2.1%) were false positive. The processing of the
alerts is displayed in figure 2.
Automated
and non-automated alerts combined, 27.0% (n = 2,850) of all alerts in 2021
resulted in a notification to the physician. This corresponds to 7.8 alerts per
day for the whole hospital. The team of clinical pharmacists performed a
retrospective follow-up and was able to determine the outcome for 76.2% (n = 2,094)
of the notifications. The acceptance rate was higher for the automated alerts (89.8%)
than for pharmacist-reviewed alerts (68.4%). Overall, the physicians accepted 1,608
notifications, which translates to an acceptance rate of 72.4%. Alerts
concerning the duplication of anticoagulants had the highest acceptance rate
(93.3%, total number of alerts = 276), whereas alerts concerning the dosing of
dabigatran had the lowest acceptance rate (33.3%, total number of alerts = 4).
Survey
The survey
was sent to 568 physicians, of which 152 participated, representing a response
rate of 26.8%. Of the participants, 113 completed the full survey, whereas 39
(24.2%) submitted only partially answered questions. The median age was 35
years (range: 25–60 years) and slightly more females participated (57.2%). Most
participants were physicians at entry level, were employed full time in the
internal medicine department, and had worked in our hospital for a medium of 3
years (range: 0–22 years). The characteristics of the participants are shown in
table 1.
Table 1Characteristics of survey participants.
| |
Participants (n =
152) |
| n |
% |
| Gender |
Female |
87 |
57.24 |
| Male |
63 |
41.45 |
| Not reported |
2 |
1.32 |
| Age |
<30 years |
31 |
20.39 |
| 30–34 years |
35 |
23.03 |
| 35–39 years |
34 |
22.37 |
| 40–44 years |
21 |
13.82 |
| ≥45 years |
26 |
17.11 |
| Not reported |
5 |
3.29 |
| Position |
Resident physician |
79 |
51.97 |
| Attending physician |
54 |
35.53 |
| Chief of service |
14 |
9.21 |
| Head of department |
5 |
3.29 |
| Not reported |
0 |
0.00 |
| Percentage of employment |
100% |
114 |
75.00 |
| 80%–99% |
16 |
10.53 |
| 50%–79% |
18 |
11.84 |
| <50% |
3 |
1.97 |
| Not reported |
1 |
0.66 |
| Time at workplace |
<1 year |
23 |
15.13 |
| 1–2 years |
30 |
19.74 |
| 2–5 years |
42 |
27.63 |
| 5–10 years |
31 |
20.39 |
| ≥10 years |
25 |
16.45 |
| Not reported |
1 |
0.66 |
Overall satisfaction with the CDSS was high, with 66.1%
being “satisfied”, 28.4% being “very satisfied”, and 5.5% being 'less satisfied'.
Forty-three
(28.3%) participants did not answer. Most participants (n = 75, 49.3%)
indicated that they had received at least one alert in the previous year,
whereas 48 (31.6%) had never received an alert and a further 29 did not
respond. Five of the six doctors who were less satisfied with the system said
that they had not received any alerts in the past year. Resident physicians received
more notifications than attending physicians and chief of service/head of
departments.
All
algorithms were considered helpful, with the algorithm addressing the
duplication of anticoagulants receiving the highest results and the algorithm
for medication errors of proton pump inhibitors receiving the lowest rating; however,
many physicians indicated not being familiar with specific algorithms (mean: 61.4%
for all algorithms). The least known algorithm was “xanthine oxidase inhibitor” which
was unknown to 86.6% of
responding physicians (n = 119). In the course of 2021, this specific algorithm
had only fired 27 alerts. An overview of all ratings is depicted in figure 3.
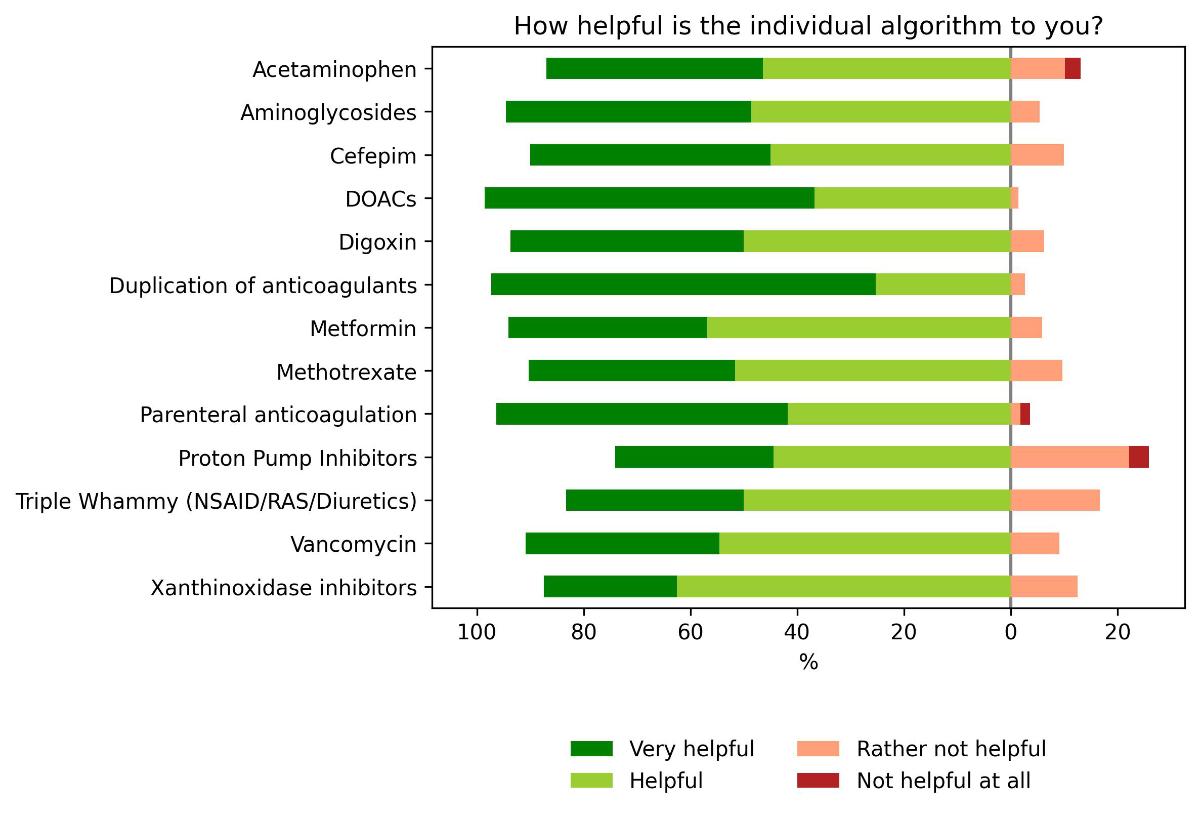
Figure 3Helpfulness of
individual alerts. Distribution of answers on the perceived helpfulness of the
individual algorithms. Helpfulness was rated using a Likert scale of 4 + 1 for
each algorithm; the answer “I do not know the algorithm” was excluded in this figure.
DOAC: direct oral anticoagulants
(apixaban, edoxaban, rivaroxaban, and dabigatran were treated as one algorithm);
NSAID: non-steroidal anti-inflammatory drugs; RAS: drugs inhibiting the
renin–angiotensin system.
Physicians'
attitudes towards relevant aspects of CDSSs were
captured by proposing several statements and asking the physicians to rate the
extent to which the statements were true in respect to our CDSS. In a
second question, the physicians rated how important this statement is for CDSSs
in general. All proposed statements were rated as rather important in a CDSS.
For an ideal CDSS, convenient timing, relevance of medication errors, and addressing
the correct health care professional received the most very important votes.
KPharm received strong confirmation that the alerts were written in an
understandable manner. Eight (10.1%, total respondents = 79) of the physicians
did not agree to the statement that the timing of the alerts was convenient.
This was the statement with the highest rate of disagreement. Overall, the
agreement rates were high (figure 4).
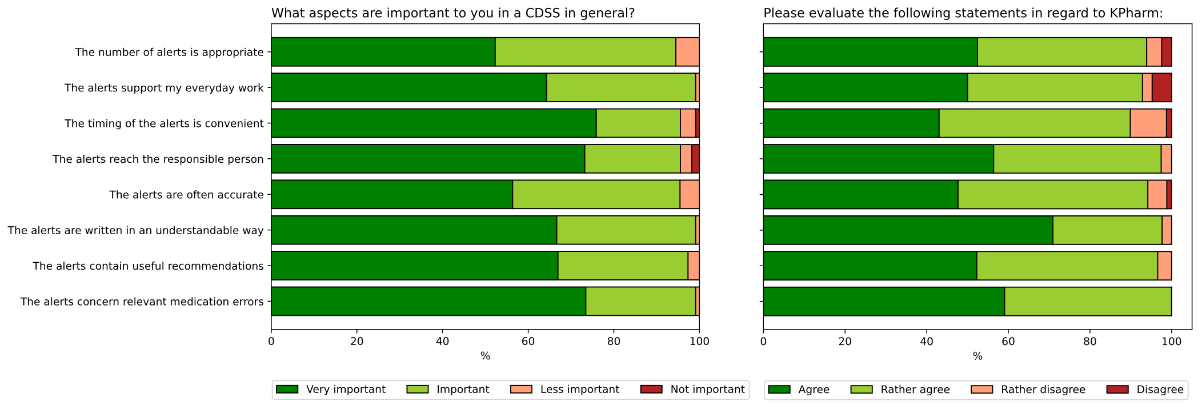
Figure 4Agreement to
satisfaction parameters for clinical decision support systems in general and
for KPharm. Distribution of answers regarding satisfaction parameters.
Physicians were asked how important each parameter is for a clinical decision
support system in general (left) and if those statements were true in regards
to KPharm (right). KPharm: semi-automated clinical decision support system.
Most
physicians indicated that they preferred “pop-ups” (38.0%) as a mode of receiving
notifications, followed by notifications in the EHR, emails, and phone calls
(28.3%, 21.2%, and 9.2%, respectively). Physicians who responded with 'other'
(3.3%) indicated that they would like the mode of notification to be adapted to
the urgency of the content. With only 27 physicians making suggestions for
future algorithms, we could not establish a clear hierarchy of topics. The most
common answer (n = 6) was the need for an allergy alert. Out of the 114 respondents,
only seven (6.1%) indicated that they already had experience with other CDSSs, obviating
further questions aimed at a comparison of
perceptions of different CDSSs.
Table 2Frequency of
alerts and satisfaction stratified by professional position.
| Questions and possible responses |
Overall |
Resident physician |
Attending physician |
Chief of service or head of department |
| n = 152 |
[%] |
n = 79 |
[%] |
n = 54 |
[%] |
n = 19 |
[%] |
| How often did you receive alerts over the past
year? |
Several times per week |
7 |
4.61 |
4 |
5.06 |
2 |
3.7 |
1 |
5.26 |
| Several times per month |
22 |
14.47 |
15 |
18.99 |
6 |
11.11 |
1 |
5.26 |
| Several times per year |
46 |
30.26 |
24 |
30.38 |
17 |
31.48 |
5 |
26.32 |
| Never |
48 |
31.58 |
18 |
22.78 |
19 |
35.19 |
11 |
57.89 |
| Not reported |
29 |
19.08 |
18 |
22.78 |
10 |
18.52 |
1 |
5.26 |
| How satisfied are you overall? |
Very satisfied |
31 |
20.39 |
17 |
21.52 |
11 |
20.37 |
3 |
15.79 |
| Satisfied |
72 |
47.37 |
35 |
44.3 |
26 |
48.15 |
11 |
57.89 |
| Less satisfied |
6 |
3.95 |
4 |
5.06 |
1 |
1.85 |
1 |
5.26 |
| Not satisfied at all |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Not reported |
43 |
28.29 |
23 |
29.11 |
16 |
29.63 |
4 |
21.05 |
Discussion
We evaluated
the performance of our clinical decision support system KPharm in terms of acceptance
rate and alert burden. The quantitative analysis revealed an overall acceptance
rate of 72.4%, which is higher than many commercially available CDSSs [19, 24–26].
Since different methods for measuring acceptance are in use, the direct
comparison of acceptance rates is difficult [27]. Recent reviews summarised the
overwrite rates of CDSSs with drug-drug interaction alerts, which can be
interpreted as the reciprocal of the acceptance rate, and found overwrite rates
between 46.2% and 98% [12, 28]. However, those reviews included CDSSs since 2000,
and advanced CDSSs with conceptualised alerts have been found to have improved
performance parameters.
A promising
approach to improving performance parameters is role tailoring. In a review by
Hussain et al. comparing 39 CDSSs with different designs,
only the four designs that involved role tailoring appeared to increase
prescriber acceptance [27]. Muylle et al. implemented clinical pharmacist
interventions to improve their CDSS [6]. Out of a total of 2,630 alerts within
8 months, 61 (2.3%) led to an intervention through clinical pharmacy. These
interventions were accepted 53 times (86.9%). Likewise, Skalafouris et al. presented
a CDSS that involves clinical pharmacists [29]. Of the 447 alerts within 132
days, 20.1% were forwarded to the physician by the clinical pharmacist. The
physicians accepted 71.0% of the suggested interventions. The acceptance of CDSS alerts
preselected by clinical pharmacists may be compared
with the acceptance of pharmacist interventions without a CDSS, which have been
studied more thoroughly [30–32]. According to the setting and medication errors
addressed, the acceptance rates vary greatly and can range between 41% and 95% [30,
33]. While the acceptance rates of CDSSs with clinical pharmacists are not
lower than those of pharmacists’ interventions, they are more time efficient [29].
With
KPharm, we combine two strategies by having high priority and specificity
alerts that go directly to the physician and semi-automated alerts that are
first evaluated by a clinical pharmacist. In our analysis, the acceptance rate
was higher for direct alerts than for pharmacist interventions. This may be due
to an underlying selection bias, since we only automated alerts that have shown
a high specificity and acceptance rate in the past.
A major
factor contributing to alert fatigue is alert burden. The more alerts a
physician receives, the less likely he will accept the intervention due to desensitisation
[11]. The comparison of alert burden between different CDSSs is often not meaningful,
since it depends largely on the number
of medication errors covered by the algorithms. Nonetheless, in a
cross-sectional survey with more than 1000 participants from 8 countries,
around half of the respondents regarded possible alert overload as a major
problem [34]. We found the alert burden of KPharm to be acceptable for both
clinical pharmacists and physicians. In our experience, up to 20 alerts a day are
very well manageable for one pharmacist, especially since the text for the
notifications is already pre-written and rarely needs adjustment. Positive
feedback from pharmacists indicates little alert fatigue on their side. Also,
the physicians’ alert burden was perceived as low, with only 4.6% of physicians
receiving several alerts per week. Moreover, 93.9% of physicians participating
in the survey agreed with the statement that the number of alerts was
appropriate.
Another
factor that largely contributes to the low alert burden is the timing of the
alerts. Taking clinical reality in hospitals into account, we introduced the
time lag of one hour, in which the physicians had the possibility to detect and
correct potential medication errors by themselves. Further, the implementation
of the CDSS in the EHR allows for an hourly re-evaluation
of the alerts, and the alert is cancelled automatically when the conditions
that trigger it no longer apply. Interestingly, even with the time lag of an
hour, a large proportion of alerts were self-limiting within the first four hours. Overall, the involvement of
pharmacists and the hourly re-evaluation of alerts effectively reduced the
alert burden of physicians by 73.4% from 10,705 alerts to 2850 alerts in 2021,
eliminating one major risk factor for alert fatigue: irrelevant alerts.
When
designing alerts with high specificity, we encountered several difficulties. In
our EHR, diagnoses are not coded until after the patient leaves the hospital. Thus,
they could not be integrated in the algorithms but would have been tremendously
helpful in some situations. For example, the algorithm detecting unnecessary
proton pump inhibitors would be better if patients with gastrointestinal
bleeding were excluded automatically. In some cases, we found workarounds: the prescription
of Entresto® (sacubitril/valsartan) or Verquvo® (vericiguat) can be used as a proxy
for the diagnosis of
heart insufficiency since this is the only indication for those drugs. Alerts
that consider the chronology of events are dependent on a timely documentation.
We measured
the physicians' satisfaction with our CDSS with an
end-user survey. The results of the online survey showed a vast support amongst
physicians for our CDSS. This is also supported by
the response rate, which is higher than in similar surveys [18, 19, 35]. Some
participants indicated that they were not receiving alerts in their daily work but
participated in the survey because they welcomed the project and considered it
relevant for drug safety.
While all algorithms
were generally well accepted, those addressing haematological medication errors
received the highest support. We assume that physicians’ perceptions of usefulness
are determined by the gravity of the medication error: a duplicated
anticoagulant is worse than receiving a proton pump inhibitor without
indication. Interestingly, many physicians indicated not being familiar with
certain algorithms. For this, we provide the following hypotheses. Firstly,
high specificity reduces the number of alerts, and the quantity of alerts
produced by the individual algorithms differs greatly. Therefore, some
physicians may never have encountered such an alert. Secondly, not every
algorithm is equally relevant to certain clinics. For example, a physician from
the clinic of ophthalmology may rarely encounter an alert for methotrexate.
Limitations
The
quantitative evaluation comes with several limitations. The CDSS does not automatically
register if a notification is read by the
physician. Therefore, it was necessary to perform a follow-up by revisiting the
patient's chart. The follow-up was performed retrospectively by a team of
clinical pharmacists. Due to the large number of different recommendations, we
had no universal standard as to what “accepted” means. When in doubt, the alert was
discussed with a colleague.
However, in cases where such consent was not sought, personal interpretations of
the clinical situation may differ. There is a possibility that measures such as
dose adjustments were done independently from the notification. We tried to
account for this by paying close attention to the chronology of events after
the notification was posted in the patient’s chart. When in doubt, the alert
was deemed ‘not assessable’ and excluded from the calculation of the acceptance
rate. Since 9.6% (275 of 2850) of notifications were lost to follow-up and could
not be evaluated, the data may be skewed. Moreover, if a patient received
several relevant alerts on the same day, they were combined in one notification
in order to reduce the “message burden”, while the others were paused. This analysis
does not account for this,
so the number of interventions is underestimated.
A
limitation to the online survey may be an underlying selection bias. Since
participation was voluntary, physicians with a positive attitude towards our CDSS
may have been more inclined to participate. However,
the response rate was high in comparison to other surveys [18, 19]. The
raffling of a free lunch voucher, valued at approx. 15 CHF, may have been a
strong incentive. Although we showed a picture of an alert and asked if
physicians had already seen such an alert, other physicians may have been
motivated to participate. The high number of physicians who claim to never have
received an alert may influence the generality of the responses. Those physicians
might be either senior physicians and/or physicians working in a ward
predominantly using another EHR. Due to the raffle incentive, the email
addresses of the participants were known to the authors, and fear of being identified
as a poor prescriber might have influenced the responses, especially regarding
the number of received alerts. Somewhat surprisingly, most of the physicians
who indicated to be less satisfied with the system also stated that they had never
received any alerts. Since we did not include a free text option to this
question, the reasoning behind the dissatisfaction remains speculative.
For the
evaluation of clinical decision support, PPV (positive predictive value) and NPV (negative
predictive value) are relevant parameters. Previous studies have
shown that the PPV of CDSSs is commonly low [36].
CDSSs that include a greater number of patients’ individual parameters tend to
have a higher PPV than automated CDSSs. We are not able to provide those
metrics at this point but are currently performing such analysis for individual
alerts and intend to publish the results in the future.
Outlook
As of May
2022, we have 20 context-based algorithms embedded in the EHR, which allow us to
find 202 individual potential drug-related problems. We plan to develop further
algorithms in the future in close collaboration with our hospitals’ physicians.
We also seek cooperation with other developers of CDSSs, as the improvement and spreading
of effective and specific contextualised
algorithms will ultimately benefit all patients. The KPharm algorithms and
their implementation into our EHR (KISIM™) will be commercially available in
the future.
We
carefully considered the feedback from the survey to find ways to further
tailor KPharm according to physicians’ needs. When comparing the physicians’
agreement in relation to the statements regarding important aspects of a CDSS, we
noticed that while timing is regarded as very
important in a CDSS in general, KPharm's rating in relation to this was
comparably low. Moreover, physicians indicated that their preferred way of
receiving notifications was a pop-up alert. Both aspects, timing and mode of
notification, were discussed within the development team. While we will
maintain our paradigm of non-interruptive alerts, we plan to automate further
alerts with high specificity and acceptance rates. This will reduce the alert delay
resulting from pharmacists assessing alerts.
Conclusion
The
development of context-based algorithms with specific algorithms resulted in
alerts with a comparably high acceptance rate. The involvement of clinical pharmacists
in a semi-automated CDSS is a promising approach to
limit the alert burden of physicians and tackle alert fatigue. The alert burden
for the physicians was low, and a vast majority indicated that the number of
alerts was appropriate. The CDSS is well accepted
amongst our physicians.
Data sharing
The
datasets are available from the authors upon reasonable request.
Acknowledgments
The authors would like to thank all
participants of the online survey. Special thanks go to all the clinical
pharmacists at the hospital who performed follow-ups on all alerts and Alison C
Zaugg for language editing the manuscript.
Authors
contributions: Conceptualisation: HD, RF, PS, CZ;
Programming and implementation: RDI, HD, CZ; Funding acquisition: RF, PS, CZ;
Data curation: HD, CZ; Formal analysis: HD; Writing – original draft: HD; Supervision, writing
– review and editing: CZ,
RF, RDI, PS. All authors have read and agreed to the final version of the
manuscript.
Hendrike Dahmke
Hospital Pharmacy
Kantonsspital Aarau
CH-5000 Aarau
hendrike.dahmke[at]ksa.ch
References
1. Aronson JK. Medication errors: definitions and classification. Br J Clin Pharmacol.
2009 Jun;67(6):599–604. 10.1111/j.1365-2125.2009.03415.x
2. Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related
problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379–407.
10.2165/00002018-200730050-00003
3. Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of Perioperative Medication
Errors and Adverse Drug Events. Anesthesiology. 2016 Jan;124(1):25–34. 10.1097/ALN.0000000000000904
4. Westbrook JI, Sunderland NS, Woods A, Raban MZ, Gates P, Li L. Changes in medication
administration error rates associated with the introduction of electronic medication
systems in hospitals: a multisite controlled before and after study. BMJ Health Care
Inform. 2020 Aug;27(3):e100170. 10.1136/bmjhci-2020-100170
5. Convertino I, Salvadori S, Pecori A, Galiulo MT, Ferraro S, Parrilli M, et al. Potential
Direct Costs of Adverse Drug Events and Possible Cost Savings Achievable by their
Prevention in Tuscany, Italy: A Model-Based Analysis. Drug Saf. 2019 03;42(3):427-44.
6. Muylle KM, Gentens K, Dupont AG, Cornu P. Evaluation of an optimized context-aware
clinical decision support system for drug-drug interaction screening. Int J Med Inform.
2021 Apr;148:104393. 10.1016/j.ijmedinf.2021.104393
7. Hardmeier B, Braunschweig S, Cavallaro M, Roos M, Pauli-Magnus C, Giger M, et al. Adverse
drug events caused by medication errors in medical inpatients. Swiss Med Wkly. 2004 Nov;134(45-46):664–70.
10.4414/smw.2004.10801
8. Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and
clinical decision support systems on medication safety: a systematic review. Arch
Intern Med. 2003 Jun;163(12):1409–16. 10.1001/archinte.163.12.1409
9. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview
of clinical decision support systems: benefits, risks, and strategies for success.
NPJ Digit Med. 2020 Feb;3(1):17. 10.1038/s41746-020-0221-y
10. Cash JJ. Alert fatigue. Am J Health Syst Pharm. 2009 Dec;66(23):2098–101. 10.2146/ajhp090181
11. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R, et al. Effects of workload,
work complexity, and repeated alerts on alert fatigue in a clinical decision support
system. BMC Med Inform Decis Mak. 2017 04;17(1):36. 10.1186/s12911-017-0430-8
12. Poly TN, Islam MM, Yang HC, Li YJ. Appropriateness of Overridden Alerts in Computerized
Physician Order Entry: systematic Review. JMIR Med Inform. 2020 Jul;8(7):e15653. 10.2196/15653
13. Nanji KC, Seger DL, Slight SP, Amato MG, Beeler PE, Her QL, et al. Medication-related
clinical decision support alert overrides in inpatients. J Am Med Inform Assoc. 2018
05 01;25(5):476-81.
14. Blecker S, Pandya R, Stork S, Mann D, Kuperman G, Shelley D, et al. Interruptive Versus
Noninterruptive Clinical Decision Support: usability Study. JMIR Human Factors. 2019 Apr;6(2):e12469.
10.2196/12469
15. Carroll AE. Averting Alert Fatigue to Prevent Adverse Drug Reactions. JAMA. 2019 Aug;322(7):601.
10.1001/jama.2019.11710
16. Chou E, Boyce RD, Balkan B, Subbian V, Romero A, Hansten PD, et al. Designing and
evaluating contextualized drug-drug interaction algorithms. JAMIA Open. 2021 Mar;4(1):ooab023.
10.1093/jamiaopen/ooab023
17. Chien SC, Chen YL, Chien CH, Chin YP, Yoon CH, Chen CY, et al. Alerts in Clinical
Decision Support Systems (CDSS): A Bibliometric Review and Content Analysis. Healthcare
(Basel). 2022 Mar;10(4):601. 10.3390/healthcare10040601
18. Baysari MT, Zheng WY, Van Dort B, Reid-Anderson H, Gronski M, Kenny E. A Late Attempt
to Involve End Users in the Design of Medication-Related Alerts: Survey Study. J Med
Internet Res. 2020 03;22(3):e14855.
19. Van De Sijpe G, Quintens C, Walgraeve K, Van Laer E, Penny J, De Vlieger G, et al. Overall
performance of a drug-drug interaction clinical decision support system: quantitative
evaluation and end-user survey. BMC Med Inform Decis Mak. 2022 02 22;22(1):48. 10.1186/s12911-022-01783-z
20. Johnson M, Sanchez P, Langdon R, Manias E, Levett-Jones T, Weidemann G, et al. The
impact of interruptions on medication errors in hospitals: an observational study
of nurses. J Nurs Manag. 2017 Oct;25(7):498–507. 10.1111/jonm.12486
21. Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array
programming with NumPy. Nature. 2020 Sep;585(7825):357–62. 10.1038/s41586-020-2649-2
22. McKinney W. Data structures for statistical computing in python. Proceedings of the
9th Python in Science Conference; 2010: Austin, TX; 2010. p. 51-6. 10.25080/Majora-92bf1922-00a
23. Hunter JD. Matplotlib: A 2D Graphics Environment. Comput Sci Eng. 2007;9(3):90–5.
10.1109/MCSE.2007.55
24. Moja L, Polo Friz H, Capobussi M, Kwag K, Banzi R, Ruggiero F, et al. Effectiveness
of a Hospital-Based Computerized Decision Support System on Clinician Recommendations
and Patient Outcomes: A Randomized Clinical Trial. JAMA Netw Open. 2019 12;2(12):e1917094.
10.1001/jamanetworkopen.2019.17094
25. Wright A, Aaron S, Seger DL, Samal L, Schiff GD, Bates DW. Reduced Effectiveness of
Interruptive Drug-Drug Interaction Alerts after Conversion to a Commercial Electronic
Health Record. J Gen Intern Med. 2018 11;33(11):1868-76.
26. Yoo J, Lee J, Rhee PL, Chang DK, Kang M, Choi JS, et al. Alert Override Patterns With
a Medication Clinical Decision Support System in an Academic Emergency Department:
Retrospective Descriptive Study. JMIR Med Inform. 2020 Nov;8(11):e23351. 10.2196/23351
27. Hussain MI, Reynolds TL, Zheng K. Medication safety alert fatigue may be reduced via
interaction design and clinical role tailoring: a systematic review. J Am Med Inform
Assoc. 2019 10;26(10):1141-9.
28. Villa Zapata L, Subbian V, Boyce RD, Hansten PD, Horn JR, Gephart SM, et al. Overriding
Drug-Drug Interaction Alerts in Clinical Decision Support Systems: A Scoping Review.
Stud Health Technol Inform. 2022 Jun;290:380–4. 10.3233/SHTI220101
29. Skalafouris C, Reny JL, Stirnemann J, Grosgurin O, Eggimann F, Grauser D, et al. Development
and assessment of PharmaCheck: an electronic screening tool for the prevention of
twenty major adverse drug events. BMC Med Inform Decis Mak. 2022 May;22(1):146. 10.1186/s12911-022-01885-8
30. Gaskin J, Conyard E. Clinical pharmacist interventions in the emergency department
and their impact on preventable adverse drug events and associated cost avoidance.
Eur J Hosp Pharm Sci Pract. 2017;24:A81.
31. Cohen V, Jellinek SP, Hatch A, Motov S. Effect of clinical pharmacists on care in
the emergency department: a systematic review. Am J Health Syst Pharm. 2009 Aug;66(15):1353–61.
10.2146/ajhp080304
32. Drovandi A, Robertson K, Tucker M, Robinson N, Perks S, Kairuz T. A systematic review
of clinical pharmacist interventions in paediatric hospital patients. Eur J Pediatr.
2018 Aug;177(8):1139–48. 10.1007/s00431-018-3187-x
33. Richardson M, Dwyer CO, Gaskin J, Conyard E, Murphy K. The potential contribution
of medicines to falls in older persons and the acceptance of pharmacist intervention.
Int J Pharm Pract. 2020;28:57–8.
34. Gallo T, Curry SC, Padilla-Jones A, Heise CW, Ramos KS, Woosley RL, et al. A computerized
scoring system to improve assessment of heparin-induced thrombocytopenia risk. J Thromb
Haemost. 2019 02;17(2):383-8.
35. Gallo T, Heise CW, Woosley RL, Tisdale JE, Antonescu CC, Gephart SM, et al. Clinician
Satisfaction With Advanced Clinical Decision Support to Reduce the Risk of Torsades
de Pointes. J Patient Saf. 2022 Sep;18(6):e1010–3. 10.1097/PTS.0000000000000996
36. Carli D, Fahrni G, Bonnabry P, Lovis C. Quality of Decision Support in Computerized
Provider Order Entry: Systematic Literature Review. JMIR Med Inform. 2018 Jan;6(1):e3.
10.2196/medinform.7170
Appendix 1
Table S1Algorithms included in the online survey and their corresponding alerts.
| Algorithm |
Alert No. |
Alert |
Automated |
| Duplication
of anticoagulants |
1 |
Duplication
of anticoagulants |
Only on
weekends |
| 2 |
Heparin/fondaparinux prescription
for therapeutic INR during/after vitamin K antagonist therapy |
Only on
weekends |
| 3 |
The heparin (or fondaparinux) may
be started at an INR >2 |
No |
| 4 |
DOAC is started too early when
switching from VKA |
Yes |
| 5 |
DOAC is started too early when
switching from fondaparinux |
No |
| 6 |
DOAC is started too early when
switching from low molecular weight heparins |
No |
| 7 |
Error in the calculation of one of
the dosages of the affected preparations |
No |
| Aminoglycoside |
1.1 |
High aminoglycoside
dosage |
No |
| 1.2 |
High aminoglycoside dosage based
on corrected weight |
No |
| 1.3 |
Aminoglycoside therapy for high
body weight; the corrected body weight cannot be calculated |
No |
| 1.4 |
No current weight available with
aminoglycoside therapy |
No |
| 2 |
Aminoglycoside and eGFR
<30ml/min |
No |
| 3 |
Aminoglycoside and dialysis |
No |
| 4 |
Drop in eGFR during aminoglycoside
therapy |
No |
| 5.1 |
No adequate renal monitoring
during aminoglycoside therapy |
No |
| 5.2 |
No adequate renal monitoring
during aminogylcoside therapy in combination with a drug that elevates the
serum creatinine |
No |
| 5.3 |
No adequate renal monitoring
during aminogylcoside therapy in pediatric patients |
No |
| 5.4 |
No adequate therapeutic drug
monitoring during aminogylcoside therapy in pediatric patients |
No |
| 6 |
Multiple aminoglycoside therapies
in the last 3 months |
No |
| 7 |
Probably not a trough level. The
blood sample was taken at the wrong time |
No |
| 8 |
Aminoglycoside in combination with
a drug that elevates the serum creatinine |
No |
| 9 |
Long duration of aminoglycoside
therapy |
No |
| 10 |
Error during one of the tests |
No |
| Apixaban |
1 |
Apixaban with a strong inductor of
CYP3A4 and P-gp |
No |
| 2 |
Apixaban dosage may be too high |
No |
| 3 |
Apixaban with dual inhibitor of
CYP3A4 / P-gp |
No |
| 4.1 |
Apixaban dosage is possibly too
low in the absence of criteria for dose reduction |
No |
| 4.2 |
Apixaban dosage is possibly too
low in the presence of only one criterion for dose reduction |
No |
| 5 |
Apixaban and eGFR <15 ml/min |
No |
| 6 |
Apixaban dosage is possibly too
high in triple anticoagulation |
No |
| 7 |
Apixaban during dual antiplatelet
therapy |
No |
| 8 |
Off-label apixaban treatment
regimen |
No |
| 9 |
Multiple apixaban
prescriptions |
No |
| 10 |
Apixaban and body weight >150
kg |
No |
| Cefepime |
1 |
Drop in eGFR during cefepime
therapy |
No |
| 2 |
Cefepime and eGFR <10 ml/min
or dialysis |
No |
| 3 |
Cefepime and GFR 10–30 ml/min/1.73
m2 |
No |
| 4 |
Cefepime and GFR 30–50 ml/min/1.73
m2 |
No |
| 5.1 |
No adequate renal monitoring
during cefepime therapy |
No |
| 5.2 |
No adequate renal monitoring
during cefepime therapy in combination with a drug that elevates the serum
creatinine |
No |
| 6 |
Cefepime in combination with a
drug that elevates the serum creatinine |
No |
| 7 |
Cefepime and
epilepsy |
No |
| 8 |
Error during one of the tests |
No |
| Dabigatran |
1 |
Dabigatran with strong inductor of
P-gp |
No |
| 2 |
Dabigatran dosage may be too high |
No |
| 3 |
Dabigatran with inhibitor of P-gp |
No |
| 4 |
Dabigatran dosage may be too low |
No |
| 5 |
Dabigatran
and eGFR <30 ml/min |
No |
| 6 |
Dabigatran dosage may be too high
during triple anticoagulation |
No |
| 7 |
Dabigatran dosage may be too high
during dual antiplatelet therapy |
No |
| 8 |
Wrong
dabigatran therapy regimen |
No |
| 9 |
Multiple
dabigatran prescriptions |
No |
| 10 |
Dabigatran
via feeding tube |
No |
| 11 |
Dabigatran and body weight >150 kg |
No |
| Digoxin |
1 |
Digoxin saturation is
reached/exceeded or daily dose is given in two doses |
Yes |
| 2 |
Digoxin dosage may be too high |
No |
| 3 |
Digoxin dosage may be too high in
geriatric patients (>65 years) |
No |
| 4 |
Digoxin dosage may be too high due
to reduced kidney function |
No |
| 5 |
Digoxin and GFR <20 ml/min |
No |
| 6 |
Risk of digoxin toxicity in the
presence of hypokalemia or hypomagnesemia |
No |
| 7 |
Digoxin and risk of hypokalemia |
No |
| 8 |
Therapeutic drug monitoring of
digoxin: the blood sample was not taken at the right time |
No |
| 9 |
Digoxin level is too high without
previous documented measure |
No |
| 10 |
Digoxin and inhibitors of P-gp |
No |
| 11 |
Digoxin and inductors of P-gp |
No |
| 12 |
Potentially inadequate monitoring
of renal function during digoxin therapy |
No |
| 13 |
An error
occured |
No |
| Edoxaban |
1 |
Edoxaban and strong inductors of
P-gp |
No |
| 2 |
Edoxaban dosage may be too high |
No |
| 3 |
Edoxaban and strong inhibitors of
P-gp |
No |
| 4 |
Edoxaban dosage may be too low |
No |
| 5 |
Edoxaban and eGFR <15 ml/min |
No |
| 6 |
Edoxaban and eGFR >95 ml/min |
No |
| 7 |
The edoxaban dosage may be too
high in triple anticoagulation with ASA and clopidogrel |
No |
| 8 |
Edoxaban dosage of 60 mg/d with
two antiplatelet agents |
No |
| 9 |
Wrong therapy
regimen |
No |
| 10 |
Multiple
edoxaban prescriptions |
No |
| 11 |
Edoxaban and body weight >150
kg |
No |
| Metformin |
1 |
Metformin and
eGFR <30 ml/min |
No |
| 2 |
Metformin and
eGFR 30–45 ml/min |
No |
| 3 |
Metformin and
eGFR 45–60 ml/min |
No |
| 4 |
No adequate renal monitoring
during metformin therapy in patients with eGFR <60 ml/min |
No |
| 5 |
Renal function unknown and
metformin therapy |
No |
| 6 |
Errors in calculating the
metformin dose |
No |
| Methotrexate |
1 |
Weekly dose of methotrexate
potentially too high or two active prescriptions |
No |
| 2 |
Inappropriate frequency of
methotrexate administration |
No |
| 3 |
Methotrexate dosage may be too
high due to reduced kidney function |
No |
| 4 |
Methotrexate and eGFR <20
ml/min |
no |
| 5 |
Lack of folic acid supplementation |
No |
| 6 |
Inappropriate
folic acid supplementation |
No |
| 7 |
Increased ALAT levels during
methotrexate therapy |
No |
| 8 |
Potential interaction with high
dose methotrexate therapy |
No |
| 9 |
Potential interaction with low
dose methotrexate therapy |
No |
| 10 |
Methotrexate in women of
childbearing age without contraception |
No |
| 11 |
Methotrexate prescription in case
of suspected infection |
No |
| 12 |
Potentially
erroneous prescription |
No |
| 13 |
Methotrexate
prescribed periodically |
No |
| 14 |
Error: one of the calculations
could not be performed |
No |
| Paracetamol (acetaminophen) |
1 |
Potential
overdose of paracetamol |
Yes |
| 2 |
The prescribed paracetamol dosage
is higher than the maximum daily dose |
Yes |
| 3 |
Paracetamol overdose when reserve
medication is exhausted |
Yes |
| 4 |
High paracetamol dosage and low
body weight (50–60 kg) |
No |
| 5 |
Paracetamol dosage too high for
body weight <50 kg |
Yes |
| 6 |
Paracetamol is dosed too high in
combination with inductor |
No |
| 7 |
Error: one of the calculations
could not be performed |
No |
| Parenterale AK |
1 |
Fondaparinux and eGFR < 16
ml/min |
No |
| 2 |
Fondaparinux and eGFR 16-30 ml/min |
No |
| 3 |
Fondaparinux thromboprophylaxis
and body weight <50 kg: use with caution |
No |
| 4 |
Fondaparinux and body weight
<50 kg: consider dose adjustments |
No |
| 5 |
Fondaparinux and body weight
51–100 kg: consider dose adjustments |
No |
| 6 |
Fondaparinux and body weight >100 kg: consider dose adjustments |
No |
| 7 |
Therapeutic dalteparin and eGFR
<20 ml/min |
No |
| 8.1 |
No monitoring of kidney function
during dalteparin prophylaxis and eGFR <15 ml/min |
No |
| 8.2 |
Monitoring of kidney function
during dalteparin prophylaxis and eGFR <15 ml/min |
No |
| 9 |
Therapy with low molecular weight
heparins and eGFR <30 ml/min |
No |
| 10 |
Thrombosis prophylaxis with low
molecular weight heparins and body weight <50 kg: dose adjustment
recommended |
Yes |
| 11 |
Thrombosis prophylaxis with low
molecular weight heparins and body weight >100 kg: consider dose
adjustment |
No |
| 12 |
Check dose of low molecular weight
heparins |
No |
| 13 |
Exceeded maximum daily dose of low
molecular weight heparins |
No |
| 14 |
An error has
occurred |
No |
| Proton pump inhibitors (PPI) |
1 |
Absence of PPI: NSAID and
antiplatelet therapy |
No |
| 2 |
Absence of PPI: NSAID and therapeutic
anticoagulation |
No |
| 3 |
Absence of PPI: NSAID and
glucocorticoid therapy |
No |
| 4 |
Absence of PPI: NSAID therapy and
other drug with GI bleeding risk |
No |
| 5 |
Absence of PPI: NSAID and age ≥65
years |
No |
| 6 |
Absence of PPI: NSAID and thrombocytes
<30 G/l |
No |
| 7 |
Absence of PPI: triple
anticoagulation |
No |
| 8 |
Absence of PPI: therapeutic
anticoagulation, low-dose ASA and additional risk factor |
No |
| 9 |
Absence of PPI: dual antiplatelet
therapy and additional risk factor |
No |
| 10 |
Absence of PPI: ASS,
glucocorticoids and age ≥65 years |
No |
| 11 |
Absence of PPI in the presence of
at least 4 risk factors |
No |
| 12 |
Prescription of PPI without risk
factors |
No |
| Rivaroxaban |
1 |
Rivaroxaban with strong CYP3A4
inductor |
No |
| 2 |
Rivaroxaban and eGFR <15
ml/min |
No |
| 3 |
Rivaroxaban and eGFR 15–29 ml/min |
No |
| 4 |
Rivaroxaban and eGFR 30–49 ml/min |
No |
| 5 |
Rivaroxaban dose of 20 mg/day may
be too high during triple anticoagulation |
No |
| 6 |
Rivaroxaban dosage may be too high
during triple anticoagulation |
No |
| 7 |
Rivaroxaban dosage may be too high
during triple anticoagulation and eGFR >50 ml/min |
No |
| 8 |
Multiple rivaroxaban prescriptions |
No |
| 9 |
Rivaroxaban with dual CYP3A4 /
P-gp inhibitor |
No |
| 10 |
Rivaroxaban dosage 20 mg/d and two
platelet aggregation inhibitors |
No |
| 11 |
Rivaroxaban and a body weight >150 kg |
No |
| Triple Whammy |
1 |
Triple Whammy and GFR <30
ml/min |
No |
| 2 |
Triple Whammy and GFR 30–60 ml/min: increased risk of kidney
failure |
No |
| 3 |
Triple Whammy and age ≥75 :
increased risk of kidney failure |
No |
| 4 |
No adequate renal monitoring
during triple whammy |
No |
| 5 |
Error in the calculation of one of
the dosages |
No |
| Vancomycin |
1 |
Drop of kidney function during
vancomycin therapy |
No |
| 2.1 |
No adequate renal monitoring
during vancomycin therapy |
No |
| 2.2 |
No adequate renal monitoring
during vancomycin therapy in combination with creatinine falsifier |
No |
| 3 |
Vancomycin levels too low |
No |
| 4 |
Vancomycin levels too high |
No |
| 5 |
Probably not a trough level. The blood
sample was taken at the wrong time |
No |
| 6 |
Vancomycin in combination with
substances that can alter creatinine levels |
No |
| 7 |
Continuous
infusion of vancomycin |
No |
| 8 |
An error has
occurred |
No |
| Xanthine oxidase inhibitors |
1 |
Xanthine oxidase inhibitors with
azathioprine or mercaptopurine |
No |
| 2 |
The dosage of the xanthine oxidase
inhibitor may be too high due to reduced kidney function |
No |
| 3 |
The dosage of the xanthine oxidase
inhibitor when combined with capecitabine |
No |
| 4 |
An error has occurred |
No |
Appendix 2
The appendix 2 is available for download as a separate file at https://doi.org/10.57187/smw.2023.40082.



