Hepatitis C prevalence and cascade of care among patients
in the decentralised opioid agonist therapy programme of the canton of St Gallen,
Switzerland:
a cross-sectional study
DOI: https://doi.org/https://doi.org/10.57187/s.3352
Kerstin Wissela,
Pietro Vernazzab,
Stefan P. Kusterb,
Katharina Hensel-Kochc,
Andrea Bregenzerbd
a Checkpoint Zurich, Zurich, Switzerland
b Division of Infectious Diseases, Infection Prevention and Travel Medicine, Cantonal
Hospital St Gallen, St Gallen, Switzerland
c Stiftung Suchthilfe, St. Gallen, Switzerland
d Department
of Infectious Diseases and Infection Prevention, Cantonal Hospital Aarau,
Aarau, Switzerland
Summary
BACKGROUND: To eliminate chronic hepatitis
C virus (HCV) infection by 2030, 90% of those infected must be diagnosed and 80%
treated. In Switzerland, >40% of the estimated 32,000
infected people are still undiagnosed. In the canton
of St Gallen, HCV prevalence and cascade of care have only been studied in the
centralised opioid agonist therapy (OAT) setting (institutions), although about
80% of OAT patients are treated decentrally (general practitioner
[GP] or pharmacy).
AIM: To describe
HCV prevalence and cascade of care among patients in the decentralised OAT programme
of the canton of St Gallen, Switzerland, and compare it to contemporaneous
data from the centralised setting.
METHODS:
For each patient receiving his/her OAT from a GP or pharmacy on
1 April 2021, the cantonal medical office sent a questionnaire to the prescribing
GP. Patient characteristics, HCV antibody (Ab)/RNA screening uptake, HCV Ab/RNA
prevalence and HCV treatment uptake were obtained and compared to those of patients
of the Medizinisch-soziale Hilfsstelle 1 in St Gallen (centralised setting).
RESULTS:
Of the 563 OAT patients under the care of 127 GPs, 107 patients from 41 GPs could
be analysed (median age: 48 years [IQR: 40–56]; ongoing intravenous drug use: 25%;
OAT provider: 66% GP, 34% pharmacy). HCV Ab screening uptake was 68% (73/107) with
an HCV Ab prevalence of 68% (50/73) among those tested. Of the HCV Ab-positive patients,
84% (42/50) were HCV RNA-tested, among whom 57% (24/42) were viraemic. HCV treatment
uptake was 83% (20/24), with 95% (19/20) achieving a sustained virological response.
Non-uptake of HCV screening and treatment tended to be higher among patients receiving
OAT at the pharmacy vs at the GP’s office: 37% vs 26% (p = 0.245)
for screening and 30% vs 7% (p = 0.139) for treatment. The proportion never HCV
Ab-tested and the proportion of HCV Ab-positives never HCV RNA-tested was significantly
higher in the decentralised compared to the centralised setting: 32% vs 3% (p <0.001)
never Ab-tested and 16% vs 0% (p = 0.002) never
RNA-tested. In contrast, HCV treatment uptake (83% vs 78%), sustained
virological response rate (95% vs 100%) and residual HCV RNA prevalence among the
HCV Ab-positive (12% vs 14%) were comparable for both settings.
CONCLUSION: In the decentralised OAT setting of the canton of St Gallen,
HCV Ab prevalence is high. Since HCV Ab and RNA screening uptake are markedly lower
than in the centralised setting, potentially >40% of patients with chronic HCV
are not diagnosed yet. HCV screening in the decentralised setting needs improvement,
e.g. by increasing awareness and simplifying testing. High HCV treatment uptake
and cure rates are possible in centralised and decentralised settings.
Introduction
Chronic hepatitis C virus (HCV) infection is
still one of the main causes of chronic liver disease with an estimated 57 million
chronically infected people worldwide [1]. In a 2020 review, Bechler et al. assume
that in Switzerland 30–50% of patients with chronic viral hepatitis are unaware
of their infection [2]. Late diagnosis of chronic hepatitis increases mortality
and morbidity and is a potential reservoir for new infections. As a result of this
global epidemic and due to the availability of well-tolerated pangenotypic direct-acting
antivirals (DAA) with cure rates of nearly 100% [3, 4], the World Health Organization
(WHO) has set a global target of eliminating HCV by 2030 [5]. Worldwide, most infections
were acquired during non-sterile medical procedures [6], whereas in Switzerland
the most common source of infection is intravenous drug use, with about 80% of newly
acquired HCV infections acquired through needle exchange among people who inject
drugs [7, 8]. Switzerland is generally considered a low-prevalence country with
an estimated HCV antibody (Ab) prevalence of 0.7% in the general population but
up to 46% in the core groups with the highest risks [7]. At the end of 2016, it
was estimated that 39,500 chronically HCV-infected people live in Switzerland [7].
This number had declined to 32,100 at the beginning of 2020 [9]. In contrast, a
study from Bertisch et al., which is also based on 2020 data but primarily uses
sources with effective HCV management, estimates the number to be significantly
lower [10–13]. According to the Federal Office
of Public Health (FOPH) reporting system and the Swiss Hepatitis
C Cohort Study (SCCS), the proportion of people who inject drugs among the Swiss
HCV population is about 60% [14–16].
Since 1993, an HCV
Ab screening test has been recommended in Switzerland for individuals with risk
factors for chronic HCV (risk-based screening approach) [17]. To date, however,
routine screening is only performed consistently for blood donors and people living
with human immunodeficiency virus (HIV) in the Swiss HIV
Cohort study (SHCS) [18]. HCV rapid tests with capillary
blood or saliva are currently not reimbursed. There is no HCV registry in Switzerland,
but all new HCV diagnoses must be reported to the Federal Office of Public
Health. Since 2014, a Swiss HCV strategy exists [19] and in 2019 the Federal
Office of Public Health published HCV guidelines for people
who use drugs (PWUD) [20]. Since 2017, DAAs are reimbursed in Switzerland irrespective
of the liver fibrosis grade. Until the end of 2021, however, DAA prescribing was
restricted to gastroenterologists, infectious disease specialists and addiction
specialists with experience in HCV treatment [21].
Of the 22,000–27,000 opioid-dependent people
in Switzerland [20], about 80% participate in an opioid agonist therapy (OAT) programme.
In 60% of them, OAT is prescribed by a general practitioner (GP).
In Switzerland, any practising physician can provide OAT with
reimbursement via health insurance to his or her patients. The only requirement
is that the patients must be registered with the cantonal authorities. Accordingly,
many GPs care for only a few OAT patients [22, 23]. For OAT,
the following substances are used: methadone, buprenorphine, slow-release morphine,
levomethadone and other opioids [24]. The prescribing of diacetylmorphine is not
part of the cantonal programme but is controlled by the Federal Office of
Public Health and restricted to 23 institutions in 14 cantons [25]. In Switzerland,
the regulations for dispensing drugs vary from canton to canton. In the canton of
St Gallen,
GPs can dispense the OAT directly to patients in their office,
so-called self-dispensing, or can deposit a prescription for their patient at a
local pharmacy [26].
A cross-sectional study conducted in 2009 in
the three largest institutions for OAT in the canton of St Gallen (Medizinisch-soziale
Hilfsstelle 1 [MSH1], Medizinisch-soziale Hilfsstelle 2 [MSH2] and the Division
of Infectious Diseases of Cantonal Hospital St Gallen [KSSG]), which offer standardised
routine HCV screening, showed a high HCV Ab prevalence of 75%, of whom 59% had detectable
HCV RNA [27]. However, 79% of OAT patients in the canton of St Gallen are not registered
with one of these large centres but are cared for in a decentralised setting by
GPs and/or pharmacies and have therefore not been investigated to date [28].
HCV screening uptake in this major group of
OAT patients in the canton of St Gallen has not been studied yet. We hypothesised
that
the HCV Ab prevalence in the group of decentrally managed OAT patients is as high
as in the three major institutions where medical assessment was performed before,
but a substantial proportion has not been tested yet. Since it must be assumed that
75% of undiagnosed HCV Ab-positive patients are chronically infected [29], a significant
number of OAT patients in the decentralised setting would still require antiviral
therapy to reduce disease burden and the spread of the disease in this core group.
The aim of our study was to describe the HCV
prevalence and the cascade of care among patients in the decentralised OAT programme
of the canton of St Gallen and compare
it to contemporaneous data from the centralised setting.
Methods
The study was conducted by the Division of Infectious
Diseases of the Cantonal Hospital St Gallen (KSSG) with support from the the canton
of St Gallenal Medical Office and the participating GPs in the canton of St Gallen.
Study population
The study was carried out in the German-speaking
canton of St Gallen, which has about 526,000 inhabitants [30] and is situated in
the eastern part of Switzerland. It is predominantly rural, with the city St Gallen
as its urban centre (81,000 inhabitants) [31].
In Switzerland, patients who receive OAT (methadone, buprenorphine, slow-release morphine,
levomethadone
or other opioid) on a regular basis outside of an acute illness must be registered
with the cantonal substitution programme. All patients who were registered in the
programme of the canton of St Gallen on 1 April 2021 and whose OAT was
prescribed by a GP formed the study population.
Data collection and analysis
For data collection, paper-based questionnaires
provided by the study team were sent by the cantonal medical office by regular mail
to the GPs (questionnaire: see appendix).
GPs were asked to complete one questionnaire for each of their OAT patients and to
return them to the study team. The questionnaires were divided into a general part
for all patients (year of birth, the place of opiate provision, current substance
use, whether an HCV Ab screening test had ever been performed, if yes: date of positive
or negative result) and a second part for those with incomplete HCV testing information.
For patients without an HCV Ab test result, the reason for non-testing was asked.
For patients with a reactive result, the result and date of an HCV RNA test were
sought. The GPs were encouraged to provide the corresponding laboratory results,
if available. For patients with a positive HCV RNA test, additional questions on
past or current HCV treatment were asked. A single reminder was sent by email to
all GPs who had not responded by June 2021. All questionnaires that were returned
by the end of September 2021 were included in the study.
Patient characteristics and the HCV cascade
of care (HCV Ab screening uptake, HCV Ab prevalence, HCV RNA testing uptake among
HCV Ab-positive patients, HCV treatment uptake, residual
HCV RNA prevalence) were compared to data from 72 OAT patients of the Medizinisch-soziale
Hilfsstelle 1 (MSH1) in St Gallen (a centralised heroin substitution programme)
that had been collected on 1 May 2021 in the framework of
an Federal Office of Public Health (FOPH) project in the Swiss Association for the
Medical Management in Substance Users (SAMMSU) cohort
[32, 33]. The SAMMSU cohort is a nationwide cohort enrolling OAT patient in eight
centres throughout Switzerland since 2014, and has been described previously [32].
In the event of a negative test result and ongoing
risk for HCV, the HCV Ab/RNA screening should be repeated annually [20]. Accordingly,
patients were considered to have a “current HCV screening test” if the data of the
screening test provided by the GP on the questionnaire were from the year 2020 or
2021. Patients were considered to have “no current screening test” if they were
at ongoing risk for acquiring HCV infection and the screening test data were
from 2019 or earlier. It should be noted that the study team did not have access
to the patient’s complete medical file, so the assessment is based solely on the
information provided in the questionnaire. HCV treatment uptake was defined as the
proportion of patients with chronic
hepatitis C (ever HCV RNA-positive) ever receiving HCV treatment irrespective of
treatment outcome. Patients with undetectable HCV RNA who were never treated were
considered to have spontaneously cleared the virus. Residual HCV RNA prevalence
among the HCV Ab-positive patients or the total population was based on still HCV
RNA-positive patients, including not-yet or not-successfully-treated chronic hepatitis
C patients and patients potentially reinfected after successful treatment or spontaneous
clearance.
To estimate the potential number of undiagnosed
chronic hepatitis C patients in our study population, we assumed that the HCV
Ab prevalence and chronification rate are the same in tested and untested patients.
First, we multiplied the HCV Ab prevalence among the tested patients with the total
number of OAT patients in the study to obtain the potential total number of HCV
Ab-positive patients. Then, we multiplied the result with the chronification rate
observed among the HCV Ab-positive patients with known HCV RNA to calculate the
potential total number of patients with ever chronic HCV. Finally, we subtracted
from the result the number of already diagnosed chronic hepatitis C patients to
yield the potential number of undiagnosed chronic hepatitis C patients. To calculate
the proportion of chronic hepatitis C patients potentially not diagnosed yet, the
potential number of undiagnosed chronic hepatitis C patients was divided by the
potential total number of patients with ever chronic HCV.
Statistical analyses
were performed with Stata Version 15.0 and OpenEpi (www.openepi.com). Categorical variables were compared using the chi-squared test. Continuous
variables were analysed with the Wilcoxon rank-sum test (Mann-Whitney U test). A
two-sided p value <0.05 was considered statistically significant. To calculate
the 95% confidence intervals, the Wilson score interval was used.
Screening and treatment offer for study patients not
yet tested or not treated
In addition to collecting epidemiological data,
we wanted our study to contribute to HCV elimination among OAT users. Hence we
offered assistance in the management of patients with incomplete screening or lack
of therapy. Support included selection and implementation of suitable test methods
e.g. saliva tests in patients who preferred not to have a blood draw as well as
telemedical assistance regarding current therapy options for chronic HCV, which
was considered to be a benefit for patients not living close to healthcare centres
or refusing referral to an external specialist in infectious diseases or hepatology.
At the time of the study, GPs could not prescribe DAAs due to a DAA prescriber restriction
in place in Switzerland to the end of 2021 [21].
Ethical considerations
The elaborate blinding implemented for the
study, including delivery of the study questionnaires to the GPs by the cantonal
medical office and return of anonymised questionnaires to the study team, as well
as minimal demographic data collection (only year of birth, not sex), guaranteed
complete anonymisation of the collected data, which could therefore not be traced
back to a specific patient by members of the study team. Given that the data for
this project were to be collected anonymously and without traceability, the local
ethics committee (Ethics Committee of Eastern Switzerland, EKOS) approved the study
without the requirement for individual written informed consent (BASEC Nr. Req-2020-00510).
Results
Setting and patient recruitment
On 1 April 2021, 563 patients managed by 127
individual GPs were registered in the OAT programme of the canton of St Gallen. Forty-eight
GPs responded, corresponding to a response rate of 38%. However, seven of them did
not provide any patient data because
they no longer had OAT patients under their care or refused participation. Thus,
data from 107 OAT patients under the care of 41 different GPs were available for
analysis. Of these 41 GPs, 44% (18) provided data for only one OAT patient, 56%
(23) for fewer than three and 44% (18) for three or more OAT patients; 12% (5) of
the 41 GPs sent data for five or more individuals, with one GP providing data for
10 OAT patients. Original lab data were provided for 15% (16/107)
of the included patients.
Patient characteristics
OAT patients with OAT prescribed by their
GP (n = 107), decentralised setting
For 105 of the 107 OAT patients, the year of
birth was available. Their median age was 48 years (IQR: 40–56) (table 1). 66% (69/104)
of the OAT patients in the decentralised OAT programme received
their OAT in their GP’s office, while for 34% (35/104), the local pharmacy was the
OAT provider (no data for 3 patients.). According to the prescribing GP, 61% (65)
of the 107 OAT patients had not engaged in concomitant intravenous drug use for
at least one year; for 7% (7), the GP knew that there had been intravenous
drug use in the past 12 months, while for another 19% (20) the GP at least
suspected intravenous drug use. Thus, a total of 25% (27) of the OAT patients were
identified with ongoing or possibly ongoing concomitant intravenous drug use. In
14% (15) of cases, the GP was unable to provide any information on current use (table
1). The reasons were either that the patient was not known well enough or that the
patient had never engaged in intravenous drug use.
Table 1Patient characteristics
| |
Decentralised
setting (general practitioners/pharmacies, April 2021) (n = 107) |
Centralised
setting (MSH1, May 2021) (n = 72) |
| Male,
in % (n) |
No data* |
79.2% (57/72) |
| Median
(IQR) age, in years |
48 (40–56), Range: 28–69 (n = 105, 2
missing) |
51 (47–55), Range: 21–74 (n = 72) |
| Age
category, in % (n) |
(n = 105, 2 missing) |
(n = 72) |
| |
≤29 years |
2.9% (3) |
4.2% (3) |
| |
30–39 years |
20.0% (21) |
9.7% (7) |
| |
40–49 years |
29.5% (31) |
23.6% (17) |
| |
50–59 years |
33.3% (35) |
56.9% (41) |
| |
60–69 years |
14.3% (15) |
4.2% (3) |
| |
≥70 years |
0% (0) |
1.4% (1) |
| Place
of OAT provision, in % (n) |
(n = 104, 3 missing) |
(n = 72) |
| |
General Practitioner’s office |
66.3% (69) |
0% (0) |
| |
Pharmacy |
33.7% (35) |
0% (0) |
| |
Institution |
0% (0) |
100% (72) |
| Concomitant
intravenous drug use (according to general practitioner’s assessment), in %
(n) |
(n = 107) |
|
| |
Ongoing concomitant intravenous drug use |
6.5% (7) |
No data** |
| |
Possible ongoing concomitant intravenous
drug use |
18.7% (20) |
| |
No longer engaging in intravenous drug
use (since at least 1 year) |
60.8% (65) |
| |
Not evaluable *** |
14.0% (15) |
OAT patients with an institution as OAT prescriber
and provider (n = 72), centralised setting
Of the 72 OAT patients of the MSH1, 79% (57)
were male. With a median age of 51 years (IQR: 47–55), the age distribution was
not significantly different to that of the decentralised setting (p = 0.246) (table
1).
HCV cascade
In the HCV cascade of care, several diagnosis-
and treatment-related gaps were identified.
HCV screening uptake and prevalence in OAT
patients of the decentralised cantonal OAT programme
An HCV Ab test was documented in only 68% (73)
of the 107 OAT patients (table 2). Thus, almost one third (32%) were never HCV Ab-screened.
Table 2Comparison of the HCV cascades in the
decentralised and the centralised OAT setting.
| |
Decentralised setting (general
practitioners /pharmacies, April 2021) |
Centralised setting (MSH1, May 2021) |
P |
| Number of patients |
107 |
72 |
|
| HCV antibody test |
68.2% (73/107) |
97.2% (70/72) |
<0.001 |
| HCV antibody-positive |
68.5% (50/73) |
81.4% (57/70) |
0.075 |
| HCV RNA if HCV antibody-positive |
84.0% (42/50) |
100% (57/57) |
0.002 |
| Ever chronic HCV |
48.0% (24/50) |
63.2% (36/57) |
0.115 |
| Ever chronic HCV if HCV antibody-positive
and HCV RNA known |
57.1% (24/42) |
63.2% (36/57) |
0.545 |
| Ever HCV treatment if ever chronic HCV |
83.3% (20/24) |
77.8% (28/36) |
0.598 |
| Cured/sustained virological response if
treated |
95% (19/20)* |
100% (28/28) |
0.232 |
| Still HCV RNA-positive (total population)
|
4.7% (5/107)** |
11.1% (8/72) |
0.104 |
| Still HCV RNA-positive if HCV antibody-positive
and HCV RNA known |
11.9% (5/42)** |
14.0% (8/57) |
0.756 |
The most frequently reported reasons for not
having a screening test were that it was never thought of (14), followed by the
wish of the patient not to be tested (8) (table 3). Some GPs have indicated that
there was never a risk of chronic HCV and some stated that transaminases were always
normal or that the patient was seen only sporadically.
Table 3Reasons for not HCV antibody screening according to the General Practitioner’s assessment
(n =
34 patients; multiple answers allowed).
| Patient did not want to be tested |
8 |
| Cost |
1 |
| Never thought of it |
14 |
| Uncertainties regarding selection of the
correct test |
0 |
| Technical problems, e.g. difficult blood
sampling |
0 |
| No risk of hepatitis C |
4 |
| Other / No answer |
8 |
Among OAT patients with an HCV Ab test, the
HCV Ab prevalence was 68% (50/73) (table 2). Of the OAT patients with a positive
HCV Ab test, only 84% (42/50) were further evaluated with an HCV RNA test. Among
the HCV Ab-positive OAT patients with known HCV RNA, 57% (24/42) had ever had documented
viraemia (table 2).
HCV treatment uptake in case of chronic HCV
infection and residual HCV RNA prevalence in OAT patients of the decentralised cantonal
OAT programme
Of the 24 patients who were ever diagnosed with
chronic HCV, 20 had received HCV therapy in the past, corresponding to an HCV treatment
uptake rate of 83% (table 2). Of those who had received therapy, 95% (19) achieved
a sustained virological response; one, who discontinued therapy before completion,
remained HCV RNA-positive. Three chronically HCV-infected patients had never received
treatment: for one, the GP reported that treatment had been planned but not yet
started; another patient refused treatment; no reason was specified for one. For
another HCV RNA-positive patient, no information regarding treatment was provided.
In total, five patients were still HCV RNA-positive and thus in need of treatment.
Gaps in HCV screening and treatment uptake
according to place of OAT provision in the decentralised setting (pharmacy versus
GP’s office)
Of the 35 patients receiving their OAT at the
pharmacy, 37% (13) had never been HCV Ab-tested compared to 26% (18) of the 69 patients
receiving their OAT at the GP’s office (p = 0.245) (figure 1).
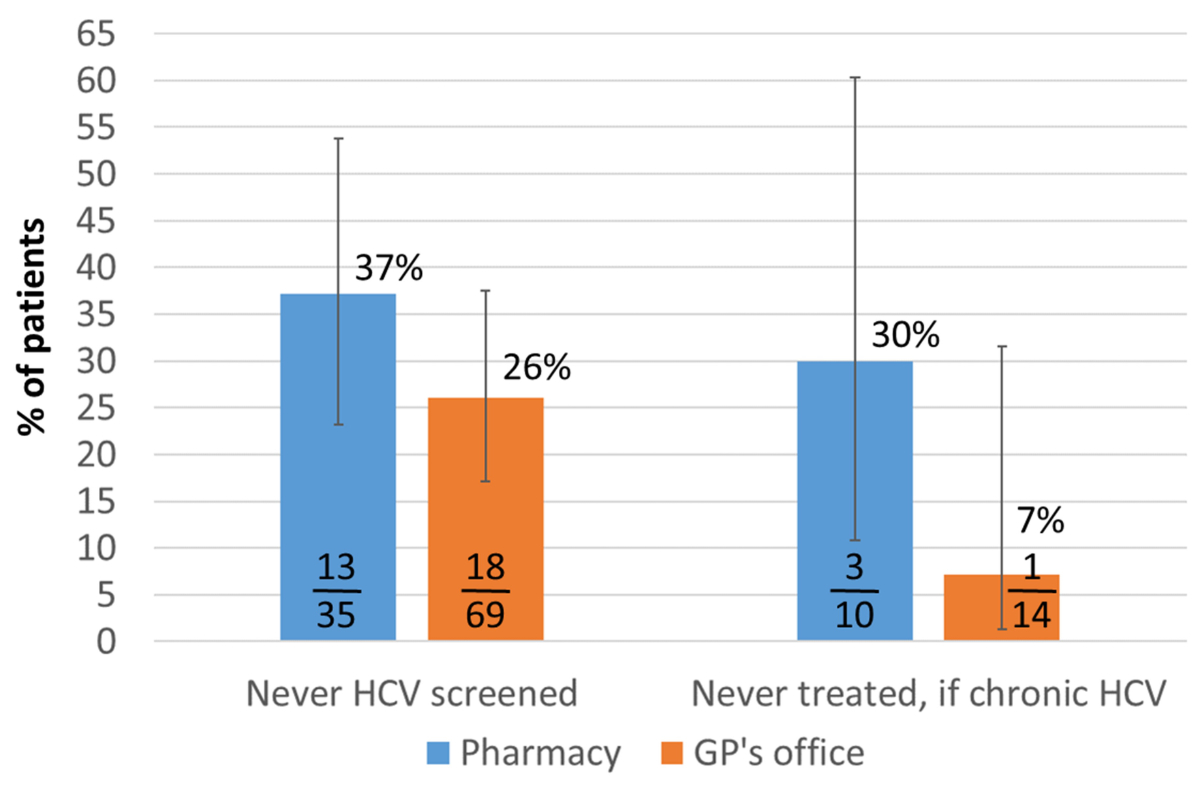
Figure 1Gaps in HCV antibody screening and treatment uptake
according to place of OAT provision in the decentralised
setting (pharmacy versus General Practitioner’s office). The error bars show the lower
and upper limits
of the 95% confidence interval (Score [Wilson]). GP: general practitioner; HCV: hepatitis
C
virus.
For 30% (3) of the 10 chronically HCV-infected
patients receiving their OAT at the pharmacy, no HCV treatment
was documented, compared to 7% (1) of the 14 chronically HCV-infected patients receiving
their OAT at the GP’s office (p = 0.139).
Screening and treatment offers for the patients
not yet tested and not treated, respectively
Sixteen (39%) individual GPs had questions about
available screening tests and/or current treatment options and, depending on the
preferred method of contact, were then contacted, given relevant additional information
and offered further support in testing and treatment.
Annual screening in patients with ongoing risk
Evaluation of the questionnaires of our study
revealed large differences regarding current screening status. Some patients had
had a recent screening test (within one year) while for other patients with ongoing
risk of acquiring HCV infection, a screening test from ≥10 years ago was provided
(data not shown).
Comparison of the HCV cascades in the decentralised
and the centralised OAT setting
Data at 1 April 2021 from the decentralised
setting (GP as OAT prescriber and either the GP or a pharmacy as OAT provider) were
compared with data at 1 May 2021 from a centralised setting (MSH1, heroin substitution
programme, institution as OAT prescriber and OAT provider) (table 2, figure 2).
The proportion of OAT patients never tested for HCV Ab was significantly higher
in the decentralised setting (32% vs 3%, p <0.001). The proportion of HCV Ab-positive
OAT patients not receiving further evaluation with an HCV RNA test was also significantly
higher in the decentralised setting (16% vs 0%, p = 0.002).
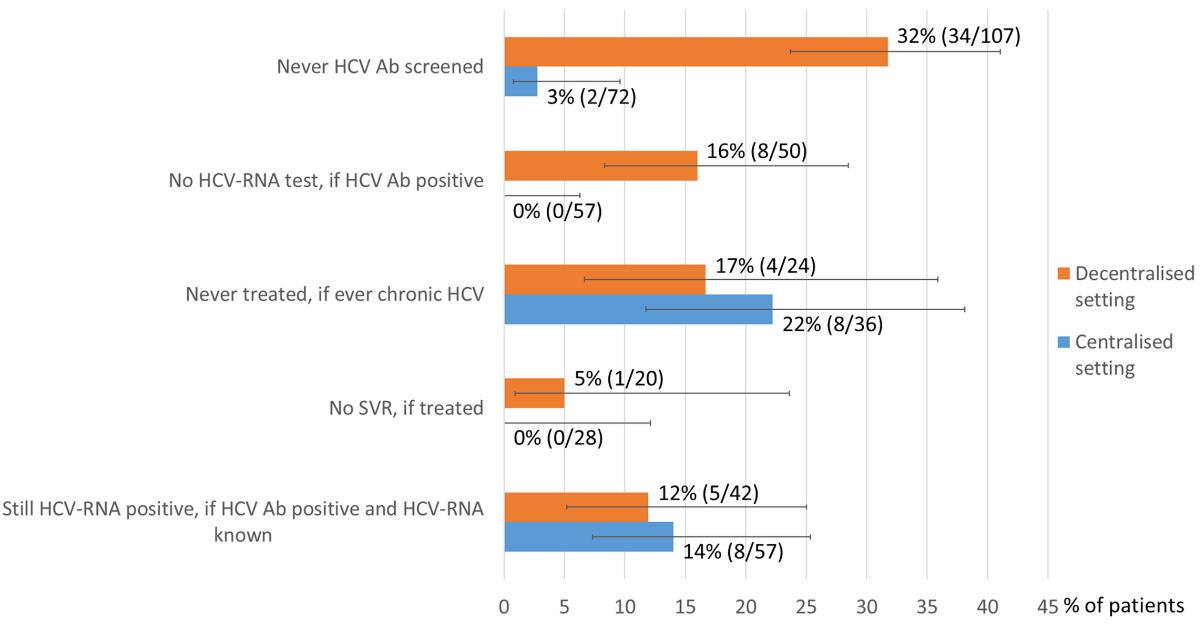
Figure 2Gaps in the HCV cascades: decentralised versus
centralised OAT setting. The error bars show the lower and upper limits
of the 95% confidence interval (Score [Wilson]). Ab: antibody; Centralised setting
(Institution, MSH1: Medizinisch-soziale Hilfsstelle 1); Decentralised setting
(general practitioner / pharmacy); HCV: hepatitis C virus; SVR: sustained
virological response (HCV RNA
undetectable ≥12 weeks after treatment).
HCV Ab prevalence was slightly lower in the
decentralised setting (68% vs 81%, p = 0.075). In contrast, there were no significant
differences regarding HCV treatment uptake among chronically HCV-infected patients
(83% vs 78%, p = 0.598), sustained virological response rate (95% vs 100%, p = 0.232)
and residual HCV RNA prevalence among HCV Ab-positive patients with known HCV RNA
status (12% vs 14%, p = 0.756).
Potential number/proportion of undiagnosed
chronic hepatitis C patients in the decentralised OAT setting
We assumed that the HCV Ab prevalence and chronification
rate are the same in tested and untested patients (figure 3).
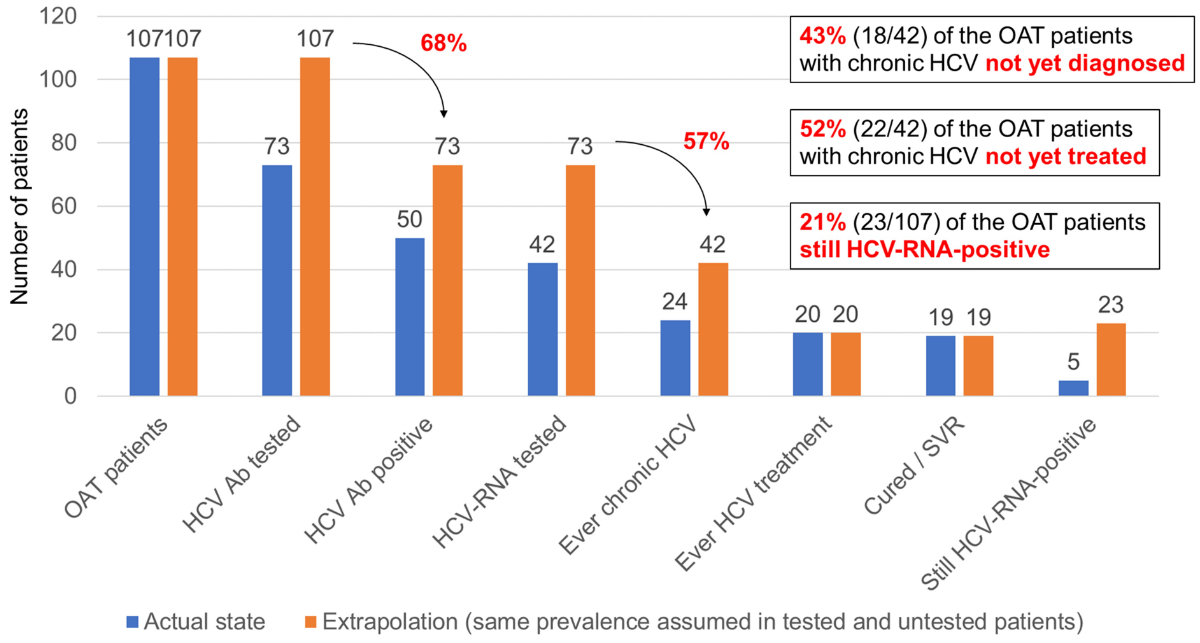
Figure 3Potential number/proportion of undiagnosed
chronic hepatitis C patients in the decentralised OAT
setting and their effect on the treatment uptake rate and HCV RNA prevalence. Ab:
antibody; HCV: hepatitis C virus; OAT:
opioid agonist therapy; SVR: sustained virological response (HCV RNA undetectable
≥12 weeks after treatment)
If we apply the HCV Ab prevalence of 68% (50/73)
we found among the HCV Ab-screened OAT patients in the decentralised setting to
all 107 OAT patients, we would expect 73 HCV Ab-positive patients overall. If we
multiply this number by the chronification rate of 57% (24/42) we found among the
HCV Ab-positive patients with known HCV RNA in this setting, we obtain a total of
42 patients with chronic HCV, of whom 24 are already diagnosed and 18 not yet diagnosed
(13 among the 34 never HCV Ab-screened patients and 5 among the 8 HCV Ab-positive
patients never HCV RNA-tested).
Thus, there are potentially still 18 undiagnosed
chronic HCV patients among the 107 OAT patients in the decentralised setting, which
would mean that 43% (18/[18+24]) of the patients with chronic HCV are not yet diagnosed
in this population. Since all undiagnosed chronic HCV patients are also untreated
and thus still HCV RNA-positive, the proportion of OAT patients with chronic HCV
not yet treated would increase from 17% (4/24) to 52% (22/42) and the proportion
of OAT patients still HCV RNA-positive from 5% (5/107) to 21% (23/107).
Discussion
Main results
For one third of the OAT patients in the decentralised
setting of the canton of St Gallen (GP as OAT prescriber, GP or pharmacy as OAT
provider) no HCV Ab test result was available, and 16% of the HCV Ab-positive OAT
patients had never been HCV RNA-tested. These diagnostic gaps in the HCV cascade
were significantly greater than in the centralised setting (institution as OAT prescriber
and provider). Lack of awareness among GPs and patients seems to play a crucial
role. In contrast, HCV treatment uptake (about 80%), sustained virological
response rate (≥95%) and residual HCV RNA prevalence among the HCV Ab-positive
patients (about 12%) were not significantly different between the decentralised
and centralised setting.
One in four OAT patients in the decentralised
setting had ongoing intravenous drug use, which justifies yearly HCV antibody and
RNA screening, respectively.
At 68%, HCV Ab prevalence in the decentralised
setting was high, but slightly lower than in the centralised setting, where high-risk
patients on heroin substitution were cared for.
Representativeness, ongoing intravenous drug use and
HCV Ab prevalence
According to cantonal OAT statistics of 2021,
50% of patients received their OAT in their GP’s office and 41% in the pharmacy
[28]. Among the 107 OAT patients investigated in our study, only one third received
their OAT in the pharmacy. We assume a response bias, because GPs dispensing in
their office may know their patients better and were therefore more willing to participate
in the study. According to national data, about 50% of OAT patients obtain their
opiates from pharmacies and only about a quarter from doctors’ offices [34].
In our study, 25% of the OAT patients had ongoing
intravenous drug use, which is in good agreement with the 27.4% proportion published
by Bruggmann et al. for Switzerland [35].
In 2009, the overall HCV Ab prevalence in the
three large opioid-dispensing institutions in the canton of St Gallen was 75%, with
82%
in MSH1 (heroin substitution programme) and 61% in MSH2 (methadone substitution
programme), respectively [27]. In our study, conducted in April 2021 in the decentralised
OAT setting of the canton of St Gallen (GP as OAT prescriber, GP or pharmacy as OAT
provider),
we found a similar HCV Ab prevalence of 68%. A contemporaneous cross-sectional study
in MSH1 in May 2021 showed a slightly higher HCV Ab prevalence of 81%, which was
stable compared to 2009 [32]. For comparison: according to national data, HCV Ab
prevalence is only 0.7% in the general Swiss population [7], but markedly higher
in oral OAT programmes (26–48%) and even higher in heroin substitution programmes
(60–80%) [36].
WHO data indicate an HCV Ab prevalence of 53%
among people who inject drugs in Western Europe [37], which is lower than what we
observed in the decentralised and centralised OAT setting of
the canton of St Gallen.
Considering data available at the end of 2020,
a recent analysis by Bertisch et al. estimates that the prevalence of chronic hepatitis
C in the general Swiss population is ≤0.1%, corresponding to 5900–9200 viraemic
people [10] which is definitely lower than former estimations [7, 9]. In their paper,
the number of viraemic people who inject drugs in Switzerland was estimated to be
2750–4750. Since data from decentralised OAT settings are scarce,
they mainly relied on published data from centralised OAT settings,
where the diagnostic work-up has been proven to be systematically better [22, 38].
This might have led to an underestimation, because among undiagnosed chronic hepatitis
C patients, HCV treatment uptake is 0%, and with a spontaneous clearance rate of
about 25% [29], 75% of the HCV Ab-positive patients undiagnosed so far must be assumed
to be still viraemic.
Diagnostic gaps in the HCV cascade of the decentralised
OAT setting
Our data demonstrate that there is still a substantial
screening gap and need for improved HCV management in this key population, which
might jeopardise the WHO goal of HCV elimination by 2030 [39].
While only 3% of the OAT patients in the centralised
setting of the heroin substitution programme of the canton of St Gallen have never
been
HCV Ab-screened, the proportion is almost one third in the decentralised OAT setting
in the same canton. These data strongly support the estimation of Swiss Hepatitis
that one third of all people infected with HCV have not yet been tested [40]. In
the centralised OAT setting, all HCV Ab-positive patients were further evaluated
with an HCV RNA test, which was not the case for 16% of the HCV Ab-positive patients
in the decentralised OAT setting. In total, 44% (47) of the 107 OAT patients in
the decentralised setting are currently not adequately diagnosed or treated (34
without an HCV Ab test, 8 HCV Ab-positive but without HCV RNA, 5 with chronic HCV
without therapy). A similar observation of suboptimal HCV management in the decentralised
OAT setting was described in a study in the canton of Aargau between 2013 and 2015
[22].
If we extrapolate the HCV Ab prevalence of 68%
we found among the OAT patients in the decentralised setting to the 34 untested
patients, it can be expected that another 23 HCV Ab-positive patients would be found
among them. If we also apply the 57% chronification rate we found in this setting,
this would correspond to 13 patients with chronic HCV. Among the 8 HCV Ab-positive
patients
without HCV RNA testing, another 5 patients with chronic HCV could be expected. Thus,
there are probably still 18 cases of undiagnosed chronic HCV among the 107 OAT patients
in the decentralised setting (i.e. 17 per 100). This would mean that 43% (18/[18+24])
of the patients with chronic HCV are not yet diagnosed in this population. This
figure is close to the estimate of Bihl et al. by 2020 (42%) [9]. Since all undiagnosed
chronic HCV patients are also untreated and thus still HCV RNA-positive, the proportion
of OAT patients with chronic HCV not yet treated would increase from 17% (4/24)
to 52% (22/42) and the proportion of OAT patients still HCV RNA-positive from 5%
(5/107) to 21% (23/107). Thus, undiagnosed chronic HCV patients lead to an overestimation
of treatment uptake and an underestimation of HCV RNA prevalence. With only 57%
of chronic HCV patients diagnosed and 48% treated, the WHO goal of 90% diagnosed
and 80% treated [39] is not met.
Annual screening in patients with ongoing risk
For patients with ongoing risk, the Federal
Office of Public Health guidelines recommend an annual HCV Ab test in HCV Ab-negative
patients and an annual HCV RNA test in HCV Ab-positive RNA-negative patients after
spontaneous clearance or successful treatment [20]. Evaluation of the questionnaires
of our study revealed large differences regarding current screening status. Some
patients had had a recent screening test (within the last year) while for other
patients with ongoing risk of acquiring HCV infection a screening test from ≥10
years ago was provided, suggesting suboptimal adherence to the annual screening
recommendation. In this context, it should be noted that the medical history regarding
ongoing intravenous drug use is often not very reliable. Even patients who were
abstinent can experience recurrent drug use, a common observation for addictive
disorders. Feelings of guilt or shame often prevent patients from admitting relapse
and lead to socially desirable answers instead [41]. Therefore, it can be discussed
whether annual screening of all OAT patients, which avoids stigmatisation, would
be more appropriate for diagnosing primary HCV infections and reinfections
early.
HCV treatment uptake
With regard to treatment uptake among patients
with known chronic HCV, we did not find any significant difference between the decentralised
and centralised OAT setting. In both settings,
the 80% treatment uptake goal of the WHO is met [5]. Nevertheless, about 20% of
the patients with known chronic HCV remain untreated. Since the introduction of
oral DAAs, treatment nowadays takes only 8–12
weeks, is well tolerated and leads to a cure in ≥95% of cases [42, 43]. Due to a
prescriber restriction in place to the end of 2021, it was necessary to refer patients
to specialised centres for HCV treatment. Some of the patients refused referral
either because of the distance but also because of a lack of trust in the centre
specialists. Since 2022, GPs are allowed to prescribe HCV therapy themselves. In
case of uncertainty regarding the correct choice of therapy, the centres can provide
support, as offered for example by HepCare in Switzerland [44]. In a global systematic
review and meta-analysis, Oru et al. were able to show that treatment uptake was
higher in settings where HCV screening and treatment were provided at the same site
as opioid substitution or in prisons compared to settings where patients diagnosed
with chronic HCV needed to be referred from their primary care providers to specialised
centres [45]. Pharmacist-led HCV treatment in OAT patients cared for in Scottish
community pharmacies is a good example of how on-site treatment improves treatment
uptake [46].
Strengths and limitations
The study has several limitations. First, the
response rate of the GPs was only 38%. This could be due to the fact that the study
was carried out during the SARS-CoV-2 pandemic. However, studies conducted with
questionnaires generally have a low response rate and postal questionnaires rarely
achieve a response rate above 20% [47].
Some GPs reported that some of their patients
received opiates for pain management and never used drugs. Thus, the number of OAT
patients provided by the canton of St Gallen might be
an overestimation. Unfortunately, it was not possible to differentiate in the cantonal
data between patients receiving opiates due to chronic pain and those receiving
opiates due to former or ongoing intravenous drug use. It might be the case
that some of the missing responses are from this patient group never considered
to be at risk for HCV by the GPs. In addition, 7 of the 127 GPs reported that they
no longer had any OAT patients under their care, reflecting the problem of a high
fluctuation rate (i.e. many new admissions to and discharges from the OAT programme
throughout the year).
Our study was insufficiently powered to detect
a significant difference in HCV screening and treatment uptake between patients
receiving their OAT at the pharmacy compared to those receiving it at the GP’s office.
Thus, studies with a larger sample size are needed to confirm our observed trend
of worse HCV management in case of OAT provision in the pharmacy.
Response bias might also have influenced results.
It can be assumed that the more engaged GPs responded to the survey. Accordingly,
HCV screening uptake might be even worse among those GPs who did not participate
in the study.
Another limitation was that the laboratory results
reported by the GPs were not verifiable, since only a minority sent the requested
laboratory values.
Clinical implication of the study
The WHO aims to eliminate viral hepatitis as
a public health threat by 2030 [39]. Switzerland actively participates in the global
elimination strategy for chronic hepatitis [40] but currently the recommended risk-based
screening for HCV in the strongly affected population
groups, mainly PWUD, men who have sex with men (MSM), people using HIV
pre-exposure prophylaxis (PrEP), sex workers, transgender people, migrant populations
from high-prevalence countries, refugees, is not
fully implemented, particularly in the decentralised OAT setting, while universal
screening of the general population might not be justified in a low-prevalence setting
[48, 49]. The elimination targets were included in 2019 in the “Roadmap for eliminating
HIV/AIDS and Hepatitis in Switzerland” of the Federal Commission for Issues relating
to
Sexually Transmitted Infections [50, 51].
So far, there is no national HCV elimination
programme, but in the summer session of 2020, Parliament passed a motion calling
for the inclusion of viral hepatitis elimination targets in the next national HIV
programme as a long overdue move [52].
Our study demonstrates the difficulty in reaching
and identifying the widely distributed group of individuals with past or current
intravenous drug use, as a majority is not attached to large institutions or even
to any institution at all. HCV Ab testing is recommended for any PWUD and can be
done during a routine check-up and, if a patient refuses blood sampling, could also
be done with alternative methods such as a saliva test [53].
The main health threat of undiagnosed chronic
HCV infection remains chronic liver disease with progression to liver cirrhosis
and hepatocellular carcinoma. Thus, patients generally benefit from early diagnosis
and treatment [54]. In Switzerland, a high-income country with a high coverage of
OAT and a needle and syringe programme since the early 1990s [55, 56], the health
burden of chronic HCV is mainly driven by its sequelae rather than by transmission
rates [57]. The cost-effectiveness of HCV treatment has already been demonstrated,
and a sustained virological response after treatment lowers liver-related mortality
[58, 59]. As the median age of our study population was 48 years, there is a high
potential for reducing the health and economic burden of chronic liver disease.
In high-income countries like Switzerland, undiagnosed chronic HCV
infections are the main barrier to controlling existing infection and allowing access
to current therapy [60–62]. No or rather unspecific symptoms like fatigue, neurocognitive
symptoms, joint pain, abdominal pain and others complicate a fast and targeted diagnosis
and achieving the elimination goal.
On the part of the GP, the most common reason,
mentioned by 14 GPs, for not performing HCV Ab screening was “never thought of it”,
while the second most common reason was “patient did not want to be tested”. Thus,
lack of awareness both among care providers and patients seems to be a crucial barrier
[63]. Although not explicitly mentioned in our study, difficult venous access after
long-term intravenous drug use is a well-known reason why patients refuse testing
[64].
In a systematic review and meta-analysis, Cunningham
et al. showed that simplified HCV screening methods like point-of-care Ab screening,
dried blood spot testing, opt-out screening and reflex RNA testing, reminders in
the patients’ medical charts and provider as well as patient education can effectively
enhance HCV screening and treatment uptake [65].
Our study itself has contributed to HCV awareness
among GPs. On several questionnaires, the GPs stated that they will perform HCV
screening in patients not tested so far. Given that in Switzerland more than half
of OAT patients obtain their opiates from a pharmacy [34], our data show a slightly
higher HCV screening gap among patients for whom the pharmacy and not the GP is
the OAT provider. Moreover offering HCV care in pharmacies has been shown to increase
screening and treatment uptake [46, 66], so pharmacies should be involved in the
implementation of a national screening campaign. A recent publication has shown
that HCV Ab screening using rapid tests with saliva is feasible in Swiss community
pharmacies [67].
The canton of St Gallen, a mainly rural area
with an urban centre, is about the Swiss average in terms of population density
(266.1 inhabitants/km2 vs 216.8 for Switzerland) [68]. Of the 8.7 million
inhabitants of Switzerland, 15,996 (0.18%) are registered in an OAT programme. In
the canton of St Gallen, the proportion is similar (0.15%) [69].
However, in the canton of St Gallen, one of around
half of cantons where self-dispensation is allowed, OAT is more often dispensed
in the GP’s practice (50% vs 26% for the whole of Switzerland). Since our study
pointed towards better screening and treatment uptake among patients receiving their
OAT in the GP’s office, HCV management might be worse outside the canton of St Gallen.
Conclusion
In the decentralised OAT
setting of the canton of St Gallen (GP as OAT prescriber, GP or pharmacy as OAT provider),
potentially >40% of patients with chronic HCV are not diagnosed yet. Thus, HCV
care and the measures taken so far by healthcare providers and health authorities
to eliminate HCV in this population are insufficient. There is a need to improve
HCV screening in the decentralised setting, e.g. by increasing awareness both among
patients and care providers and simplifying testing (e.g. with point-of-care tests
with saliva/capillary blood, dried blood spot testing, reflex RNA testing, opt-out
screening and screening in pharmacies). One in four OAT patients in the decentralised
setting had ongoing intravenous drug use, which justifies annual HCV Ab and RNA
screening, as recommended by national and international guidelines.
High HCV treatment uptake (80%) and cure rates
(≥95% sustained virological response) with consecutive HCV RNA prevalence reduction
are possible both in centralised and decentralised OAT settings.
Acknowledgments
The authors
thank the Cantonal medical office St. Gallen, especially Dr. med. Karen Peier, for
supporting the project by sending the questionnaires to the OAT prescribing physicians.
We also thank all OAT prescribing
physicians who participated.
Kerstin Wissel
Checkpoint
Zurich
Limmatstrasse 25
CH-8005 Zurich
Kerstin.Wissel[at]cpzh.ch
References
1.Blach S, Terrault NA, Tacke F, Gamkrelidze I, Craxi A, Tanaka J, et al.; Polaris Observatory
HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care
between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022 May;7(5):396–415.
10.1016/S2468-1253(21)00472-6
2.Bechler A, Schmassmann A. Update Virushepatitis B und C: Der Beitrag der Schweiz zur
weilweiten Krankheitselimination „Teil 1: Übersicht Hepatitis B und C sowie -Hepatitis
C im Fokus“. Swiss med. Forum. 2020;20(4344):597–601.
3.Brown RS Jr, Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, et al. Glecaprevir/pibrentasvir
for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated
cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020 Mar;72(3):441–9. 10.1016/j.jhep.2019.10.020
4.Miller MM. Sofosbuvir-velpatasvir: A single-tablet treatment for hepatitis C infection
of all genotypes. Am J Health Syst Pharm. 2017 Jul;74(14):1045–52. 10.2146/ajhp60632
5.World Health Organisation (WHO). GLOBAL HEALTH SECTOR STRATEGY ON VIRAL HEPATITIS
2016–2021: TOWARDS ENDING VIRAL HEPATITIS. World Health Organization; 2016. Available
from: https://apps.who.int/iris/handle/10665/246177 (Accessed 11 January 2023)
6.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection.
Nat Rev Gastroenterol Hepatol. 2013 Sep;10(9):553–62. 10.1038/nrgastro.2013.107
7.Zahnd CB, Bertisch B, Giudici F, Keiser O. Situationsanalyse zu Hepatitis B und C
in der Schweiz. BAG 2017. Available from: https://www.bag.admin.ch/bag/de/home/dasbag/publikationen/forschungsberichte/forschungsberichte-uebertragbarekrankheiten/situationsanalyse-hepatitis.html (Accessed 11 January 2023).
8.Broers B, Helbling B, François A, Schmid P, Chuard C, Hadengue A, et al.; Swiss Association
for the Study of the Liver (SASL 18). Barriers to interferon-alpha therapy are higher
in intravenous drug users than in other patients with acute hepatitis C. J Hepatol.
2005 Mar;42(3):323–8. 10.1016/j.jhep.2004.11.018
9.Bihl F, Bruggmann P, Castro Batänjer E, Dufour JF, Lavanchy D, Müllhaupt B, et al. HCV
disease burden and population segments in Switzerland. Liver Int. 2022 Feb;42(2):330–9.
10.1111/liv.15111
10.Bertisch B, Schaetti C, Schmid P, Peter L, Vernazza P, Isler M, et al. Chronic hepatitis
C virus infections in Switzerland in 2020: lower than expected and suggesting achievement
of WHO elimination targets. J Viral Hepat. 2023 Aug;30(8):667–84. 10.1111/jvh.13842; Online ahead of print.
11.Blach S, Bregenzer A, Bruggmann P, Cerny A, Maeschli B, Müllhaupt B, et al. Assessing
the hepatitis C epidemiology in Switzerland: it’s not that trivial. J Viral Hepat.
2023 Aug. 10.1111/jvh.13879; Epub ahead of print.
12.Hepatitis C epidemiology in Switzerland: Our position on new estimates. Available
from: https://hepatitis-schweiz.ch/news/hepatitis-c-epidemiologie-in-der-schweiz-unsere-stellungnahme-zu-neuen-schaetzungen (Accessed 21 september 2023)
13.List of concerns. Available from: https://hepatitis-schweiz.ch/data/download/81680/List-of-concerns-re-Bertisch-et-al-2023_17_07_23.pdf (Accessed 21 september 2023)
14.Sexually transmitted infections and Hepatitis B/C in Switzerland in 2021: An epidemiological
overview, https://www.bag.admin.ch/dam/bag/de/dokumente/mt/p-und-p/hiv-sti-statistiken-analysen-und-trends/hiv-sti-epizahlen-2021.pdf.download.pdf/hiv-sti-epizahlen-2021.pdf (Accessed 13 september 2023)
15.Prasad L, Spicher VM, Zwahlen M, Rickenbach M, Helbling B, Negro F; Swiss Hepatitis
C Cohort Study Group. Cohort Profile: the Swiss Hepatitis C Cohort Study (SCCS). Int
J Epidemiol. 2007 Aug;36(4):731–7. 10.1093/ije/dym096
16. SCCS Interim Annual Report. Available from: ex11NegroAN50_annual_report.pdf (swisshcv.org)
17.Publique BA. Hépatite C en Suisse: Pour une information et un conseil individualisé.
Bulletin BAG/OFSP 2001; 46:877-881. Available from: https://www.admin.ch/gov/fr/start/dokumentation/medienmitteilungen.msg-id-4212.html (Accessed 11 January 2023).
18.Wandeler G, Gsponer T, Bregenzer A, Günthard HF, Clerc O, Calmy A, et al.; Swiss HIV
Cohort Study. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly
evolving epidemic. Clin Infect Dis. 2012 Nov;55(10):1408–16. 10.1093/cid/cis694
19.Swiss Hepatitis Strategy 2014 – 2030. Available from: https://hepatitis-schweiz.ch/data/download/2871/Process_Paper_14_02_2019.pdf (Accessed 13 september 2023)
20.Bundesamt für Gesundheit (BAG). Hepatitis C bei Drogenkonsumierenden: Richtlinien
mit settingspezifischen Factsheets. 2019. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/infektionskrankheiten/hepatitisc/richtlinien-hepatitis-c-drogen.pdf (Accessed 11 January 2023).
21.Maeschli B, Bruggmann P. Hausärztinnen und Hausärzte dürfen neu Hepatitis-C Behandlungen
selbst verschreiben. Prim Hosp Care. 2022;22(12):372–6. 10.4414/phc-d.2022.10512
22.Bregenzer A, Conen A, Knuchel J, Friedl A, Eigenmann F, Näf M, et al. Management of
hepatitis C in decentralised versus centralised drug substitution programmes and minimally
invasive point-of-care tests to close gaps in the HCV cascade. Swiss Med Wkly. 2017 Nov;147:w14544.
23.Brunner N, Falcato L, Bruggmann P, Senn O, Seidenberg A.Qualität der Hepatitis-C-Abklärung
bei Patienten in Opioid-Substitutionstherapie im Kanton Zürich. Suchtmedizin in Forschung
und Praxis. 2015;17:259–64.
24.National statistics on opioid agonist substitution treatments.Results 2021.Available
from: https://www.bag.admin.ch/dam/bag/de/dokumente/npp/sucht/suchtberatung-therapie/act-info-berichte/2021/act-info-nationalestatistik-substitutionsbehandlung.pdf.download.pdf/7d_Rapport_2021DE_Substitution.pdf
25.Substitution-assisted treatment with diacetylmorphine. Available from: https://www.bag.admin.ch/bag/de/home/gesund-leben/sucht-und-gesundheit/suchtberatung-therapie/substitutionsgestuetzte-behandlung/heroingestuetzte-behandlung.html (Accessed 13 september 2023)
26.Facts and figures Swiss pharmacies 2020 Available from: https://www.pharmasuisse.org/data/docs/de/19076/Fakten-und-Zahlen-2020.pdf (Accessed 13 september 2023)
27.Witteck A, Schmid P, Hensel-Koch K, Thurnheer MC, Bruggmann P, Vernazza P; Swiss Hepatitis
C and HIV Cohort Studies. Management of hepatitis C virus (HCV) infection in drug
substitution programs. Swiss Med Wkly. 2011 May;141:w13193. 10.4414/smw.2011.13193
28.Jährliche Statistik - substitution.ch.2018. Available from: https://www.substitution.ch/de/jahrliche_statistik.html&year=2018&canton=sg (Accessed 11 January 2023).
29.Grebely J, Page K, Sacks-Davis R, van der Loeff MS, Rice TM, Bruneau J, et al.; InC3
Study Group. The effects of female sex, viral genotype, and IL28B genotype on spontaneous
clearance of acute hepatitis C virus infection. Hepatology. 2014 Jan;59(1):109–20.
10.1002/hep.26639
30.Structure of the permanent resident population by canton, 1999-2022 Available from:
https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/stand-entwicklung.assetdetail.26565154.html (Accessed 13 september 2023)
31.The city in numbers. Available from: https://www.stadt.sg.ch/home/verwaltung-politik/stadt-zahlen.html (Accessed 13 september 2023)
32.Bregenzer A, Bruggmann P, Castro E, Moriggia A, Rothen M, Thurnheer MC, et al. Hepatitis
C virus elimination in Swiss opioid agonist therapy programmes - the SAMMSU cohort.
Swiss Med Wkly. 2021 Mar;151(910):w20460. 10.4414/smw.2021.20460
33.Studie zu Hepatitis C. im Rahmen SAMMSU-Kohorte 2020-2022. 2022. Available from: https://www.aramis.admin.ch/Texte/?ProjectID=45343,https://www.aramis.admin.ch/Texte/?ProjectID=40779 (Accessed 11 January 2023).
34.Jährliche Statistik - substitution.ch. 2021. Available from: https://www.substitution.ch/de/jahrliche_statistik.html&year=2021&canton=ch (Accessed 12 March 2023).
35.Bruggmann P, Blach S, Deltenre P, Fehr J, Kouyos R, Lavanchy D, et al. Hepatitis C
virus dynamics among intravenous drug users suggest that an annual treatment uptake
above 10% would eliminate the disease by 2030. Swiss Med Wkly. 2017 Nov;147:w14543.
10.4414/smw.2017.14543
36.Cominetti FS, Dubois-Arber F, Gervasoni JP. IUMSP, Schaub M., ISGF, Monnat M., SSP.
Analyse der Hepatitis-C-Situation bei den drogenkonsumierenden Personen in der Schweiz.
Lausanne, Institut universitaire de medicine sociale et preventive 2014. Available
from: https://www.aramis.admin.ch/Default?DocumentID=14464&Load=true (Accessed 11 January 2023).
37.World Health Organisation (WHO). Accelerating access to hepatitis C diagnostics and
treatment. 2020. Available from: https://www.who.int/publications-detailredirect/9789240019003 (Accessed 15 January 2023).
38.Schürch S, Fux CA, Dehler S, Conen A, Knuchel J, Friedl A, et al. Management of hepatitis
C in opioid agonist therapy patients of the Swiss canton Aargau within and outside
the cohort study. Swiss Med Wkly. 2020 Aug;150(3132):w20317. 10.4414/smw.2020.20317
39.World Health Organization (WHO). Combating hepatitis B and C to reach elimination
by 2030: advocacy brief. 2016. Available from: https://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.pdf?seq uence=1&isAllowed=y (Accessed 26 April 2023)
40.Blindenbacher R, Maeschli B, Bruggmann P. The Swiss Hepatitis Strategy as a model
for facing future health policy challenges. Health Policy. 2019 Jul;123(7):681–7.
10.1016/j.healthpol.2019.05.010
41.Schmid P. Nachsorge nach erfolgreicher Hepatitis-C-Therapie. Swiss med. Forum. 2021;21(1112):177–8.
42.Thompson WW, Symum H, Sandul A, Gupta N, Patel P, Nelson N, et al.; DHSc. Vital Signs:
Hepatitis C Treatment Among Insured Adults - United States, 2019-2020. MMWR Morb Mortal
Wkly Rep. 2022 Aug;71(32):1011–7. 10.15585/mmwr.mm7132e1
43.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral
Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review.
Ann Intern Med. 2017 May;166(9):637–48. 10.7326/M16-2575
44.HepCare - Für Grundversorger. Available from: https://www.hepcare.ch/de/grundversorger.php (Accessed 15 January 2023).
45.Oru E, Trickey A, Shirali R, Kanters S, Easterbrook P. Decentralisation, integration,
and task-shifting in hepatitis C virus infection testing and treatment: a global systematic
review and meta-analysis. Lancet Glob Health. 2021 Apr;9(4):e431–45. 10.1016/S2214-109X(20)30505-2
46.Radley A, de Bruin M, Inglis SK, Donnan PT, Hapca A, Barclay ST, et al. Clinical effectiveness
of pharmacist-led versus conventionally delivered antiviral treatment for hepatitis
C virus in patients receiving opioid substitution therapy: a pragmatic, cluster-randomised
trial. Lancet Gastroenterol Hepatol. 2020 Sep;5(9):809–18. 10.1016/S2468-1253(20)30120-5
47.Diekmann A. Empirische Sozialforschung. 2007. Available from: https://www.zvab.com/buch-suchen/titel/empirische-sozialforschung/autor/andreasdiekmann/antiquarisch/ (Accessed 15 January 2023).
48.Sadeghimehr M, Bertisch B, Schaetti C, Wandeler G, Richard JL, Scheidegger C, et al. Modelling
the impact of different testing strategies for HCV infection in Switzerland. J Virus
Erad. 2019 Nov;5(4):191–203. 10.1016/S2055-6640(20)30036-4
49.Girardin F, Painter C, Hearmon N, Eddowes L, Kaiser S, Negro F, et al. Hepatitis C
prevalences in the psychiatric setting: cost-effectiveness of scaling-up screening
and direct-acting antiviral therapy. JHEP Rep Innov Hepatol. 2021 Mar;3(3):100279.
10.1016/j.jhepr.2021.100279
50.Federal Commission for Issues relating to Sexually Transmitted Infections (FCSTI).
Available from: https://www.bag.admin.ch/bag/en/home/das-bag/organisation/ausserparlamentarische-kommissionen/eidgenoessische-kommission-fuer-sexuelle-gesundheit-eksg.html (Accessed 13 september 2023)
51.Roadmap for eliminating HIV/AIDS and Hepatitis in Switzerland. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/p-und-p/eksg/roadmap-for-eliminating-hiv-aids-hepatitis-in-switzerland-fcsh.pdf.download.pdf/roadmap-for-eliminating-hiv-aids-hepatitis-in-switzerland-fcsh.pdf (Accessed 13 september 2023)
52.Motion 19.3743: Eliminating hepatitis belongs in a national program on sexually and
bloodborne infectious diseases. Available from: https://www.parlament.ch/de/ratsbetrieb/suche-curia-vista/geschaeft?AffairId=20193743#:~:text=Der%20Bundesrat%20wird%20aufgefordert%2C%20das,HIV%20bis%202030%20zu%20eliminieren (Accessed 13 september 2023)
53.Tang W, Chen W, Amini A, Boeras D, Falconer J, Kelly H, et al. Diagnostic accuracy
of tests to detect Hepatitis C antibody: a meta-analysis and review of the literature.
BMC Infect Dis. 2017 Nov;17(S1 Suppl 1):695. 10.1186/s12879-017-2773-2
54.Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al.; EASL
HEPAHEALTH Steering Committee. Burden of liver disease in Europe: epidemiology and
analysis of risk factors to identify prevention policies. J Hepatol. 2018 Sep;69(3):718–35.
10.1016/j.jhep.2018.05.011
55. Herzig M Wolf M. Inside Switzerland’s Radical Drug Policy Innovation. Stanford Social
Innovation Review 2019.
56.Uchtenhagen A. Heroin-assisted treatment in Switzerland: a case study in policy change.
Addiction. 2010 Jan;105(1):29–37. 10.1111/j.1360-0443.2009.02741.x
57.Ansaldi F, Orsi A, Sticchi L, Bruzzone B, Icardi G. Hepatitis C virus in the new era:
perspectives in epidemiology, prevention, diagnostics and predictors of response to
therapy. World J Gastroenterol. 2014 Aug;20(29):9633–52. 10.3748/wjg.v20.i29.9633
58.Wong JB. Hepatitis C: cost of illness and considerations for the economic evaluation
of antiviral therapies. PharmacoEconomics. 2006;24(7):661–72. 10.2165/00019053-200624070-00005
59.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis
C in the United States. Hepatology. 2009 Dec;50(6):1750–5. 10.1002/hep.23220
60.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis
C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology.
2013 Apr;57(4):1333–42. 10.1002/hep.26141
61.Grebely J, Dore GJ. What is killing people with hepatitis C virus infection? Semin
Liver Dis. 2011 Nov;31(4):331–9. 10.1055/s-0031-1297922
62.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge
of hepatitis C, and medical follow-up among individuals testing positive for hepatitis
C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012 Jun;55(6):1652–61.
10.1002/hep.25556
63.Harris M, Ward E, Gore C. Finding the undiagnosed: a qualitative exploration of hepatitis
C diagnosis delay in the United Kingdom. J Viral Hepat. 2016 Jun;23(6):479–86. 10.1111/jvh.12513
64.Bajis S, Maher L, Treloar C, Hajarizadeh B, Lamoury FM, Mowat Y, et al.; LiveRLife
Study Group. Acceptability and preferences of point-of-care finger-stick whole-blood
and venepuncture hepatitis C virus testing among people who inject drugs in Australia.
Int J Drug Policy. 2018 Nov;61:23–30. 10.1016/j.drugpo.2018.08.011
65.Cunningham EB, Wheeler A, Hajarizadeh B, French CE, Roche R, Marshall AD, et al. Interventions
to enhance testing, linkage to care, and treatment initiation for hepatitis C virus
infection: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022 May;7(5):426–45.
10.1016/S2468-1253(21)00471-4
66.Wade AJ. Can community pharmacists treat hepatitis C virus? Lancet Gastroenterol Hepatol.
2020 Sep;5(9):790–1. 10.1016/S2468-1253(20)30184-9
67.Stämpfli D, Imfeld-Isenegger TL, Hersberger KE, Messerli M. Hepatitis C virus screening
in community pharmacies: results on feasibility from a Swiss pilot. BMC Infect Dis.
2023 Jun;23(1):384. 10.1186/s12879-023-08362-1
68.Population density in Switzerland by canton on December 31, 2021. Available from:
https://de.statista.com/statistik/daten/studie/412044/umfrage/bevoelkerungsdichte-in-der-schweiz-nach-kantonen/ (Accessed 13 september 2023)
69.Substitution.ch.https://www.substitution.ch/de/jahrliche_statistik.html&year=2021&canton=ch). (Accessed 13 september 2023)
Appendix
The appendix is available for download as a separate file at https://doi.org/10.57187/s.3352.


