
Figure 1Patient flow chart.
DOI: https://doi.org/10.57187/smw.2023.40090
Vaccination is considered a key element for both individual protection and epidemiological control during the current severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic, but vaccine antibody responses are highly heterogeneous even in the general population [1]. Patients with multiple myeloma are at an increased risk of severe courses of coronavirus disease 19 (COVID-19), and there is accumulating substantial evidence that the humoral immune responses to currently available SARS-CoV-2 vaccines are reduced in this vulnerable patient group [2]. Apart from specific antibody responses, SARS-CoV-2-specific T-cells are an integral part of the immune response following both infection [3] and vaccination [4]. It has been suggested that virus-specific T-cells reacting against the spike (S) protein may provide protection against severe COVID-19 for patients with haematological malignancies who develop insufficient anti-S antibodies [5]. While several large-scale studies on serologic responses to booster vaccination have been published, only few studies have addressed the effects on T-cell responses, especially when enzyme-linked immunospot (ELISpot) assay is used to quantify SARS-CoV-2-specific T-cells. Herein, we report the humoral and cellular immune responses following booster vaccination with BNT162b2 messenger ribonucleic acid (mRNA)-based vaccine (Pfizer/BioNTech) and persistence of antibodies up to 6 months post-booster in patients with multiple myeloma.
For this prospective cohort study, we recruited patients with known multiple myeloma at the Department of Medical Oncology and Haematology of the Cantonal Hospital St Gallen, Switzerland. For enrolment, patients had to have received two doses of the approved SARS-CoV-2 mRNA-based vaccine BNT162b2. Patients were excluded when they had any other comorbidity known to be associated with immunosuppression (e.g. human immunodeficiency virus infection), when they received immunosuppressive treatment for a reason other than neoplastic disease or when they had known previous COVID-19 (either symptomatic or asymptomatic and documented on the basis of positive anti-nucleocapsid [N] titres). Information about patient demographics, disease characteristics, anti-myeloma treatment including steroids and potential SARS-CoV-2 breakthrough infections was obtained by reviewing clinical records and actively interviewing patients.
For comparison and assessment of the validity of the pre-booster results, a cohort of 22 healthy volunteers within the same age range was recruited.
To assess vaccine-induced immunity and to exclude natural immunity, we measured specific antibodies against the viral proteins S (anti-S) and N (anti-N) via electro-chemiluminescence immunoassay (ECLIA; Elecsys®, Roche, Switzerland) at baseline (T1, about 3 months following the second dose of BNT162b2), T2 (pre-booster with BNT162b2) in comparison with T3 (+1 month after the booster dose), T4 (+3 months after the booster dose) and T5 (+6 months after the booster dose), as shown in figure 1. The antibody concentrations were reported in binding antibody units (BAUs) per millilitre, and seroconversion was defined as an anti-S level of ≥0.8 BAU/ml. Test performance has been described previously [6].

Figure 1Patient flow chart.
The anti-S levels that provide clinically meaningful protection against COVID-19 are still unclear. For patients with cancer, the following classification has been suggested: (i) responders: anti-S level of >260 BAU/ml, (ii) low responders: anti-S level of 40–260 BAU/ml and (iii) non-responders: anti-S level of <40 BAU/ml [7].
In addition to the anti-N and anti-S antibodies, a surrogate virus neutralisation test (Genscript, USA) with enzyme-linked immunosorbent assay was used at T1–T5 to detect neutralising antibodies blocking the interaction between the viral receptor-binding domain of the S glycoprotein (wild type and variants) and the human cell surface receptor angiotensin-converting enzyme-2 [8]. A cut-off value of ≥30% inhibition as recommended by the manufacturer indicates the presence of SARS-CoV-2 neutralising antibodies.
The presence of SARS-CoV-2-specific T-cells was detected using enzyme-linked immunospot (ELISpot) assay on cryopreserved peripheral blood mononuclear cells at T1–T3. The peripheral blood mononuclear cells were rapidly thawed, incubated overnight and stimulated for 19 h with 15 mers of overlapping peptides (Peptide Solutions, JPT, Germany) of the SARS-CoV-2 S protein. The number of interferon-gamma-producing cells was quantified as spot-forming units per 106 of peripheral blood mononuclear cells according to the manufacturer's protocol (Mabtech AB, Sweden).
For patients with multiple myeloma under active treatment, measurements were performed on day 1 of the respective treatment cycle before exposure to any multiple myeloma medication. Volunteers in the same age range but without cancer and/or autoimmune disease served as controls at T1.
Categorical variables were analysed using frequency tables and compared using the χ2 test; continuous variables were described as medians and interquartile range (IQRs) and compared using the Mann–Whitney U test (comparison of two groups) or the Kruskal–Wallis test (comparison of three or more groups), since the data did not follow a normal distribution. Correlations between continuous variables were assessed using Spearman correlation coefficients. Differences between two time points were tested using the Wilcoxon paired-samples test. For graphical analyses, the anti-S levels were plotted on a logarithmic scale because of the skewed distribution. All analyses were performed using IBM SPSS Statistics for Windows (version 25.0., IBM Corp., USA) or Prism (version 9, GraphPad, USA).
This research project was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Ethical approval was granted for the project and use of clinical patient data (BASEC 2021-01062) before data collection and analysis, and written informed consent was obtained from the patients for participation. The study is listed in the Registry of All Projects in Switzerland (RAPS) (https://raps.swissethics.ch, BASEC ID 2021-01062). The protocol is detailed in the supplementary material.
From January to April 2021, 89 patients with multiple myeloma treated at our institution received two doses of BNT162b2 (median of 37 days between doses) within the cantonal vaccination campaign. Of them, 59 (67%) were eligible for inclusion to the study. The participant flow chart illustrates the distribution of the patients within the study at each time point (figure 1). The patient characteristics are detailed in table 1.
Table 1Patient characteristics.
| Sex | Female, n (%) | 20 (34) |
| Male, n (%) | 39 (66) | |
| Age (median, IQR), year | 69 (63–74) | |
| Type of myeloma (n, %) | IgG | 36 (61.0) |
| IgA | 7 (11.9) | |
| Light chain | 12 (20.3) | |
| IgM | 1 (1.7) | |
| IgD | 1 (1.7) | |
| AL amyloidosis | 2 (3.4) | |
| Stage according to the R-ISS (n, %) | Missing | 7 (11.9) |
| Stage I | 13 (22) | |
| Stage II | 32 (54.2) | |
| Stage III | 7 (11.9) | |
| Remission state (n, %) | PD | 6 (10.2) |
| SD | 3 (5.1) | |
| PR | 17 (28.8) | |
| VGPR | 8 (13.6) | |
| Serological CR | 25 (42.4) | |
| High-risk cytogenetics (n, %) | Missing, n = 7 | |
| t(4;14) | 7 (11.9) | |
| t(14;16) | 1 (1.7) | |
| del(17p) | 10 (16.9) | |
| Gain 1q | 9 (17.3) | |
| Treatments (n, %) | Watch and wait | 13 (22.0) |
| (Re)induction | 18 (30.5) | |
| Maintenance | 28 (47.5) | |
| Autologous transplant (n, %) | None | 20 (33.9) |
| Single | 33 (55.9) | |
| Tandem | 6 (10.2) | |
AL: amyloid light-chain; CR: complete remission; Ig: immunoglobulin; IQR: interquartile range; n: number; PD: progressive disease; PR: partial response; R-ISS: Revised International Staging System; SD: stable disease; VGPR: very good partial response
At baseline (T1), a median of 100 (IQR = 92–107) days post-second vaccine dose, seroconversion was detectable in 54/59 (91.5%) patients and in all 22 (100%) controls. The patients with multiple myeloma had significantly lower anti-S levels than the controls (median anti-S level = 166 BAU/ml vs 929 BAU/ml, p <0.001; figure 2A). The neutralising antibodies were detectable in 30/59 (50.8%) patients and 20/22 controls (91%; χ2 = 10.886, df = 1, p = 0.001).
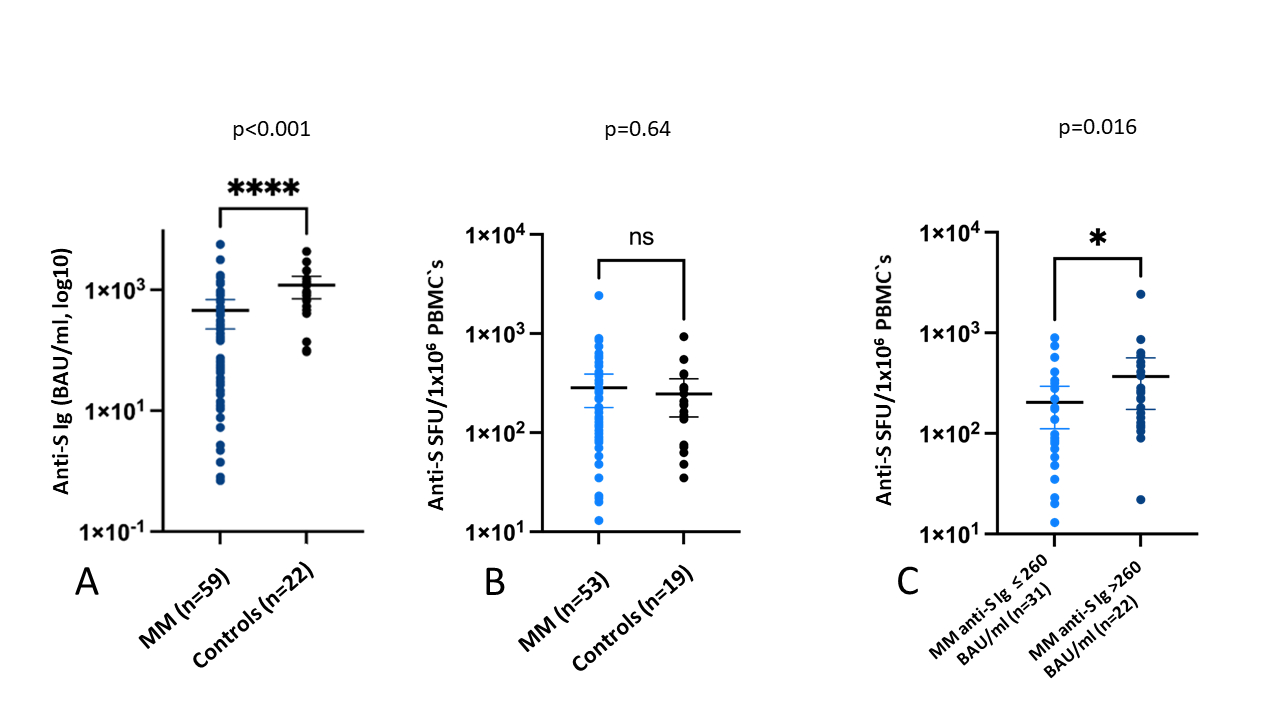
Figure 2P value indicates the significance of the difference in the Wilcoxon rank sum test. (A) Humoral response following baseline vaccination with BNT162b2 between the patients with myeloma (median anti-S level = 166 BAU/ml, IQR = 14–517) and healthy controls (median anti-S level = 929 BAU/ml, IQR = 497–1435). (B) T-cell response following baseline vaccination with BNT162b2 between the patients with myeloma (median anti-SFU/106 of PBMCs = 175, IQR = 88–325) and healthy controls (median anti-S SFU/106 of PBMCs = 193, IQR = 75–288). (C) T-cell response in the patients with myeloma according to the serological responder status. Responders (n = 22): median anti-SFU/106 of PBMCs = 255 (IQR = 120–482), low responders/non-responders (n = 31): median anti-SFU/106 of PBMCs = 128 (IQR = 58–280).
BAU: binding antibody unit; SFU: spot-forming unit; PBMC: peripheral blood mononuclear cell.
The treatment intensity during vaccination was associated with the degree of humoral response, with the lowest anti-S level (median = 13 BAU/mL) observed in the patients receiving (re)induction therapy (table 2 and figure 3A). All 18 patients vaccinated during (re)induction therapy were non-responders (anti-S level <40 BAU/ml). Furthermore, an age of ≥75 years, a dexamethasone dose of ≥20 mg/month, an absolute lymphocyte count of <1.0 × 109/l, a CD4+ T-cell count of <0.25 × 109/l, a CD19+ B-cell count of <0.03 × 109/l and an immunoglobulin (Ig) G concentration of <4 g/l were associated with significantly lower anti-S Ig levels (table 2).
Table 2Response to baseline vaccination according to patient-related factors and disease characteristics.
| After second vaccination (T1) | ||||||
| n | Anti-S level (BAU/ml) (median, IQR) | p | n | Anti-S SFU/10 of peripheral blood mononuclear cells (median, IQR) | p | |
| All | 59 | 166 (14–517) | 53 | 175 (88–325) | ||
| Female sex | 20 | 279 (31–610) | 0.501 | 17 | 115 (56–152) | 0.017 |
| Male sex | 39 | 153 (12–469) | 36 | 223 (90–457) | ||
| <75 years of age | 45 | 190 (32–674) | 0.021 | 41 | 180 (90–299) | 0.588 |
| ≥75 years of age | 14 | 16 (1.25–265) | 12 | 117 (49–511) | ||
| R-ISS stage I | 13 | 190 (19–493) | 0.259 | 12 | 268 (103–485) | 0.072 |
| R-ISS stage II | 32 | 173 (26–654) | 31 | 130 (70–265) | ||
| R-ISS stage III | 7 | 14 (5–43) | 5 | 570 (135–744) | ||
| At least CR | 25 | 190 (32–674) | 0.211 | 23 | 180 (90–280) | 0.795 |
| Less than CR | 34 | 69 (7–443) | 30 | 141 (77–383) | ||
| Watch and wait | 13 | 641 (388–1230) | <0.001 | 13 | 180 (112–492) | 0.384 |
| Reinduction | 18 | 13 (2.6–48) | 14 | 88 (67–424) | ||
| Maintenance | 28 | 207 (39–632) | 26 | 180 (96–281) | ||
| Daratumumab unexposed | 43 | 166 (8–641) | 0.811 | 40 | 200 (86–400) | 0.145 |
| Daratumumab exposed | 16 | 124 (37–390) | 13 | 128 (80–198) | ||
| <20 mg dexamethasone/month | 34 | 388 (72–822) | <0.001 | 32 | 223 (116–330) | 0.079 |
| ≥20 mg dexamethasone/month | 25 | 22 (4–145) | 21 | 90 (64–298) | ||
| ALC of <1.0 G/l | 26 | 20 (2–265) | 0.001 | 22 | 129 (78–229) | 0.206 |
| ALC of ≥1.0 G/l | 33 | 385 (65–674) | 31 | 225 (90–408) | ||
| CD4+ T-cell count of <0.25 G/l | 19 | 27 (2–179) | 0.009 | 16 | 134 (84–220) | 0.418 |
| CD4+ T-cell count of ≥0.25 G/l | 35 | 251 (43–676) | 32 | 168 (90–400) | ||
| CD19+ B-cell count of <0.03 G/l | 21 | 11 (1–38) | <0.001 | 19 | 130 (80–375) | 0.736 |
| CD19+ B-cell count of ≥0.03 G/l | 33 | 391 (160–824) | 29 | 175 (98–262) | ||
| IgG level of ≥4 g/l | 41 | 306 (47–748) | 0.004 | 38 | 220 (103–383) | 0.047 |
| IgG level of <4 g/l | 17 | 19 (7–185) | 15 | 90 (53–180) | ||
ALC: absolute lymphocyte count; BAU: binding antibody units; CR: complete remission; IgG: immunglobuline G; IQR: interquartile range; n: number; R-ISS: Revised International Staging System; SFU: spot forming unit
No significant difference in the SARS-CoV-2-specific T-cells was detected between the controls and patients with multiple myeloma (figure 2B). Among the patients with myeloma, neither treatment intensity, age nor markers of cellular and humoral immunity influenced the T-cell response (figure 3B and table 2).
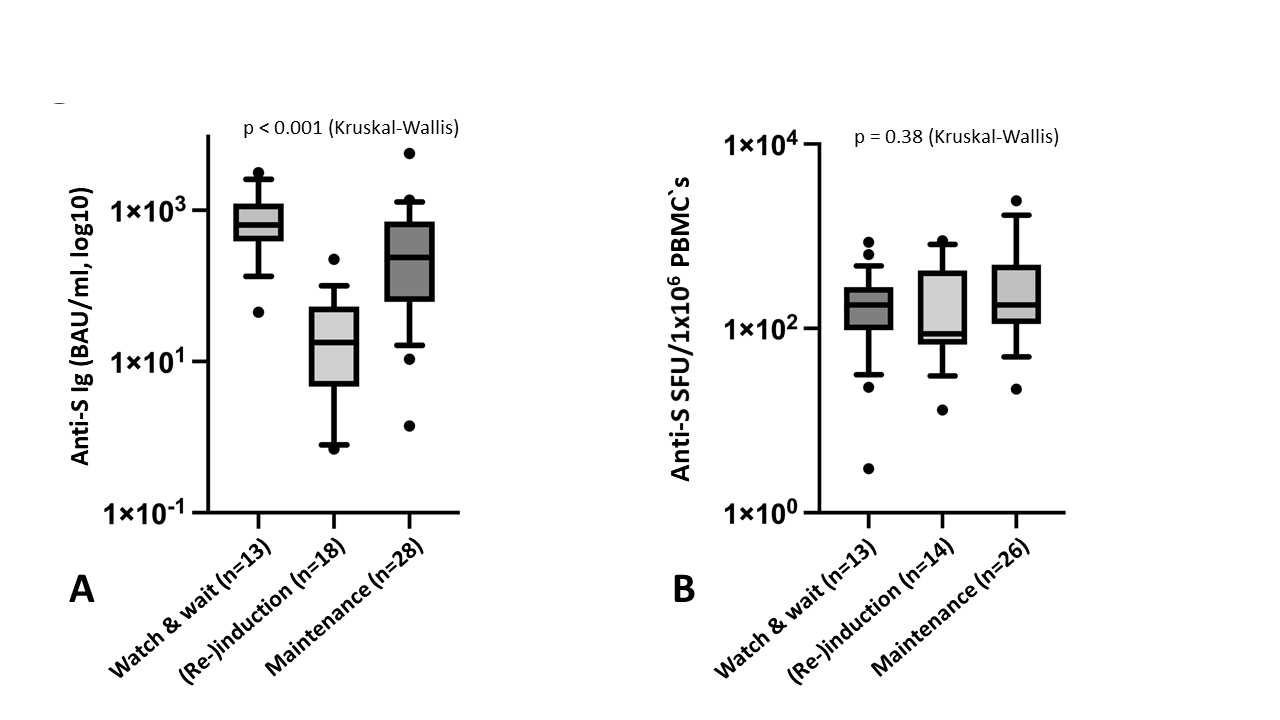
Figure 3P value indicates the significance of the difference in the Kruskal-Wallis test. (A) Humoral response in the patients with multiple myeloma following baseline vaccination with BNT162b2 according to the treatment strategy: watch and wait: median anti-S level = 641 BAU/ml, (re)induction: median anti-S level = 13 BAU/ml and maintenance: median anti-S level = 207 BAU/ml. (B) T-cell response in the patients with multiple myeloma following baseline vaccination with BNT162b2 according to the treatment strategy: watch and wait (n = 13): median anti-S SFU/106 of PBMCs = 180, (re)induction (n = 14): median anti-S SFU/106 of PBMCs = 88 and maintenance (n = 26): median anti-S SFU/106 of PBMCs = 180.
BAU: binding antibody unit; PBMC: peripheral blood mononuclear cell; SFU: spot-forming unit
There was no correlation between the anti-S IgG level and anti-S spot-forming unit (Spearman correlation coefficient = 0.24, p = 0.08). However, the responders (anti-S IgG level >260 BAU/l, n = 22) had a significantly higher specific T-cell count than the non-responders and low responders (anti-S Ig level ≤260 BAU/l, n = 31, figure 2C).
Of the 59 patients with multiple myeloma, 56 (95%) received a booster vaccine at a median of 214 (IQR = 184–262) days post-second vaccine dose, and 50 (85%) were evaluable according to the protocol.
Pre-booster immunity (T2) was assessed at a median of 92 (IQR = 76–108) days after T1. During this period, the median anti-S Ig level decreased significantly from 153 to 41 BAU/ml (p <0.0001), and the anti-S level decreased from >260 BAU/ml at baseline to below 260 BAU/ml in 9/24 (38%) patients (figure 4A). In contrast, the S-specific T-cell count slightly increased (figure 5).
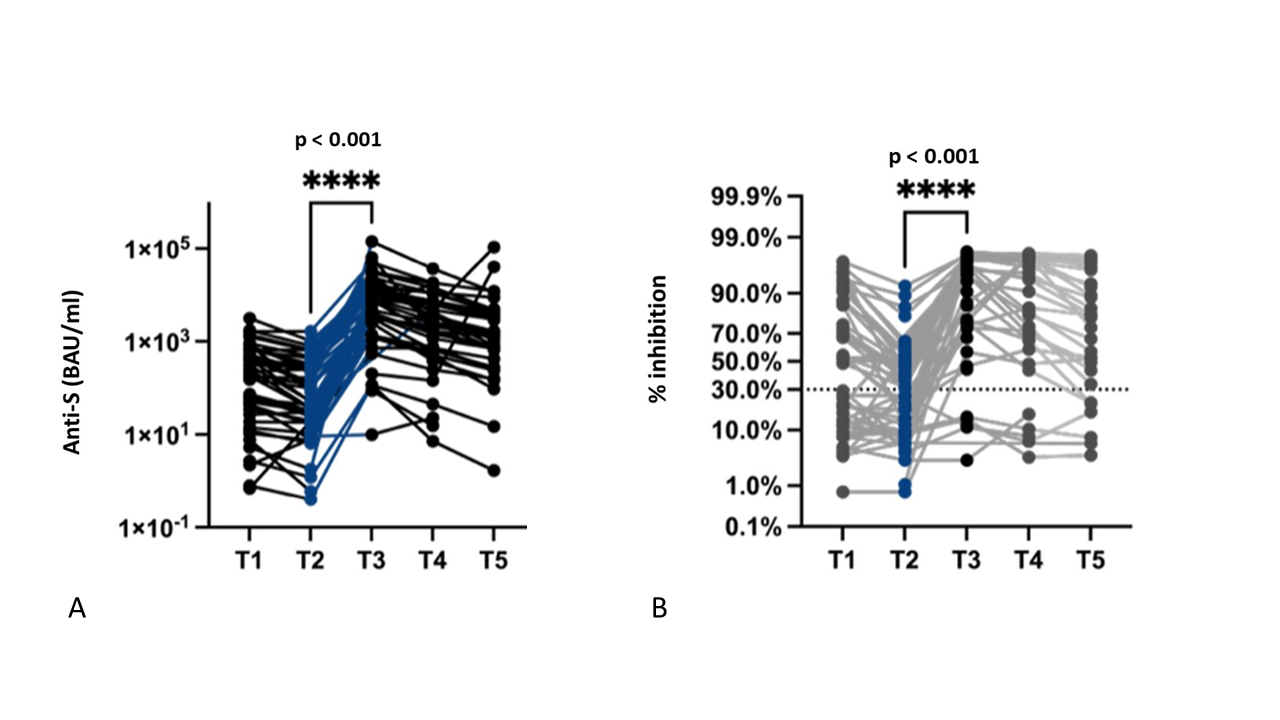
Figure 4P value indicates the significance of the difference in the Wilcoxon matched-pairs signed rank test. (A) Humoral response in the patients with multiple myeloma following the third booster dose of BNT162b2 (T1, baseline; T2, pre-booster; T3, 4 weeks; T4, 3 months; and T5, 6 months after booster vaccination): median anti-S level pre-booster (T2) = 41 (IQR = 9–312) BAU/ml, median anti-S level 4 weeks post-booster (T3) = 3901 (IQR = 1304–13,748) BAU/ml. (B) Neutralising antibody response against the wild type and variants of the virus: median inhibition pre-booster = 19% (IQR = 6–47), median inhibition post-booster = 97% (IQR = 65–98).
Post-booster immunity (T3) was assessed at a median of 34 (IQR = 28–40) days post-booster. Both serological (anti-S and neutralising antibodies) and cellular responses showed significant increases compared with those at T2(table 3 and figures 4A/B and 5).
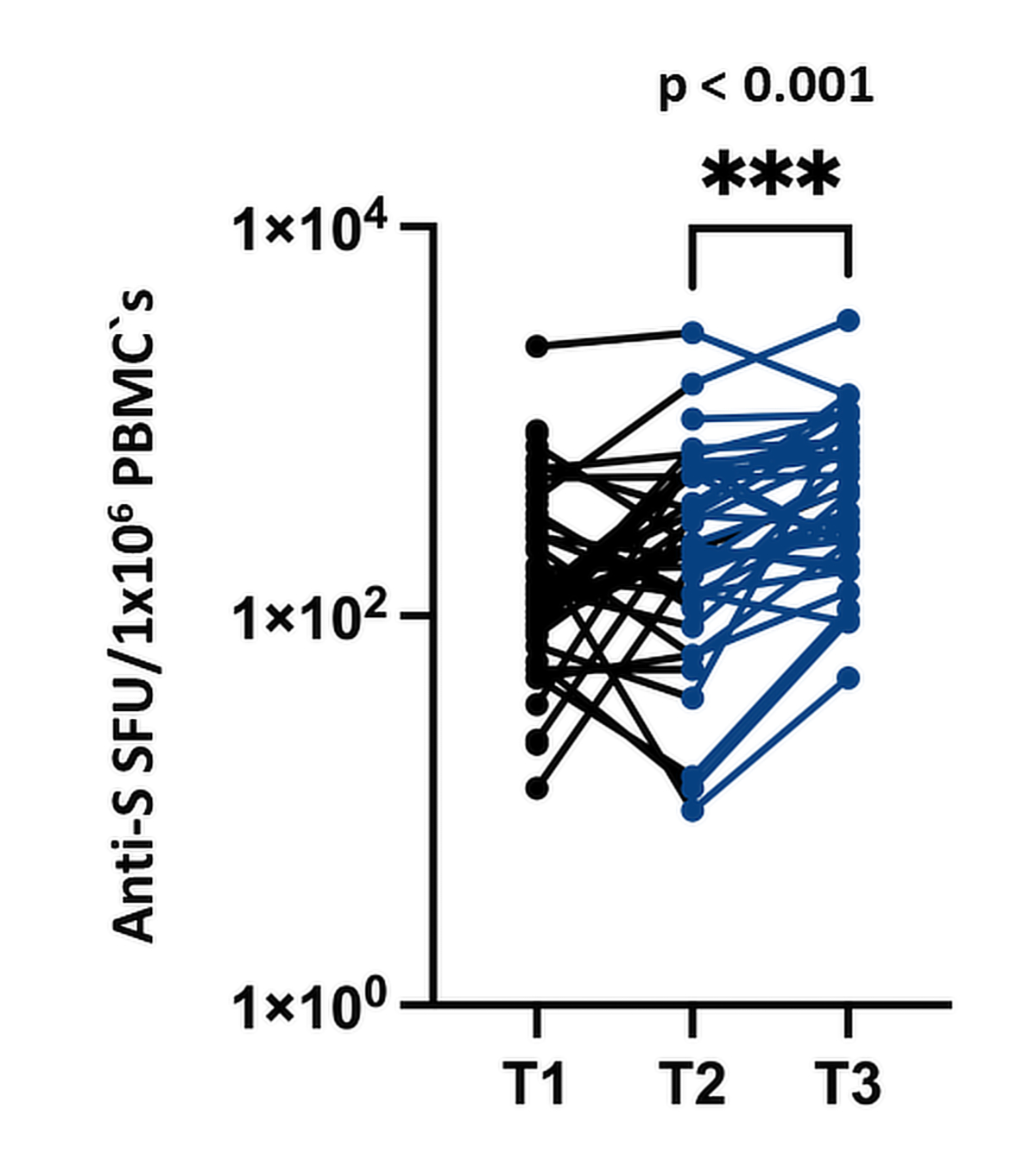
Figure 5T-cell response to the third booster dose of BNT162b2 in the patients with multiple myeloma (T1, baseline; T2, pre-booster; T3, 4 weeks following booster): median anti-S SFU/106 of PBMCs pre-booster (T2) = 235 (IQR = 128–570), median anti-S SFU/106 of PBMCs post-booster (T3) = 443 (IQR = 209–814), p <0.001.P value indicates the significance of the difference in the Wilcoxon paired-samples test.
PBMC: peripheral blood mononuclear cell; SFU: spot-forming unit
The treatment intensity was associated with lower anti-S levels under maintenance and (re)induction therapies (median anti-S level = 3678 and 1949 BAU/ml, respectively) than under watch and wait (median anti-S level = 15185 BAU/ml, p = 0.017; table 3). The difference between (re)induction and maintenance therapies was not significant (p = 0.145). Notably, four of five (80%) patients with no initial serological response developed anti-S antibodies after booster vaccination but at low levels (median anti-S level post-booster = 88 BAU/ml, IQR = 5–373) compared with the initial responders (median anti-S level = 6565 BAU/ml, IQR = 2555–14,044, p = 0.001). In contrast, the cellular responses were affected neither by the treatment intensity nor by the markers of cellular and/or humoral immunity measured (table 3).
Table 3Response to a third booster dose according to patient-related factors and disease characteristics.
| After booster vaccination (T3) | ||||||
| n | Anti-S IgG level (BAU/ml (median, IQR) | p | n | Anti-S SFU/10 of peripheral blood mononuclear cells (median, IQR) | p | |
| All | 50 | 3902 (1304–13748) | 37 | 443 (209–814) | ||
| Female sex | 20 | 6068 (1602–15497) | 0.526 | 15 | 343 (230–788) | 0.516 |
| Male sex | 30 | 3678 (1274–13748) | 22 | 598 (184–1069) | ||
| <75 years of age | 38 | 5090 (1388–13478) | 0.286 | 27 | 443 (230–798) | 0.694 |
| ≥75 years of age | 12 | 2792 (223–17590) | 10 | 396 (155–889) | ||
| R-ISS stage I | 11 | 3684 (1109–7480) | 0.790 | 9 | 690 (358–1224) | 0.178 |
| R-ISS stage II | 27 | 3672 (1330–12800) | 19 | 308 (133–788) | ||
| R-ISS stage III | 5 | 13600 (365–78489) | 3 | 1065 (230– .) | ||
| At least CR | 23 | 3684 (472–12800) | 0.566 | 14 | 633 (230–1087) | 0.280 |
| Less than CR | 27 | 4119 (1330–16178) | 23 | 423 (133–798) | ||
| Watch and wait | 12 | 15185 (4605–25670) | 0.017 | 4 | 687 (240–1257) | 0.692 |
| Reinduction | 16 | 1949 (528–7968) | 15 | 423 (108–1080) | ||
| Maintenance | 22 | 3678 (1344–12627) | 18 | 393 (236–692) | ||
| Daratumumab unexposed | 33 | 4119 (1278–16325) | 0.602 | 19 | 448 (170–798) | 0.799 |
| Daratumumab exposed | 17 | 3684 (1256–8920) | 18 | 433 (236–900) | ||
| <20 mg dexamethasone/month | 29 | 7120 (1395–7120) | 0.123 | 20 | 376 (199–696) | 0.493 |
| ≥20 mg dexamethasone/month | 21 | 2813 (634–8840) | 17 | 528 (204–1073) | ||
| ALC of <1.0 G/l | 22 | 2772 (198–02627) | 0.123 | 17 | 423 (209–760) | 0.831 |
| ALC of ≥1.0 G/l | 28 | 6591 (1820–16399) | 20 | 488 (187–893) | ||
| CD4+ T-cell count of <0.25 G/l | 31 | 7210 (2813–16473) | 0.025 | 13 | 423 (161–739) | 0.459 |
| CD4+ T-cell count of ≥0.25 G/l | 16 | 2613 (133–7911) | 23 | 528 (230–925) | ||
| CD19+ B-cell count of <0.03 G/l | 17 | 1330 (116–7862) | 0.002 | 16 | 655 (175–1006) | 0.836 |
| CD19+ B-cell count of ≥0.03 G/l | 30 | 7300 (3133–21717) | 20 | 393 (232–761) | ||
| IgG level of ≥4 g/l | 37 | 7210 (1943–16325) | 0.081 | 11 | 283 (230–1080) | 0.594 |
| IgG level of <4 g/l | 12 | 2072 (589–8331) | 25 | 528 (208–814) | ||
ALC: absolute lymphocyte count; BAU: binding antibody unit; CR: complete remission; IgG: immunoglobulin G; IQR: interquartile range; R-ISS: Revised International Staging System; SFU: spot-forming unit
The anti-S antibody concentrations declined significantly over time to a median of 2055 (IQR = 504–6170) BAU/ml 3 months post-booster (T4, n = 45, p <0.001) and 1050 (IQR = 269–4198) BAU/ml 6 months post-booster (T5, n = 39, p <0.001). Nevertheless, 32/39 (82%) patients maintained neutralising antibody positivity at T5 (figure 4A and B).
During the study and follow-up periods, 21 (35%) patients acquired COVID-19. Of them, 17 were diagnosed via PCR testing following symptoms and four via detection of new anti-N antibodies. In Eastern Switzerland, the period from October to December 2021 was mainly Delta-dominant [9], while the period thereafter was Omicron-dominant. Two patients (one before and one after booster vaccination) acquired the infection during the Delta-dominant period and 19 during the Omicron-dominant period. Infections occurred at a median of 231 (IQR = 114–320) days post-booster vaccination.
Most infections were either asymptomatic (n = 4/21) or with mild flu-like symptoms (n = 12/21) such as fever, headache, fatigue, sore throat, cough and muscle ache, which resolved after 2–14 weeks and were managed with symptomatic treatment in an outpatient setting. Nevertheless, 5/21 patients required hospitalisation for a median of 6 (IQR = 3–16) days. Supplemental oxygen was necessary in 4/5, remdesivir in 4/5 and antibiotics for suspected bacterial superinfection in 2/5 patients. No patient required intensive care or died of COVID-19.
In this study, we assessed the humoral and cellular immune responses after two and three doses of the mRNA-based vaccine BNT162b2 (Pfizer/BioNTech) in a monocentric cohort of patients with multiple myeloma under watch and wait, maintenance and (re)induction therapies with different regimens.
After two doses of the vaccine, most patients with multiple myeloma responded via mounting of an S-specific humoral immunity; however, the immune responses were highly heterogeneous with vaccination failure occurring irrespective of the treatment intensity or remission status. We noted a significant impact of the treatment intensity applied during vaccination and disease- or treatment-associated immunosuppression on the humoral immune responses only. These observations are in line with other reports, which have been subject of several meta-analyses [10–12].
The third booster dose was effective on the serological level even in the patients with failed prior two-dose vaccination, albeit leading to low concentrations. Generally, the booster dose reversed the negative impact of several treatment modalities, and most patients with multiple myeloma achieved a serological response in the range of the healthy controls following booster vaccination. This finding is in line with several previous reports [13–20], which are shown in detail in table 4. In 7/8 studies, a significant increase in the antibody levels after the booster has been reported, with therapies directed against B-cell maturation antigen remaining as the sole treatment modality clearly associated with a reduced response.
Table 4Summary of existing analyses after SARS-CoV-2 booster vaccinations in patients with multiple myeloma.
| Reference | Vaccine type | Time point of analysis after the third dose | Humoral response | Neutralising antibodies | T-cell response | Assessment method for T-cell response | Number of patients with multiple myeloma | Healthy controls | Main findings |
| Frankel et al., Med Res Arch 2022 [13] | BNT162b2 and mRNA-1273 | 1 month | Yes | Yes | No | n.a. | 12/160 | None | Only minor serological response following booster vaccinations in patients with myeloma |
| Ntanasis-Stathopoulos et al., Hemasphere 2022 [14] | BNT162b2 | 1 and 3 months | Yes | Yes | No | n.a. | 201 | None | Booster vaccination with BNT162b2 resulted in a substantially improved humoral response against SARS-CoV-2 in patients with multiple myeloma. Anti-BCMA treatment remained an adverse predictive factor for neutralising antibody response. |
| Terpos et al., Blood 2022 [15] | BNT162b2 | 30 days | Yes | Yes | No | n.a. | 167 | None | A booster dose enhanced the neutralising antibody response significantly. However, several patients did not achieve sufficient antibody response, especially those receiving treatment with anti-BCMA therapeutics. |
| Enssle et al., Cancer Cell 2022 [16] | BNT162b2 | 1–3 months | Yes | Yes | Yes | Flow cytometry and ELISpot assay | 71 | Yes | High immunogenicity of a third booster dose on the serological level; strong CD4+ T-cell response against the wild type and Delta variant without difference between patients with multiple myeloma and controls; not significantly diminished T-cell responses towards Omicron |
| Re et al., Nature Communications 2022 [17] | BNT162b2 | 3–5 weeks (median = 27 days) | Yes | Yes | Yes | IGRA | 16/45 patients with lymphoid malignancies | None | A third booster dose increased antibody titres and neutralising antibody levels in patients with multiple myeloma. A third dose increased the median secreted IFN-γ level in the whole cohort and induced IFN-γ secretion in a fraction of seronegative patients. However, the results for patients with multiple myeloma were not reported separately. |
| Storti et al., OncoImmunology 2022 [18] | Booster vaccination with mRNA-1273 following baseline vaccination with BNT162b2 | 14 days | Yes | Yes | Yes | Flow cytometry | 16/40 patients with plasma cell dyscrasias | None | Heterologous booster immunisation improved SARS-CoV-2 spike humoral and cellular responses in patients newly diagnosed with multiple myeloma and in most, but not all, patients with relapsed multiple myeloma. |
| Wagner et al., Front Immunol 2022 [19] | BNT162b2 and mRNA-1273 | 4 weeks | Yes | Yes | Yes | Cytokine release assay | 70 patients with myeloma of a mixed cohort and cellular response and 9 patients with multiple myeloma | Booster vaccination increased antibody levels by >8-fold in seroresponders and induced anamnestic responses even in those with undetectable pre-booster antibody levels. T-cell response was detectable in all patient groups assessed but reported in nine patients with multiple myeloma only. | |
| Aleman et al., Cancer Cell 2022 [20] | BNT162b2 and mRNA-1273 | At least 1 week | Yes | Yes | Yes | Cytokine release assay | 261 patients with a cellular response and 31 patients with multiple myeloma | Yes | A third mRNA-based vaccine dose significantly augmented cellular and humoral immune responses against SARS-CoV-2, including the antigenically distinct Omicron variant, in patients with multiple myeloma. The third dose induced seroconversion in more than 80% of patients with multiple myeloma with undetectable anti-S IgG. |
In contrast to serological data, data on the T-cell responses following both baseline and booster vaccinations are still scarce and are generally based on much smaller patient samples. Since different methods have been used to detect and quantify SARS-CoV-2-specific T-cells, it is difficult to directly compare results between studies.
However, our observation of a largely preserved T-cell response after baseline vaccination in the patients with multiple myeloma without a difference from the healthy controls matches the majority of previous reports [21–25]. Notably, in all studies, T-cell responses were detectable even in the majority of serological non-responders. Only two studies reported an impairment of the T-cell response in patients with myeloma in the early phase following baseline vaccination [26, 27].
Administration of a booster dose has been uniformly reported to augment the T-cell response in all studies (table 4), including in follow-up reports on the two populations, wherein the T-cell response has been reported to be impaired following baseline vaccination [16, 20].
Notably, the post-booster results obtained via ELISpot assay as a measure of the functional T-cell response reported by Enssle et al. [16] are similar to our results: No difference was observed between the patients and healthy controls in terms of the T-cell responses against the receptor-binding domain, and no correlation was found between the serological and T-cell responses.
As in other studies [28–30], we observed a substantial number of symptomatic and asymptomatic breakthrough infections (35% of the cohort) even after three doses of vaccination. Breakthrough infections occurred mainly during the Omicron-dominant period [9], and the disease courses were mostly mild with only influenza-like symptoms. Only a minor proportion of the patients required hospital admission, but no patient needed mechanical ventilation or died.
In our monocentric study, several limitations must be considered. First, the number of patients was relatively small. However, this sample size allowed us to repeatedly perform complex and elaborate diagnostics such as the quantification of specific T-cells and to compare the respective results with the humoral vaccine responses on the background of different disease characteristics and treatment strategies. Thus, this study could also be described as exploratory. Second, a healthy control group was available at baseline only. Accordingly, any statement on the persistence of immune reactions could not be made. Third, our assay for the determination of neutralising antibodies does not allow for differentiation of the inhibition of different SARS-CoV-2 variants. This prevented us from describing vaccine-induced neutralising antibodies against new variants currently prevalent in the community, such as the XBB.1.5 sub-lineage. However, we uniformly assessed specific T-cell and humoral responses including overall neutralising antibodies in a population reflecting real-world myeloma treatment. As detection of specific T-cells is substantially laborious, we could evaluate the serological response only at later time points (3 and 6 months after booster) but were nevertheless able to demonstrate the early effect of booster vaccination on the T-cell response. Based on our data, the specific T-cell response seems to be more stable than the humoral response at least in the early phase following (booster) vaccination. However, larger-scale studies with longer follow-ups are needed to confirm the persistence of specific T-cells in the long term.
Current evidence suggests that in the majority of patients, a third dose (i.e. booster) is capable of overcoming the impairments of the initial two-dose vaccine response. This is mainly a consequence of anti-myeloma therapy but also of the underlying disease.
A lower initial humoral vaccine response value was associated with a significantly lower booster vaccine response value, and the decline in humoral immunity was even more pronounced in patients over time. This finding suggests that serological vaccination failures occur even after third dose vaccination in some patients. Therefore, our data further support individual assessment of the humoral vaccine response in patients with multiple myeloma [7, 31, 32] to identify patients who might benefit not only from further protective measures, such as social distancing, mask protection or vaccination of household contacts (cocooning), but also from pre-exposure prophylaxis via passive immunisation [33], which has been proven effective in highly immunocompromised patients [34]. Finally yet importantly, the possibility of enhancing the T-cell responses even in serological non-responders should be considered once vaccination strategies using vaccines adapted to newer virus variants for immunocompromised hosts are developed.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
We thank our patients for participating in the study, our colleagues at the Cantonal Hospital St. Gallen (Theresa Fehr, Dagmar Hess, Felicitas Hitz, Michael Baumann, Martin Fehr, Martin Gramatzki, Thomas Lehmann and Marcus Schittenhelm) for including patients into the study and Dorothea Hillmann, Centre for Laboratory Medicine Dr. Risch, Vaduz, Liechtenstein for performing the antibody measurements.
Authors' contributions: TS, CRK, WCA, CD and SF designed the study; TS acquired funding; TS, CD, SF and RD organised patient recruitment; SN and CRK performed the laboratory investigations; TS and SF collected clinical data, TS and CRK analysed data and wrote the original draft of the manuscript; all authors reviewed and edited the paper; SF supervised the research.
The project was funded by the Swiss Cancer Foundation (project ID 21–03).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. WCA has participated in advisory boards for MSD, Sanofi, Pfizer, GSK, OM Pharma and Jannsen outside the scope of this manuscript with reimbursement paid to his institution and has received honararia for lectures, presentations and educational events by Pfizer, Medscape, GSK and MSD with reimbursement paid to his institution. No other potential conflict of interest related to the content of this manuscript was disclosed.
1. Tomalka JA, Suthar MS, Deeks SG, Sekaly RP. Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses. Nat Immunol. 2022 Mar;23(3):360–70. 10.1038/s41590-022-01130-4
2. Ludwig H, Sonneveld P, Facon T, San-Miguel J, Avet-Loiseau H, Mohty M, et al. COVID-19 vaccination in patients with multiple myeloma: a consensus of the European Myeloma Network. Lancet Haematol. 2021 Dec;8(12):e934–46. 10.1016/S2352-3026(21)00278-7
3. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022 Feb;23(2):186–93. 10.1038/s41590-021-01122-w
4. Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021 Jul;595(7868):572–7. 10.1038/s41586-021-03653-6
5. Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021 Jul;27(7):1280–9. 10.1038/s41591-021-01386-7
6. Riester E, Findeisen P, Hegel JK, Kabesch M, Ambrosch A, Rank CM, et al. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J Virol Methods. 2021 Nov;297:114271.
7. Barrière J, Carles M, Audigier-Valette C, Re D, Adjtoutah Z, Seitz-Polski B, et al. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: should humoral responses be monitored? A position article. Eur J Cancer. 2022 Feb;162:182–93.
8. Murray MJ, McIntosh M, Atkinson C, Mahungu T, Wright E, Chatterton W, et al. Validation of a commercially available indirect assay for SARS-CoV-2 neutralising antibodies using a pseudotyped virus assay. J Infect. 2021 May;82(5):170–7.
9. Hodcroft, E. Overview of Variants in Countries.
10. Gagelmann N, Passamonti F, Wolschke C, Massoud R, Niederwieser C, Adjallé R, et al. Antibody response after vaccination against SARS-CoV-2 in adults with hematological malignancies: a systematic review and meta-analysis. Haematologica. 2022 Aug;107(8):1840–9.
11. Ito Y, Honda A, Kurokawa M. COVID-19 mRNA Vaccine in Patients With Lymphoid Malignancy or Anti-CD20 Antibody Therapy: A Systematic Review and Meta-Analysis. Clin Lymphoma Myeloma Leuk. 2022 Aug;22(8):e691–707.
12. Wang, X.; Sima, L. Antibody Response after Vaccination against SARS–CoV–2 in Adults with Hematological Malignancies: A Systematic Review and Meta–Analysis. J Infect 2022, S0163–4453(22)00674–0, doi:.
13. Frankel AE, Capozzola T, Andrabi R, Ahn C, Zhou P, He WT, et al. The Effects of an mRNA Covid-19 Vaccine Booster on Immune Responses in Cancer-Bearing Veterans. Med Res Arch. 2022 Jul;10(7):
14. Ntanasis-Stathopoulos I, Karalis V, Gavriatopoulou M, Malandrakis P, Sklirou AD, Eleutherakis-Papaiakovou E, et al. Second Booster BNT162b2 Restores SARS-CoV-2 Humoral Response in Patients With Multiple Myeloma, Excluding Those Under Anti-BCMA Therapy. HemaSphere. 2022 Jul;6(8):e764.
15. Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. Booster BNT162b2 optimizes SARS-CoV-2 humoral response in patients with myeloma: the negative effect of anti-BCMA therapy. Blood. 2022 Mar;139(9):1409–12.
16. Enssle JC, Campe J, Büchel S, Moter A, See F, Grießbaum K, et al. Enhanced but variant-dependent serological and cellular immune responses to third-dose BNT162b2 vaccination in patients with multiple myeloma. Cancer Cell. 2022 Jun;40(6):587–9.
17. Re D, Seitz-Polski B, Brglez V, Carles M, Graça D, Benzaken S, et al. Humoral and cellular responses after a third dose of SARS-CoV-2 BNT162b2 vaccine in patients with lymphoid malignancies. Nat Commun. 2022 Feb;13(1):864. 10.1038/s41467-022-28578-0
18. Storti P, Marchica V, Vescovini R, Franceschi V, Russo L, Notarfranchi L, et al. Immune response to SARS-CoV-2 mRNA vaccination and booster dose in patients with multiple myeloma and monoclonal gammopathies: impact of Omicron variant on the humoral response. OncoImmunology. 2022 Sep;11(1):2120275.
19. Wagner A, Garner-Spitzer E, Schötta AM, Orola M, Wessely A, Zwazl I, et al. SARS-CoV-2-mRNA Booster Vaccination Reverses Non-Responsiveness and Early Antibody Waning in Immunocompromised Patients - A Phase Four Study Comparing Immune Responses in Patients With Solid Cancers, Multiple Myeloma and Inflammatory Bowel Disease. Front Immunol. 2022 May;13:889138.
20. Aleman A, Van Oekelen O, Upadhyaya B, Beach K, Kogan Zajdman A, Alshammary H, et al.; PVI/MM/Seronet Study Group. Augmentation of humoral and cellular immune responses after third-dose SARS-CoV-2 vaccination and viral neutralization in myeloma patients. Cancer Cell. 2022 May;40(5):441–3.
21. Chung A, Banbury B, Vignali M, Huang CY, Asoori S, Johnson R, et al. Antibody and T-cell responses by ultra-deep T-cell receptor immunosequencing after COVID-19 vaccination in patients with plasma cell dyscrasias. Br J Haematol. 2022 Nov;199(4):520–8.
22. Zaleska, J.; Kwasnik, P.; Paziewska, M.; Purkot, J.; Szabelak, A.; Jurek, M.; Masny, N.; Dziatkiewicz, I.; Pronobis–Szczylik, B.; Piebiak, A.; et al. Response to Anti–SARS–CoV–2 MRNA Vaccines in Multiple Myeloma and Chronic Lymphocytic Leukemia Patients. International Journal of Cancer n/a, doi:.
23. Ramasamy K, Sadler R, Jeans S, Weeden P, Varghese S, Turner A, et al. Immune response to COVID-19 vaccination is attenuated by poor disease control and antimyeloma therapy with vaccine driven divergent T-cell response. Br J Haematol. 2022 May;197(3):293–301.
24. Marasco V, Carniti C, Guidetti A, Farina L, Magni M, Miceli R, et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2022 Feb;196(3):548–58.
25. Rouhani SJ, Yu J, Olson D, Zha Y, Pezeshk A, Cabanov A, et al. Antibody and T cell responses to COVID-19 vaccination in patients receiving anticancer therapies. J Immunother Cancer. 2022 Jun;10(6):e004766. 10.1136/jitc-2022-004766
26. Enßle JC, Campe J, Schwenger A, Wiercinska E, Hellstern H, Dürrwald R, et al. Severe impairment of T-cell responses to BNT162b2 immunization in patients with multiple myeloma. Blood. 2022 Jan;139(1):137–42.
27. Aleman A, Upadhyaya B, Tuballes K, Kappes K, Gleason CR, Beach K, et al.; PVI/Seronet Study Group. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell. 2021 Nov;39(11):1442–4.
28. Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 Breakthrough Infection and Hospitalization in Fully Vaccinated Patients With Multiple Myeloma. JAMA Netw Open. 2021 Nov;4(11):e2137575.
29. Wang L, Kaelber DC, Xu R, Berger NA. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: A clarion call for maintaining mitigation and ramping-up research. Blood Rev. 2022 Jul;54:100931.
30. Pinato DJ, Aguilar-Company J, Ferrante D, Hanbury G, Bower M, Salazar R, et al.; OnCovid study group. Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022 Jul;23(7):865–75. 10.1016/S1470-2045(22)00273-X
31. Terpos E, Rajkumar SV, Leung N. Neutralizing Antibody Testing in Patients With Multiple Myeloma Following COVID-19 Vaccination. JAMA Oncol. 2022 Feb;8(2):201–2.
32. Federal Vaccination Commission (FVC/EKIF/CFV); Swiss Society of Infectious Diseases (SSI/SGINF) Position Paper on the Use of Monoclonal Antibodies against SARS–CoV–2 as Passive Immunisation Treatments in Severely Immunocompromised Persons in Switzerland.
33. Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al.; PROVENT Study Group. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med. 2022 Jun;386(23):2188–200.
34. Nguyen Y, Flahault A, Chavarot N, Melenotte C, Cheminant M, Deschamps P, et al.; AP-HP-Centre Monoclonal Antibodies Working Group. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect. 2022 Dec;28(12):1654.e1–4.
The protocol is available for download as a separate file at https://doi.org/10.57187/smw.2023.40090.