Hepatitis C antibody test frequencies and positive rates in Switzerland from
2007 to 2017: a retrospective longitudinal study
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40085
Rosario Agosti-Gonzaleza,
Luis Falcatoa,
Thomas Grischottb,
Oliver Sennb,
Philip Bruggmannab
a Arud Centre for Addiction Medicine, Zurich,
Switzerland
b Institute of Primary Care, University Hospital
Zurich, University of Zurich, Switzerland
Summary
BACKGROUND
AND AIMS: The prevalence of chronic hepatitis C in Switzerland is currently
estimated at approximately 32,000 affected individuals (0.37% of the permanent
resident population). An estimated 40% of affected individuals in Switzerland is
undiagnosed. The Swiss Federal Office of Public Health requires laboratories to
report all positive hepatitis C virus (HCV) test results. Approximately 900
newly diagnosed cases are reported annually. The number of HCV tests performed,
however, is not collected by the Federal Office of Public Health and positive
rates are therefore unknown. The aim of this study was to describe the
longitudinal course of the numbers of hepatitis C antibody tests and of positive
rates in Switzerland for the years 2007 to 2017.
METHODS: Twenty
laboratories were asked to provide the number of HCV antibody tests performed
and the number of positive antibody tests per year. Using data from the Federal
Office of Public Health reporting system for the years 2012 to 2017, we
calculated a factor to correct our values for multiple tests of the same
person.
RESULTS: The
annual number of HCV antibody tests performed tripled linearly from 2007 to
2017 (from 42,105 to 121,266) while the number of positive HCV antibody test
results increased by only 75% over the same period (from 1360 to 2379). The HCV
antibody test positive rate steadily decreased from 3.2% in 2007 to 2.0%
in 2017. After correction for multiple tests per person, the person-level HCV antibody
tested positive rate decreased from 2.2% to 1.7% from 2012 to 2017.
CONCLUSION:
In the Swiss laboratories considered, more HCV antibody tests were performed
each year in the period (2007–2017) before and during the approval of the new hepatitis
C drugs. At the same time, the HCV antibody
positive rates decreased, both on a per-test as well as a per-person level.
This study is the first to describe the evolution of tests performed and of positive
rates for HCV antibody in Switzerland at the national level over several years.
In order to more accurately guide future measures to achieve the goal of
eliminating hepatitis C by 2030, we recommend annual collection and publication
of positive rates by health authorities, along with mandatory reporting of numbers
of tests and people treated.
Introduction
Worldwide,
58 million people are living with hepatitis C. The World Health Organization estimated
that in 2019 approximately 290,000 people died from hepatitis C [1]. The
prevalence of chronic hepatitis C virus (HCV) infection in Switzerland is
estimated at approximately 32,000 (0.37%) affected individuals [2]. An
estimated 40% of those affected in Switzerland are undiagnosed [2]. The
estimates are based on subpopulation studies and
epidemiological modelling, as well as on data from the Federal Office of Public
Health reporting system [3]. It must be emphasised though that no systematic
prevalence studies in the general population are available for Switzerland.
The Federal
Office of Public Health reporting system requires laboratories to report all
positive HCV test results: an anti-HCV antibody reaction confirmed by another
test – usually an
immunoblot test, HCV core antigen detection or HCV ribonucleic
acid (RNA) detection. There are even fewer reliable data on incidence
than on prevalence in Switzerland. The incidence rate in 2016 was estimated in
one study to be 32–39 cases per 100,000 inhabitants [4].
According to the Federal Office of Public Health, the incidence of acute
hepatitis C in 2020 was 0.2 per 100,000 inhabitants [5]. At the same time, hepatitis
C notification rates have been
declining in Switzerland for 20 years [5].
According
to information from the Federal Office of Public Health bulletin, approximately
900 newly diagnosed cases are reported annually [6].
This number has been decreasing slightly for several years. The underlying HCV antibody
or HCV RNA positive rates are not known. “HCV positive rate” is defined as the
proportion of positive HCV tests in either the total number of tests performed
in a defined time period or the corresponding number of tested individuals. Positive
rates are most likely to be informative about the effectiveness of the testing
strategy. Efficient testing aims to achieve the highest possible positive rates,
while a low positive rate indicates that testing is being done broadly [7].
The
epidemiological situation regarding hepatitis C in Switzerland is unclear, as
many figures are based on estimates. In order to better classify the existing
data, additional parameters such as the number of tests performed and the
positive rates may be helpful. Therefore the evolution of testing practices in
our country over the last few years is of interest for the assessment of the
epidemiological situation. Currently, there are no data for Switzerland for either
the number of HCV tests performed annually or positive rates.
The aim of
this retrospective study was to determine the number of HCV antibody tests
performed and the HCV antibody positive rates at all large medical laboratories
in Switzerland and to describe antibody testing practices from 2007 to 2017.
Materials and
methods
Upon
request, the Federal Office of Public Health provided a list of the 20 medical
laboratories with the highest numbers of hepatitis C reports. All 20 laboratories
were asked to provide the following information for the years 2007‒2017: the number
of HCV antibody
tests performed per year throughout Switzerland (one laboratory also reported
data from Lichtenstein), the number of positive antibody tests per year and the
number of positive HCV RNA tests following positive antibody tests. While the
numbers of antibody tests performed and positive antibody tests were easily available
from most laboratories, none of the contacted laboratories could provide the
HCV RNA data associated with the positive antibody tests because data preparation
or linkage was perceived as too time-consuming. Therefore, the calculation of the
annual HCV test positive rates had to be based solely on the numbers of HCV antibody
tests performed and positive HCV antibody tests, as available from the primary
data collection.
The
laboratories provided aggregated totals of the numbers of performed tests and
of positive tests. No patient-related individual data were provided, so anonymisation
was not necessary. For reasons of data protection, the names of the
laboratories are not disclosed. The project was approved by the Ethics
Committee of the Canton of Zurich (Basec No.: Req-2018-00789).
HCV antibody test
positive rates
The annual
HCV antibody test positive rate was calculated by dividing the total number (over
all laboratories) of positive HCV antibody tests per year by the total number
of HCV antibody tests performed in the same year.
HCV antibody tested
positive rates
For the
years 2012 to 2017, the Federal Office of Public Health provided yearly numbers
of confirmed HCV antibody tests reported and the yearly numbers corrected for
multiple reports for one person. No person-related data were provided by the Federal
Office of Public Health. Using the provided data, we corrected the HCV antibody
test positive rates for multiple testing
of the same person as follows: For every year from 2012 to 2017, a correction
factor (multiple testing factor) was calculated as the ratio of the number of confirmed
positive antibody tests per year and the number of individuals with at least
one confirmed positive antibody test in that same year, or – equivalently – as the
average
number of confirmed positive antibody tests per patient in a specific year. By
dividing the HCV antibody test positive rates from our laboratory data
by these correction factors, we extrapolated Swiss-wide person-level HCV antibody
tested positive rates, i.e. average
proportions of positively tested individuals per antibody test. Correction for
multiple positive tests in different years was neither possible nor sought,
given our aim of calculating yearly positive rates. Supplementary table S1
summarises all variables and calculations.
We used
non-parametric Mann-Kendall tests to search for monotonic trends in the antibody
test numbers and positive rates. The significance threshold was set at p ˂0.05.
The use of modified
Mann-Kendall tests accounting for serial correlations resulted in identical
decisions with equal or smaller p values, and for the sake of brevity we chose
not to report these.
Finally, we
explored the between-laboratory variability of the antibody positive rates both
graphically and by means of non-robust (relative standard deviation [rsd]) and
robust (quartile coefficient of dispersion [qcd]) variability measures and
assessed the representativeness of our study sample by estimating the analysed
proportion of the total HCV antibody test volume in Switzerland. Full details
on between-laboratory variability and the representativeness of our data are
provided in the Appendix.
Results
Of the 20
laboratories contacted, 13 provided the annual numbers of HCV antibody tests
performed as well as the proportions with positive results, but not all 13
laboratories did so for every year between 2007 and 2017 (supplementary table S2).
For the years 2007‒2008, only 10 laboratories contributed their data. For the years
2009‒2010, 11 laboratories provided their
data; for the years 2011‒2017, data were available from 13 laboratories, which, as
the comparison
with nationwide the Federal Office of Public Health data shows, account for an
estimated 80% of the total HCV antibody test volume in Switzerland (supplementary
methods 2).
The annual
number of HCV antibody tests performed between 2011 and 2017 showed a linear
increase of about 55% from 78,141 to 121,266 (p = 0.003; figure 1). Note that
only the figures from 2011 to 2017 cover all 13 laboratories, and thus the figures
from previous years are not directly comparable. The absolute number of
positive HCV antibody test results showed a slight but significant increase by
14% over the same period, from 2090 tests in 2011 to 2379 tests in 2017 (p = 0.007).
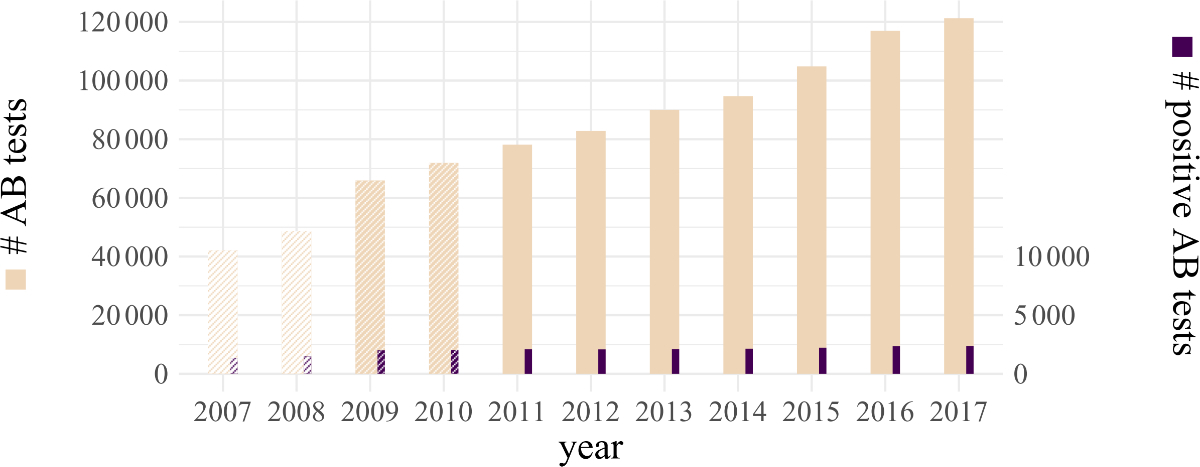
Figure 1Total numbers
of HCV antibody tests and of positive HCV antibody
tests in the years 2007‒2017 as reported by 13 large
laboratories in Switzerland. Years with fewer laboratories are shaded (see main
body text). Column heights indicate numbers of tests (left vertical axis) and
positive tests (right vertical axis), and area ratios between dark and light
columns represent antibody test positive rates.
AB: antibody.
The HCV antibody
test positive rate (i.e. without correction) was 3.2% in 2007 and
continuously decreased to 2.0% in 2017 (p <0.001; figure 2). After correcting
our laboratory data for multiple testing, the HCV antibody tested positive
rate (i.e. after correction) decreased from 2.2% in 2012 to 1.7% in 2017 (p = 0.02;
figure 2).
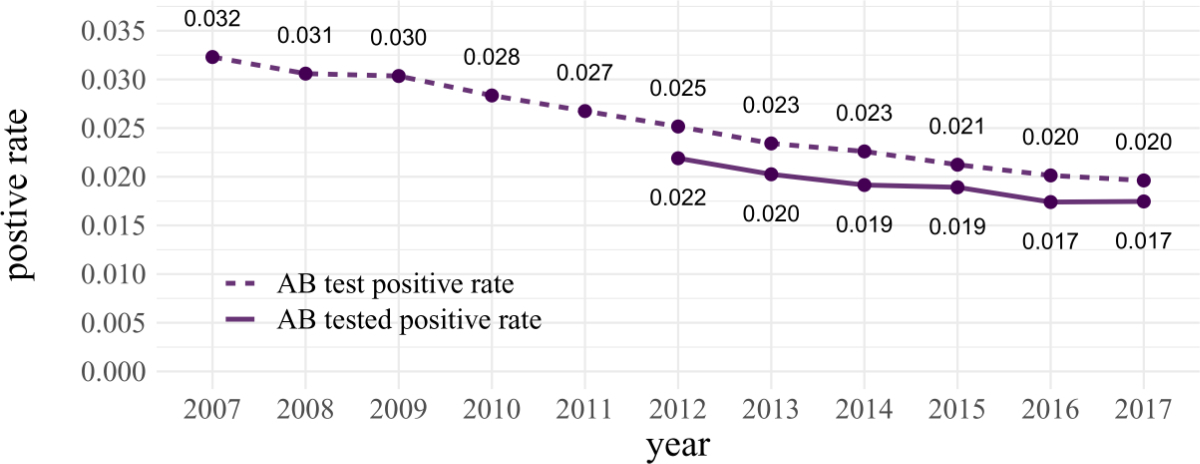
Figure 2HCV antibody
test positive rate and the HCV antibody tested positive rate (with correction
for multiple testing of individuals). The correction factor was not available
before 2012.
AB: antibody.
All data underlying
figures 1 and 2 are presented in supplementary table S2.
Discussion
In our study,
the annual numbers of HCV antibody tests performed in 13 large Swiss
laboratories between 2011 and 2017 showed a linear increase of about 55%, while
the number of positive HCV antibody test results over the same time increased by
about 14%. The HCV antibody test positive rate decreased by 22% between 2012
and 2017. To our knowledge, these are the first published historical data on national
hepatitis C test counts and positive rates.
The reasons
for the increase in the number of antibody tests performed per year may be seen
in the growing evidence on the disease burden and the gaps in the hepatitis C cascade
of care in recent years [8, 9], as well
as in the introduction of new hepatitis C drugs in 2014 and the increased awareness
of hepatitis C in the preceding years following the breakthrough results from pivotal
studies [10, 11]. Another possible cause might
be the Swiss Hepatitis Strategy, which was launched in 2014 and has conducted annual
awareness campaigns since then [12].
The
absolute number of positive HCV antibody test results showed a slight increase.
Our results thus show a trend similar to the numbers of positive antibody tests
reported to the Federal Office of Public Health, which remained approximately
stable over the same period (supplementary table S2).
In contrast
to the number of tests performed, antibody positive rates have been decreasing over
time, possibly due to changes in testing strategies over the years. Given the
increased attention to hepatitis C, the various potential HCV transmission
routes and the Swiss Hepatitis Strategy’s goal of eliminating viral hepatitis
by 2030, it is conceivable that the focus of HCV testing shifted from utmost
efficiency to broader testing. Thus, increasingly frequent antibody testing may
have taken place in populations with lower prevalence than among the commonly
known HCV high-risk groups, namely intravenous substance users and recipients
of blood transfusions before 1990 [13]. Broader
testing in such populations would explain a decrease in positive rates.
Moreover,
the decreasing antibody positive rates could also be due to a decreasing number
of untested HCV antibody-positive people in the population. To verify this
hypothesis, it would be necessary to know in which populations testing rates
have increased.
This
retrospective study has several limitations. The 13 laboratories involved in
the study are distributed in all parts of the country and all language regions
and are among the 20 (out of a total of 43) laboratories that report most cases
to the Federal Office of Public Health. They include the largest providers of
medical laboratory tests in the country. Even though
the catchment area of many of these laboratories covers the entire country, it
may differ from their locations. Although we expect our data to cover about 80%
of the Switzerland-wide HCV antibody testing volume, it is not possible to
specify exactly how representative our study sample is.
In
addition, due to the unfeasible efforts needed for data preparation, none of
the respondent laboratories could provide the HCV RNA data. It is therefore not
possible to relate our positive antibody tests to actual cases of hepatitis C
or to extrapolate from our positive rates to actual hepatitis C prevalence.
However, all laboratories involved in the study used 3rd-generation enzyme
immune assays (EIA) for HCV antibody detection which, in general, have very
high sensitivities and specificities [14].
Lastly, the correction factor for multiple testing of the same individual was
determined from the total number of reports to the Federal Office of Public
Health, whereas the antibody test positive rates were based on data from selected
laboratories only.
One particular
strength of this study is that it is the first to comprehensively describe nationwide
HCV antibody test frequencies and positive rates over several years, thus
providing new information on the epidemiological situation of hepatitis C in
Switzerland. We are not aware of any such published data internationally
either, so these results may also be of interest to countries with comparable
hepatitis C epidemiology.
Conclusion
The yearly number
of reported positive HCV antibody tests in the Federal Office of Public Health
reporting system, which remained relatively stable during the study period, was
accompanied by an increasing number of HCV antibody tests performed over the
years and corresponding decreases in both the antibody test and tested positive
rates. To achieve the goal of eliminating hepatitis C
in Switzerland, the number of HCV antibody-positive people who have not yet
been tested must be significantly reduced. In general, a high tested positive rate can be seen as a
marker for an efficient testing strategy. This is especially true for
infectious diseases with very clearly defined risk populations. However, as the
transmission route is unknown in a substantial proportion of hepatitis C
patients in Switzerland [13], a broader
testing strategy has to be discussed, which inevitably coincides with a
reduction of the HCV tested positive rate.
For
monitoring of hepatitis C elimination targets by 2030, we recommend annual
collection and publication of testing and positive rates along with mandatory
reporting and treatment counts by public health authorities. Decreasing numbers
of newly reported cases accompanied by rapidly declining positive and treatment
rates at stable testing rates would then indicate successful implementation of
the hepatitis C elimination roadmap.
Acknowledgement
The authors
thank the Federal Office of Public Health for the data from the hepatitis C
reporting system.
Prof. Philip Bruggmann, MD
Arud Centre for Addiction Medicine
Schützengasse 31
CH-8001
Zurich
p.bruggmann[at]arud.ch
References
1. Hepatitis C. World Health Organization (WHO). https://www.who.int/news/item/24-06-2022-WHO-publishes-updated-guidance-on-hepatitis-C-infection
2. Bihl F, Bruggmann P, Castro Batänjer E, Dufour JF, Lavanchy D, Müllhaupt B, et al. HCV
disease burden and population segments in Switzerland. Liver Int. 2022 Feb;42(2):330–9.
10.1111/liv.15111
3. Zahnd C, Brezzi M, Bertisch B, Giudici F, Keiser O. Situationsanalyse zu Hepatitis
B und C in der Schweiz 2017.
4. Mühlberger N, Schwarzer R, Lettmeier B, Sroczynski G, Zeuzem S, Siebert U. HCV-related
burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity,
and mortality. BMC Public Health. 2009 Jan;9(1):34. 10.1186/1471-2458-9-34
5. Sexuell übertragene Infektionen und Hepatitis B/C in der Schweiz im Jahr 2020: eine
epidemiologische Übersicht. BAG-Bulletin 48 vom 29. November 2021 2021.
6. Meldepflichtige Infektionskrankheiten – Wöchentliche Fallzahlen. https://www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-zu-infektionskrankheiten/meldepflichtige-infektionskrankheiten---woechentliche-fallzahlen.html [
7. Welche Corona-Kennziffer bedeutet was? https://www.lungenaerzte-im-netz.de/news-archiv/meldung/article/welche-corona-kennziffer-bedeutet-was/ [
8. Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden
of liver disease in Europe: a review of available epidemiological data. J Hepatol.
2013 Mar;58(3):593–608. 10.1016/j.jhep.2012.12.005
9. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection.
Nat Rev Gastroenterol Hepatol. 2013 Sep;10(9):553–62. 10.1038/nrgastro.2013.107
10. Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir
for previously untreated chronic hepatitis C infection. N Engl J Med. 2013 May;368(20):1878–87.
10.1056/NEJMoa1214853
11. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS,
et al.; POSITRON Study; FUSION Study. Sofosbuvir for hepatitis C genotype 2 or 3 in
patients without treatment options. N Engl J Med. 2013 May;368(20):1867–77. 10.1056/NEJMoa1214854
12. It is Time to Act Now! Process Paper – A Living Document, https://hepatitis-schweiz.ch/data/download/1893/Process_Paper_14_02_2019_neu.pdf [
13. Richard JL, Schaetti C, Basler S, Mäusezahl M. The epidemiology of hepatitis C in
Switzerland: trends in notifications, 1988-2015. Swiss Med Wkly. 2018 Apr;148:w14619.
14. Chevaliez S, Pawlotsky JM. Hepatitis C virus serologic and virologic tests and clinical
diagnosis of HCV-related liver disease. Int J Med Sci. 2006;3(2):35–40. 10.7150/ijms.3.35
Appendix
The appendix is available for download as a separate file at https://doi.org/10.57187/smw.2023.40085.

