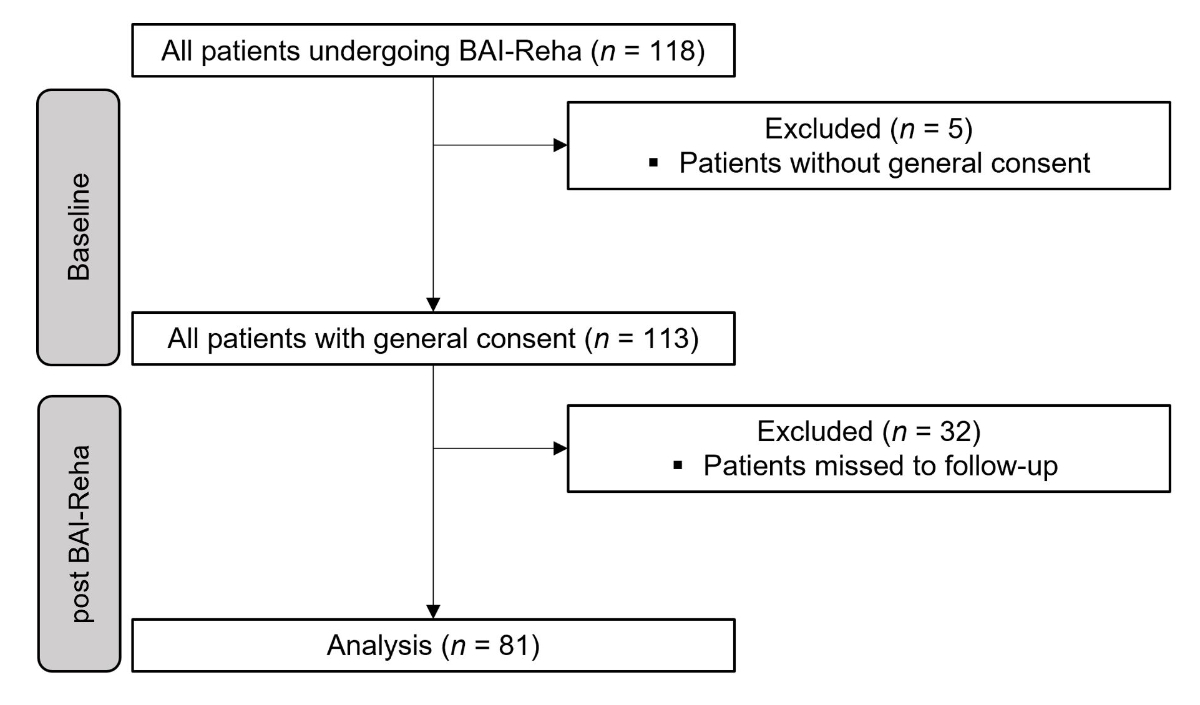
Figure 1Flowchart of patient inclusion.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40083
Chronic musculoskeletal pain is a major public health problem worldwide. Prevalence in the general population ranges from 0% to 24%, with most estimates between 10% and 15% [1–3]. In Switzerland, 16% of the general population and 18.6% of the older population are affected [4, 5]. Primary and secondary chronic musculoskeletal pain are subcategories of chronic pain and affect the musculoskeletal system. Chronic musculoskeletal pain is defined as pain that persists or recurs for more than 12 weeks in joints, bones, muscles, or tendons. It is linked with emotional distress (e.g., depressed mood, anxiety, anger, or frustration) and/or functional impairments [6]. Chronic musculoskeletal pain is not just a nociception of prolonged duration, but a unique, individualised experience, consisting of dynamic interactions of physiological, psychological, and social factors [7].
Patients with chronic musculoskeletal pain report a reduction in both self-reported functional capacity and self-perceived health status [8–12]. Functional capacity refers to a person's maximum potential to perform a functional activity in a standardised environment, such as walking, lifting, or maintaining a body position [13]. It can be measured using strictly defined performance-based test batteries [14]. Impaired functional capacity can lead to substantial limitations in activity and participation (e.g., job demands, leisure, and household responsibilities) [15]. Self-perceived health status summarises all aspects of a person's subjectively assessed health, such as physical functioning in everyday life, specific illness characteristics (severity and prognosis), life circumstances, and cultural background [16].
The spectrum of treatment modalities for people with chronic pain is broad. It includes medication, individual interventions such as information, education, physiotherapy and occupational therapy, surgical procedures and placebo therapies, but also interprofessional rehabilitation programmes [25]. In the current literature, the definition and use of the terms interprofessional, multidisciplinary, and/or interdisciplinary rehabilitation may vary, making comparisons difficult. Interprofessional rehabilitation is congruent with an interdisciplinary setting as defined by Körner and Bengel [26].
In the last decade, several randomised controlled trials have been published showing that interprofessional interventions are more effective than a single intervention for people with chronic pain (e.g., [27–30]). The Bern Ambulatory Interprofessional Rehabilitation (BAI-Reha) was developed based on evidence and international recommendations for interprofessional interventions for persons with chronic pain. The chosen teamwork approach best fits the characteristics of a transdisciplinary team model [31]. With the professions involved, the outpatient setting and the patient-centred and goal-oriented approach, BAI-Reha is in line with current recommendations from the literature and is comparable in content to other effective interprofessional treatment programmes, although the duration is longer [18, 20, 32].
Previous studies on interprofessional rehabilitation for patients with chronic musculoskeletal pain showed low to moderate effects on self-reported outcome measurements such as psychological symptoms, activity and participation, health, and quality of life [17–21]. In previous studies investigating the effects of rehabilitation on functional capacity in patients with chronic musculoskeletal pain, the reduction was mostly self-reported instead of objectively measured [8, 22]. However, self-reported outcome measurements carry the risk of recall bias or biased estimation [23]. In contrast, standardised functional tests (e.g., six-minute-walk test [6MWT], lifting, or sitting tests), support the understanding of underlying factors for restrictions in activity and participation in patients with chronic musculoskeletal pain [24].
The effectiveness of BAI-Reha for patients with chronic musculoskeletal pain was shown in a previous study with a different cohort of patients in terms of return to work, quality of life, and burden of suffering [33]. In this study, functional capacity was not objectively assessed. Exercise proved to be a safe way to improve functional capacity over time in patients with chronic conditions [34] and exercise is an essential component of BAI-Reha, since patients spend a substantial part of their rehabilitation exercising. Therefore, BAI-Reha lends itself to also assessing functional capacity. Furthermore, studies in the field of cancer or lung disease show that functional capacity and self-perceived health status are linked [35, 36]. Consequently, the two factors should be assessed together in patients with chronic musculoskeletal pain.
Therefore, the aim of this study is to assess the amount of change over time and its clinical meaningfulness in functional capacity and self-perceived health status of patients with chronic musculoskeletal pain undergoing BAI-Reha.
This study is a register-based longitudinal cohort study. We collected data prospectively and submitted it to the hospital's chronic pain registry. We consecutively collected data at baseline and post-BAI-Reha (i.e., at 4 months). The intervention team entered the functional capacity data into the registry, while the self-perceived health status data came directly from the patients. We extracted the coded data from the registry. Study data is accessible on request and has been shared for Master’s and PhD Students in the fields of Occupational and Physical Therapy and Social Work.
The research related to human use complies with all the relevant national regulations and institutional policies, was performed in accordance with the tenets of the Helsinki Declaration and has been approved by the Ethics Review Board Bern, Switzerland (Project ID: 2018-01583).
After the referral of potential patients by general practitioners, the specialist physicians of the Department of Rheumatology and Immunology, University Hospital (Inselspital) and University of Bern, Switzerland carried out an initial broad suitability screening. Subsequently, the definitive suitability for participation in the BAI-Reha was assessed during a 3-day inpatient stay at the Department of Rheumatology and Immunology. The intervention team, consisting of physiotherapists, occupational therapists, nurses, psychologists, clinical social workers, and physicians, assessed patients with chronic musculoskeletal pain using validated subjective and objective assessments and complete medical histories. The consensus discussions on patients' suitability are embedded in the regular daily ward meetings during their inpatient stay. The heads of the interprofessional team (see setting) meet monthly to discuss challenges and possible solutions regarding the programme (e.g., dealing with insurance issues, implementing the latest research, etc.). In addition, the intervention team receives monthly case supervision by a psychosomatic specialist to deal with challenging patient situations and to strengthen their professional mindsets. Finally, the team members interact daily in meetings, in the corridor, and/or during breaks.
For this study, we retrieved coded data from the Inselspital Department of Rheumatology and Immunology chronic musculoskeletal pain registry at the end of May 2020. We included all datasets from April 2018 to May 2020 with baseline and postevaluations (i.e., at 4 months). Data from patients who were excluded from BAI-Reha or who had not given consent to take part in a scientific study were excluded from the analysis. We performed baseline and follow-up measurements at the Bern University Hospital, Inselspital, Department of Physiotherapy, Insel Gruppe, Bern, Switzerland, and the Department of Rheumatology and Immunology.
To be included, patients had to be over the age of 18, suffering from chronic musculoskeletal pain with psychological and/or psychosocial impairments, and willing to take part in active rehabilitation. They also had to already have tried monoprofessional physiotherapy without success. This last condition renders inclusion criteria stricter than in comparable programmes [37, 38]. We excluded patients with primary psychological disorders or who were unable to take part in group discussions held in German. Patients with ongoing investigations into financial compensations under disability insurance or insufficient pain control by medication were also excluded from participation.
Informed consent was obtained from all patients included in this study.
BAI-Reha is an interprofessionally organised intervention that lasts 12 weeks and is divided into three phases of 4 weeks each. The programme consists of individual and group interventions, as well as independent, self-directed exercises derived from evidence-based practice and international guidelines. Individual goals for patients are set in interprofessional team meetings with review and adjustment of goals with and without patients (before the start and at weeks 2, 4, and 9 of BAI-Reha). The content and duration of the interventions are listed in table 1.
Table 1Content of interprofessional BAI-Reha: Description and duration of interventions. Note: Time needed for independent self-directed exercises was variable.
| Description of interventions | Duration (h) | ||
| Phase one: 1st to 4th week | Individual interventions | Medical treatment | |
| Physiotherapy | 2 | ||
| Occupational therapy | 4 | ||
| Behavioural therapy | 4 | ||
| Social worker`s intervention | 4 | ||
| Interprofessional meeting | 2 | ||
| Independent self-directed exercises | 2 | ||
| Group interventions | Group exercise | 10 | |
| Behavioural therapy group intervention | 6 | ||
| Occupational therapy groups | 12 | ||
| Basic Body Awareness group (BBAT) | 4 | ||
| Aquatic exercise | 2 | ||
| Nordic walking | 4 | ||
| Total | 56 | ||
| Phase two: 5th to 8th week | Individual interventions | Individualised interventions based on the patients' specific needs (medical treatment, physiotherapy, occupational, and/or behavioural therapy) | |
| Workplace visits and interventions | 4 | ||
| Independent self-directed exercises | |||
| Group interventions | Group exercise | 10 | |
| Supervision of independent self-directed exercise programme | 2 | ||
| Total | 24 | ||
| Phase three: 9th to 12th week | Individual interventions | Individualised interventions based on the patients' specific needs (medical treatment, physiotherapy, occupational, and/or behavioural therapy) | |
| Interprofessional meeting | 1 | ||
| Independent self-directed exercises | |||
| Group interventions | Group exercise | 8 | |
| Supervision of independent self-directed exercise programme | 2 | ||
| Total | 17 | ||
| All phases | Total | 97 | |
Specific to the BAI-Reha is its interprofessional nature. This means that more than one profession provides intervention and collaborate on a daily basis in each phase of the intervention (e.g., goal setting, intervention). The team has regular meetings with and without the patients (at baseline and at 2, 4, and 9 weeks). Furthermore, there is an integrated system similar to a primary nursing system with defined contact persons for the patients. Members of the interprofessional team work together as equals without organisational and professional leadership.
Patients are requested not to engage in other interventions, especially during the first 4 weeks (e.g., additional physiotherapy, occupational therapy, massages, complementary, or/and alternative medicine). After BAI-Reha, they are advised to proceed with the self-directed exercises and, if necessary, to continue with individual therapy (e.g., psychotherapy, physiotherapy for coaching and exercise adaptation).
To measure functional capacity, we used two standardised tests from the functional capacity evaluation (FCE) [39]. We selected the six-minute-walk test and safe maximum floor-to-waist lift (SML) to be representative of upper and lower extremity or back problems. We used the European Quality of Life and Health measure (EuroQoL) visual analogue scale (EQ VAS) to measure the self-perceived health status. Primary outcome variables are walking distance with the six-minute-walk test (m), weight lifted with the SML (kg), and points in the EQ VAS. Secondary outcome variables are heart rate pre- and post-test of the six-minute-walk test (bpm), time to complete the SML (sec), and heart rate pre- and post-test of the SML (bpm).
We determined the thresholds for clinically meaningful changes over time in the primary outcome variables a priori, taking clinical considerations, the current minimal clinically important difference literature, and normative values [40–45] into account. We set the corresponding thresholds at 50 m for the six-minute-walk test, 7 kg for the SML, and 10 points for EQ VAS. The threshold for the SML is rather high at 7 kg because there is no minimal clinically important difference for this test, which is why we could only consider the existing limits of agreement [46].
The walking test was executed on a 50-metre-distance track. Patients with chronic musculoskeletal pain were instructed to walk as far as possible for 6 minutes. Pausing was allowed, but the time kept running. Every 2 minutes, the patients were informed of the remaining time without additional encouragement. The walking distance was measured in metres. Heart rate was measured before and after the test with a polar heart rate sensor (chest strap). Previous studies indicate very good test-retest reliability of the six-minute-walk test (ICC >0.9) [47,48].
The lifting test was carried out with a standardised wooden crate (40 × 30 × 26 cm) of 2.5 kg. Weight increments of at least 2.5 kg were added until the maximum weight that could be handled safely was reached. The time it took for the patient to lift the weight five times from the table to the floor and back again, with a 90° rotation of the body, was measured. The patient’s hands remained on the crate during the test (time limit ≤90 sec). Heart rate was measured before and after the test with a polar heart rate sensor (chest strap). Previous studies indicate an excellent test-retest reliability of the SML (ICC 0.92) with limits of agreement from –8.0 to 6.7 kg [46]. In addition, the FCE lifting tests appear to be behavioural tests with several influencing factors (e.g., physical ability, beliefs, and perceptions) [49].
The EuroQoL is a short questionnaire assessing self-perceived health-related quality of life. It is based on an index with five items (EQ-5D) and a visual analogue scale (EQ VAS) [50]. The vertical EQ VAS is 20 cm long and patients marked their current self-perceived health status. Measuring overall health, 100 points is labelled as “best imaginable health status”, and 0 points is equivalent to “worst imaginable health status” [51]. Previously, the test-retest reliability of the EQ VAS, measured in patients with rheumatoid arthritis, showed good results: (ICC 0.82, 95% CI [0.74, 0.88]) [51]. The EQ VAS seems to be more responsive to mild self-perceived health status impairments than the EQ-5D [51]. The EQ VAS is interval scaled and is more accurate in detecting subtle changes without the ceiling effect present in the EQ-5D [52]. Therefore, only EQ VAS was analysed in this study.
All primary and secondary outcome variables were measured at baseline and post-BAI-Reha. Depending on the waiting time between the suitability assessment and the start of rehabilitation, the duration between baseline and postevaluation may have varied and was, on average, 4 months. Graduated physiotherapists or fourth-year physiotherapy students who had received training from certified FCE therapists measured functional capacity. They performed refresher training at least annually to guarantee standardised testing. To measure self-perceived health status, the patients completed the EQ VAS electronically on a tablet.
We used descriptive statistical methods to describe patient characteristics.
For each dependent variable, we fitted a linear mixed model to the data with time point, age, sex, body mass index, visual analogue scale (VAS) of 0–100 for pain today, and VAS of 0–100 for pain in the last week as fixed effects and subjects as random intercepts. The quantity of interest was the adjusted time-effect (point estimate and 95% confidence interval [CI] as well as p-value) for the test of the null hypothesis of no change. We performed a residual analysis to check if there was evidence against model assumptions, that is, the normality of random intercepts and residuals. We performed all analyses using the “lme4” package within the R statistical software R version 3.6.3 [53]. We assumed a level of significance of α= 0.05 and assessed if the mean value changes over time were clinically meaningful, using predefined thresholds for each primary outcome variable (see above).
We included a total of 81 patients with chronic musculoskeletal pain between 22 and 77 years of age (mean value 43.06, standard deviation (SD) 10.89 years) in this study. The flowchart of patient inclusion is shown in figure 1.

Figure 1Flowchart of patient inclusion.
There were no dropouts from the programme during the evaluated period. We excluded 32 patients who missed post-data collection entirely. Figure 2 and table 3 show that there are missing values for several patients. This was the case either because they had decided not to participate in the measurements or because the transfers from the separate rehabilitation documentation to the registry were not complete. Descriptive values are presented as mean value and (SD). A total of 65.4% of patients were women, with a BMI of 26.84 (5.54), VAS pain today of 58.25 (19.08), and VAS pain over the last week of 66.25 (16.51).
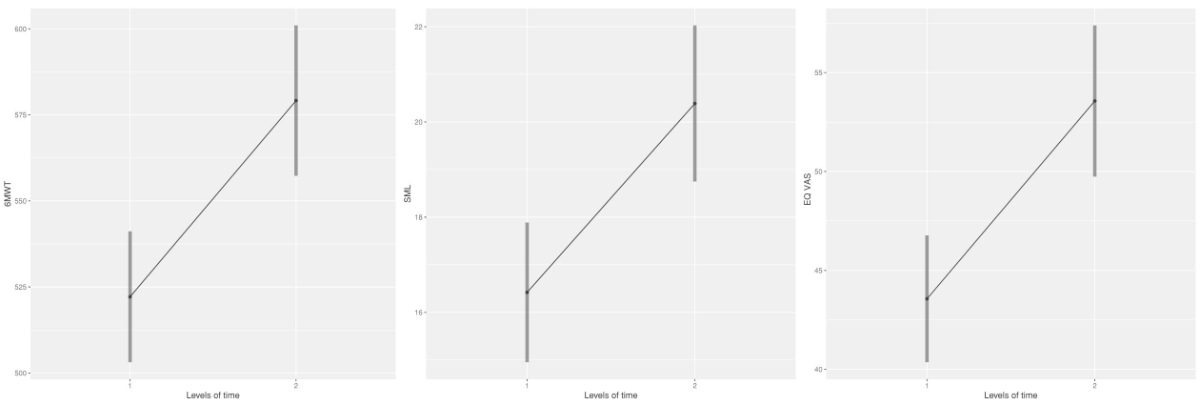
Figure 2Change of mean value over time for the primary outcome variables 6MWT (m), SML (kg), and EQ VAS (points). 6MWT: Six-minute-walk test, baseline n = 78; post-BAI-Reha, n = 66. SML: safe maximum floor-to-waist lift, baseline n = 77; post-BAI-Reha, n = 63. EQ VAS: European Quality of Life and Health measure visual analogue scale, baseline n = 80; post-BAI-Reha, n = 76.
Patient characteristics at baseline are shown in table 2.
Table 2Sociodemographic characteristics of patients at baseline.
| Baseline characteristics | n | % | |
| Sex | Men | 28 | 34.6 |
| Women | 53 | 65.4 | |
| CMPS-detail * | Associated with actual and/or potential tissue damage | 10 | 12.3 |
| Associated with tissue damage and a mental disorder | 60 | 74.1 | |
| Indicators of significant impairment in psychosocial functions | 11 | 13.6 | |
| Marital status | Married with children | 22 | 27.2 |
| Married | 13 | 16.1 | |
| Single | 13 | 16.1 | |
| Couple | 10 | 12.3 | |
| Couple with children | 7 | 8.6 | |
| Other | 15 | 18.5 | |
| N/A | 1 | 1.2 | |
| Educational level ** | Lower secondary education | 12 | 14.8 |
| Upper secondary education | 40 | 49.4 | |
| Bachelor’s or equivalent level | 14 | 17.3 | |
| Master’s or equivalent level | 4 | 4.9 | |
| Doctorate or equivalent level | 2 | 2.5 | |
| Other *** | 8 | 9.9 | |
| N/A | 1 | 1.2 | |
| Employment status | Employed | 35 | 43.2 |
| Unemployed | 11 | 13.6 | |
| Unfit | 17 | 21.0 | |
| Homemaker | 7 | 8.6 | |
| Self-employed | 5 | 6.2 | |
| Other | 4 | 4.9 | |
| N/A | 2 | 2.5 | |
* Chronic musculoskeletal pain syndrome (CMPS)-detail based on ICD-10.
** Educational levels are based on International Standard Classification of Education (ISCED 2011) levels of education.
*** Other levels of education: early childhood education, primary education, or postsecondary education.
As shown in table 3, the linear mixed model analysis showed a statistically significant change over time for all three primary outcome variables: six-minute-walk test, SML, and EQ VAS. A clinically meaningful change over time after BAI-Reha was achieved for the six-minute-walk test. EQ VAS just failed to reach the threshold for clinically meaningful change over time, while SML did not reach the predefined threshold for clinical meaningfulness.
Table 3Change of mean value over time, averaged over the levels of sex, for the primary outcome variables six-minute-walk test (m), SML (kg), and EQ VAS.
| Baseline | 4-months post-BAI-Reha | |||||||||
| n | Mean value | (SD) | Median | n | Mean value | (SD) | Median | Mean value change (95% CI) | Clinically meaningful threshold * | |
| 6MWT (m) | 78 | 521.92 | 93.61 | 520.00 | 66 | 579.53 | 94.53 | 576.50 | 56.08 **,†[36.13, 76.03] | 50 |
| SML (kg) | 77 | 15.81 | 7.92 | 15.00 | 63 | 19.64 | 8.61 | 20.00 | 3.92** [2.66, 5.19] | 7 |
| EQ VAS | 80 | 43.34 | 16.90 | 41.00 | 76 | 53.25 | 20.51 | 54.00 | 9.58** [4.87, 14.28] | 10 |
* Clinically meaningful threshold was defined a priori of analysis, BMI: Body Mass Index; VAS: visual analogue scale from 0–100. Time effects were adjusted for age, sex, BMI, VAS pain today, VAS pain over the last week.
** statistically significant p <0.001, † clinically meaningful change.
6MWT: six-minute-walk test; SML: safe maximum floor-to-waist lift; EQ VAS: European Quality of Life and Health measure visual analogue scale; CI: confidence interval.
In the secondary outcome variables, only the six-minute-walk test heart rate post-test (mean value change 6.98 bpm, 95% CI [2.82, 11.12]; p = 0.001 showed a statistically significant change over time. All other secondary outcome variables, i.e., time to complete the SML (mean value change –0.69 sec, 95% CI [–4.93, 3.56], p = 0.747), heart rate pretest SML (mean value change –2.19 bpm, 95% CI [–6.26, 1.88], p = 0.287), heart rate post-test SML (mean value change 1.67 bpm, 95% CI [–3.82, 7.15], p = 0.546), and heart rate pretest six-minute-walk test (mean value change –0.88 bpm, 95% CI [–5.57, 3.80], p = 0.708) did not show statistically significant changes over time.
The aim of this study was to assess the amount of change over time in functional capacity and self-perceived health status in patients with chronic musculoskeletal pain undergoing BAI-Reha. The results revealed a statistically significant and clinically meaningful change over time in walking distance, and a statistically significant change over time in the weight lifted and self-perceived health status.
To our knowledge, this is the first study showing objectively measured improvements in functional capacity and self-perceived health status for patients with chronic musculoskeletal pain after interprofessional rehabilitation. While previous studies [8, 22] mostly used self-reported outcome measurements for functional capacity, it is potentially more objective to assess them with functional testing. Patients tend to underestimate their functional capacity with self-reported outcome measurements because it can be difficult to rate a capacity that has not been performed recently [54]. Therefore, in previous studies using subjective measurements, the rehabilitation effects in functional capacity may have been underestimated. Also, the patients’ direct experience of their functional capacity through performance-based testing may have a direct therapeutic effect [54]. Therefore, this study can provide valuable information for patients, clinicians, healthcare insurance providers, employers, and researchers (e.g., how much further patients with chronic musculoskeletal pain can walk on average after BAI-Reha).
Based on current literature, we expected the patients in this study to improve after BAI-Reha in the six-minute-walk test. Our findings confirm the results of previous studies that showed an improvement in walking distance through interprofessional rehabilitation [20, 55]. The regular exercise therapy (individual and group) that is an essential part of BAI-Reha is, in our opinion, one of the crucial factors for this outcome. In addition, some patients return to walking longer distances several times a week during BAI-Reha (e.g., commuting between home and the hospital as well as between the different therapy locations), which could have an additional training effect. However, based on our study, it is not possible to draw firm conclusions about causation and effect of individual interventions.
Clinically, an increase of 0.5 km/h walking speed, which corresponds to a gain of 50 m in the six-minute-walk test, seems to be very meaningful in the daily life of a person. For example, it enables a wider range of independent mobility and can make a difference when it comes to keeping up with others while walking in public spaces. The patients in our study achieved this improvement in walking distance.
Despite the statistically significant changes over time, the change in patients’ lifting capacity measured with the SML, does not seem to be clinically meaningful. We expected these results. In the absence of a previously established minimal clinically important difference for the SML, it was important to us not to choose a value that was below the smallest detectable change. However, from a clinical perspective, 7 kg as an minimal clinically important difference appears to be quite high in patients with chronic musculoskeletal pain. Unfortunately, with the present change in the SML (3.92 kg), we do not know if a true change is present.
This line of thought could be challenged by the existing minimal clinically important difference of the similar Progressive Isoinertial Lift Evaluation test (PILE). For the PILE, an minimal clinically important difference of 4 kg for patients with chronic musculoskeletal pain, which had been suggested previously, was almost achieved in this study [41]. Two previous studies on patients with chronic back pain also found statistically significant improvements in lifting capacity with the PILE test [56, 57]. These studies used relative values in relation to body mass or a multilevel testing procedure and did not examine the clinical meaningfulness of the observed improvement. Defining the threshold as an absolute weight may seem convenient, but a relative value with reference to the baseline measurement could also be useful. Another reason for not reaching the set threshold with the SML could be the enormous heterogeneity in the baseline measurements (min 2.50 kg, max 37.50 kg, SD 7.92 kg). For a patient who only lifted 2.5 kg at the beginning, an increase of 7 kg would mean lifting more than three times the initial weight. If we look at the relative values, our patients improved by 24.79% compared to the initial mean value. An increase of 7 kg would be equivalent to a 44.28% improvement relative to our baseline values. Looking at these numbers, a threshold of 7 kg seems to be very high for our sample.
Also, the ability to lift more weight was rarely set as an individual goal by patients. Most patients do not reach the stage of high-impact strength training during BAI-Reha that would be required for a substantial improvement in lifting capacity, or they already have sufficient lifting capacity and had no need for further improvement. A subgroup analysis between low versus high capacity at the beginning of the programme could also be very interesting and could possibly deliver valuable prognostic factors for patients’ lifting capacity.
Surprisingly, time to lift did not improve statistically significantly after BAI-Reha (estimate –0.69 sec). We expected the patients to move more efficiently and, therefore, increase movement speed in the test situation. One reason for not becoming faster could be that patients move more consciously after BAI-Reha.
Self-perceived health improved statistically significantly and almost reached the threshold for clinical meaningfulness of 10 points post-BAI-Reha, as expected. A change of 9.58 points is enough to be noticed in daily life and not to be judged by patients as part of their usual ups and downs.
Interprofessional rehabilitation targets different aspects of self-perceived health status. Physical functioning in everyday life is one of them, which can be linked to an improvement in functional capacity. By increasing the functional capacity, certain parts of everyday life (e.g., household duties) can be carried out more easily. Previous studies have reported similar results to our study on changes in self-perceived health status after interprofessional rehabilitation in patients with chronic pain [19, 58]. A similar amount of change compared to our study was found in the EQ VAS with data from the Swedish Quality Register for Pain Rehabilitation. At baseline, patients with chronic musculoskeletal pain had a mean value EQ VAS of 41.22 points and improved to 50.99 points immediately after interprofessional pain rehabilitation. They continued to improve slightly and had a mean value of 52.96 points at a 12-month follow-up, which is almost equivalent to a modest long-term effect size (Cohen's d = 0.46) [59]. We would expect similar results with BAI-Reha at a 12-month follow-up.
There are many strengths and challenges within interprofessional rehabilitation [60]. Therefore, we would like to highlight some points that are specific to the BAI-Reha programme and that have been taken into account in its implementation. A flat hierarchy is practised within the BAI intervention team, which leads to everyone feeling accountable. Through shared working spaces of the intervention team, a high level of communication is facilitated through numerous spontaneous and planned meetings. This way, synergies are promoted, and parallel work is reduced.
As the same staff members are responsible for both assessment and intervention, continuity is automatically provided for the patients. The team members involved jointly deliberate and there must be consensus on the inclusion of patients in the programme. This increases the patients’ awareness that the intervention team is committed to their rehabilitation potential and recovery. This effect is further enhanced by the use of a system similar to a primary nursing system.
By far, the biggest challenges in the BAI-Reha are its high case complexity of patients and the associated resource requirements.
Since this is a registry-based study, there is no control group, and some data are missing. The data came from a clinical setting, and the outcome values of functional capacity had to be entered separately in the clinical documentation system and in the registry. Data may have been lost in the process, or patients may have refused to take certain measurements. Blinding of healthcare professionals and patients during interventions and functional capacity testing was not possible. Furthermore, the examiner was usually the physiotherapist treating the respective patient. This could result in the patient feeling pressured to perform better to increase treatment success. Also, one of the first authors is substantially involved in the BAI-Reha, as she is part of the intervention team and could therefore have certain biases or be routine-blinded (e.g., prioritisation of physiotherapeutic interventions and outcome variables). The time between baseline and postmeasurements varied with respect to the start of the BAI-Reha after the suitability assessment. Depending on the activity level of the patients and ongoing therapeutic interventions, the baseline measurements could have changed in the meantime.
Moreover, the suitability assessment before the BAI-Reha might induce a selection bias and therefore prevent generalisation to a larger population. Without a control group, we cannot exclude the possibility that phenomena such as regression to the mean may affect the results. The highly individualised treatment approach due to the heterogenous presentation of patients with chronic musculoskeletal pain might affect the external validity of this study. Even though BAI-Reha is standardised by means of defined treatment schedules, the interventions themselves are not standardised but targeted to the individual needs of the patients with chronic musculoskeletal pain. This reflects current best practice in treating patients with chronic musculoskeletal pain and is, therefore, at the same time a strength of the BAI-Reha [61, 62].
A further strength of this study is its comprehensive dataset deriving from a real-life setting. It is increasingly seen that data analysis based on real-life conditions is a way to enable healthcare decision-making to be more responsive to the needs of individual patients, leading to more personalised and, thus, effective healthcare [63]. The selected outcome variables are well established and provide reference and normative values so that clinicians can compare them with their own patient population. These outcome variables can be collected easily and with minimal equipment. The research question was asked with a clinical background; hence it was important to establish whether the changes over time were clinically meaningful besides being statistically significant. This makes them transferable to clinical work with patients affected by chronic musculoskeletal pain.
Potential future research questions based on the same registry data could focus on further defining the minimal clinically important difference of functional capacity and self-perceived health status in patients with chronic musculoskeletal pain. Once sufficient one- and two-year follow-up data from the BAI-Reha are available, the long-term change in functional capacity and self-perceived health status will be analysed.
Patients walk further, lift more weight, and feel healthier post-BAI-Reha. Hence it can be concluded that interprofessional patient-centred and goal-oriented rehabilitation can make a difference in the lives of patients with chronic musculoskeletal pain.
These findings confirm and add to previous results. Our study provides objectively measured values for convenient outcome measurements of functional capacity and self-perceived health status. We encourage other providers of rehabilitation for patients with chronic musculoskeletal pain to measure functional capacity with objective outcome variables and to use self-reported outcome measures in addition to self-perceived health status. The well-established assessments used in this study are suitable for this purpose.
We would like to thank the whole team of BAI-Reha for the implementation of the programme and the support in data collection. Colette Widmer, Hannu Luomajoki, Fabian Pfeiffer, Tom Friedli, Andrea Hausheer, Rita Morf, Ursula Stutz, Mara De Zanet, Samuel Wieland and Andreas Haffter for their support.
Authors’ contributions: The two first authors Franziska Schütz and Eva Haffter made significant contributions to the conception and design, data collection and analysis/interpretation of the data. Both engaged in the writing of the article and critically revised it for important intellectual content. Brigitte E. Gantschnig supported conception and design as a supervisor and provided input on the analysis and interpretation of the data. André Meichtry advised on statistical matters and created the statistical model for the data analysis. Balz Winteler provided professional advice with his expertise in physiotherapy and the conception and interpretation of the results and helped to select the suitable literature.
The Chronic Pain registry at the Department of Rheumatology and Immunology at the University Hospital (Inselspital) Bern is mainly financed by an internal funding and is additionally supported by the Foundation Johanna Dürmüller-Bol and by Sandoz Pharmaceuticals AG.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Andrews P, Steultjens M, Riskowski J. Chronic widespread pain prevalence in the general population: A systematic review. Eur J Pain. 2018 Jan;():5–18.
2. Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016 Jan;():55–64.
3. van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013 Jul;():13–8.
4. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006 May;():287–333.
5. Cimas M, Ayala A, Sanz B, Agulló-Tomás MS, Escobar A, Forjaz MJ. Chronic musculoskeletal pain in European older adults: cross-national and gender differences. Eur J Pain. 2018 Feb;():333–45.
6. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019 Jan;():19–27.
7. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007 Jul;():581–624.
8. Björnsdóttir SV, Jónsson SH, Valdimarsdóttir UA. Functional limitations and physical symptoms of individuals with chronic pain. Scand J Rheumatol. 2013;():59–70.
9. Bernfort L, Gerdle B, Rahmqvist M, Husberg M, Levin LÅ. Severity of chronic pain in an elderly population in Sweden—impact on costs and quality of life. Pain. 2015 Mar;():521–7.
10. Molander P, Dong HJ, Äng B, Enthoven P, Gerdle B. The role of pain in chronic pain patients’ perception of health-related quality of life: a cross-sectional SQRP study of 40,000 patients. Scand J Pain. 2018 Jul;():417–29.
11. Mäntyselkä PT, Turunen JH, Ahonen RS, Kumpusalo EA. Chronic pain and poor self-rated health. JAMA. 2003 Nov;():2435–42.
12. Pérez C, Margarit C, Sánchez-Magro I, de Antonio A, Villoria J. Chronic Pain Features Relate to Quality of Life More than Physiopathology: A Cross-Sectional Evaluation in Pain Clinics. Pain Pract. 2017 Sep;():866–78.
13. Bui KL, Nyberg A, Maltais F, Saey D. Functional Tests in Chronic Obstructive Pulmonary Disease, Part 1: Clinical Relevance and Links to the International Classification of Functioning, Disability, and Health. Ann Am Thorac Soc. 2017 May;():778–84.
14. Gross DP. Are functional capacity evaluations affected by the patient’s pain? Curr Pain Headache Rep. 2006 Apr;():107–13.
15. Tseli E, Boersma K, Stålnacke BM, Enthoven P, Gerdle B, Äng BO, et al. Prognostic Factors for Physical Functioning After Multidisciplinary Rehabilitation in Patients With Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Clin J Pain. 2019 Feb;():148–73.
16. Maniscalco L, Miceli S, Bono F, Matranga D. Self-Perceived Health, Objective Health, and Quality of Life among People Aged 50 and Over: Interrelationship among Health Indicators in Italy, Spain, and Greece. Int J Environ Res Public Health. 2020 Apr;():2414.
17. Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015 Feb; feb18 5:h444–444.
18. Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008 May;():670–8.
19. Hållstam A, Löfgren M, Benson L, Svensén C, Stålnacke BM. Assessment and treatment at a pain clinic: A one-year follow-up of patients with chronic pain. Scand J Pain. 2017 Oct;():233–42.
20. Kurklinsky S, Perez RB, Lacayo ER, Sletten CD. The Efficacy of Interdisciplinary Rehabilitation for Improving Function in People with Chronic Pain. Pain Res Treat. 2016;:7217684.
21. Banerjee S, Argáez C. Multidisciplinary Treatment Programs for Patients with Chronic Non-Malignant Pain: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019.
22. Cheng JO, Cheng ST. Effectiveness of physical and cognitive-behavioural intervention programmes for chronic musculoskeletal pain in adults: A systematic review and meta-analysis of randomised controlled trials. PLoS One. 2019 Oct;():e0223367.
23. Taylor AM, Phillips K, Patel KV, Turk DC, Dworkin RH, Beaton D, et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain. 2016 Sep;():1836–50.
24. Wittink H. Functional capacity testing in patients with chronic pain. Clin J Pain. 2005;():197–9.
25. Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al.; COST B13 Working Group on Guidelines for Chronic Low Back Pain. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006 Mar;( Suppl 2):S192–300.
26. Körner M, Bengel J. [Teamwork and team success in multi- and interdisciplinary teams in medical rehabilitation]. Rehabilitation (Stuttg). 2004 Dec;():348–57.
27. Henchoz Y, de Goumoëns P, So AK, Paillex R. Functional multidisciplinary rehabilitation versus outpatient physiotherapy for non specific low back pain: randomized controlled trial. Swiss Med Wkly. 2010 Dec;:w13133.
28. Kjeken I, Bø I, Rønningen A, Spada C, Mowinckel P, Hagen KB, et al. A three-week multidisciplinary in-patient rehabilitation programme had positive long-term effects in patients with ankylosing spondylitis: randomized controlled trial. J Rehabil Med. 2013 Mar;():260–7.
29. Monticone M, Ferrante S, Rocca B, Baiardi P, Dal Farra F, Foti C. Effect of a long-lasting multidisciplinary program on disability and fear-avoidance behaviors in patients with chronic low back pain: results of a randomized controlled trial. Clin J Pain. 2013 Nov;():929–38.
30. Nazzal ME, Saadah MA, Saadah LM, Al-Omari MA, Al-Oudat ZA, Nazzal MS, et al. Management options of chronic low back pain. A randomized blinded clinical trial. Neurosciences (Riyadh). 2013 Apr;():152–9.
31. Schwarz B, Neuderth S, Gutenbrunner C, Bethge M. Multiprofessional teamwork in work-related medical rehabilitation for patients with chronic musculoskeletal disorders. J Rehabil Med. 2015 Jan;():58–65.
32. Wade DT. What is rehabilitation? An empirical investigation leading to an evidence-based description. Clin Rehabil. 2020 May;():571–83.
33. Gantschnig BE, Heigl F, Widmer Leu C, Bütikofer L, Reichenbach S, Villiger PM. Effectiveness of the Bern Ambulatory Interprofessional Rehabilitation (BAI-Reha) programme for patients with chronic musculoskeletal pain: a cohort study. Swiss Med Wkly. 2017 May;():w14433. Available from: https://doi.org/doi
34. Pasanen T, Tolvanen S, Heinonen A, Kujala UM. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomised controlled trials. Br J Sports Med. 2017 Oct;():1459–65.
35. Dürr S, Zogg S, Miedinger D, Steveling EH, Maier S, Leuppi JD. Daily physical activity, functional capacity and quality of life in patients with COPD. COPD. 2014 Dec;():689–96.
36. José A, Dal Corso S. Inpatient rehabilitation improves functional capacity, peripheral muscle strength and quality of life in patients with community-acquired pneumonia: a randomised trial. J Physiother. 2016 Apr;():96–102.
37. Gerdle B, Molander P, Stenberg G, Stålnacke BM, Enthoven P. Weak outcome predictors of multimodal rehabilitation at one-year follow-up in patients with chronic pain-a practice based evidence study from two SQRP centres. BMC Musculoskelet Disord. 2016 Nov;():490.
38. Hållstam A, Löfgren M, Svensén C, Stålnacke BM. Patients with chronic pain: one-year follow-up of a multimodal rehabilitation programme at a pain clinic. Scand J Pain. 2016 Jan;():36–42.
39. Isernhagen SJ. Functional capacity evaluation: Rationale, procedure, utility of the kinesiophysical approach. J Occup Rehabil. 1992 Sep;():157–68. Available from: https://doi.org/doi
40. Oesch P, Meyer K, Jansen B, Kool J. Functional Capacity Evaluation: Performance of Patients with Chronic Non-specific Low Back Pain Without Waddell Signs. J Occup Rehabil. 2015 Jun;():257–66.
41. Benaim C, Blaser S, Léger B, Vuistiner P, Luthi F. “Minimal clinically important difference” estimates of 6 commonly-used performance tests in patients with chronic musculoskeletal pain completing a work-related multidisciplinary rehabilitation program. BMC Musculoskelet Disord. 2019 Jan;():16.
42. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017 Apr;():377–81.
43. Grochtdreis T, Dams J, König HH, Konnopka A. Health-related quality of life measured with the EQ-5D-5L: estimation of normative index values based on a representative German population sample and value set. Eur J Health Econ. 2019 Aug;():933–44.
44. Whynes DK, McCahon RA, Ravenscroft A, Hodgkinson V, Evley R, Hardman JG. Responsiveness of the EQ-5D health-related quality-of-life instrument in assessing low back pain. Value Health. 2013;():124–32.
45. Soer R, de Vries HJ, Brouwer S, Groothoff JW, Geertzen JH, Reneman MF. Do workers with chronic nonspecific musculoskeletal pain, with and without sick leave, have lower functional capacity compared with healthy workers? Arch Phys Med Rehabil. 2012 Dec;():2216–22.
46. Trippolini MA, Reneman MF, Jansen B, Dijkstra PU, Geertzen JH. Reliability and safety of functional capacity evaluation in patients with whiplash associated disorders. J Occup Rehabil. 2013 Sep;():381–90.
47. Hamilton DM, Haennel RG. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. J Cardiopulm Rehabil. 2000;():156–64.
48. King MB, Judge JO, Whipple R, Wolfson L. Reliability and responsiveness of two physical performance measures examined in the context of a functional training intervention. Phys Ther. 2000 Jan;():8–16.
49. Gross DP, Battié MC. Factors influencing results of functional capacity evaluations in workers’ compensation claimants with low back pain. Phys Ther. 2005 Apr;():315–22. 10.1093/ptj/85.4.315
50. N’Goran AA, Déruaz-Luyet A, Haller DM, Zeller A, Rosemann T, Streit S, et al. Comparing the self-perceived quality of life of multimorbid patients and the general population using the EQ-5D-3L. PLoS One. 2017 Dec;():e0188499.
51. Brooks R, Rabin R, de Charro F, editors. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective: Evidence from the EuroQol BIOMED Research Programme. Springer Netherlands; 2003.
52. Hinz A, Klaiberg A, Brähler E, König H-H. Der Lebensqualitätsfragebogen EQ-5D: Modelle und Normwerte für die Allgemeinbevölkerung. PPmP - Psychother · Psychosom · Med Psychol 2005;56:42–8. https://doi.org/.
53. R Core Team. R: A Language and Environment for Statistical Computing 2020.
54. Schindl M, Wassipaul S, Wagner T, Gstaltner K, Bethge M. Impact of Functional Capacity Evaluation on Patient-Reported Functional Ability: An Exploratory Diagnostic Before-After Study. J Occup Rehabil. 2019 Dec;():711–7.
55. Peppin JF, Marcum S, Kirsh KL. The chronic pain patient and functional assessment: use of the 6-Minute Walk Test in a multidisciplinary pain clinic. Curr Med Res Opin. 2014 Mar;():361–5.
56. Smeets RJ, Vlaeyen JW, Hidding A, Kester AD, van der Heijden GJ, van Geel AC, et al. Active rehabilitation for chronic low back pain: cognitive-behavioral, physical, or both? First direct post-treatment results from a randomized controlled trial [ISRCTN22714229]. BMC Musculoskelet Disord. 2006 Jan;():5.
57. Caby I, Olivier N, Janik F, Vanvelcenaher J, Pelayo P. A Controlled and Retrospective Study of 144 Chronic Low Back Pain Patients to Evaluate the Effectiveness of an Intensive Functional Restoration Program in France. Healthcare (Basel). 2016 Apr;():23.
58. Tseli E, LoMartire R, Vixner L, Grooten WJ, Gerdle B, Äng BO. What Is the Effectiveness of Different Duration Interdisciplinary Treatment Programs in Patients with Chronic Pain? A Large-Scale Longitudinal Register Study. J Clin Med. 2020 Aug;():2788.
59. Ringqvist Å, Dragioti E, Björk M, Larsson B, Gerdle B. Moderate and Stable Pain Reductions as a Result of Interdisciplinary Pain Rehabilitation-A Cohort Study from the Swedish Quality Registry for Pain Rehabilitation (SQRP). J Clin Med. 2019 Jun;():905.
60. Müller C, Zimmermann L, Körner M. Förderfaktoren und Barrieren interprofessioneller Kooperation in Rehabilitationskliniken—Eine Befragung von Führungskräften. Rehabilitation (Stuttg). 2014 Dec;():390–5.
61. Hutting N, Caneiro JP, Ong’wen OM, Miciak M, Roberts L. Patient-centered care in musculoskeletal practice: key elements to support clinicians to focus on the person. Musculoskelet Sci Pract. 2021;:
62. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021 May;():2082–97.
63. Liossi C, Johnstone L, Lilley S, Caes L, Williams G, Schoth DE. Effectiveness of interdisciplinary interventions in paediatric chronic pain management: a systematic review and subset meta-analysis. Br J Anaesth. 2019 Aug;():e359–71.