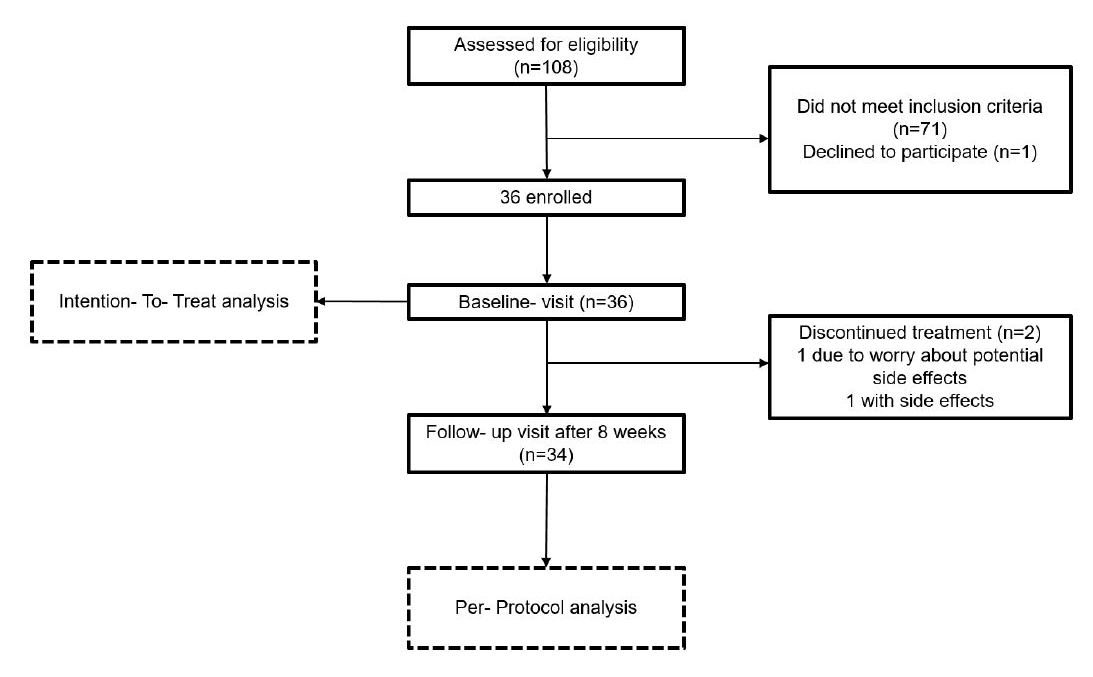
Figure 1Study flow diagram.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40079
Iron deficiency is the most common cause of anaemia affecting over 1 billion people worldwide [1], while iron deficiency without anaemia is probably even more common and often not diagnosed. In the global South, low iron intake is the major cause of iron deficiency, which is often aggravated by chronic infection. However, iron deficiency without anaemia is very common in developed countries with good healthcare facilities, with a prevalence ranging from 15.6% to 22.7% in premenopausal women [2, 3]. In other areas, such as Lebanon in the Middle East, it is as high as 57.5% in women aged 18–50 years old according to a recent study [4]. Iron deficiency without anaemia leads to fatigue [5–8], cognitive impairment [8, 9] and poor physical endurance [10]. Iron therapy in non-anaemic iron-deficient women improves fatigue [5] and the physical performance of athletes [11] and is recommended for those suffering from restless legs syndrome [12] or heart failure [13]. Thus, iron deficiency should be prevented.
The major reasons for iron deficiency are inadequate intake, impaired or reduced intestinal absorption and chronic blood loss due to heavy menstruation, all resulting in low iron stores, especially in young women. A recent review of dietary iron intake in women of reproductive age, including 49 surveys from 29 European countries, reported an iron intake below the recommended 15 mg/d in 61–97% of women [14], further worsening iron balance in women.
Iron deficiency is usually treated with daily oral iron doses of 60–180 mg elemental iron [15] and recent guidelines from the British Society of Gastroenterology recommend daily doses of 50–100 mg [16]. Unfortunately, standard oral iron supplementation is frequently associated with gastrointestinal side effects [17] with a frequency of up to 47% [18] due to insufficient intestinal iron absorption with residual amounts of unabsorbed iron leading to intestinal epithelial cells irritation and altered microbiome composition [15, 19]. Only about 10% of the normal iron dose is absorbed [20], even less for higher iron doses [21] and intestinal irritation provoked by unabsorbed iron is more likely, therefore, a therapy with few side effects is desirable.
Consequently, this study was designed to determine whether a low iron dose with potentially better iron absorption efficacy can replenish iron stores and have fewer side effects. We investigated whether low-dose iron supplementation with 6 mg twice daily over 8 weeks can increase serum ferritin levels to within the normal range in healthy iron-deficient non-anaemic premenopausal women.
This study was conducted at the University Hospital of Zurich in the Department of Endocrinology according to the Declaration of Helsinki and the Guidelines of Good Clinical Practice. The protocol and amendments were approved by the local ethics committee. The study participants and the public were not involved in the design, conduct, reporting, or dissemination of this research but all participants provided written informed consent. The study was registered in the Swiss National Clinical Trials Portal and at ClinicalTrials.gov: 202002599 and NCT04636060.
This study involved healthy iron-deficient non-anaemic premenopausal women defined as having a serum ferritin concentration ≤30 µg/l and haemoglobin ≥117 g/l. Further inclusion criteria were: age >18 years, regular menstrual cycle (defined as a cycle with a duration of 23 to 35 days), body-mass-index (BMI) of 18–25 kg/m2, no intake of dietary supplements for at least 4 weeks, not pregnant, no hypermenorrhoea (more than 5 sanitary pads or 5 tampons per day or more than 80 ml blood loss during one menstruation), no chronic inflammatory disease, no psychiatric disorders, no hypersensitivity to iron-supplements, no chronic kidney disease (creatinine ≤80 µmol/l), no hypo/hyperthyroidism (Thyroid Stimulating Hormone (TSH), 0.16–4.25 mU/l) and no intake of drugs interacting with oral iron supplementation (e.g. PPI, antacids, calcium channel blocker, antibiotics such as ciprofloxacin or tetracyclines, bisphosphonates, levothyroxine).
Adverse events reported by the participants were assessed with detailed history by the investigators at each visit and immediately afterwards classified as “serious” or “not serious” and as “drug-associated” or “not drug-associated”. Information regarding the event was recorded in a proforma including the symptoms, date of beginning, duration, intensity, the relationship to the study procedure and actions taken. In the case of a serious adverse event, the sponsor investigator was informed immediately and all further steps were evaluated thoroughly.
Of the 108 participants screened, 36 met the inclusion criteria (see figure 1).

Figure 1Study flow diagram.
The study consisted of two visits after enrolment (baseline and follow-up visit) and there was no control group. At both visits, participants were fasting, vital signs were measured, and a blood sample was obtained for the determination of serum ferritin, haemoglobin, C-reactive protein (CRP), and plasma hepcidin. At baseline, we rechecked for concomitant medications, asked about the occurrence of any adverse events since the screening visit and distributed the iron supplement; two boxes containing sixty 6 mg for a total of 120 coated tablets of elemental iron (corresponding to 18.6 mg dried iron sulphate, produced by Streuli Pharma AG, 8730 Uznach, Switzerland). The participants were asked to take two tablets per day, one in the morning and one in the evening, 60 minutes before or after a meal for eight weeks. Since there were no visits during the eight-week intervention, the participants were contacted every two weeks by telephone, mail, or online messages to monitor compliance and ask about any adverse events. The data were obtained from 36 participants in 2 visits, with the first visit being the baseline and the second visit after 8 weeks of taking the study medication. At the follow-up visit, the participants were asked about adverse events and the remaining iron supplements were counted as a further measure of compliance. Any change in their general health status was assessed eight weeks after treatment initiation by asking participants to categorise their current health status compared to the baseline visit: improved (much or moderately), unchanged, or worsened (little, moderately, or much) (adapted from Anker et al. [22]).
The study medication was donated by Streuli Pharma AG (Bahnhofstrasse 7, 8730 Uznach, Switzerland). The low-dose vegan iron supplement was in the form of coated tablets containing 6 mg of elemental iron (corresponding to 18.6 mg ferrous sulphate).
The plasma for the measurement of hepcidin was stored immediately after collection on dry ice in a locked refrigerator at -80°C without freeze-thaw cycles until shipment to the laboratory. Two months after study termination, the frozen samples were sent to the Laboratory of Translational Metabolic Laboratory (TML), Department of Laboratory Medicine, Radboud University Medical Center, Nijmegen, the Netherlands for analysis using a validated but not standardised c-ELISA method (DRG-international Hepcidin25 (bioactive) HS ELISA (#EIA-5782) Lots 314K111 and 314K051-2). Haemoglobin, iron, serum ferritin, transferrin, CRP, creatinine, alanine aminotransferase and TSH were measured at the Institute of Clinical Chemistry, University Hospital of Zurich, directly after the blood samples were obtained. Haemoglobin was measured on an ABL-90 blood gas analyser (Radiometer, Den-mark) and other serum analytes were measured using a Cobas 8000 (502c and e801) analyser (Roche Diagnostics, Mannheim, Germany).
All data were analysed using SAS 9.4. Normally distributed data are represented as mean values (± SD) and non-normally distributed data as median values. Comparisons of pre and post-intervention values were performed using the paired T-test or Wilcoxon test. Changes in health status were statistically compared under a null hypothesis of unchanged status and analysed using the sign test. All tests were performed at the 5% significance level (p = 0.05). Two women discontinued the study medication but returned for the follow-up visit. For the power calculation, we assumed a serum ferritin difference after 8 weeks of treatment of 5 ng/ml with an SD of 10, therefore, we assumed an effect size of 0.5 and a sample size of 34 probands. The data of all included participants were calculated according to the intention-to-treat (ITT) principle and the per-protocol (PP) principle.
Out of 108 premenopausal women screened, 36 were included in the study and had an average age of 28 ± 6 years and a mean BMI of 21 ± 2 kg/m2. They presented with a median serum ferritin of 18 (15, 23) ng/ml, average haemoglobin of 135 ± 9 g/l, and a median CRP of 1 (0, 3) mg/l. Of the 72 measured CRP values, 66 (92%) were ≤5 mg/l and 5 (7%) ≤10 ng/ml. There was one CRP value above 10 mg/l. The median plasma hepcidin level at baseline was 2.9 (1.4, 4.2) ng/ml. See table 1 for further baseline parameters.
Table 1Baseline characteristics.
| Age | 28 ± 6 years |
| Body mass index | 21 ± 2 kg/m2 |
| Systolic blood pressure | 114 ± 9 mmHg |
| Diastolic blood pressure | 78 ± 6 mmHg |
| Haemoglobin | 135 ± 9 g/l |
| Serum ferritin | 18 (15, 23) ng/ml |
| Transferrin saturation | 19 ± 9% |
| Serum creatinine | 65 ± 9 µmol/l |
| Alanine aminotransferase | 15 ± 5 U/l |
| Thyroid-stimulating hormone | 1.8 ± 0.7 mU/l |
| Plasma hepcidin | 2.9 (1.4, 4.2) ng/ml |
| C-reactive protein | 1 (0, 3) mg/l |
Results are presented as mean values (± 1 standard deviation) or median values (quartiles Q1, Q3).
The changes in blood parameters are shown in table 2. After 8 weeks of 6 mg of iron twice daily, the median serum ferritin increased from 18 ng/ml to 33 (24, 43) ng/ml (p <0.001) (figure 2) and haemoglobin increased by 3 g/l to a mean value of 138 ± 8 g/l (p = 0.014). Systolic blood pressure increased by 5 mmHg to an average value of 120 ± 10 mmHg (p = 0.003) and plasma hepcidin increased by 2 ng/ml to 5.6 (2.9, 10.0) ng/ml after 8 weeks (p = 0.004). One woman had a CRP value above 10 mg/l but did not report any complaints. For all changes of blood parameters, see table 2. Figure 2 shows serum ferritin values before and after the low-dose iron therapy. In two women, serum ferritin decreased by 1 ng/ml and 2 ng/ml respectively and one of these women discontinued therapy after 4 weeks due to side effects (see adverse events). Serum ferritin remained unchanged during therapy in one woman (3%). Serum ferritin increased in 33 women (92%).
Table 2Blood parameters and blood pressure at start and 8 weeks after low dose iron treatment (6 mg of elemental iron twice daily) for n = 36.
| Low dose iron treatment | ||||
| 0 weeks | 8 weeks | Change | p-value | |
| Systolic blood pressure, mmHg | 114 ± 9 | 120 ± 10 | 5 ± 10 | 0.003 |
| Diastolic blood pressure, mmHg | 78 ± 6 | 80 ± 9 | 2 ± 7 | NS |
| Hemoglobin, g/l | 135 ± 9 | 138 ± 8 | 3 ± 8 | 0.014 |
| Serum ferritin, ng/ml | 18 (15, 23) | 33 (24, 43) | 16 (8, 26) | <0.001 |
| Plasma hepcidin ng/ml | 2.9 (1.4, 4.2) | 5.6 (2.9, 10.0) | 2.0 (0.0, 5.9) | 0.004 |
| C-reactive protein, mg/l | 1 (0, 3) | 1 (0, 2) | 0 (–1.0, 0) | NS |
Results are presented as mean values (± 1 standard deviation) or median values (quartiles Q1, Q3). P-values were calculated by paired T-test or Wilcoxon test, ns: not significant and no trend (p >0.10)
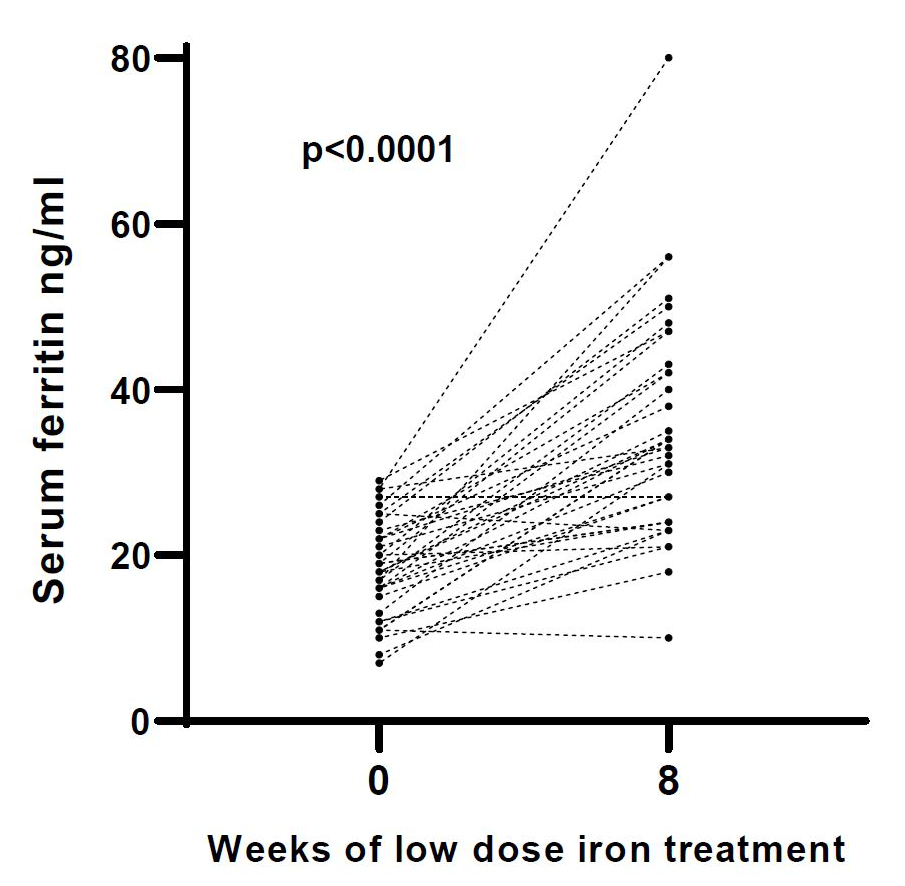
Figure 2Change of serum ferritin for each woman (n = 36) after 8 weeks of low dose iron treatment (6 mg of elemental iron twice daily).
The self-reported health status improved significantly after 8 weeks of low-dose iron treatment (p <0.0001), with most women (n = 24, 67%) reporting an improved health status. One woman who stopped taking the study medication felt worse after the study. The per-protocol analysis (n = 34, see table S1 in the appendix) showed identical results, apart from a significant increase in diastolic blood pressure after iron therapy.
Two women reported adverse events, one of which was related to the iron supplement. The woman paused the intake of the study medication as she complained of constipation and abdominal discomfort, which were resolved after changing the time of intake to 60 minutes after a meal. The other woman was concerned about potential side effects and discontinued the therapy after three weeks.
This study showed for the first time that low-dose oral iron therapy for eight weeks significantly improved iron stores in iron-deficient non-anaemic premenopausal women. The low-dose therapy of 6 mg of elemental iron twice daily was very well tolerated with only one woman reporting side effects.
Studies with lower-dose iron supplements have been performed in children and the elderly [23–25]. Daily doses of 20 mg of iron in children aged 6 to 43 months living in Mexico resulted in a significant increase in serum ferritin after 4 months, but therapy with lower iron doses such as 12.5 mg or 6.7 mg daily had no effect [23]. An oral supplement of 15 mg of elemental iron daily in iron-deficient non-anaemic elderly (>80 years, baseline Hb 10 g/dl) resulted in a significant increase in serum ferritin and haemoglobin after 60 days [25]. In the present study, treatment with only 6 mg of elemental iron twice a day for eight weeks lead to a highly significant improvement in iron stores in non-anaemic women, possibly because of effective iron absorption. Moretti et al. showed that in non-anaemic healthy females with depleted iron stores (defined as serum ferritin below 20 ng/ml) that a daily dose of 60–240 mg elemental iron increased plasma hepcidin with consecutive reduced iron absorption on the next day [21]. In the present study, therapy with only 6 mg of elemental iron twice a day for 8 weeks lead to significantly improved iron stores, suggesting effective iron absorption due to less plasma hepcidin production. The recommended dietary allowance for premenopausal women in Switzerland is the same as in the US, 15 mg/d. In a previous study of women with a mean age of 23 years in Zurich, the average dietary iron intake was only 10.8 mg/d [26]. Interestingly, as well as a similar age, the women also had a similar BMI of 21.7 kg/m2, therefore we assume that our study population had a similarly low and insufficient iron intake. In addition, we excluded women with substantial menstrual iron loss due to hypermenorrhoea. Taken together, the iron deficiency in our study population may be primarily due to an insufficient iron intake, not from increased losses. Additional oral iron supplementation would therefore have a beneficial effect on iron stores, as observed in the present study.
The current standard iron therapy often leads to side effects caused by gastrointestinal toxicity, with a frequency of up to 47% [18]. In addition, there is a risk of an unfavourable change in the microbiome if iron therapy is inadequate with an excess of unabsorbed iron [27, 28]. Unpleasant side effects result in treatment discontinuation and unnecessary, expensive intravenous iron therapy. In the present study, only one woman reported gastrointestinal side effects in the form of abdominal pain and constipation, which were resolved after she started taking the iron pill 60 minutes after a meal instead of before. Thus, the low-dose iron therapy of 6 mg twice a day appears to be well tolerated. Low-dose iron therapy per se leads to a reduced proportion of non-absorbed iron, which could be even lower because of improved iron absorption.
This study has some limitations including the small sample and that the study population was selected to exclude overweight women. Excess weight and obesity are often associated with low-grade inflammation leading to fundamental changes in iron metabolism, hence a higher plasma hepcidin concentration and impaired absorption of oral iron [29]. It was not possible to determine which daily low-dose iron therapy works best or whether a single morning dose in the low-dose iron range would be sufficient. It would certainly be advantageous to have a single iron dose in terms of treatment compliance. Low-dose iron therapy in iron-deficient women has not yet been investigated.
Accordingly, this study aimed to examine the effects of low-dose iron therapy per se and not to compare it with standard daily iron therapy of 60–100 mg. This should now be considered in a further study, as well as the low prevalence of side effects observed under low-dose iron therapy in a placebo-controlled trial. Most women reported an improvement in their self-reported health status at the end of the study. Since iron therapy, as we have reported in an earlier study [5], has a substantial placebo effect, a blinded control group would have been helpful for the assessment of clinical efficacy. However, this was not the intention of our study because it has already been shown that iron treatment to improve iron stores is clinically beneficial in iron-deficient non-anaemic women [5–7]. Interestingly, there was a significant increase in systolic blood pressure after ameliorating iron stores but whether the increase in systolic blood pressure is due to an improved cardiac muscular function, while intriguing, remains unclear. However, it is well known that iron administration improves cardiac performance in participants with heart failure [22].
In summary, low-dose oral iron therapy with 6 mg of elemental iron twice a day is effective with negligible side effects in iron-deficient non-anaemic premenopausal women. Due to the known intestinal irritation (and potential damage to the microbiome) and associated clinical side effects of standard iron treatment, low-dose iron treatment is a valuable therapy option for iron-deficient non-anaemic premenopausal women with a normal BMI and menstruation. Further placebo-controlled studies with a larger number of participants are needed to confirm these results.
Authors` contribution to manuscript: Design/Conception of study: SS, MK, SK, AN,D M, FB, PS, LS, PAK
Protocol, recruitment, data measurement: SS, MK, SK, DM, DS, LS, PAK
Data analysis: SS, MK, MS, DS, PAK.
Study supervision: AN, FB, PS, PAK
Writing: all authors
Statistical support was provided by Acomed Statistik (Leipzig, Germany).
The study was supported financially by the foundation “Stiftung für klinische Forschung”, Universitätsspital Zürich, Zürich, Switzerland.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Prof Krayenbuehl receives consulting fees from Streuli Pharma (Uznach, Switzerland). All other authors declare no conflict of interests.
1. Kassebaum NJ, Collaborators GA; GBD 2013 Anemia Collaborators. The Global Burden of Anemia. Hematol Oncol Clin North Am. 2016 Apr;():247–308. 10.1016/j.hoc.2015.11.002
2. Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003-2006. Am J Clin Nutr. 2009 May;():1334–42. 10.3945/ajcn.2008.27151
3. Galan P, Yoon HC, Preziosi P, Viteri F, Valeix P, Fieux B, et al. Determining factors in the iron status of adult women in the SU.VI.MAX study. SUpplementation en VItamines et Minéraux AntioXydants. Eur J Clin Nutr. 1998 Jun;():383–8. 10.1038/sj.ejcn.1600561
4. Abuaisha M, Itani H, El Masri R, Antoun J. Prevalence of Iron Deficiency (ID) without anemia in the general population presenting to primary care clinics: a cross-sectional study. Postgrad Med. 2020 Apr;():282–7. 10.1080/00325481.2020.1715701
5. Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011 Sep;():3222–7. 10.1182/blood-2011-04-346304
6. Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A, et al. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women—PREFER a randomized, placebo-controlled study. PLoS One. 2014 Apr;():e94217. 10.1371/journal.pone.0094217
7. Verdon F, Burnand B, Stubi CL, Bonard C, Graff M, Michaud A, et al. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ. 2003 May;():1124. 10.1136/bmj.326.7399.1124
8. Scott SP, Murray-Kolb LE. Iron Status Is Associated with Performance on Executive Functioning Tasks in Nonanemic Young Women. J Nutr. 2016 Jan;():30–7. 10.3945/jn.115.223586
9. Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet. 1996 Oct;():992–6. 10.1016/S0140-6736(96)02341-0
10. Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol (1985). 2000;88(3):1103-1111.
11. Rubeor A, Goojha C, Manning J, White J. Does Iron Supplementation Improve Performance in Iron-Deficient Nonanemic Athletes? Sports Health. 2018;():400–5. 10.1177/1941738118777488
12. Winkelman JW, Armstrong MJ, Allen RP, Chaudhuri KR, Ondo W, Trenkwalder C, et al. Practice guideline summary: Treatment of restless legs syndrome in adults: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016 Dec;():2585–93. 10.1212/WNL.0000000000003388
13. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021 Dec;():4901. 10.1093/eurheartj/ehab670
14. Milman NT. Dietary Iron Intake in Women of Reproductive Age in Europe: A Review of 49 Studies from 29 Countries in the Period 1993-2015. J Nutr Metab. 2019 Jun;:7631306. 10.1155/2019/7631306
15. Richards T, Breymann C, Brookes MJ, Lindgren S, Macdougall IC, McMahon LP, et al. Questions and answers on iron deficiency treatment selection and the use of intravenous iron in routine clinical practice. Ann Med. 2021 Dec;():274–85. 10.1080/07853890.2020.1867323
16. Snook J, Bhala N, Beales IL, Cannings D, Kightley C, Logan RP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021 Nov;():2030–51. 10.1136/gutjnl-2021-325210
17. Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015 Feb;():e0117383. 10.1371/journal.pone.0117383
18. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, Haya-Palazuelos J, Ciria-Recasens M, Manasanch J, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013 Apr;():291–303. 10.1185/03007995.2012.761599
19. Ribeiro M, Fonseca L, Anjos JS, Capo-Chichi JC, Borges NA, Burrowes J, et al. Oral iron supplementation in patients with chronic kidney disease: can it be harmful to the gut microbiota? Nutr Clin Pract. 2022 Feb;():81–93. 10.1002/ncp.10662
20. Cook JD, Reddy MB. Efficacy of weekly compared with daily iron supplementation. Am J Clin Nutr. 1995 Jul;():117–20. 10.1093/ajcn/62.1.117
21. Moretti D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015 Oct;():1981–9. 10.1182/blood-2015-05-642223
22. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al.; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009 Dec;():2436–48. 10.1056/NEJMoa0908355
23. Rosado JL, González KE, Caamaño MC, García OP, Preciado R, Odio M. Efficacy of different strategies to treat anemia in children: a randomized clinical trial. Nutr J. 2010 Sep;():40. 10.1186/1475-2891-9-40
24. Mozaffari-Khosravi H, Noori-Shadkam M, Fatehi F, Naghiaee Y. Once weekly low-dose iron supplementation effectively improved iron status in adolescent girls. Biol Trace Elem Res. 2010 Jun;():22–30. 10.1007/s12011-009-8480-0
25. Rimon E, Kagansky N, Kagansky M, Mechnick L, Mashiah T, Namir M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005 Oct;():1142–7. 10.1016/j.amjmed.2005.01.065
26. Andersson M, Hurrell R, Zimmermann MB. Impact evaluation of food fortification with iron in school children and women of reproductive age. Laboratory of Human Nutrition, Institute of Food, Nutrition and Health. Vol. Doctor of Sciences. Zürich: ETH; 2010. p. 241.
27. Kortman GA, Raffatellu M, Swinkels DW, Tjalsma H. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev. 2014 Nov;():1202–34. 10.1111/1574-6976.12086
28. Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr. 2017 Dec; Suppl 6:1688S–93S. 10.3945/ajcn.117.156067
29. Stoffel NU, El-Mallah C, Herter-Aeberli I, Bissani N, Wehbe N, Obeid O, et al. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int J Obes. 2020 Jun;():1291–300. 10.1038/s41366-020-0522-x
Table S1Blood parameters and blood pressure at start and 8 weeks after low dose iron treatment (6 mg of elemental iron twice daily) according to per protocol (n = 34).
| Low dose iron treatment | ||||
| 0 weeks | 8 weeks | Change | p-value | |
| Systolic blood pressure, mmHg | 115 ± 8 | 120± 10 | 6± 10 | 0.003 |
| Diastolic blood pressure, mmHg | 78 ± 6 | 80± 8 | 3± 7 | 0.025 |
| Haemoglobin, g/l | 136 ± 9 | 139 ± 8 | 3 ± 8 | 0.033 |
| Serum ferritin, ng/ml | 18 (15, 22) | 34 (26, 44) | 16 (9, 26) | <0.001 |
| Plasma hepcidin ng/ml | 2.9 (1.3, 4.3) | 5.7 (2.9, 10.8) | 2.0 (0.1, 6.1) | 0.004 |
| C-reactive protein, mg/l | 1 (0, 3) | 1 (0, 2) | 0 (-1.0, 0) | NS |
Results are presented as mean values (± 1 standard deviation) or median values (quartiles Q1, Q3). P-values were calculated by paired T-test or Wilcoxon test, ns: not significant and no trend (p >0.10)