Combined use of intraoperative MRI and awake tailored microsurgical resection to respect functional neural networks: preliminary experience
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40072
Constantin
Tuleascaabcd, Henri-Arthur
Leroya, Ondine
Strachowskia, Benoit
Derrea, Claude-Alain
Mauragee, Iulia
Peciu-Florianua, Nicolas
Reynsa
aCentre Hospitalier Regional Universitaire de Lille, Roger Salengro
Hospital, Neurosurgery
and Neurooncology Service, Lille, France
bDepartment of Clinical
Neurosciences, Neurosurgery Service and Gamma Knife Center, Lausanne University
Hospital (CHUV), Lausanne, Switzerland
cFaculty of Biology and Medicine, University of
Lausanne, Lausanne, Switzerland
dSignal Processing Laboratory (LTS
5), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
eCentre Hospitalier Regional
Universitaire de Lille, Roger Salengro Hospital, Pôle de
Biologie-Pathologie-Génétique, Lille, France
* Equal contribution as first authors
** Equal contribution as senior authors
Summary
INTRODUCTION:
The combined use of intraoperative MRI and awake surgery is a tailored
microsurgical resection to respect functional neural networks (mainly the
language and motor ones). Intraoperative MRI has been classically considered to
increase the extent of resection for gliomas, thereby reducing neurological
deficits. Herein, we evaluated the combined technique of awake microsurgical
resection and intraoperative MRI for primary brain tumours (gliomas,
metastasis) and epilepsy (cortical dysplasia, non-lesional, cavernomas).
PATIENTS AND
METHODS: Eighteen patients were treated with the commonly used “asleep awake
asleep” (AAA) approach at Lille University Hospital, France, from November 2016
until May 2020. The exact anatomical location was insular with various
extensions, frontal, temporal or fronto-temporal in 8 (44.4%), parietal in 3
(16.7%), fronto-opercular in 4 (22.2%), Rolandic in two (11.1%), and the supplementary
motor area (SMA) in one (5.6%).
RESULTS: The
patients had a mean age of 38.4 years (median 37.1, range 20.8−66.9). The mean surgical duration
was 4.1 hours (median 4.2, range 2.6−6.4) with a mean duration of intraoperative MRI of 28.8 minutes (median
25, range 13−55). Overall, 61% (11/18) of patients underwent further resection, while
39% had no additional resection after intraoperative MRI. The mean preoperative
and postoperative tumour volumes of the primary brain tumours were 34.7 cc
(median 10.7, range 0.534−130.25) and 3.5 cc (median 0.5, range 0−17.4), respectively. Moreover, the proportion
of the initially resected tumour volume at the time of intraoperative MRI
(expressed as 100% from preoperative volume) and the final resected tumour
volume were statistically significant (p= 0.01, Mann-Whitney test). The tumour
remnants were commonly found posterior (5/9) or anterior (2/9) insular and in proximity
with the motor strip (1/9) or language areas (e.g. Broca, 1/9). Further
resection was not required in seven patients because there were no remnants (3/7),
cortical stimulation approaching eloquent areas (3/7) and non-lesional epilepsy
(1/7). The mean overall follow-up period was 15.8 months (median 12, range 3−36).
CONCLUSION: The
intraoperative MRI and awake microsurgical resection approach is feasible with
extensive planning and multidisciplinary collaboration, as these methods are
complementary and synergic rather than competitive to improve patient
oncological outcomes and quality of life.
Introduction
Primary central nervous system (CNS) tumours are heterogeneous
and derived from cells within the CNS. They can be benign or malignant, while
the most malignant tumours are gliomas with a 5-year overall survival (OS) not
greater than 35% [1]. The resection extent is associated with OS [2–4], with a
larger resection reducing tumour recurrence and further malignant
transformation of low-grade gliomas [5, 6]. The introduction of fluorescence-guided
microsurgical resection further improved the extent of resection [7, 8]. Initially,
intraoperative MRI was considered to lead to a 20% increase in resection extent
[9, 10], particularly for low-grade gliomas, but more recent series indicate that
this benefit is much higher [11, 12].
CNS pathologies located within eloquent areas pose a
specific challenge, particularly to their gross-total microsurgical resection.
The primary goal remains neurological function preservation while maximising
the extent of resection to optimise long-term neurooncological outcomes and
neurological function. In such instances, intraoperative electrical stimulation
helps to define cortical areas underlying eloquent function [13−15].
Conscious or awake craniotomy has a long neurosurgical track
record and was initially recommended for patients at risk of language function
and intractable epilepsy [16]. The current goal of awake craniotomy is to
preserve motor, language and cognitive neurological functions for patients with
any type of pathology anatomically located near or within eloquent areas of the
brain [17]. Consequently, there is a need for the combined use of
intraoperative MRI and awake microsurgical resection for both primary brain tumours
and epilepsy of various origins to achieve a tailored microsurgical resection
to respect functional neural networks [18].
In most of the available literature with regards to the
combined management using awake intraoperative MRI and awake microsurgical
resection for primary brain tumours, there is heterogeneity in terms of
complete initial tumour resection, further resection, no further resection, or
complete resection post-surgery [17, 19−25]. Complete initial resection was
reported between 10 to 58.3%, while further resection after intraoperative MRI
was 36.7% and the final rate of complete resection was between 40.5% and 70% [17,
19−25].
There is also heterogeneity in terms of final results, as some authors describe
this as resection to an eloquent margin, subtotal, gross total, near-total or
complete resection [17, 19−25], which may confuse the interpretation of the published
data.
Our hospital has benefited from the first intraoperative MRI
installation in France in 2014, so we sought to review our experience as a
referral centre for the operative techniques of combined awake craniotomy and intraoperative
MRI. Herein, we present a detailed overview of our data using standardised
scales and complete patient information to provide a broader view of combined
management awake microsurgical resection and intraoperative MRI in patients with
gliomas as well as other pathologies. Moreover, we illustrate some of the
indications also covering various eloquent anatomical areas and describe our
asleep-awake-asleep technique.
Methods
Study design
The study was a retrospective, non-randomised,
case series. The case report form for each patient was retrospectively analysed.
All patients provided informed consent and this historical case series review
was approved by the Lille University Hospital Ethical Committee. Initially, we
published a series of 56 cases with lesions adjacent to eloquent areas managed
by intraoperative MRI [26] but herein, we focus on those benefiting from the combined
management of awake microsurgical resection and intraoperative MRI. The
CNIL number was 791.
Inclusion and exclusion criteria
The inclusion criteria were patients aged
more than 18 years, able to provide written informed consent, with either a primary
brain tumour or epileptic focus anatomically located within a motor and/or
language area (table 1; the illustrative cases are shown in figures 1 and 2).
Exclusion criteria were patients aged less than 18 years at the time of
surgery, unable to provide written informed consent, minimal residual motor
function, pronounced aphasia, a score under 23 on the Mini Mental Status
examination, and those with an apathic/disorganised comportment, large vascular
lesions or potential airway difficulties [27].
Table 1Basic demographic data.
|
Pts
|
Sex
|
Age (yrs)
|
Side
|
Anatomical location
|
Preoperative symptom
|
Preoperative deficit
|
Histological type
|
Further treatment
|
Follow-up (months)
|
| #1 intraoperative MRI |
F |
36.4 |
L |
Anterior insular |
Partial seizures |
None |
Cortical dysplasia type IIb |
Surgery 30 months later (epilepsy) |
36 |
| F |
38.9 |
L |
Anterior insular |
Partial seizures |
None |
Cortical dysplasia type IIb |
None |
12 |
| #2 intraoperative MRI |
M |
37.7 |
L |
Fronto-temporo-insular |
Partial seizures |
None |
Oligoastrocytoma WHO II (diagnosed on biopsy 7 y before with further
chemotherapy) |
Surgery 21.6 months later (epilepsy & volumetric progression) |
36 |
| M |
39.4 |
L |
Temporo-polar; insular; |
Partial seizures |
None |
Anaplastic astrocytoma, IDH-1 mutated (WHO III, WHO IV foci) |
Chemo (6 cycles of Temodal)- plus radiotherapy |
24 |
| #3 |
M |
23.4 |
L |
Frontal, precentral (SMA) |
Generalised seizures |
None |
Cortical dysplasia, schizencephaly |
None |
12 |
| #4 |
F |
37 |
L |
Temporo-insular |
Partial seizure with language arrest |
None |
Anaplastic astrocytoma, IDH 1 mutated, WHO III |
Chemo (6 cycles of Temodal) - plus radiotherapy |
29 (remnant stability) |
| #5 |
F |
37.1 |
L |
Rolandic and parietal |
Headache (incidental discovery, Chiari type I surveillance) |
None |
Oligodendroglioma, WHO II on a previous biopsy (4 months before surgery);
astrocytoma IDH 1 mutated, WHO II, with anaplastic foci, Chr 7, no 1p19q
codelation |
Chemo (6 cycles of Temodal) - plus radiotherapy |
24 (no remnant) |
| #6 |
F |
36.7 |
L |
Fronto-temporo-insular |
Generalised seizure |
None |
Astrocytoma WHO II, IDH 1 mutated, absence chr 13,19, no codelation 1p
19q |
Chemo (6 cycles of Temodal) - plus radiotherapy |
21 (remnant stability) |
| #7 |
F |
38.9 |
L |
Parietal |
Partial motor seizure |
None |
Oligodendroglioma WHO II, IDH 1 mutated, 1p 19q codelation |
None |
18 months(no remnant) |
| #8 |
F |
20.8 |
L |
Frontal |
Headaches |
None |
Oligoastrocytoma WHO II IDH 1 mutated, 1p19q codelated(previously operated 5 y before) |
None |
20 (no remnant) |
| #9 |
F |
66.6 |
L |
Rolandic area |
Headaches |
Right superior upper limb deficit |
Metastasis, breast adenocarcinoma(previously 2 Gamma Knife, 1 Cyber Knife and 4 surgeries, including 1
awake without intraoperative MRI) |
Further Gamma Knife on remnant |
12 (remnant stability) |
| F |
66.94 |
L |
Rolandic area |
Right superior upper limb deficit |
Right superior upper limb deficit |
Metastasis, breast adenocarcinoma(previously 2 Gamma Knife, 1 Cyber Knife and 4 surgeries, including 1
awake with intraoperative MRI) |
None |
Stability |
| #10 |
F |
26.4 |
L |
Inferior parietal |
Drug-resistant epilepsy (SEEG previously performed, with thermocoagulation on relevant
electrodes) |
None |
Discrete gliosis/ dysplasia without further details being possible |
None |
12 |
| #11 |
F |
59.1 |
L |
Fronto-temporo-insular |
Partial seizures |
NoneCognitive decline |
Oligodendroglioma WHO II, IDH 1 mutated (also by biopsy 5 years before
awake surgery) |
Chemo (6 cycles of Temodal) - plus radiotherapy |
24 (remnant stability) |
| #12 |
M |
27.7 |
L |
Temporo-insular |
Generalised seizures |
None |
Astrocytoma WHO II (majority, some foci of III), IDH 1 mutated |
Chemo (6 cycles of Temodal) - plus radiotherapy |
12 (remnant stability) |
| #13 |
M |
20.8 |
L |
Opercula |
Generalised seizures (recurrence) |
None |
Pleomorphic
xanthoastrocytoma WHO II (previously operated twice in another country, no
details) |
None |
6 (remnant stability) |
| #14 |
M |
46.4 |
L |
Fronto-opercular |
Partial seizures |
None |
Glioblastoma WHO IV (previously biopsy- anaplastic gangliogliomas without
further details) |
EORTC 1709 (Stupp protocol plus Marizomib) |
6 (remnant stability) |
| #15 |
F |
31.1 |
L |
Fronto-opercular |
Partial seizures |
None |
Astrocytoma WHO II, IDH 1 mutated |
Chemo (6 cycles of Temodal) - plus radiotherapy |
3 months (remnant stability) |
| #16 |
F |
46.9 |
L |
Anterior insular |
Partial seizures |
None |
Astrocytoma WHO II, IDH 1 mutated |
Chemo (6 cycles of Temodal) - plus radiotherapy |
3 months (remnant stability) |
| #17 |
M |
41.6 |
L |
Fronto-opercular |
Partial and generalised seizures |
None |
Non-lesional epilepsy |
None |
3 months |
| #18 |
M |
27.5 |
L |
Inferior parietal |
Partial seizures |
None |
Cavernoma |
None |
3 months |

Figure 1 Illustrative cases benefiting
from the combined use of awake surgery and intraoperative MRI. Case 1: Preoperative evolutive primary brain tumor (insular
astrocytoma IDH 1 mutated) WHO II, in contact on its medium pole with the inferior
fronto-occipital fasciculus (IFOF). Case 2: Recurrent (multioperated and
irritiated) brain metastasis, localized in the central area, in contact with
the corticospinal tract (in red, interrupted by the tumor).

Figure 2 Illustrative cases benefiting
from the combined use of awake surgery and intraoperative MRI. Case 3: Cortical
dysplasia, with structural T1 preoperative MRI (left), functional task-based
fMRI showing that the lesion is in contact with premotor and motor areas of the
hand (center) and diffusion tensor imaging showing the motor tract (in blue,
left). Case 4: cavernous malformation, located at the junction between the
inferior parietal and the posterior temporal lobe (Wernicke area).
Patient population
Eighteen patients were treated with this
approach at Lille University Hospital, France, from November 2016 until the end
of May 2020 (see table 1 for patient details) and all procedures were performed
by the senior neurosurgeon (NR). The mean patient age was 38.4 years (median
37.1, range 20.8−66.9) and the male-to-female
ratio was 7:11. The mean overall follow-up period was 15.8 months (median 12,
range 3−36) and the preoperative symptoms
included partial seizure in 11 patients (61.1%), generalized seizure in 4 patients
(22.2%), a motor deficit in 1 patient (5.6%), headaches in 1 patient (5.6%) and
incidental in 1 patient (5.6%). In terms of preoperative neurological deficits,
one patient had a right superior upper limb deficit. The exact anatomical
location was insular with various extensions, frontal, temporal or
fronto-temporal in 8 patients (44.4%), parietal in 3 patients (16.7%),
fronto-opercular in 4 patients (22.2%), Rolandic in two patients (11.1%), and
the supplementary motor area (SMA) in 1 patient (5.6%).
Pre- and postoperative clinical assessment
Clinical assessment was performed by a
board-certified neurosurgeon preoperatively and at 3, 6, and 12 months
postoperatively using the Karnofsky performance score (KPS). The neuropsychological
exam was performed by a specialized neuropsychologist (co-author OS) for
patients' task-based functional MRI (fMRI) to check for their dominant
hemisphere. Complementary diffusion tensor imaging by fibre tracking (DTI) was
performed to evaluate the position of major fascicles including, but not
limited to, the corticospinal tract, arcuate fasciculus, inferior
fronto-occipital fasciculus (IFOF), and arcuate fasciculus (AF) [28]. Postoperative
MRI was usually performed within the first 48 hours after surgery and at 3 and
6 months.
The awake surgery technique
The most common procedure performed in our
institution is the combination of general anaesthesia and an awake technique, referred
to as “asleep awake asleep” (AAA). During this approach, the patient is placed
under general anaesthesia before and after brain mapping and/or finalised
resection. Usually, there is an initial phase of general anaesthesia, followed
by intraoperative awakening and then back to general anaesthesia.
All patients in this case series underwent
craniotomy under general anaesthesia. The opening of the dura was performed in the
awake condition after local anaesthesia with Lidocaine (without noradrenaline).
The intraoperative MRI was generally performed in the awake condition with patients
remaining awake during the second resection if required. They were placed back
to sleep if no further resection was performed for their comfort, except for in
three cases who expressed the desire to remain awake.
The electrical stimulation mapping was
performed using a bipolar electrical stimulator probe (5 mm between tips)
connected to a pulse generator (Ojemann Cortical Stimulator, Integra
LifeSciences), which was placed outside the 5-gauss field to further prevent
electrical interference. Typically, stimulation was performed over the entire
exposed cortical surface using biphasic square wave pulses (0.5 msec per pulse,
50 Hz, 2-second duration) starting at 2.5 mA. The identification of eloquent
areas during cortical stimulation was as follows: somatosensory cortex – paresthesia appeared, receptive language cortex (Wernicke area) - speech arrest
and anomia, motor language cortex (Broca area) – speech arrest during number
counting. Cortex points that did not elicit an effect on stimulation were
deemed non-eloquent. Identification sterile tags were not routinely placed over
the brain area, only in selected cases as presented in the literature.
Intraoperative evaluation of microsurgical
resection
For primary brain tumours, tumour removal
was performed by dissecting circumferentially around the tumour border, as
determined by the registered preoperative MR images displayed on the
neuronavigation system. Microsurgical resection was guided by the surgeon’s
intraoperative observation and assessment of tumour margins. An intraoperative
MRI was performed as described below.
Intraoperative MRI: general considerations and
specific sequences
A new intraoperative MRI was performed when
the neuronavigation became insufficiently accurate and/or to assess the
presence of a residual tumour.
Before translating the patient into the
MRI, a checklist was completed to ensure the absence of metallic material in
the surgical site that could interfere with the magnetic field.
Before performing the intraoperative MRI
evaluation to assess the extent of resection, the dura mater and skin were approximated.
A sterile field was placed over the operative field and the head was held using
a Mayfield MR/X-Ray Skull clamp with Excite 3.0T Adaptor (Integra Lifescience,
New Jersey, USA). All patients benefitted from an intraoperative 1.5 Tesla MRI
(General Electric®, Boston, MA) and the imaging sequences for neuronavigation
were 3D T1 after gadolinium injection with 1-mm slice thickness. Additional
sequences, such as 3D FLAIR (especially for low-grade gliomas), diffusion
(B1000 and ADC), and T2 with gradient echo, were also performed if required. An
intraoperative tractography was performed when the gliomas were close to
functional areas (e.g., corticospinal tract). The neuronavigation data update
procedure was performed using the automatic coregistration provided by
Brainlab® Munich, Germany, with the quality and accuracy of the coregistration
double-checked by an imaging engineer and the board-certified neurosurgeon [28].
The MRI was discussed with the neuroradiologist to evaluate the extent of
resection.
The surgical microscope (OPMI Pentero®
Zeiss, Germany) was connected to the imaging network and could be used for neuronavigation.
Volumetric assessment of primary brain tumours
Two independent neurosurgeons measured all
volumetric primary brain tumour volumes using the Intellispace Portal
(Philips®, Amsterdam, Netherlands) module BTumour tracking software.The lesions without contrast
enhancement or slightly enhanced after gadolinium infusion were contoured using
the T2/FLAIR hypersignal MR sequences and highly contrast-taking tumours were
segmented using the T1 hypersignal after gadolinium infusion sequences. After
segmentation, volumes were calculated in cubic centimetres (cc). For a
discrepancy > 10% between the two observers, both segmentations were
compared to achieve a consensus volume. The extent of resection was reported in
both cc and percentages.
Histological analysis of primary brain tumours
For each patient, the definitive histologic
subtype was reviewed by a senior neuropathologist based on the 2016 WHO
classification.
The diagnosis in the present series (n= 13,
primary brain tumours, table 1) included astrocytoma WHO grade II in 5 patients
(27.8%), oligoastrocytoma WHO grade II in 1 patient (5.6%), oligodendroglioma
WHO grade II in 2 patients (11.1%), pleomorphic xantoastrocytoma WHO grade II
in 1 patient (5.6%), anaplastic astrocytoma WHO grade III in 2 patients (11.1%),
glioblastoma WHO grade IV and brain metastasis in 1 patient (5.6%) each,
respectively.
Short review of the literature
A short review of the literature is presented
in table 2 and further detailed in the results
section [11, 17, 20−25, 29−31].
Table 2Short review of the literature for the use of awake surgery and intraoperative
MRI for primary brain tumours.
|
Series
|
Intraoperative
MRI complete resection
|
Further
resection
|
No further
resection
(n,
percentage)
|
Complete
resection (details)
|
| Whiting et
al. (2020) |
14/62 (22.6%) |
41/48 (85.4%) |
7/48 (14.6%) |
27/63 (42.8%) |
| White et al.
(2018) |
12/36 (33%) |
18/36 (50%) |
16/24 (66.7%) |
− |
| Motomura et
al. (2017) |
9/25 (36%) |
7/16 (43.8%) |
9/16 (56.2%) |
− |
| Mehdorn et
al. (2017) |
− |
− |
− |
− |
| Zhuang et al.
(2016) |
16/30 (53%)
GTR |
− |
− |
23/30 (77%) |
| Ghinda et al.
(2016) |
44/106
(41.5%) |
30/62 (48.4%) |
32/62 (51.6%) |
64/106
(60.4%) |
| Coburger et
al. (2015) |
− |
|
− |
17/26 (65.4%) |
| Maldaun et al. (2014) |
− |
17/42 (40.5%) |
25/42 (59.5%) |
17/42 gross total
(40.5%) (grace to intraoperative
MRI 7/17 [41%]) |
| Tuominen et al. (2013) |
− |
|
− |
10/20 (50%)
complete |
| Lu et al. (2012) |
11/30 (36.7%) |
11/30 (36.7%) |
19/30 (63.3%) |
18/30 (60%)
complete (grace to intraoperative
MRI 7/18 [60%]) |
| Leuthardt et
al. (2011) |
7/12 (58.3%) |
6/12 (50%) |
5/12 (41.7%) |
5/12 (41.7%)
complete |
| 2/12 (16.7%)
nearly total |
| 5/12 (41.7%)
subtotal |
| Weingarten et
al. (2009) |
1/10 (10%) |
7/9 (77.8%) |
2/9 (22.2%) |
7/10 (70%)
complete 3/10 (30%) to
an eloquent margin |
Intractable epilepsy: a multidisciplinary
assessment using SEEG
For patients with intractable epilepsy or
unknown or doubtful origin, SEEG was used before any microsurgical resection is
performed, even in the case of an identified structural lesion on preoperative
MRI. This is the standard approach together with the specialised team in our
hospital.
The histological diagnosis for cases with
intractable epilepsy (n= 5) in the present series was cortical dysplasia in 3
(16.7%), gliosis and cavernoma in 1 (5.6%) each, respectively.
Results
The mean operative time and duration of intraoperative
MRI, lesion remnant, pre- and postoperative deficits are presented in table 3,
showing a mean duration of microsurgery of 4.1 hours
(median 4.2, range 2.6−6.4) and intraoperative
MRI of 28.8 minutes (median 25, range 13−55; figure 3). The tumour remnants were most commonly located posterior
(5/9) or anterior (2/9) insular and in proximity with the motor strip (1/9) or
language areas (e.g. Broca, 1/9).
Table 3Operative time and duration of intraoperative
MRI, lesion remnants, pre- and postoperative deficits.
|
Pts
|
Total operative time (hours)
|
Scanning time for intraoperative MRI (min)
|
Remnant anatomical location during intraoperative
MRI
|
Further surgery after intraoperative MRI
|
Remnant at the end of the surgery
|
Preoperative deficit
|
Postoperative deficit
|
| #1 |
3h:23 |
0h:42 |
Left frontal, in contact with the lenticular nucleus |
Yes, approaching the intraoperative MRI residual part |
Insular, anterior and superior |
None |
None |
| #2 |
5h:48 |
0h:32 |
Insular and frontal internal |
Yes, approaching the internal part |
Insular posterior and temporo-polar |
None |
Transient right hemiparesis |
| #3 |
3h:05 |
0h:28 |
Posterior, at the interface with the motor cortex |
Yes, optimizing the resection |
Posterior, at the interface with the motor cortex |
None |
Transient supplementary motor area syndrome |
| #4 |
5h:16 |
0h:19 |
- |
Yes, optimising the extent of resection |
Insular, behind the superior temporal gyrus |
None |
None. No postoperative partial seizures (under Vimpat 400 mg/day) (Engel IA) |
| #5 |
4h:14 |
0h:25 |
Anterior, within the inferior part of the intraparietal sulcus area where
paresthesias were encountered during cortical stimulation |
Yes, optimising the extent of resection |
None |
Right upper limb ataxia |
Preexistent right upper limb ataxia became more important. Profound sensibility problems. Neuropathic pain (left lower limb). Partial motor seizures (Engel IIB) |
| #6 |
4h:29 |
0h:18 |
Insular and fronto-basal |
Yes, approaching the fronto-basal part |
Insular |
None |
None. Seizure disappearance (Engel IA) |
| #7 |
4h:04 |
0h:21 |
Minimal, between postcentral&cingulate sulcus |
Yes, approaching the remnant part |
None |
None |
Ataxia, well alleviated. Dysgraphia, disappeared |
| #8 |
- |
0h:21 |
None |
Not applicable (no remnant) |
None |
None |
None. Emotional issues due to the awake surgery |
| #9 |
4h:40 |
0h:43 |
Anterior and inferior at the level of the surgical cavity |
Not possible as per intraoperative cortical stimulation |
Anterior and inferior at the level of the surgical cavity |
Right superior upper limb deficit |
Worsening of right superior upper limb deficit |
| 3h:31 |
|
Anterior and inferior at the level of the surgical cavity |
Not possible as per intraoperative cortical stimulation |
Anterior and inferior at the level of the surgical cavity |
Right superior upper limb deficit |
Stability |
| #10 |
4h:04 |
0h:13 |
Left inferior parietal |
Yes, approaching the remnant part |
None |
None |
Postoperative stability of neuropsychological exam |
| #11 |
5h:16 |
0h:55 |
Left frontal superior and temporoinsular |
Yes, approaching the left frontal superior |
Temporo-insular |
None |
None |
| #12 |
6h:38 |
0h:25 |
Left posterior T1 and insular |
Yes, approaching both parts |
Temporo-insular |
None |
None |
| #13 |
4h:50 |
0h:25 |
None |
Not applicable |
Not applicable |
None |
Transient right-hand hypoesthesia. Decrease in seizure frequency (Engel IIB) |
| #14 |
5h:30 |
0h:52 |
Intraventricular (plus Flair remnant, left untouched due to its anatomical location) |
Yes, approaching the intraventricular part |
Flair remnant in functional areas (Broca); contrast-enhancing part
completely resected) |
None |
None |
| #15 |
2h:58 |
0h: 32 |
Minimal left fronto-opercular remnant due to transient language arrest |
Not possible as per intraoperative cortical stimulation |
Left fronto-opercular due to transient language arrest |
None |
None |
| #16 |
4h:29 |
0h:19 |
The vast majority of the lesion (microsurgical resection just at the
anterior pole of it) |
Yes, approaching the lesion |
None |
None |
None |
| #17 |
3h:13 |
0h:26 |
Non-lesional epilepsy |
No, unnecessary |
Not applicable |
None |
None |
| #18 |
2h:56 |
0h:22 |
None |
Not applicable |
Not applicable |
None |
None |
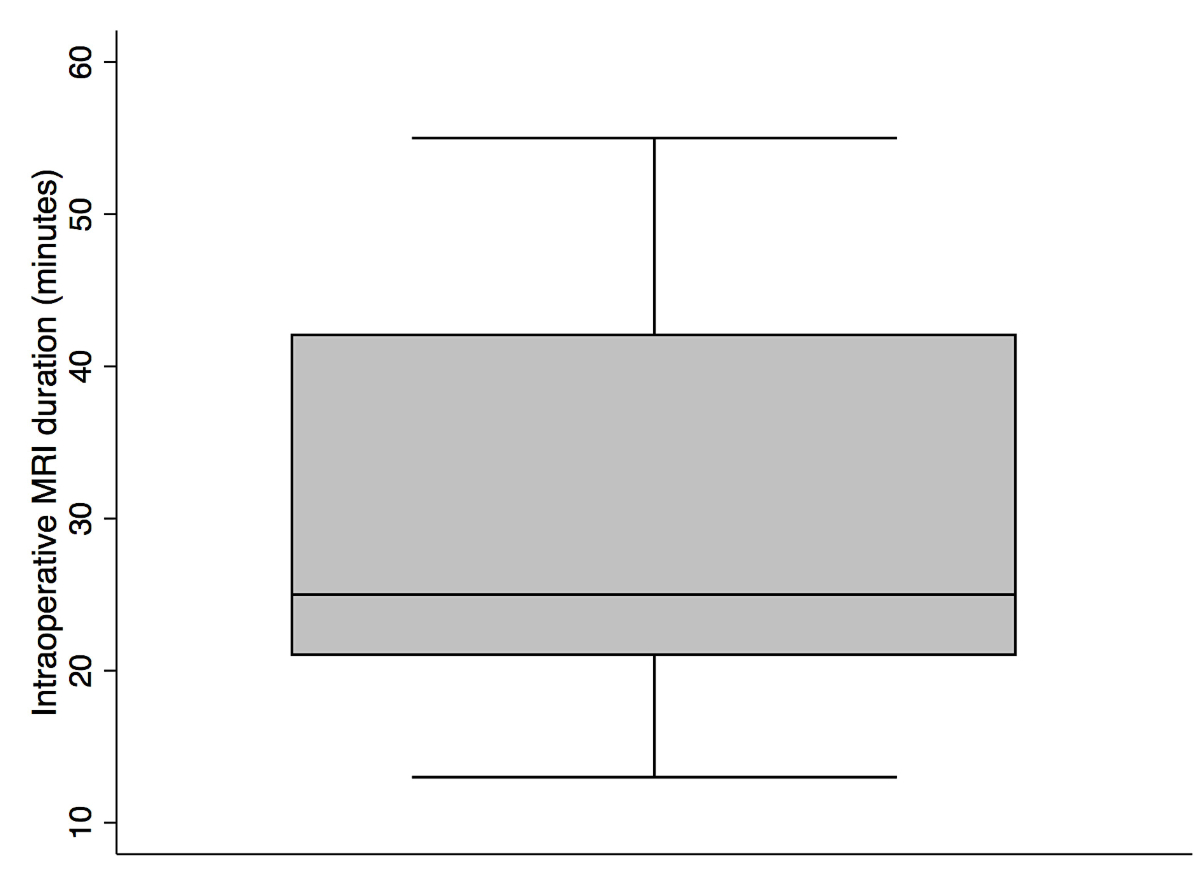
Figure 3 Intraoperative MRI duration (minutes).
Microsurgery for primary brain tumours: further
resection after the first intraoperative MRI and potential adjuvant therapies
The mean preoperative tumour volume of the primary
brain tumours was 34.7 cc (median 10.7, range 0.534−130.25; standard deviation 41.2 cc).
Overall, 61% (11/18) of cases underwent
further resection, with no further resection in seven patients because there
were no remnants (3/7), cortical stimulation approaching eloquent areas (3/7)
and non-lesional epilepsy (1/7).
The mean postoperative volume was 3.8 cc
(median 0.5, range 0−17.4; standard
deviation 5.6 cc; figure 4, upper level, absolute values, in cc; lower level,
in percentages, according to the reference preoperative volume of 100%). A
Mann-Whitney test was performed to compare the mean perioperative tumour
volumes (in cc) resected at the time of intraoperative MRI and the final
resected tumour volumes showing that they were not statistically significant
(p= 0.6, figure 4, upper part). However, the total tumour volumes resected at the
time of intraoperative MRI (as by 100% tumour volumes preoperatively) compared
to the final results were statistically significant (p= 0.01, figure 4, lower
part).
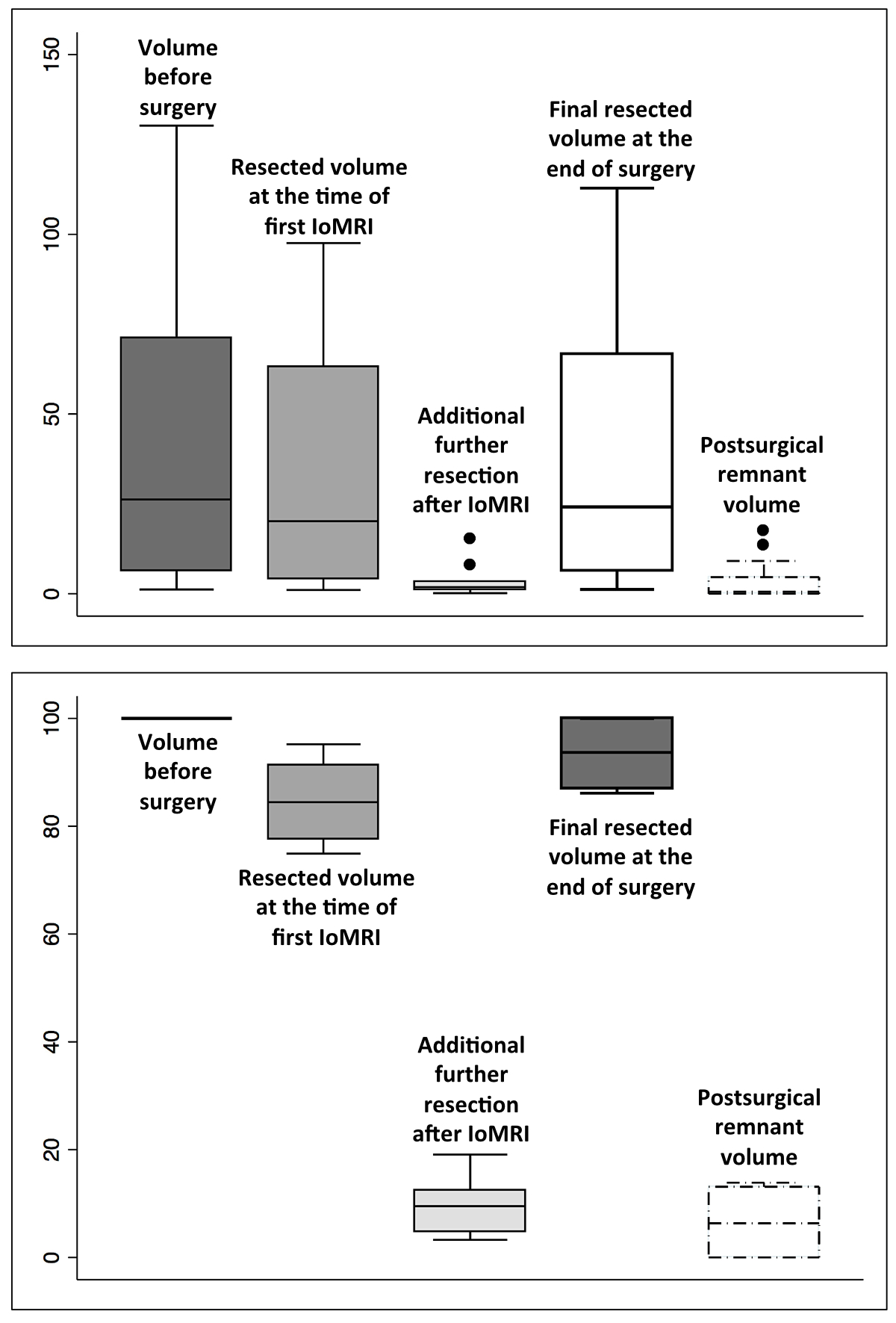
Figure 4 The impact of intraoperative MRI on the
microsurgical resection in terms of volumes (as cc, upper side; as percentages,
lower side).
After multidisciplinary discussion, 8
(8/13, 61.5%) cases benefitted from a standard protocol of combined
radiotherapy (50.4 Gy) and six cycles of Temozolomide, 1 patient (1/13, 7.7%)
was involved in the EORTC 1709 (the Stupp protocol plus Marizomib) and two
(2/13, 15.4%) cases were subsequently followed-up.
At the last follow-up, 12 (12/13, 92.3%) patients
had volumetric tumour stability or a decreased volume. One (7.7%) patient with
oligoastrocytoma WHO II required further surgery at 21.6 months after the first
resection due to volumetric progression and drug-resistant epilepsy, with LPFS achieved
(see figure 5).

Figure 5 Illustrative case of an oligodendroglioma
WHO II, 1p19q co-deleted.
A short review of the literature on this
topic is provided in table 2, presenting the initial complete resection rates,
as well as those at the end of the surgery. The mean initial resection rate at
the time of the first intraoperative MRI was 36.4% (median 36.3, range 10−58.3) and at the end of surgery was 56.4% (median 60, range 40.5−77).
Microsurgery for epilepsy: further resection
after the first intraoperative MRI and clinical outcome
One case with cortical dysplasia type IIb underwent
further surgery after the initial procedure.
The patients with cortical dysplasia had, among the epilepsy cases, the
longest follow-up in the present series and all experienced a major decrease in
seizure frequency and duration (see table 3 for the Engel classes).
The patient with non-lesional epilepsy
(number 17) experienced no crisis to date (3-month follow-up, previously
several crises per week) and the patient with cavernoma had no seizures after 3
months of follow-up.
Neurological complications
Transient neurological complications were
encountered in 3/18 (16.7%) cases, each one with transient hemiparesis, ataxia
and dysgraphia, and right-hand hypoesthesia.
Definitive neurological complications occurred
in 2/18 (11.1%) patients, each with neuropathic pain and partial motor seizures
and right-hand hypoesthesia, respectively.
Neuropsychological exam and Karnofsky
performance status
A neuropsychological exam was performed in 5
cases with no statistically significant difference between the pre-and
postoperative assessment (table 4). Also, there was no statistically
significant difference between the pre-and postoperative Karnofsky performance
status (table 4).
Table 4Pre- and postoperative neuropsychological exam
and pre- and postoperative OMS and Karnofsky performed depending on the
pathology.
|
Pts
|
Preoperative
neuropsychological exam
|
Postoperative
neuropsychological exam
|
Pre- and postoperative
OMS
(when
relevant)
|
Pre- and
postoperative Karnofsky
(when
relevant)
|
| #1 |
IQ = 91, VIQ = 86, PIQ = 100 |
IQ = 94, VIQ = 81, PIQ = 114 |
– |
– |
| –:
verbal LTM, verbal WM, VA, PS |
–: verbal LTM, verbal WM, PS |
| #2 |
–: verbal WM |
– |
1–1 |
100–90 |
| #3 |
IQ = 89, VIQ = 102, PIQ = 72 |
IQ = 86, VIQ = 92, PIQ = 81 |
– |
– |
| –: VC,
VF, PS |
–: VC,
VF, PS |
| #4 |
– |
– |
0–0 |
100–100 |
| #5 |
MoCA: 27/30 |
MoCA: 26/30 |
0–0 |
100–100 |
| –:
verbal WM |
–: IC, PS |
| #6 |
MoCA: 27/30 |
MoCA: 25/30 |
0–0 |
100–100 |
| –:
verbal WM, IC, VF, PS |
–: verbal
WM, IC, PS |
| #7 |
MoCA: 29/30 |
MoCA: 28/30 |
0–0 |
100–90 |
| Normal |
–: DA |
| #8 |
MoCA: 28/30 |
MoCA: 27/30 |
0–0 |
100–90 |
| –: verbal
WM, IC, VF |
–: verbal
WM, VF, PS |
| #9 |
– |
– |
|
|
| #10 |
IQ = 80, VIQ = 78, PIQ = 83 |
IQ = 85, VIQ = 76, PIQ = 100 |
– |
– |
| –:
verbal WM, IC, VF |
–:
verbal WM, VF |
| #11 |
MoCA: 28/30 |
– |
0–1 |
100–80 |
| –: verbal
LTM |
| #12 |
MoCA: 22/30 |
– |
0–0 |
90–90 |
| –: VC, LTM,
IC, VF, PS |
| #13 |
– |
– |
1–1 |
90–90 |
| #14 |
MoCA: 26/30 |
MMSE: 26/30 |
0–0 |
100–100 |
| –: verbal
LTM, verbal WM, IC, VF |
– |
| #15 |
–: VC,
verbal WM, S, IC, DA |
– |
0–0 |
90–90 |
| #16 |
MoCA: 26/30 |
– |
0–0 |
100–100 |
| normal |
| #17 |
IQ = 76, VIQ = 76, PIQ = 82 |
– |
|
|
| –: verbal
LTM, verbal WM, IC, VF, PS |
| #18 |
MoCA: 30/30 |
MoCA: 28/30 |
|
|
| Normal |
Normal |
Discussion
The present study evaluated our preliminary
results of the combined technique of awake microsurgical resection and intraoperative
MRI for primary brain tumours (including gliomas and brain metastasis) and
epilepsy surgery (including cortical dysplasia, non-lesional epilepsy, and
cavernoma). The combined intraoperative MRI and awake microsurgical resection
is a challenging approach and the patient needs to be cooperative and motivated
to participate in all language and/or motor tasks. The role of the
anesthesiologist is crucial, both preoperatively (determining if the patient is
a suitable candidate, medically and emotionally) and intraoperatively, while
maintaining anT appropriate level of anaesthesia and analgesia. There is also
much real-time information for the surgeon to process, so this approach
requires a skilled and cohesive operative team and a compliant patient.
The anaesthetic technique in an awake brain
surgical procedure varies from light or deep sedation [32] to general anaesthesia
with an endotracheal tube [33] or laryngeal mask [34]. The anaesthetic goals
are mainly to maintain an awake and cooperative patient during cortical mapping
while ensuring the safety of the airway, and providing adequate cerebral
perfusion and brain relaxation [35]. Inherent challenges are desaturation or
hypercapnia during surgery and it is possible that this awake-asleep-awake
(AAA) paradigm would potentially interfere with intra-operative testing of the respective
eloquent areas due to anaesthesia remnants. Therefore, to avoid such
interference, we use a laryngeal mask and Remifentanil, a short-acting
synthetic opioid analgesic. The advantage of such a drug is that the
context-sensitive half-life remains at 4 minutes after, for example, 4 hours of
administration [36] and it is also associated with fewer seizures compared with
other opioids. In general, factors that might potentially modulate the effects
of anaesthetic agents include but are not limited to, patient age, physical and
medical conditions, and pharmacogenetics. Also, dural local anaesthetics are
used once the patient is awake. To date, no randomised trials comparing our
approach with the awake tumour resection under local anaesthetic [37] have been
performed but it is acknowledged that awake tumour resection can be completed
using only local anaesthesia [38]. Nevertheless, in our experience, using
sedative drugs can be particularly helpful for craniotomy and closure. Due to a
lack of comparative studies, there is no evidence showing the superiority of
one technique over the other [39].
Of note, the craniotomy is performed when
the patient is asleep and the dura mater is not opened during this first step.
Once the patient is awake, completely stable and calm, the dura mater is
infiltrated to further proceed to its opening, thereby avoiding increased intraoperative
intracranial pressure in the transition from asleep to awake with its potential
inherent consequences.
The
entry cortical point is always selected using the neuronavigation system and if
this point is formally contraindicated after cortical stimulation, we proceed
to further changes. Indeed, the response of the cortical stimulation provides multiple
possibilities for cortical routes and the cranial flap is designed to provide
enough room. Naropein-immersed cottonwoods are not used on the dura for local
anaesthesia [40], rather the dura is indirectly infiltrated [41]. Importantly,
the brain is not pain sensitive, while the basal dura and larger vessel
manipulation could potentially engender pain [42].
Cortical stimulation can generate seizures,
with an incidence of 5−20% [43]. In our centre, these episodes can be managed
by cold Ringer’s lactate solution irrigation of the cortex and patience without
the need for intravenous antiepileptic medication [44].
The interruption time due to the intraoperative
MRI is usually small, around 30 minutes (15 minutes couch in, 15 minutes couch
out).
With
regards to the use of such an approach for primary brain tumours and
particularly for gliomas, the literature review [11, 17, 20−25, 29−31] shows a clear
benefit, increasing the mean extent of resection by approximately 20% for
primary brain tumours. Tuominen et al. [24] showed that this approach reduces
the risk of postoperative impairment following resection of tumours located in
or near speech and motor areas, whilst Mehdorn et al. [23] suggested that there
was a slight preponderance in redo surgeries for tumour remnants in the first
(11.2%, before the use of intraoperative MRI) compared to the second period (7.4%,
after the use of intraoperative MRI). Moreover, interestingly, patients with
low-grade gliomas in the second series (using intraoperative MRI) did not
experience recurrences as frequently as those in the first series.
The aim of epilepsy surgery is either
complete excision or disconnection of the epileptic network while conserving
the structure and function of the eloquent cortex [45]; we consider that
presurgical correct diagnosis is mandatory for this. Modern epileptologists can
use multimodal diagnostic tools, including semiology analysis,
electrophysiological recordings, functional testing and complex neuroimaging
techniques [45], complementary modalities that define the location and
boundaries of the epileptic zone. In challenging cases,
stereo-electroencephalography (SEEG) by depth electrodes is considered [46]. The
existence of a lesion does not automatically relate to an epileptogenic network
solely driven by that specific lesion. Moreover, a lesion can recruit and
further drive other epileptogenic zones in the brain, making diagnosis even
more challenging [47, 48]. In our experience, SEEG is used when necessary to establish
a preoperative project, which proposes an area of microsurgical resection
including the electrodes for which intraoperative MRI can refine the
pre-planned resection area and evaluate the extent of resection.
Together with subcortical mapping and other aiding tools, the surgeon can
determine whether further resection is required. The awake component
remains crucial for function preservation, thus complete resection becomes
strictly related to an SEEG preoperative project.
The infection rate in these particular
procedures involving intraoperative MRI and additional staff is approximately
1.2%, which is similar to our overall cohort in classical brain surgeries.
The
present study has several inherent limitations. First, the retrospective nature
of this study with all the inherent bias. Second, there may be small variations
in the volumetric analysis depending on the slice thickness etc. A third
limitation is the inclusion of multiple pathologies such as gliomas, brain
metastasis, cavernomas, cortical dysplasia etc. However, they all share the
same goal, which is for infiltrative brain tumours, the gross-total resection
and for others, microsurgical resection while respecting functional neural
networks present at their boundaries.
Conclusion
Intraoperative MRI and awake microsurgical
resection is a feasible approach with extensive planning and multidisciplinary
collaboration. These two methods can be considered complementary and synergic
rather than competitive, aimed at improving patient oncological outcomes and
quality of life. This case series shows a statistical significance when
comparing the percentage initially resected at the time of intraoperative MRI
and the final extent of resection for primary brain tumours and although the
series is small, these results might translate to a clear neurooncological
benefit if extended to a large population of patients.
Constantin Tuleasca, MD-PhD
University
of Lausanne
Faculty of
Biology and Medicine and Centre Hospitalier Universitaire Vaudois (CHUV)
Department
of Clinical Neurosciences
Neurosurgery
Service and Gamma Knife Center
CH-1011 Lausanne
constantin.tuleasca[at]chuv.ch
References
1.
Lapointe S
,
Perry A
,
Butowski NA
. Primary brain tumours in adults. Lancet. 2018 Aug;392(10145):432–46. https://doi.org/10.1016/S0140-6736(18)30990-5
2.
Berger MS
,
Rostomily RC
. Low grade gliomas: functional mapping resection strategies, extent of resection, and outcome. J Neurooncol. 1997 Aug;34(1):85–101. https://doi.org/10.1023/A:1005715405413
3.
Laws ER
,
Parney IF
,
Huang W
,
Anderson F
,
Morris AM
,
Asher A
, et al.; Glioma Outcomes Investigators
. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003 Sep;99(3):467–73. https://doi.org/10.3171/jns.2003.99.3.0467
4.
Sanai N
,
Berger MS
. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008 Apr;62(4):753–64. https://doi.org/10.1227/01.neu.0000318159.21731.cf
5.
Berger MS
,
Deliganis AV
,
Dobbins J
,
Keles GE
. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994 Sep;74(6):1784–91. https://doi.org/10.1002/1097-0142(19940915)74:6<1784::AID-CNCR2820740622>3.0.CO;2-D
6.
Martin C
,
Alexander E 3rd
,
Wong T
,
Schwartz R
,
Jolesz F
,
Black PM
. Surgical treatment of low-grade gliomas in the intraoperative magnetic resonance imager. Neurosurg Focus. 1998 Apr;4(4):e8. https://doi.org/10.3171/foc.1998.4.4.11
7.
Stummer W
,
Novotny A
,
Stepp H
,
Goetz C
,
Bise K
,
Reulen HJ
. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000 Dec;93(6):1003–13. https://doi.org/10.3171/jns.2000.93.6.1003
8.
Stummer W
,
Pichlmeier U
,
Meinel T
,
Wiestler OD
,
Zanella F
,
Reulen HJ
; ALA-Glioma Study Group
. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006 May;7(5):392–401. https://doi.org/10.1016/S1470-2045(06)70665-9
9.
Oh DS
,
Black PM
. A low-field intraoperative MRI system for glioma surgery: is it worthwhile? Neurosurg Clin N Am. 2005 Jan;16(1):135–41. https://doi.org/10.1016/j.nec.2004.07.010
10.
Schulder M
,
Carmel PW
. Intraoperative magnetic resonance imaging: impact on brain tumor surgery. Cancer Contr. 2003;10(2):115–24. https://doi.org/10.1177/107327480301000203
11.
Coburger J
,
Merkel A
,
Scherer M
,
Schwartz F
,
Gessler F
,
Roder C
, et al.
Low-grade Glioma Surgery in Intraoperative Magnetic Resonance Imaging: Results of a Multicenter Retrospective Assessment of the German Study Group for Intraoperative Magnetic Resonance Imaging. Neurosurgery. 2016 Jun;78(6):775–86. https://doi.org/10.1227/NEU.0000000000001081
12.
Coburger J
,
Wirtz CR
. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: a systematic review. J Neurooncol. 2019 Feb;141(3):533–46. https://doi.org/10.1007/s11060-018-03052-4
13.
Berger MS
,
Kincaid J
,
Ojemann GA
,
Lettich E
. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989 Nov;25(5):786–92. https://doi.org/10.1227/00006123-198911000-00015
14.
Duffau H
. Intraoperative cortico-subcortical stimulations in surgery of low-grade gliomas. Expert Rev Neurother. 2005 Jul;5(4):473–85. https://doi.org/10.1586/14737175.5.4.473
15.
Ojemann G
,
Ojemann J
,
Lettich E
,
Berger M
. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989 Sep;71(3):316–26. https://doi.org/10.3171/jns.1989.71.3.0316
16.
Zanello M
,
Meyer B
,
Still M
,
Goodden JR
,
Colle H
,
Schichor C
, et al.
Surgical resection of cavernous angioma located within eloquent brain areas: international survey of the practical management among 19 specialized centers. Seizure. 2019 Jul;69:31–40. https://doi.org/10.1016/j.seizure.2019.03.022
17.
Motomura K
,
Natsume A
,
Iijima K
,
Kuramitsu S
,
Fujii M
,
Yamamoto T
, et al.
Surgical benefits of combined awake craniotomy and intraoperative magnetic resonance imaging for gliomas associated with eloquent areas. J Neurosurg. 2017 Oct;127(4):790–7. https://doi.org/10.3171/2016.9.JNS16152
18.
Tuleasca C
,
Leroy HA
,
Peciu-Florianu I
,
Strachowski O
,
Derre B
,
Levivier M
, et al.
Impact of combined use of intraoperative MRI and awake microsurgical resection on patients with gliomas: a systematic review and meta-analysis. Neurosurg Rev. 2021 Dec;44(6):2977–90. https://doi.org/10.1007/s10143-021-01488-3
19.
Ghinda D
,
Zhang N
,
Lu J
,
Yao CJ
,
Yuan S
,
Wu JS
. Contribution of combined intraoperative electrophysiological investigation with 3-T intraoperative MRI for awake cerebral glioma surgery: comprehensive review of the clinical implications and radiological outcomes. Neurosurg Focus. 2016 Mar;40(3):E14. https://doi.org/10.3171/2015.12.FOCUS15572
20.
Leuthardt EC
,
Lim CC
,
Shah MN
,
Evans JA
,
Rich KM
,
Dacey RG
, et al.
Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: preliminary experience. Neurosurgery. 2011;69(1):194-205; discussion -6. https://doi.org/10.1227/NEU.0b013e31821d0e4c
21.
Lu J
,
Wu J
,
Yao C
,
Zhuang D
,
Qiu T
,
Hu X
, et al.
Awake language mapping and 3-Tesla intraoperative MRI-guided volumetric resection for gliomas in language areas. J Clin Neurosci. 2013 Sep;20(9):1280–7. https://doi.org/10.1016/j.jocn.2012.10.042
22.
Maldaun MV
,
Khawja SN
,
Levine NB
,
Rao G
,
Lang FF
,
Weinberg JS
, et al.
Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: analysis of 42 cases. J Neurosurg. 2014 Oct;121(4):810–7. https://doi.org/10.3171/2014.6.JNS132285
23.
Mehdorn HM
,
Schwartz F
,
Becker J
. Awake Craniotomy for Tumor Resection: Further Optimizing Therapy of Brain Tumors. Acta Neurochir Suppl (Wien). 2017;124:309–13. https://doi.org/10.1007/978-3-319-39546-3_45
24.
Tuominen J
,
Yrjänä S
,
Ukkonen A
,
Koivukangas J
. Awake craniotomy may further improve neurological outcome of intraoperative MRI-guided brain tumor surgery. Acta Neurochir (Wien). 2013 Oct;155(10):1805–12. https://doi.org/10.1007/s00701-013-1837-3
25.
Whiting BB
,
Lee BS
,
Mahadev V
,
Borghei-Razavi H
,
Ahuja S
,
Jia X
, et al.
Combined use of minimal access craniotomy, intraoperative magnetic resonance imaging, and awake functional mapping for the resection of gliomas in 61 patients. J Neurosurg. 2019:1–9.
26.
Reyns N
,
Leroy HA
,
Delmaire C
,
Derre B
,
Le-Rhun E
,
Lejeune JP
. Intraoperative MRI for the management of brain lesions adjacent to eloquent areas. Neurochirurgie. 2017 Jun;63(3):181–8. https://doi.org/10.1016/j.neuchi.2016.12.006
27.
Nabavi A
,
Goebel S
,
Doerner L
,
Warneke N
,
Ulmer S
,
Mehdorn M
. Awake craniotomy and intraoperative magnetic resonance imaging: patient selection, preparation, and technique. Top Magn Reson Imaging. 2009 Jan;19(4):191–6. https://doi.org/10.1097/RMR.0b013e3181963b46
28.
Leroy HA
,
Delmaire C
,
Le Rhun E
,
Drumez E
,
Lejeune JP
,
Reyns N
. High-field intraoperative MRI and glioma surgery: results after the first 100 consecutive patients. Acta Neurochir (Wien). 2019 Jul;161(7):1467–74. https://doi.org/10.1007/s00701-019-03920-6
29.
Weingarten DM
,
Asthagiri AR
,
Butman JA
,
Sato S
,
Wiggs EA
,
Damaska B
, et al.
Cortical mapping and frameless stereotactic navigation in the high-field intraoperative magnetic resonance imaging suite. J Neurosurg. 2009 Dec;111(6):1185–90. https://doi.org/10.3171/2009.5.JNS09164
30.
White T
,
Zavarella S
,
Jarchin L
,
Nardi D
,
Schaffer S
,
Schulder M
. Combined Brain Mapping and Compact Intraoperative MRI for Brain Tumor Resection. Stereotact Funct Neurosurg. 2018;96(3):172–81. https://doi.org/10.1159/000488991
31.
Zhuang DX
,
Wu JS
,
Yao CJ
,
Qiu TM
,
Lu JF
,
Zhu FP
, et al.
Intraoperative Multi-Information-Guided Resection of Dominant-Sided Insular Gliomas in a 3-T Intraoperative Magnetic Resonance Imaging Integrated Neurosurgical Suite. World Neurosurg. 2016 May;89:84–92. https://doi.org/10.1016/j.wneu.2016.01.067
32.
Hervey-Jumper SL
,
Li J
,
Lau D
,
Molinaro AM
,
Perry DW
,
Meng L
, et al.
Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg. 2015 Aug;123(2):325–39. https://doi.org/10.3171/2014.10.JNS141520
33.
Huncke K
,
Van de Wiele B
,
Fried I
,
Rubinstein EH
. The asleep-awake-asleep anesthetic technique for intraoperative language mapping. Neurosurgery. 1998 Jun;42(6):1312–6. https://doi.org/10.1097/00006123-199806000-00069
34.
Piccioni F
,
Fanzio M
. Management of anesthesia in awake craniotomy. Minerva Anestesiol. 2008;74(7-8):393–408.
35.
Meng L
,
Berger MS
,
Gelb AW
. The Potential Benefits of Awake Craniotomy for Brain Tumor Resection: An Anesthesiologist’s Perspective. J Neurosurg Anesthesiol. 2015 Oct;27(4):310–7. https://doi.org/10.1097/ANA.0000000000000179
36.
Komatsu R
,
Turan AM
,
Orhan-Sungur M
,
McGuire J
,
Radke OC
,
Apfel CC
. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007 Dec;62(12):1266–80. https://doi.org/10.1111/j.1365-2044.2007.05221.x
37.
Potters JW
,
Klimek M
. Local anesthetics for brain tumor resection: current perspectives. Local Reg Anesth. 2018 Feb;11:1–8. https://doi.org/10.2147/LRA.S135413
38.
Danks RA
,
Rogers M
,
Aglio LS
,
Gugino LD
,
Black PM
. Patient tolerance of craniotomy performed with the patient under local anesthesia and monitored conscious sedation. Neurosurgery. 1998;42(1):28-34; discussion -6. https://doi.org/10.1097/00006123-199801000-00006
39.
Danks RA
,
Aglio LS
,
Gugino LD
,
Black PM
. Craniotomy under local anesthesia and monitored conscious sedation for the resection of tumors involving eloquent cortex. J Neurooncol. 2000 Sep;49(2):131–9. https://doi.org/10.1023/A:1026577518902
40.
Chowdhury T
,
Singh GP
,
Zeiler FA
,
Hailu A
,
Loewen H
,
Schaller B
, et al.
Anesthesia for Awake Craniotomy for Brain Tumors in an Intraoperative MRI Suite: challenges and Evidence. Front Oncol. 2018 Nov;8:519. https://doi.org/10.3389/fonc.2018.00519
41.
Duffau H
. Awake surgery for nonlanguage mapping. Neurosurgery. 2010 Mar;66(3):523–8. https://doi.org/10.1227/01.NEU.0000364996.97762.73
42.
Fontaine D
,
Almairac F
,
Santucci S
,
Fernandez C
,
Dallel R
,
Pallud J
, et al.
Dural and pial pain-sensitive structures in humans: new inputs from awake craniotomies. Brain. 2018 Apr;141(4):1040–8. https://doi.org/10.1093/brain/awy005
43.
Sartorius CJ
,
Wright G
. Intraoperative brain mapping in a community setting—technical considerations. Surg Neurol. 1997 Apr;47(4):380–8. https://doi.org/10.1016/S0090-3019(96)00340-0
44.
Sartorius CJ
,
Berger MS
. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer's lactate to the cortex. Technical note. J Neurosurg. 1998 Feb;88(2):349–51. https://doi.org/10.3171/jns.1998.88.2.0349
45.
Rosenow F
,
Lüders H
. Presurgical evaluation of epilepsy. Brain. 2001 Sep;124(Pt 9):1683–700. https://doi.org/10.1093/brain/124.9.1683
46.
Thorsteinsdottir J
,
Vollmar C
,
Tonn JC
,
Kreth FW
,
Noachtar S
,
Peraud A
. Outcome after individualized stereoelectroencephalography (sEEG) implantation and navigated resection in patients with lesional and non-lesional focal epilepsy. J Neurol. 2019 Apr;266(4):910–20. https://doi.org/10.1007/s00415-019-09213-3
47.
Luders HO
,
Najm I
,
Nair D
,
Widdess-Walsh P
,
Bingman W
. The epileptogenic zone: general principles. Epileptic disorders : international epilepsy journal with videotape. 2006;8 Suppl 2:S1-9.
48.
Abel TJ
,
Woodroffe RW
,
Nourski KV
,
Moritani T
,
Capizzano AA
,
Kirby P
, et al.
Role of the temporal pole in temporal lobe epilepsy seizure networks: an intracranial electrode investigation. J Neurosurg. 2018 Jul;129(1):165–73. https://doi.org/10.3171/2017.3.JNS162821




