Burden of disease in patients hospitalised with COVID-19 during the first and second pandemic wave in Switzerland: a nationwide cohort study
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40068
Claudia
Gregorianoa, Kris
Rafaiszab, Philipp
Schuetzab, Beat
Muellerab, Christoph A.
Fuxac, Anna
Conenac, Alexander
Kutzad
a Medical University Department, Division of General
Internal and Emergency Medicine, Cantonal Hospital Aarau, Aarau, Switzerland
b Department
of Clinical Research, University Hospital Basel, University of Basel, Basel,
Switzerland
cDepartment of Infectious Diseases and Infection Prevention, Cantonal Hospital Aarau,
Aarau, Switzerland
d Division
of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham
and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA
*
Equally contributing first authors
Summary
AIM OF THE STUDY: The first and second waves of
the COVID-19 pandemic led to a tremendous burden of disease and influenced
several policy directives, prevention and treatment strategies as well as lifestyle
and social behaviours. We aimed to describe trends of hospitalisations with
COVID-19 and hospital-associated outcomes in these patients during the first
two pandemic waves in Switzerland.
METHODS: In this nationwide retrospective cohort
study, we used in-hospital claims data of patients hospitalised with COVID-19
in Switzerland between January 1st and December 31st, 2020. First, stratified
by wave (first wave: January to May, second wave: June to December), we
estimated incidence rates (IR) and rate differences (RD) per 10,000
person-years of COVID-19-related hospitalisations across different age groups
(0–9, 10–19, 20–49, 50–69, and ≥70 years). IR was calculated by counting the
number of COVID-19 hospitalisations for each patient age stratum paired with the
number of persons living in Switzerland during the specific wave period. Second,
adjusted odds ratios (aOR) of outcomes among COVID-19 hospitalisations were
calculated to assess the association between COVID-19 wave and outcomes,
adjusted for potential confounders.
RESULTS: Of 36,517 hospitalisations with
COVID-19, 8,862 (24.3%) were identified during the first and 27,655 (75.7%)
during the second wave. IR for hospitalisations with COVID-19 was highest
during the second wave and among patients above 50 years (50–69 years: first
wave: 31.49 per 10,000 person-years; second wave: 62.81 per 10,000 person-years;
RD 31.32 [95% confidence interval [CI]: 29.56 to 33.08] per 10,000 person-years;
IRR 1.99 [95% CI: 1.91 to 2.08]; ≥70 years: first wave: 88.59 per 10,000
person-years; second wave: 228.41 per 10,000 person-years; RD 139.83 [95% CI: 135.42
to 144.23] per 10,000 person-years; IRR 2.58 [95% CI: 2.49 to 2.67]). While
there was no difference in hospital readmission, when compared with the first
wave, patients hospitalised during the second wave had a lower probability of death
(aOR 0.88 [95% CI: 0.81 to 0.95], ARDS (aOR 0.56 [95% CI: 0.51 to 0.61]), ICU
admission (aOR 0.66 [95% CI: 0.61 to 0.70]), and need for ECMO (aOR 0.60 [95%
CI: 0.38 to 0.92]). LOS was –16.1 % (95% CI: –17.8 to –14.2) shorter during
the second wave.
CONCLUSION: In this nationwide cohort study,
rates of hospitalisations with COVID-19 were highest among adults older than 50
years and during the second wave. Except for hospital readmission, the
likelihood of adverse outcomes was lower during the second pandemic wave, which
may be explained by advances in the understanding of the disease and improved treatment
options.
Introduction
In 2020, Switzerland faced two waves of the
severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic in spring
and winter, respectively. At the beginning of the first wave, many European
countries reached their capacity limits of acute and/or intensive care beds for
COVID-19 affected patients requiring inpatient care [1]. Therefore, hospitals
throughout Europe were forced to restore their capacity by postponing elective
treatments and non-emergency surgeries [2, 3]. To address the threat, Swiss authorities
introduced public health demands and restrictions to limit the spread of the
new virus. While during the first wave, policy interventions included
non-pharmacological mitigation measures such as national closures of borders,
schools, non-essential stores and businesses with a nationwide lockdown mid of March
2020, [4] during the second wave, the main non-pharmacological measures
included social distancing, increased testing, and restricted mobility [5, 6].
With cumulating evidence during the second
wave, the antiviral remdesivir was prescribed more frequently [7–9]. In
addition, dexamethasone was prescribed to most hospitalised patients needing
oxygen therapy during the second wave [10]. Moreover, different strategies were
applied regarding the hospitalisation of infected people. While, during the
first wave, most infected people were hospitalised, even with only minor
symptoms, this was no longer the case during the second wave, where criteria
for hospitalisation were far more restrictive and based on clinical parameters [11].
Regardless, given the many infected people, the absolute number of hospitalisations
during the second wave were higher.
As both the
number of hospitalisations with COVID-19 and treatment options varied substantially
between the first and second wave [12], we sought to evaluate epidemiological trends
of COVID-19 related hospitalisations and corresponding in-hospital outcomes across
different age groups using clinical routine data from Switzerland.
Methods
Study design
This
analysis was conducted using a nationwide cohort of patients hospitalised with COVID-19
in Switzerland between January 1st and December 31st
2020. Hospitalisation data was provided by the Swiss Federal Statistical Office
(FSO, Neuchâtel, Switzerland), based on a nationwide compulsory full census of
Swiss hospitals. The dataset includes all Swiss inpatient discharge records
from general hospitals for acute somatic care. Individual-level data on patient
demographics, healthcare utilisation, hospital typology, medical diagnoses, clinical
procedures, and in-hospital patient outcomes were provided. A multi-step anonymisation
procedure ensured patient confidentiality, and an unique patient identifier was
used to ascertain rehospitalisations. Medical diagnoses were coded using the
International Classification of Disease version 10, German Modification
(ICD-10-GM) codes. Open-source census data from the FSO on the Swiss population
size, stratified by age and year, and data from the Swiss Federal Office of
Public Health (FOPH) on the number of positive tests for SARS-CoV-2, stratified
by age and period were obtained to calculate population-based incidence rates (IR)
of hospitalisation with COVID-19. The institutional review board of
Northwestern and Central Switzerland (EKNZ) waived the need for an ethical authorisation
due to the use of exclusively anonymised data (EKNZ Project-ID:
Req-2021-01397). This study adheres to the “Strengthening The Reporting of Observational
Studies in Epidemiology (STROBE)” statement [13].
Case ascertainment and study variables
For this
analysis, we included all hospitalisations that were treated with or for COVID-19.
Hospitalisations in special clinics (psychiatric clinics, rehabilitation
clinics, and other special clinics) were excluded. To identify hospitalisations
with COVID-19, we used the following International Statistical Classification
of Diseases and Related Health Problems, German Modification (ICD-10-GM) discharge
codes at any position: U07.1 and U07.2. Details on all ICD-10-GM codes and
Swiss operation classification (CHOP) codes used for the analysis are summarised
in Table S1-S3 in the appendix. Comorbidities were measured using the
Elixhauser Comorbidity Index [14], and frailty was measured using the Hospital
Frailty Score [15].
Outcomes
The
primary outcome was the IR of hospitalisation with COVID-19 per 10,000
person-years (PY) with corresponding 95% confidence intervals (CIs) during the
first and second waves across the age spectrum. Secondary outcomes comprised of
the occurrence of the following in-hospital outcomes during the first and second
wave: all-cause in-hospital mortality, acute respiratory distress syndrome
(ARDS), length of hospital stay (LOS), intensive care unit (ICU) admission,
length of ICU stay, mechanical ventilation, duration of mechanical ventilation,
extracorporeal membrane oxygenation (ECMO), and 30-day all-cause hospital
readmission. Hospitalisation with COVID-19 and ARDS was defined using ICD-10-GM
codes, and the need for ECMO was defined using CHOP codes (table S1-S3 in the appendix).
The remaining outcomes were applicable in the dataset provided by the FSO. Analyses
of hospital outcomes were stratified by different age groups (0–9, 10–19, 20–49,
50–69, and ≥70 years).Out-of-hospital mortality data was not
available in the dataset and could not be linked to the national death
registry.
Statistical analysis
Descriptive statistic was calculated for
patient demographics, including age, sex, nationality, and insurance status.
All baseline characteristics are expressed as mean (standard deviation [SD]),
median (interquartile range [IQR]), or frequency (%). Graphical illustration of
hospitalisation IR over age spectrum was performed using locally estimated
scatterplot smoothing (LOESS). Stratified by waves (first wave: January to May,
second wave: June to December), we estimated IR per 10,000 PY with 95% CI, rate
differences (RD), and incidence rate ratios (IRR). IR was calculated, allowing
multiple hospitalisations for a single person (more than 95% of the patients
were hospitalised only once) [16]. These estimates were calculated as the
number of individuals with a COVID-19 hospitalisation divided by the sum of “person-time”
population at risk in Switzerland, represented by the population size
multiplied by the 5-month follow-up during the first wave and by the 7-month
follow-up, according to age. To assess the IR of hospitalisation among the
overall Swiss population, the denominator was the standard population in 2020
during the first or second waves, respectively. We used the number of people living in
Switzerland at the end of 2020 as a surrogate. This information is publicly
available and published by the FSO [17]. We aggregated COVID-19 hospitalisations
per year of patient age. In detail, we counted the number of COVID-19 hospitalisations
for each patient age stratum paired with the published number of persons during
the respective wave in Switzerland. Thus, we assumed 5/12 of one person-year for every resident during the first wave and 7/12 of one person-year for every resident during
the second wave, ignoring people dying or moving in and out of the country. IR of hospitalisation among those who had a proven SARS-CoV-2 infection
were calculated using the overall positively tested population in the
denominator. These analyses were performed within the above-mentioned age
groups, and the first wave was denoted as a reference.
To explore differences in binary hospital
outcomes between the waves and by age groups, we estimated adjusted odds ratios
(aOR) and corresponding 95% CIs using a multivariable logistic regression
model. For continuous right-skewed outcomes, we assessed changes in percentage
and corresponding 95% CIs using a multiple linear generalised log-gamma
regression model. All models were adjusted for age, sex and Elixhauser
comorbidity index. The selection of covariates was based on the research
question at hand and on knowledge such as what was important to guide hospitalisation
criteria during the waves in Switzerland.
We evaluated for heterogeneity in OR
estimates across age groups using the Wald test for homogeneity. We performed a
risk factor analysis for mortality using univariable and multivariable analyses.
All p-values are two-sided and have not been adjusted for multiple testing.
Results were considered statistically significant at p <0.05. Statistical
analyses were performed with STATA 15.1 (STATA Corp., College Station, TX,
USA).
Results
Characteristics of the cohort
From January 1st to December 31st
2020, we identified 36,517
hospitalisations with COVID-19, 8862 (24.3%) during
the first and 27,655 (75.7%) during the second wave. Baseline characteristics
of the eligible study population stratified by age groups are shown in table 1.
Baseline characteristics stratified by waves are summarised in table S4 in the appendix.
Overall, the median age was 72 years (IQR, 58 to 82), 57.3% were male, 74.8% were
Swiss residents, and 13.8% had supplementary health care insurance. The
majority of hospitalisations was observed in patients aged older than 45 years.
Among those, we observed an overall high burden of comorbidities, with similar
distribution during the first and second waves.
Table 1Baseline characteristics stratified by age
groups.
| |
<10 years
|
10–19 years
|
20–49 years
|
50–69 years
|
≥70 years
|
| Hospitalisations, n |
297 |
275 |
4,338 |
11,278 |
20,329 |
| Wave, n (%)
|
First wave |
73 (24.6) |
72 (26.2) |
1333 (30.7) |
2974 (26.4) |
4410 (21.7) |
| Second wave |
224 (75.4) |
203 (73.8) |
3005 (69.3) |
8304 (73.6) |
15,919 (78.3) |
| Demographics |
Age, median (IQR)
[years] |
0 (0, 2) |
16 (14, 18) |
40 (32, 46) |
61 (56, 65) |
80 (75, 86) |
| Male sex, n (%) |
161 (54.2) |
133 (48.4) |
2321 (53.5) |
7244 (64.2) |
11,052 (54.4) |
| Swiss nationality,
n (%) |
181 (60.9) |
178 (64.7) |
2277 (52.5) |
7622 (67.6) |
17,058 (83.9) |
| Supplementary
insurance, n (%) |
20 (6.7) |
32 (11.6) |
308 (7.1) |
1248 (11.1) |
3432 (16.9) |
| Admission data |
Emergency
admission, n (%) |
215 (72.4) |
221 (80.4) |
3692 (85.1) |
9831 (87.2) |
16,576 (81.5) |
| Admission from
home, n (%) |
277 (93.3) |
246 (89.5) |
3857 (88.9) |
9617 (85.3) |
14,675 (72.2) |
| Admission to tertiary
care hospital: university hospital, n (%) |
116 (39.1) |
122 (44.4) |
1472 (33.9) |
2803 (24.9) |
4507 (22.2) |
| Admission to tertiary
care hospital: non-university hospital, n (%) |
168 (56.6) |
119 (43.3) |
2190 (50.5) |
6338 (56.2) |
11,657 (57.3) |
| Admission to secondary
care hospital, n (%) |
13 (4.4) |
34 (12.4) |
676 (15.6) |
2137 (18.9) |
4165 (20.5) |
| Comorbidities, n
(%) |
Hypertension |
2 (0.7) |
4 (1.5) |
444 (10.2) |
4596 (40.8) |
12,481 (61.4) |
| Dyslipidemia |
0 (0.0) |
0 (0.0) |
128 (3.0) |
1731 (15.3) |
4119 (20.3) |
| Obesity (BMI ≥ 30.0
kg/m2) |
2 (0.7) |
6 (2.2) |
197 (4.5) |
612 (5.4) |
559 (2.7) |
| Coronary artery
disease |
2 (0.7) |
1 (0.4) |
63 (1.5) |
1220 (10.8) |
4336 (21.3) |
| Atrial fibrillation |
0 (0.0) |
1 (0.4) |
31 (0.7) |
820 (7.3) |
5269 (25.9) |
| Congestive heart
failure |
6 (2.0) |
9 (3.3) |
64 (1.5) |
476 (4.2) |
3094 (15.2) |
| Peripheral arterial
disease |
0 (0.0) |
0 (0.0) |
5 (0.1) |
181 (1.6) |
1012 (5.0) |
| Cerebrovascular
disease |
1 (0.3) |
2 (0.7) |
43 (1.0) |
357 (3.2) |
1233 (6.1) |
| Chronic obstructive
pulmonary disease |
0 (0.0) |
0 (0.0) |
17 (0.4) |
582 (5.2) |
1889 (9.3) |
| Bronchial asthma |
9 (3.0) |
12 (4.4) |
247 (5.7) |
557 (4.9) |
618 (3.0) |
| Obstructive sleep
apnoea syndrome |
1 (0.3) |
1 (0.4) |
95 (2.2) |
669 (5.9) |
889 (4.4) |
| Chronic kidney disease
stage 3 & 4 |
0 (0.0) |
1 (0.4) |
36 (0.8) |
458 (4.1) |
4066 (20.0) |
| Chronic kidney disease
stage 5 & hemodialysis |
1 (0.3) |
1 (0.4) |
42 (1.0) |
294 (2.6) |
495 (2.4) |
| Solid organ
transplant recipient |
1 (0.3) |
4 (1.5) |
64 (1.5) |
199 (1.8) |
84 (0.4) |
| Solid tumour |
5 (1.7) |
3 (1.1) |
88 (2.0) |
560 (5.0) |
1296 (6.4) |
| Liver disease,
including cirrhosis |
2 (0.7) |
6 (2.2) |
202 (4.7) |
693 (6.1) |
660 (3.2) |
| Diabetes mellitus
type 2 |
0 (0.0) |
1 (0.4) |
231 (5.3) |
2584 (22.9) |
5172 (25.4) |
| Diabetes mellitus
type 1 |
1 (0.3) |
11 (4.0) |
27 (0.6) |
57 (0.5) |
45 (0.2) |
| Haematological
malignancy |
8 (2.7) |
7 (2.5) |
60 (1.4) |
216 (1.9) |
367 (1.8) |
| Rheumatoid
arthritis |
0 (0.0) |
1 (0.4) |
22 (0.5) |
131 (1.2) |
324 (1.6) |
| Human
immunodeficiency virus infection |
0 (0.0) |
0 (0.0) |
31 (0.7) |
78 (0.7) |
32 (0.2) |
| Elixhauser
comorbidity index, median (IQR) |
0 (0, 1) |
0 (0, 1) |
1 (0, 2) |
2 (1, 3) |
3 (2, 4) |
| Hospital frailty score,
n (%) |
<5 points |
275 (92.6) |
249 (90.5) |
3870 (89.2) |
8390 (74.4) |
9647 (47.5) |
| 5–15 points |
21 (7.1) |
26 (9.5) |
445 (10.3) |
2642 (23.4) |
8987 (44.2) |
| >15 points |
1 (0.3) |
0 (0.0) |
23 (0.5) |
246 (2.2) |
1695 (8.3) |
| Outcomes |
In-hospital
mortality, n (%) |
2 (0.7) |
0 (0.0) |
39 (0.9) |
526 (4.7) |
3725 (18.3) |
| ICU admission, n
(%) |
23 (7.7) |
29 (10.5) |
479 (11.0) |
2012 (17.8) |
2080 (10.2) |
| ICU LOS, median
(IQR) [days] |
3.5 (1.7, 7.6) |
2.1 (0.8, 5.8) |
3.8 (1.5, 10.5) |
6.9 (2.5, 15.7) |
4.9 (1.6, 12.6) |
| Need for mechanical
ventilation, n (%) |
14 (4.7) |
11 (4.0) |
282 (6.5) |
1484 (13.2) |
1430 (7.0) |
| Duration of mechanical
ventilation, median (IQR) [days] |
2.2 (1.0, 7.5) |
2.0 (1.0, 6.3) |
7.0 (2.7, 12.3) |
8.7 (3.7, 16.0) |
6.7 (2.0, 14.3) |
| 30-days rehospitalisation,
n (%) |
17 (5.7) |
13 (4.7) |
203 (4.7) |
485 (4.3) |
1049 (5.2) |
Hospitalisation rates of patients with COVID-19
While IR of hospitalisations during the
first and second waves were generally low in children and adolescents, they
increased at around the age of 40 years, with a peak in older patients during
both waves (figure 1a).

Figure 1a Age-dependent
incidence rates for hospitalisations for COVID-19 among the overall Swiss population
per 10,000 person-years during the first wave (dark blue) and second wave
(light blue), using locally estimated scatterplot smoothing (LOESS).
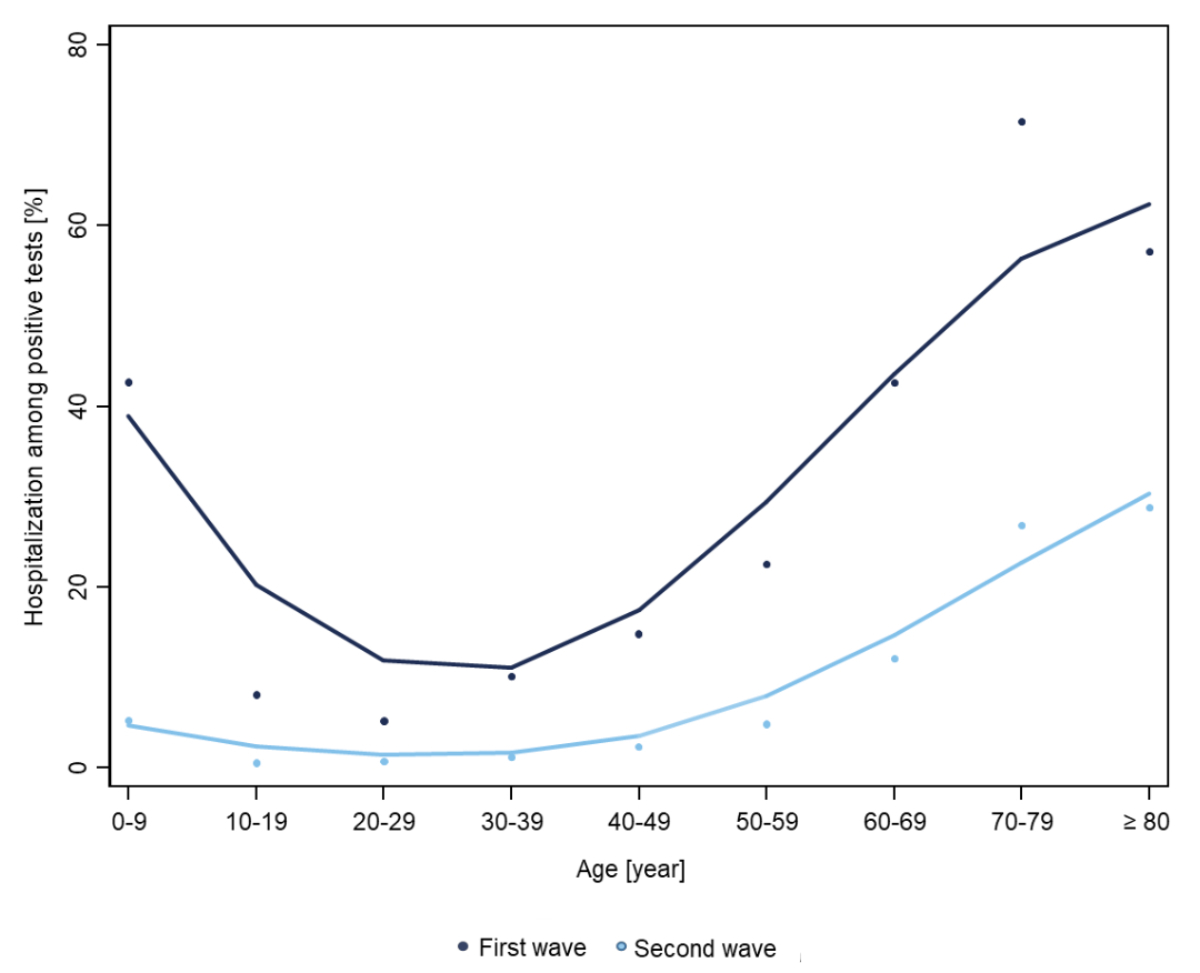
Figure 1b Age-dependent
incidence rates for hospitalisations for COVID-19 among the overall SARS-CoV-2
positive tested population per 10,000 person-years during the first wave (dark
blue) and second wave (light blue), using locally estimated scatterplot
smoothing (LOESS).
Considering the overall standard population in the
denominator, IR were higher during the second wave compared with the first wave.
COVID-19 hospitalisation rates were highest in patients above 50 years (50–69
years: first wave: 31.49 per 10,000 person-years; second wave: 62.81 per 10,000 person-years; RD 31.32 [95% CI: 29.56 to 33.08] per 10,000 person-years;
≥70 years: first wave: 88.59 per 10,000 person-years; second wave: 228.41 per 10,000 person-years; RD 139.83 [95% CI: 135.42 to 144.23] per 10,000 person-years)
with a strong
predominance during the second wave (table 2).
Table 2Differences in
absolute and relative risk between the first and second COVID-19 waves across
age groups.
|
|
Age 0–9
years
|
Age 10–19
years
|
Age 20–49
years
|
Age 50–69
|
Age ≥70
|
p of
interaction
|
| First
wave |
Second
wave |
First
wave |
Second
wave |
First
wave |
Second
wave |
First
wave |
Second
wave |
First
wave |
Second
wave |
|
| Hospitalisations,
n |
73 |
224 |
72 |
203 |
1333 |
3005 |
2974 |
8304 |
4410 |
15,919 |
|
| Person-years |
365,335 |
511,468 |
353,958 |
495,541 |
1,451,184 |
2,031,657 |
944,334 |
1,322,068 |
497,815 |
696,941 |
|
| Incidence
rate per 10,000 PY |
1.99 |
4.38 |
2.03 |
4.10 |
9.19 |
14.79 |
31.49 |
62.81 |
88.59 |
228.41 |
|
| Incidence
rate difference (95% CI) |
Ref. |
2.38 (1.65
to 3.12) |
Ref. |
2.06 (1.32
to 2.80) |
Ref. |
5.61 (4.88
to 6.33) |
Ref. |
31.32 (29.56
to 33.08) |
Ref. |
139.83 (135.42
to 144.23) |
p <0.001 |
| Incidence
rate ratio (95% CI) |
Ref. |
2.19 (1.65
to 3.12) |
Ref. |
2.01 (1.53
to 2.67) |
Ref. |
1.61 (1.51
to 1.72) |
Ref. |
1.99 (1.91
to 2.08) |
Ref. |
2.58 (2.49
to 2.67) |
p <0.001 |
Illustration of hospitalisation IR
across the age spectrum among those who were tested positive for SARS-CoV-2 showed
different patterns for the first and second wave, with a first peak of
hospitalisations among children aged 0 to 9 year and a second peak with a
continuous increase of hospitalisations among adults beyond age 50 (figure 1b).
While rates of hospitalisation among the overall Swiss population were higher
during the second wave (figure 1a), the proportion of hospitalised people among
positively tested individuals was higher during the first wave (figure 1b).
In-hospital outcomes between the first
and second wave
Among the overall population, 1028 (11.6%)
died in hospital during the first wave and 3264 (11.8%) during the second wave,
corresponding to lower odds of in-hospital mortality during the second wave
(aOR 0.88 [95% CI: 0.81 to 0.95]). Similarly, compared with the first wave, we
observed lower numbers of patients with ARDS (1056 [11.9%] vs. 1945 [7.0%], aOR
0.56 [95% CI: 0.51 to 0.61]), ICU admission (1463 [16.5%] vs. 3160 [11.4%], aOR
0.66 [95% CI: 0.61 to 0.70]), in need for mechanical ventilation (1101 [12.4%]
vs. 2120 [7.7%], aOR 0.67 [95% CI: 0.58 to 0.78]), and in need for ECMO (38 [0.4%]
vs. 46 [0.2%], aOR 0.60 [95% CI: 0.38 to 0.92]) during the second wave. The odds
for 30-day all-cause hospital readmission remained similar during the first and
second wave (427 [4.8%] vs. 1340 [4.9%], aOR of 0.99 [95% CI: 0.88 to 1.10])
(figure 2). Compared with the first wave, LOS (7.4 [SD 2.7] vs. 6.7 [SD 2.5]) and
ICU LOS (6.2 [SD 4.0] vs. 4.1 [SD 3.9]) were shorter during the second wave with
a reduction of –16.1% (95% CI: –17.8 to –14.2) and –32.7% (95% CI: –37.2 to –27.9),
respectively (figure 3). There was no evidence for effect modification by age
for all hospital outcomes of interest (p of interaction >0.05).
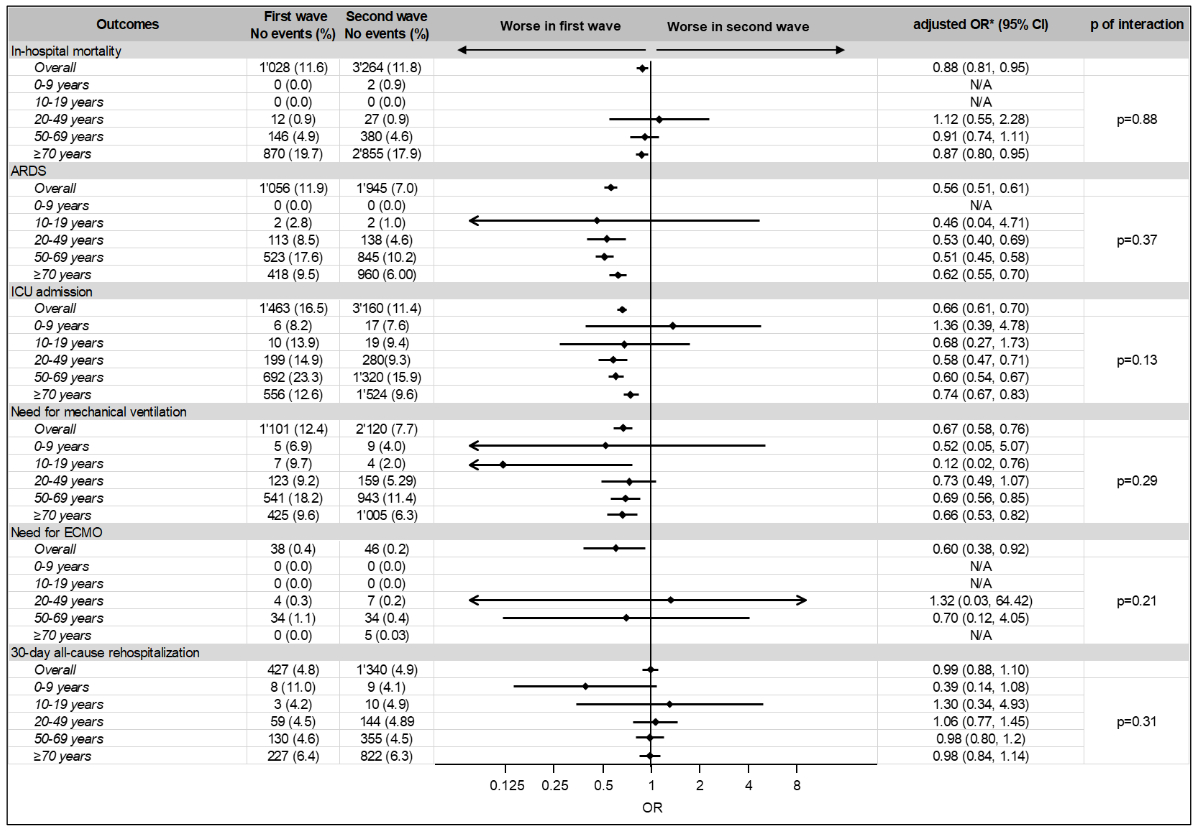
Figure 2 Odds ratios and 95%
confidence intervals for adverse outcomes in prespecified age subgroups. First wave was chosen as a reference. There was no
evidence for effect modification by age for all hospital outcomes of interest
(p of interaction >0.05).
* adjusted for age, sex and Elixhauser comorbidity Index
ARDS: acute respiratory distress
syndrome; CI: confidence interval; ECMO: extracorporeal membrane oxygenation;
ICU: intensive care unit; N/A: not applicable; no: number; OR: odds ratio
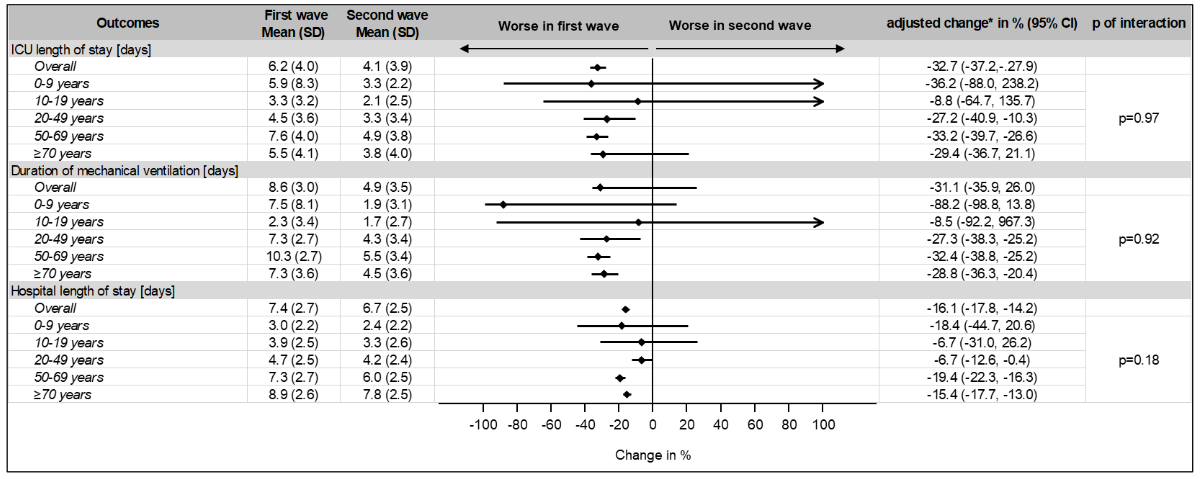
Figure 3 Changes in frequency
for adverse outcomes in prespecified age subgroups.
First wave was chosen as a reference. There was no
evidence for effect modification by age for all hospital outcomes of interest
(p of interaction >0.05).
* adjusted for age, sex and Elixhauser comorbidity Index
CI: confidence interval; ICU: intensive
care unit; SD: standard deviation
Predictors for mortality
Table 3 and 4 show baseline risk factors for in-hospital mortality during
the first and second waves. Among the adjusted regression analysis, the main
risk factors were the prevalence of chronic kidney disease stage 5 and/or
hemodialysis (first wave: aOR 3.26 [95% CI: 2.47 to 4.31), p <0.001];
second wave: aOR 4.02 [95% CI: 3.31 to 4.89], p <0.001) and haematological
malignancy (first wave: aOR 3.03 [95% CI: 1.97 to 4.66)], p <0.001; second
wave: aOR 2.39 [95% CI: 1.92 to 2.98], p <0.001).
Table 3Risk factor
analysis for in-hospital mortality during the first pandemic wave.
|
Factor
|
Survivors
(n = 7,834)
|
Non-survivors
(n = 1,028)
|
Unadjusted
OR (95% CI), p-value
|
Adjusted
OR* (95% CI), p-value
|
| Demographics |
Age,
median (IQR) [years] |
67
(54,78) |
81
(73,86) |
1.07
(1.06, 1.08), p <0.001
|
1.07 (1.06, 1.08), p
<0.001
|
| Male
sex, n (%) |
4493
(57.4) |
700
(68.1) |
1.59
(1.38, 1.82), p <0.001
|
1.96 (1.69, 2.28), p
<0.001
|
| Swiss
nationality, n (%) |
5750
(73.4) |
836
(81.3) |
1.58
(1.34, 1.86), p <0.001
|
0.97 (0.81, 1.16),
p = 0.728 |
| Supplementary
insurance, n (%) |
952
(12.2) |
109
(10.6) |
0.86
(0.70, 1.06), p = 0.151 |
0.74 (0.60, 0.93), p
= 0.008
|
| Admission
data |
Emergency
admission, n (%) |
6410
(81.8) |
883
(85.9) |
1.35
(1.12, 1.63), p = 0.001
|
1.68 (1.38, 2.04), p
<0.001
|
| Admission
from home, n (%) |
5988
(76.4) |
724
(70.4) |
0.73
(0.64, 0.85), p <0.001
|
1.16 (1.00, 1.36),
p = 0.054 |
| Admission
to tertiary care hospital: University
hospital, n (%) |
2520
(32.2) |
287
(27.9) |
0.82
(0.71, 0.94), p = 0.006
|
0.84 (0.72, 0.98), p
= 0.027
|
| Admission
to tertiary care hospital: Non-university
hospital, n (%) |
3789
(48.5) |
562
(54.7) |
1.28
(1.12, 1.46), p <0.001
|
1.29 (1.12, 1.48), p
<0.001
|
| Admission
to secondary care hospital, n (%) |
1516
(19.4) |
179
(17.4) |
0.88
(0.74, 1.04), p = 0.137 |
0.84 (0.70, 1.00),
p = 0.055 |
| Comorbidities,
n (%) |
Hypertension,
n (%) |
3437
(43.9) |
579
(56.3) |
1.65
(1.45, 1.88), p <0.001
|
0.67 (0.57, 0.78), p
<0.001
|
| Dyslipidemia,
n (%) |
1256
(16.0) |
178
(17.3) |
1.10
(0.92, 1.30), p = 0.294 |
0.74 (0.61, 0.88), p
= 0.001
|
| Obesity
(BMI ≥30.0 kg/m2), n (%) |
241
(3.1) |
34
(3.3) |
1.08
(0.75, 1.55), p = 0.688 |
1.39 (0.94, 2.07),
p = 0.100 |
| Coronary
artery disease, n (%) |
971
(12.4) |
253
(24.6) |
2.31
(1.97, 2.70), p <0.001
|
1.21 (1.02, 1.43), p
= 0.030
|
| Atrial
fibrillation, n (%) |
1043
(13.3) |
296
(28.8) |
2.63
(2.27, 3.06), p <0.001
|
1.15 (0.97, 1.37),
p = 0.104 |
| Congestive
heart failure, n (%) |
604
(7.7) |
223
(21.7) |
3.32
(2.80, 3.93), p <0.001
|
1.47 (1.21, 1.80), p
<0.001
|
| Peripheral
arterial disease, n (%) |
210
(2.7) |
56
(5.4) |
2.09
(1.55, 2.83), p <0.001
|
0.89 (0.64, 1.23),
p = 0.488 |
| Cerebrovascular
disease, n (%) |
305
(3.9) |
81
(7.9) |
2.11
(1.64, 2.72), p <0.001
|
1.35 (1.03, 1.77), p
= 0.029
|
| Chronic
obstructive pulmonary disease, n (%) |
467
(6.0) |
114
(11.1) |
1.97
(1.59, 2.44), p <0.001
|
1.20 (0.95, 1.51),
p = 0.132 |
| Bronchial
asthma, n (%) |
379
(4.8) |
19
(1.8) |
0.37
(0.23, 0.59), p <0.001
|
0.52 (0.32, 0.85), p
= 0.008
|
| Obstructive
sleep apnoea syndrome, n (%) |
361
(4.6) |
68
(6.6) |
1.47
(1.12, 1.92), p = 0.005
|
1.37 (1.03, 1.83), p
= 0.029
|
| Chronic
kidney disease stage 3 & 4, n (%) |
649
(8.3) |
196
(19.1) |
2.61
(2.19, 3.11), p <0.001
|
1.02 (0.84, 1.24),
p = 0.851 |
| Chronic
kidney disease stage 5 & hemodialysis, n (%) |
212
(2.7) |
93
(9.0) |
3.58
(2.78, 4.61), p <0.001
|
3.26 (2.47, 4.31), p
<0.001
|
| Solid
organ transplant recipients, n (%) |
65
(0.8) |
8 (0.8) |
0.94
(0.45, 1.96), p = 0.864 |
1.42 (0.65, 3.13),
p = 0.389 |
| Solid
tumor, n (%) |
328
(4.2) |
97
(9.4) |
2.38
(1.88, 3.02), p <0.001
|
1.87 (1.44, 2.42), p
<0.001
|
| Liver
disease including cirrhosis, n (%) |
427
(5.5) |
85
(8.3) |
1.56
(1.23, 1.99), p <0.001
|
1.86 (1.43, 2.42), p
<0.001
|
| Diabetes
mellitus type 2, n (%) |
1447
(18.5) |
265
(25.8) |
1.53
(1.32, 1.78), p <0.001
|
0.97 (0.82, 1.15),
p = 0.758 |
| Diabetes
mellitus type 1, n (%) |
27
(0.3) |
4 (0.4) |
1.13
(0.39, 3.23), p = 0.821 |
1.75 (0.56, 5.44),
p = 0.335 |
| Haematological
malignancy, n (%) |
98
(1.3) |
34
(3.3) |
2.70
(1.82, 4.01), p <0.001
|
3.03 (1.97, 4.66), p
<0.001
|
| Rheumatoid
arthritis, n (%) |
81
(1.0) |
16
(1.6) |
1.51
(0.88, 2.60), p = 0.133 |
1.45 (0.82, 2.56),
p = 0.203 |
| Human
immunodeficiency virus infection, n (%) |
37
(0.5) |
2 (0.2) |
0.41
(0.10, 1.71), p = 0.221 |
0.67 (0.16, 2.91),
p = 0.597 |
| Elixhauser
comorbidity index, median (IQR) |
2 (1,
4) |
3 (2,
5) |
1.27
(1.24, 1.31), p <0.001
|
1.13 (1.10, 1.17), p
<0.001
|
| Hospital
frailty score |
<5 points, n (%) |
5061
(64.6) |
449
(43.7) |
0.42
(0.37, 0.48), p <0.001
|
0.92 (0.79, 1.08),
p = 0.304 |
| 5–15 points,
n (%) |
2377
(30.3) |
507
(49.3) |
2.23
(1.96, 2.55), p <0.001
|
1.22 (1.06, 1.41),
p = 0.006
|
| >15
points, n (%) |
396
(5.1) |
72
(7.0) |
1.41
(1.09, 1.83), p = 0.009
|
0.64 (0.49, 0.85), p
= 0.002
|
Table 4Risk factor
analysis for in-hospital mortality during the second pandemic wave.
|
Factor
|
Survivors
(n = 24,391)
|
Non-Survivors
(n = 3,264)
|
unadjusted
OR (95% CI),
p-value
|
adjusted
OR* (95% CI), p-value
|
| Demographics |
Age,
median (IQR) [years] |
71
(58,81) |
82 (75,
87) |
1.07
(1.06, 1.07), p <0.001
|
1.07 (1.06, 1.07), p
<0.001
|
| Male
sex, n (%) |
13,595
(55.7) |
2123
(65.0) |
1.48
(1.37, 1.59), p <0.001
|
1.79 (1.65, 1.94), p
<0.001
|
| Swiss
nationality, n (%) |
18,069
(74.1) |
2661
(81.5) |
1.54
(1.41, 1.69), p <0.001
|
0.94 (0.85, 1.04),
p = 0.238 |
| Supplementary
insurance, n (%) |
3448
(14.1) |
531
(16.3) |
1.18
(1.07, 1.30), p = 0.001
|
0.94 (0.85, 1.05),
p = 0.310 |
| Admission
data |
Emergency
admission, n (%) |
20,420
(83.7) |
2822
(86.5) |
1.24
(1.12, 1.38), p <0.001
|
1.48 (1.33, 1.66), p
<0.001
|
| Admission
from home, n (%) |
19,609
(80.4) |
2351
(72.0) |
0.63
(0.58, 0.68), p <0.001
|
0.91 (0.83, 0.99),
p = 0.031
|
| Admission
to tertiary care hospital |
|
|
|
|
| University
hospital, n (%) |
5555
(22.8) |
658
(20.2) |
0.86
(0.78, 0.94), p = 0.001
|
0.92 (0.84, 1.01),
p = 0.093 |
| Non-university
hospital, n (%) |
14,087
(57.8) |
2025
(62.0) |
1.20
(1.11, 1.29), p <0.001
|
1.16 (1.07, 1.25), p
<0.001
|
| Admission
to secondary care hospital, n (%) |
4749
(19.5) |
581
(17.8) |
0.90
(0.81, 0.99), p = 0.023
|
0.87 (0.79, 0.96), p
= 0.006
|
| Comorbidities,
n (%) |
Hypertension,
n (%) |
11,700
(48.0) |
1811 (55.5) |
1.35
(1.26, 1.46), p <0.001
|
0.58 (0.53, 0.63), p
<0.001
|
| Dyslipidemia,
n (%) |
4008
(16.4) |
536
(16.4) |
1.00
(0.91, 1.10), p = 0.998 |
0.69 (0.62, 0.76), p
<0.001
|
| Obesity
(BMI ≥ 30.0 kg/m2), n (%) |
968
(4.0) |
133
(4.1) |
1.03
(0.85, 1.24), p = 0.771 |
1.08 (0.89, 1.33),
p = 0.430 |
| Coronary
artery disease, n (%) |
3563
(14.6) |
835
(25.6) |
2.01
(1.84, 2.19), p <0.001
|
1.13 (1.03, 1.25), p
= 0.008
|
| Atrial
fibrillation, n (%) |
3703
(15.2) |
1079
(33.1) |
2.76
(2.54, 3.00), p <0.001
|
1.27 (1.16, 1.39), p
<0.001
|
| Congestive
heart failure, n (%) |
2050
(8.4) |
772
(23.7) |
3.38
(3.08, 3.70), p <0.001
|
1.63 (1.46, 1.81), p
<0.001
|
| Peripheral
arterial disease, n (%) |
752
(3.1) |
180
(5.5) |
1.83
(1.55, 2.17), p <0.001
|
0.90 (0.75, 1.07),
p = 0.224 |
| Cerebrovascular
disease, n (%) |
1015
(4.2) |
235
(7.2) |
1.79
(1.54, 2.07), p <0.001
|
1.11 (0.95, 1.30),
p = 0.184 |
| Chronic
obstructive pulmonary disease, n (%) |
1528
(6.3) |
379
(11.6) |
1.97
(1.74, 2.21), p <0.001
|
1.26 (1.11, 1.43), p
<0.001
|
| Bronchial
asthma, n (%) |
962
(3.9) |
83
(2.5) |
0.64
(0.51, 0.80), p <0.001
|
0.67 (0.53, 0.85), p
= 0.001
|
| Obstructive
sleep apnoea syndrome, n (%) |
1068
(4.4) |
158
(4.8) |
1.11
(0.94, 1.32), p = 0.229 |
0.98 (0.82, 1.18),
p = 0.870 |
| Chronic
kidney disease stage 3 & 4, n (%) |
2891
(11.9) |
825
(25.3) |
2.52
(2.30, 2.75), p <0.001
|
1.07 (0.97, 1.18),
p = 0.201 |
| Chronic
kidney disease stage 5 & hemodialysis, n (%) |
328
(1.3) |
200
(6.1) |
4.79
(4.00, 5.73), p <0.001
|
4.02 (3.31, 4.89), p
<0.001
|
| Solid
organ transplant recipients, n (%) |
252
(1.0) |
27
(0.8) |
0.80
(0.54, 1.19), p = 0.270 |
1.21 (0.80, 1.84),
p = 0.368 |
| Solid
tumor, n (%) |
1201
(4.9) |
326
(10.0) |
2.14
(1.88, 2.44), p <0.001
|
1.61 (1.40, 1.85), p
<0.001
|
| Liver
disease including cirrhosis, n (%) |
861
(3.5) |
190
(5.8) |
1.69
(1.44, 1.99), p <0.001
|
1.88 (1.57, 2.25), p
<0.001
|
| Diabetes
mellitus type 2, n (%) |
5366
(22.0) |
910
(27.9) |
1.37
(1.26, 1.49), p <0.001
|
0.95 (0.87, 1.05),
p = 0.321 |
| Diabetes
mellitus type 1, n (%) |
104
(0.4) |
6 (0.2) |
0.43
(0.19, 0.98), p = 0.045
|
0.61 (0.26, 1.43),
p = 0.253 |
| Haematological
malignancy, n (%) |
402
(1.6) |
124
(3.8) |
2.36
(1.92, 2.89), p <0.001
|
2.39 (1.92, 2.98), p
<0.001
|
| Rheumatoid
arthritis, n (%) |
324
(1.3) |
57
(1.7) |
1.32
(0.99, 1.75), p = 0.055 |
1.07 (0.80, 1.44),
p = 0.655 |
| Human immunodeficiency
virus infection, n (%) |
98
(0.4) |
4 (0.1) |
0.30
(0.11, 0.83), p = 0.020
|
0.56 (0.20, 1.56),
p = 0.271 |
| Elixhauser
comorbidity index, median (IQR) |
2 (1,
4) |
3 (2,
5) |
1.27
(1.25, 1.29), p <0.001
|
1.16 (1.14, 1.18), p
<0.001
|
| Hospital
frailty score
|
<5
points, n (%) |
15,645
(64.1) |
449
(43.7) |
0.36
(0.33, 0.39), p <0.001
|
0.70 (0.64, 0.76), p
<0.001
|
| 5–15
points, n (%) |
7551
(31.0) |
1686
(51.7) |
2.38
(2.21, 2.57), p <0.001
|
1.40 (1.29, 1.51),
p = 0.006
|
| >15
points, n (%) |
1195
(4.9) |
302
(9.3) |
1.98
(1.73, 2.26), p <0.001
|
0.97 (0.84, 1.12), p
= 0.678
|
Discussion
This nationwide cohort study of hospitalised
COVID-19 patients in Switzerland revealed several key findings: First, hospitalisation
rates in patients with COVID-19 were highest among adults older than 50 years
and more pronounced during the second wave. Second, while the hospitalisation rate was higher
among the overall Swiss population during the second wave, it was lower among
positively tested individuals during the same period. Third, except for readmission, the risk for hospital-associated adverse
outcomes was lower during the second wave, irrespective of patient age.
The highest hospitalisation rates among
the overall Swiss population were observed in patients aged 50 years and older,
both during the first and second wave. However, the peak of hospitalisations during
the second wave was much higher than the first wave. These results are
plausible since we observed significantly more infections during the second
wave, resulting in more hospitalisations. Although data must be interpreted
carefully, as hospitalisation and test criteria differed between the two waves,
we observed a lower proportion of hospitalised people among the overall
positively tested population during the second wave.
Improvement of patient management was a
further important key component which was achieved during the second wave. Overall,
the odds of adverse outcomes decreased during the second wave, except for hospital
readmission. The expected decrease of in-hospital adverse events during the
second wave is probably explained by the improved understanding of the treating
physicians and nursing staff about the management of COVID-19 patients [18]. Importantly,
the widespread administration of dexamethasone during the second wave may have contributed
to a stronger reduction in in-hospital mortality, as already shown by previous
studies [19]. Consistent with other studies [20–24], we observed a lower risk of
in-hospital mortality during the second wave (aOR 0.88 [95% CI: 0.81 to 0.95]).
While, as compared with findings from clinical trials, a relative risk
reduction of 12% may seem small, hospitalised patients during the second wave tended
to be older and more comorbid, both characteristics known to independently increase
the risk of COVID-19-related mortality [25–28].
Similarly, corticosteroids may also have
reduced the rate of progression to severe COVID-19 due to an attenuation of the
inflammation, resulting in lower rates of ARDS, admission to ICU, need for mechanical
ventilation and ECMO support as well as a 16% shorter LOS of 7.4 vs. 6.7 days [29].
In addition, the reduction in LOS during the second wave could also be due to improved
discharge processes and earlier transfer to rehabilitation facilities. This was
possible since many rehabilitation facilities were obligated to unburden acute
care hospitals by accepting still-infectious COVID-19 patients [30].
We did not observe a change in readmission
rates between the two waves. This can be explained by the fact that COVID-19 is
an acute disease and may not lead to readmissions per se, whereas the burden of comorbidities as a potential driver for
readmission tended to be higher during the second wave and may have diluted any
beneficial effect on hospital readmission. These data must be interpreted in
the context of the study design. As COVID-19 hospitalisations were identified
using ICD-10-GM codes used for billing purposes, thus, misclassification and
underreporting are possible, especially during the beginning of the pandemic when
no specific ICD-10 codes were available. In line, the
database provided by the FSO revealed larger numbers of hospitalisations as
compared with data from the CH-SUR database. Since algorithms based on the U07.1 code had high
sensitivity among hospitalised patients but at the expense of low specificity [31],
it is likely that the number of hospitalisation
with COVID-19 as provided by the FSO might be overestimated. However, it can
also not be excluded that some hospitalisations with COVID-19 in the FSO-database
were not lab-confirmed during the hospital stay but diagnosed based on an
out-of-hospital test or clinical features. Second,
unmeasurable confounding like smoking status, genetic susceptibility or home
medication must be considered, as the used dataset does not include any
information in this regard. Third, due to the study’s retrospective design, no
causal inference is possible. Fourth, we did not account for potential
within-patient/hospital clustering. However, we do not think that accounting for clustering
relevantly changes the conclusion of our manuscript, as the number of clusters
(hospitals, hospital admissions per patient) were comparable between the first
and second waves of the pandemic. Fifth, medical
treatments received during the hospitalisation for COVID-19 could not be analysed,
as this information is missing in the analysed dataset. However, there are
several strengths of note. This analysis was based on nationwide hospital care
data with high external validity, strong statistical power and high generalisability
across all regions in Switzerland. Moreover, this study provides insights into
different age groups that were not comprehensively addressed in earlier studies
in Switzerland.
Conclusion
In this nationwide cohort study, hospitalisation
rates of COVID-19 patients were highest among adults older than 50 years and
during the second wave. Except for readmission, the risk of hospital adverse
outcomes was decreased during the second wave, regardless of the patient`s age.
Data sharing statement
The data supporting this study’s findings
are available upon request from the Swiss Federal Statistical Office
(Neuchâtel, Switzerland). Restrictions apply to the availability of these data,
which were used under license for this study. Data are available as part of the
data on “Medizinische Statistik der Krankenhäuser” with the permission of the
Swiss Federal Statistical Office, Section Health Services and Population
Health.
Acknowledgement
We
thank the Swiss Federal Statistics Office (Neuchâtel, Switzerland) for the
acquisition and provision of data.
Claudia Gregoriano, PhD
Medical University Department of Medicine
Kantonsspital Aarau
Tellstrasse 25
CH-5001 Aarau
c.gregoriano[at]gmail.com
References
1.
Verelst F
,
Kuylen E
,
Beutels P
. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID-19) cases, March 2020. Euro Surveill. 2020 Apr;25(13):2000323. https://doi.org/10.2807/1560-7917.ES.2020.25.13.2000323
2.
Winkelmann J
,
Webb E
,
Williams GA
,
Hernández-Quevedo C
,
Maier CB
,
Panteli D
. European countries’ responses in ensuring sufficient physical infrastructure and workforce capacity during the first COVID-19 wave. Health Policy. 2022 May;126(5):362–72. https://doi.org/10.1016/j.healthpol.2021.06.015
3.
Webb E
,
Hernández-Quevedo C
,
Williams G
,
Scarpetti G
,
Reed S
,
Panteli D
. Providing health services effectively during the first wave of COVID-19: A cross-country comparison on planning services, managing cases, and maintaining essential services. Health Policy. 2022 May;126(5):382–90. https://doi.org/10.1016/j.healthpol.2021.04.016
4.
Zimmermann BM
,
Fiske A
,
McLennan S
,
Sierawska A
,
Hangel N
,
Buyx A
. Motivations and Limits for COVID-19 Policy Compliance in Germany and Switzerland. Int J Health Policy Manag. 2021 Apr;11(8):1342–53. https://doi.org/10.34172/ijhpm.2021.30
5.
Salathé M
,
Althaus CL
,
Neher R
,
Stringhini S
,
Hodcroft E
,
Fellay J
, et al.
COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020 Mar;150:w20225. https://doi.org/10.4414/smw.2020.20225
6.
Flaxman S
,
Mishra S
,
Gandy A
,
Unwin HJ
,
Mellan TA
,
Coupland H
, et al.; Imperial College COVID-19 Response Team
. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020 Aug;584(7820):257–61. https://doi.org/10.1038/s41586-020-2405-7
7.
Cao B
,
Wang Y
,
Wen D
,
Liu W
,
Wang J
,
Fan G
, et al.
A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020 May;382(19):1787–99. https://doi.org/10.1056/NEJMoa2001282
8.
Boulware DR
,
Pullen MF
,
Bangdiwala AS
,
Pastick KA
,
Lofgren SM
,
Okafor EC
, et al.
A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020 Aug;383(6):517–25. https://doi.org/10.1056/NEJMoa2016638
9.
Wang Y
,
Zhang D
,
Du G
,
Du R
,
Zhao J
,
Jin Y
, et al.
Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May;395(10236):1569–78. https://doi.org/10.1016/S0140-6736(20)31022-9
10.
Horby P
,
Lim WS
,
Emberson JR
,
Mafham M
,
Bell JL
,
Linsell L
, et al.; RECOVERY Collaborative Group
. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021 Feb;384(8):693–704. https://doi.org/10.1056/NEJMoa2021436
11.
Biswas M
,
Rahaman S
,
Biswas TK
,
Haque Z
,
Ibrahim B
. Association of Sex, Age, and Comorbidities with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Intervirology. 2020 Dec;1–12.
12.
Bundesamt für Statistik
. Auswirkungen der Covid-19-Pandemie auf die Gesundheitsversorgung im Jahr 2020. 2021.
13.
von Elm E
,
Altman DG
,
Egger M
,
Pocock SJ
,
Gøtzsche PC
,
Vandenbroucke JP
; STROBE Initiative
. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007 Oct;335(7624):806–8. https://doi.org/10.1136/bmj.39335.541782.AD
14.
Elixhauser A
,
Steiner C
,
Harris DR
,
Coffey RM
. Comorbidity measures for use with administrative data. Med Care. 1998 Jan;36(1):8–27. https://doi.org/10.1097/00005650-199801000-00004
15.
Gilbert T
,
Neuburger J
,
Kraindler J
,
Keeble E
,
Smith P
,
Ariti C
, et al.
Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018 May;391(10132):1775–82. https://doi.org/10.1016/S0140-6736(18)30668-8
16.
Bundesamt für Gesundheit (BAG)
. Coronavirus: Monitoring [Available from: https://www.bag.admin.ch/bag/de/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/situation-schweiz-und-international/monitoring.html#19385953 ], last access: 23.12.2022.
17.
Bundesamt für Statistik
. Permanent resident population by age, sex and category of citizenship, 2010-2021 2010-2021 [Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/stand-entwicklung/alter-zivilstand-staatsangehoerigkeit.assetdetail.23064709.html ], last access: 23.12.2022.
18.
World Health Organization (WHO)
. Clinical management of COVID-19: Living guideline, 23 June 2022 2022 [Available from: WHO-2019-nCoV-Clinical-2022.1-eng.pdf.], last access, 23.12.2022.
19.
van Paassen J
,
Vos JS
,
Hoekstra EM
,
Neumann KM
,
Boot PC
,
Arbous SM
. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020 Dec;24(1):696. https://doi.org/10.1186/s13054-020-03400-9
20.
Oladunjoye O
,
Gallagher M
,
Wasser T
,
Oladunjoye A
,
Paladugu S
,
Donato A
. Mortality due to COVID-19 infection: A comparison of first and second waves. J Community Hosp Intern Med Perspect. 2021 Nov;11(6):747–52. https://doi.org/10.1080/20009666.2021.1978154
21.
Saito S
,
Asai Y
,
Matsunaga N
,
Hayakawa K
,
Terada M
,
Ohtsu H
, et al.
First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics. J Infect. 2021 Apr;82(4):84–123. https://doi.org/10.1016/j.jinf.2020.10.033
22.
Di Fusco M
,
Shea KM
,
Lin J
,
Nguyen JL
,
Angulo FJ
,
Benigno M
, et al.
Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–17. https://doi.org/10.1080/13696998.2021.1886109
23.
Mallow PJ
,
Belk KW
,
Topmiller M
,
Hooker EA
. Outcomes of Hospitalized COVID-19 Patients by Risk Factors: Results from a United States Hospital Claims Database. J Health Econ Outcomes Res. 2020 Sep;7(2):165–74. https://doi.org/10.36469/jheor.2020.17331
24.
Iftimie S
,
López-Azcona AF
,
Vallverdú I
,
Hernández-Flix S
,
de Febrer G
,
Parra S
, et al.
First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021 Mar;16(3):e0248029. https://doi.org/10.1371/journal.pone.0248029
25.
Hothorn T
,
Bopp M
,
Günthard H
,
Keiser O
,
Roelens M
,
Weibull CE
, et al.
Assessing relative COVID-19 mortality: a Swiss population-based study. BMJ Open. 2021 Mar;11(3):e042387. https://doi.org/10.1136/bmjopen-2020-042387
26.
Williamson EJ
,
Walker AJ
,
Bhaskaran K
,
Bacon S
,
Bates C
,
Morton CE
, et al.
Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020 Aug;584(7821):430–6. https://doi.org/10.1038/s41586-020-2521-4
27.
Bravi F
,
Flacco ME
,
Carradori T
,
Volta CA
,
Cosenza G
,
De Togni A
, et al.
Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS One. 2020 Jun;15(6):e0235248. https://doi.org/10.1371/journal.pone.0235248
28.
Maximiano Sousa F
,
Roelens M
,
Fricker B
,
Thiabaud A
,
Iten A
,
Cusini A
, et al.; Ch-Sur Study Group
. Risk factors for severe outcomes for COVID-19 patients hospitalised in Switzerland during the first pandemic wave, February to August 2020: prospective observational cohort study. Swiss Med Wkly. 2021 Jul;151(2930):w20547. https://doi.org/10.4414/smw.2021.20547
29.
Ye Z
,
Wang Y
,
Colunga-Lozano LE
,
Prasad M
,
Tangamornsuksan W
,
Rochwerg B
, et al.
Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020 Jul;192(27):E756–67. https://doi.org/10.1503/cmaj.200645
30.
Liebl ME
,
Gutenbrunner C
,
Glaesener JJ
,
Schwarzkopf S
,
Best N
,
Lichti G
, et al.
Early Rehabilitation in COVID-19 – Best Practice Recommendations for the Early Rehabilitation of COVID-19 Patients. Phys Med Rehabilmed Kurortmed. 2020 Jun;30(3):129–34. https://doi.org/10.1055/a-1162-4919
31.
Brown CA
,
Londhe AA
,
He F
,
Cheng A
,
Ma J
,
Zhang J
, et al.
Development and Validation of Algorithms to Identify COVID-19 Patients Using a US Electronic Health Records Database: A Retrospective Cohort Study. Clin Epidemiol. 2022 May;14:699–709. https://doi.org/10.2147/CLEP.S355086
Appendix
Table S1ICD-10 codes for inclusion criteria.
|
Name
|
ICD-10 diagnosis code
|
Code position
|
| COVID-19, virus confirmed |
U071 |
Any |
| COVID-19, virus not confirmed |
U072 |
Any |
Table S2ICD-10 codes for baseline characteristics
(comorbidities) and outcomes.
|
Name of group
|
ICD-10 diagnosis code
|
Code position: comorbidities
|
| Hypertension |
I10–I13, I15, I674 |
Any |
| Dyslipidemia |
E78 |
Any |
| Obesity (BMI ≥ 30.0 kg/m2) |
E65–E66
|
Any |
| Coronary artery disease |
I20–I25 |
Any |
| Congestive heart failure |
I50, I110, I130, I132 |
Any |
| Peripheral arterial disease |
I702 |
Any |
| Cerebrovascular disease |
I60–I67, I69 |
Any |
| Chronic obstructive pulmonary disease |
J44 |
Any |
| Bronchial asthma |
J45–J46 |
Any |
| Obstructive sleep apnoea syndrome |
G473
|
Any |
| Acute respiratory distress syndrome |
J80 |
Any |
| Chronic kidney disease stage 3 & 4 |
N183–N184 |
Any |
| Chronic kidney disease stage 5 &
hemodialysis |
N185, T824, T8571,Z491–Z492, Z992 |
Any |
| Solid organ transplant recipients |
T861–T864, T8681–T8682, T8688, T869, Z940–Z944,
Z9488, Z949
|
Any |
| Solid tumor |
C0–C7 |
Any |
| Liver disease including cirrhosis |
K7, K703, K717, K744, K746 K7470–K7472 |
Any |
| Diabetes mellitus type 1 |
E10
|
Any |
| Diabetes mellitus type 2 |
E11 |
Any |
| Haematological malignancy |
C81–88, C90–C96) |
Any |
| Rheumatoid
arthritis |
M05–M06, M08 |
Any |
| Human immunodeficiency virus infection |
B20–B24, F024, O987, U60–U61, U85, Z21 |
Any |
Table S3CHOP codes baseline characteristics
(comorbidities) and outcomes.
|
Name
|
CHOP code
|
Code position
|
| Haemo- and peritoneal dialysis |
3895, 3927, 3942–3943, 39951–39954, 3995I1–3995I2, 5498 |
Any |
| Solid organ transplant |
0091*–0093*,335*–336*,
3751*,4194*,4697*,505*,528*,556* |
Any |
| Installation and revision of portosystemic
shunt |
391100, 391199 |
Any |
| Extracorporeal membrane oxygenation |
37698*, 3769A*, 376A61*– 376A62*, 376A71*–376A73*,
376B61*– 376B62*, 376B71*–376B73*, 376C61*, 376C62*, 376C71*–376C73*, 376D*,
376D31*, 376D41* |
Any |
Table S4Baseline characteristics stratified by waves.
| |
First wave
|
Second wave
|
p-value
|
| Hospitalisations, n |
8862 |
27,655 |
|
| Demographics |
Age, median (IQR)
[years] |
69 (55, 80) |
73 (60, 82) |
<0.001 |
| Age groups, n (%) |
|
|
|
| <10 years |
73 (0.8) |
224 (0.8) |
<0.001 |
| 10–19 years |
72 (0.8) |
203 (0.7) |
|
| 20–49 years |
1333 (15.0) |
3005 (10.9) |
|
| 50–69 years |
2974 (33.6) |
8304 (30.0) |
|
| ≥70 years |
4410 (49.8) |
15,919 (57.6) |
|
| Male sex, n (%) |
5,193 (58.6) |
15,718 (56.8) |
0.004 |
| Swiss nationality,
n (%) |
6586 (74.3) |
20,730 (75.0) |
0.23 |
| Supplementary
insurance, n (%) |
1061 (12.0) |
3979 (14.4) |
<0.001 |
| Admission data |
Emergency
admission, n (%) |
7293 (82.3) |
23,242 (84.0) |
<0.001 |
| Admission from
home, n (%) |
6712 (75.7) |
21,960 (79.4) |
<0.001 |
| Admission to tertiary
care hospital, n (%) |
|
|
<0.001 |
| University hospital |
2807 (31.7) |
6213 (22.5) |
|
| Non-university
hospital |
4360 (49.2) |
16,112 (58.3) |
|
| Admission to secondary
care hospital, n (%) |
1695 (19.1) |
5330 (19.3) |
|
| Comorbidities, n
(%) |
Hypertension |
4016 (45.3) |
13,511 (48.9) |
<0.001 |
| Dyslipidemia |
1434 (16.2) |
4544 (16.4) |
0.58 |
| Obesity (BMI ≥ 30.0
kg/m2) |
275 (3.1) |
1101 (4.0) |
<0.001 |
| Coronary artery disease |
1224 (13.8) |
4398 (15.9) |
<0.001 |
| Atrial fibrillation |
1339 (15.1) |
4782 (17.3) |
<0.001 |
| Congestive heart
failure |
827 (9.3) |
2822 (10.2) |
0.017 |
| Peripheral arterial
disease |
266 (3.0) |
932 (3.4) |
0.090 |
| Cerebrovascular
disease |
386 (4.4) |
1250 (4.5) |
0.52 |
| Chronic obstructive
pulmonary disease |
581 (6.6) |
1907 (6.9) |
0.27 |
| Bronchial asthma |
398 (4.5) |
1,045 (3.8) |
0.003 |
| Obstructive sleep
apnea syndrome |
429 (4.8) |
1226 (4.4) |
0.11 |
| Chronic kidney
disease stage 3 & 4 |
845 (9.5) |
3716 (13.4) |
<0.001 |
| Chronic kidney
disease stage 5 & hemodialysis |
305 (3.4) |
528 (1.9) |
<0.001 |
| Solid organ
transplant recipients |
73 (0.8) |
279 (1.0) |
0.12 |
| Solid tumor |
425 (4.8) |
1527 (5.5) |
0.008 |
| Liver disease including
cirrhosis |
512 (5.8) |
1051 (3.8) |
<0.001 |
| Diabetes mellitus
type 2 |
1712 (19.3) |
6276 (22.7) |
<0.001 |
| Diabetes mellitus
type 1 |
31 (0.3) |
110 (0.4) |
0.53 |
| Haematological
malignancy |
132 (1.5) |
526 (1.9) |
0.011 |
| Rheumatoid arthritis |
97 (1.1) |
381 (1.4) |
0.041 |
| Human
immunodeficiency virus infection |
39 (0.4) |
102 (0.4) |
0.35 |
| Elixhauser
comorbidity index, median (IQR) |
2 (1, 4) |
2 (1, 4) |
<0.001 |
| Hospital frailty score,
n (%) |
<5 points |
5510 (62.2) |
16,921 (61.2) |
0.25 |
| 5–15 points |
2884 (32.5) |
9237 (33.4) |
|
| >15 points |
468 (5.3) |
1497 (5.4) |
|
| Outcomes |
In-hospital
mortality, n (%) |
1028 (11.6) |
3264 (11.8) |
0.61 |
| ICU admissions, n
(%) |
1463 (16.5) |
3160 (11.4) |
<0.001 |
| ICU LOS, median
(IQR) [days] |
8.3(2.1, 18.3) |
4.9 (1.8, 11.7) |
<0.001 |
| Need for mechanical
ventilation, n (%) |
1101 (12.4) |
2120 (7.7) |
<0.001 |
| Duration of mechanical
ventilation, median (IQR) [days] |
10.7 (5.0, 18.3) |
6.3 (2.0, 13.0) |
<0.001 |
| 30-days rehospitalisation,
n (%) |
427 (4.8) |
1340 (4.8) |
0.92 |



