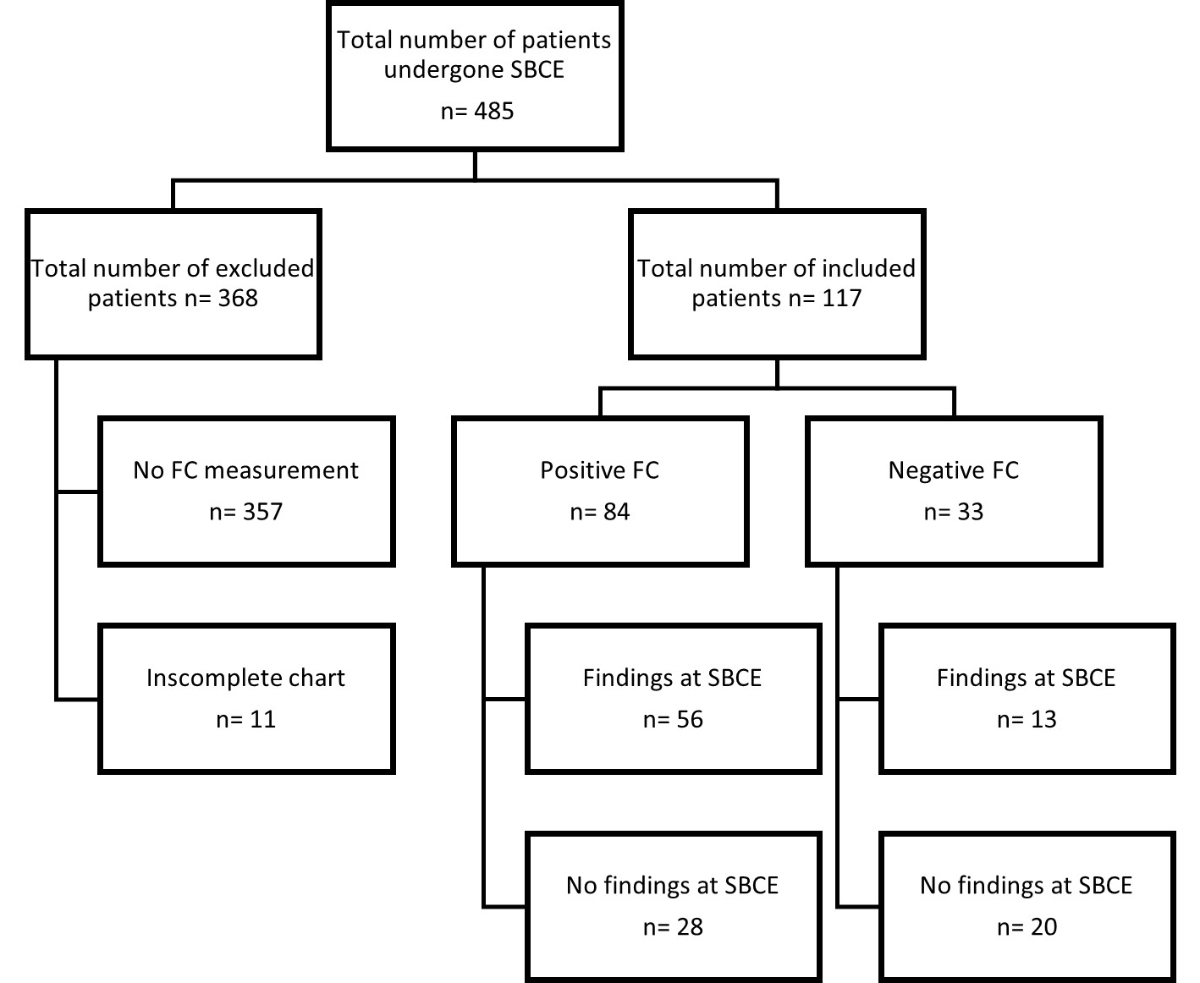
Figure 1 Study flow chart of patients. SBCE: small bowel capsule endoscopy; FC: faecal calprotectin.
DOI: https://doi.org/10.57187/smw.2023.40050
The small intestine can be affected by various infectious and inflammatory diseases, be the origin of primary bowel malignancies, and be the source of gastrointestinal bleeding. In obscure gastrointestinal bleeding, bidirectional endoscopy may identify >90% of bleeding sources in the upper or lower gastrointestinal tract. However, 75% of the remaining ~10% of cases are estimated to originate from the small bowel [1, 2]. In inflammatory bowel disease (IBD), about two-thirds of patients with Crohn’s disease have small bowel involvement, mainly in the terminal ileum [3, 4]. In a minority of patients, Crohn’s disease may only affect small bowel segments beyond the reach of a standard endoscope [5]. Patients with small bowel Crohn’s disease usually present with chronic abdominal pain, potentially overlapping with symptoms from irritable bowel syndrome, which may delay diagnosis and early treatment for IBD [6].
The evaluation of the small bowel remains challenging despite technological advances in imaging modalities and device-assisted enteroscopy. Small bowel capsule endoscopy is the most sensitive technique for detecting lesions in the small intestine and allows direct visualisation of the mucosa. It is becoming widely available and has a low complication rate. Several societies have recommended small bowel capsule endoscopy for evaluating small bowel bleeding and suspected IBD after negative upper and lower endoscopy [2, 5, 7, 8]. However, this method has limitations since its diagnostic yield of small bowel capsule endoscopy is highly variable depending on the indication, ranging from 4–44% in patients with abdominal pain [5, 9], 45–62% in patients with obscure gastrointestinal bleeding [10–14], and 38–86% in patients with Crohn’s disease [15, 16].
Additionally, interpreting findings related to clinical significance can sometimes be challenging since findings suggestive of disease may be observed even in healthy individuals [17, 18]. Therefore, selecting patients for small bowel capsule endoscopy is difficult since most gastrointestinal symptoms are nonspecific, very common in the general population, and often due to irritable bowel syndrome. Finally, since small bowel capsule endoscopy is time-consuming and expensive, it should be prioritised in patients likely to benefit from this investigation.
Family physicians and general practitioners are often consulted first rather than a gastroenterologist in cases with gastrointestinal symptoms. Therefore, a decision tool to guide physicians on the need for further investigations or referral to a secondary care centre is greatly needed.
Calprotectin is a cytosolic protein present in granulocytes. When measured in stool, it correlates well with neutrophil infiltration into the intestinal mucosa. It has been established as a non-invasive intestinal inflammation biomarker in IBD and other gastrointestinal diseases, including of the small bowel. Some studies have reported an association between higher faecal calprotectin levels and small bowel inflammation in IBD [19–24] and have shown correlations between small bowel capsule endoscopy activity scores and faecal calprotectin levels in Crohn’s disease [25–27], while others have reported conflicting results [28–32]. In addition, 20–42% of patients investigated for obscure gastrointestinal bleeding had inflammatory lesions that might be detectable with faecal calprotectin measurement [12–14]. However, none had their faecal calprotectin measured. One study even reported elevated faecal calprotectin levels with non-steroidal anti-inflammatory drug (NSAID)-induced small bowel pathologies after a short course of diclofenac [18]. In summary, data on the value of faecal calprotectin measurements in patients suspected of small bowel pathology are scarce. This study aimed to investigate the value of faecal calprotectin in detecting small bowel lesions in unselected patients undergoing small bowel capsule endoscopy.
We performed a multicenter retrospective data analysis of consecutive patients who underwent small bowel capsule endoscopy and had faecal calprotectin measurements.
The study was conducted in the Department of Gastroenterology and Hepatology at the University Medical Clinic of the Cantonal Hospital Baselland and in the Division of Gastroenterology and Hepatology at the University Hospital Basel in Switzerland. All patients who had undergone small bowel capsule endoscopy between September 2010 and November 2018 were assessed. Exclusion criteria were age <18 years, current infection, abdominal surgery within the last three months, pregnancy, and no faecal calprotectin measurement.
This study was conducted according to the principles of the Declaration of Helsinki, and the local ethics committees (EKNZ–Ethikkommission Nordwest und Zentralschweiz, Switzerland; project number: 2019-01855) approved its protocol. Informed consent was not required for this retrospective analysis.
The primary endpoint was the presence of a clinically significant finding in the small bowel, defined as mucosal erosions, mucosal ulcers, adenoma/carcinoma, and angiodysplasia. The secondary endpoint was the presence of inflammatory mucosal breaks (erosions or ulcers).
Faecal calprotectin was measured in a single stool sample from all patients. Patients were instructed to collect the sample at home 24 hours before bowel preparation for endoscopy. Samples were delivered on the day of the investigation and sent to the study laboratory (Rothen Medical Laboratories, Basel, Switzerland) for analysis within 48 hours. Faecal calprotectin is stable at room temperature for ≤3 days [33]. Faecal calprotectin was measured using a commercially available enzyme-linked immunosorbent assay (Bühlmann Laboratories AG, Schönenbuch, Switzerland). The laboratory personnel performing the analysis were blinded to the patients’ clinical histories and endoscopic findings.
All patients had undergone esophagogastroduodenoscopy and colonoscopy before referral for small bowel evaluation. In Switzerland, this is a prerequisite for applying for reimbursement for small bowel capsule endoscopy. All small bowel capsule endoscopies were performed using the PillCam® SB1/SB2 (Given Imaging Ltd, Yokneam, Israel). Pre-procedure small bowel preparation used a 2-litre polyethylene glycol regimen. Using the standard methodology, experienced board-certified gastroenterologists blind to the faecal calprotectin results analysed the video capsule sequences. All indicative small bowel capsule endoscopy findings were recorded.
Two experienced board-certified gastroenterologists reviewed all investigations and adjudicated all endoscopic findings using pre-specified lists for esophagogastroduodenoscopy (normal findings, erosion, reflux esophagitis, ulceration, carcinoma, or other), colonoscopy (normal findings, colitis, polyp, carcinoma, angiodysplasia, or other), and small bowel capsule endoscopy (normal, erosion, ulceration, carcinoma, angiodysplasia, or other).
Where appropriate, numerical data results are presented as mean (standard deviation) or median (interquartile range [IQR]). Numerical data were compared using a Mann-Whitney U-test (for two independent groups) or a Kruskal–Wallis H-test (for >2 independent groups). Categorical data were compared using a Chi-square test. A p-value <0.05 was considered statistically significant. Receiver operating characteristic (ROC) analyses were used to determine the optimal faecal calprotectin cut-off value based on the Youden index and to calculate the faecal calprotectin test’s characteristics for identifying small bowel capsule endoscopy findings. The test characteristics presented are sensitivity, specificity, positive (LR+) and negative (LR−) likelihood ratios, and positive (PPV) and negative (NPV) predictive values. The test’s overall accuracy was calculated according to the following formula: (true positive test results + true negative test results) / total population. We anticipated an area under the ROC curve (AUC) of 0.750 for faecal calprotectin to identify clinically significant findings during small bowel capsule endoscopy and a ratio of 3 for the negative and positive groups’ sample sizes (type 1 error = 0.05, type 2 error = 0.1), which returned a sample size of 72 subjects. Considering an AUC of 0.630 and a ratio of 0.7, the calculated sample size was 155 (type 1 error = 0.05, type 2 error = 0.2). Statistical analyses were performed using MedCalc for Windows, version 20.027 (MedCalc Software, Ostend, Belgium).
A total of 485 patients had small bowel capsule endoscopy during the study period, of which 117 (24.1%) were included in the study (figure 1). Of the 368 patients (75.9%) excluded, no faecal calprotectin value was available for 357, and chart review was insufficient to adjudicate a final diagnosis for 11 (figure 1).

Figure 1 Study flow chart of patients. SBCE: small bowel capsule endoscopy; FC: faecal calprotectin.
Table 1 presents the baseline characteristics of our study population.
Table 1Baseline characteristics.
| Variable | Value |
| Age, median (IQR) | 56.4 (42.4–68.2) |
| Female, n (%) | 58 (49.6) |
| Laboratory test values | |
| Haemoglobin (g/l), median (IQR) | 125.0 (96.0–142.0) |
| Leucocytes (×109/l), median (IQR) | 6.8 (5.3–8.8) |
| Thrombocytes (×109/l), median (IQR) | 283 (231–332) |
| C-reactive protein (mg/l), median (IQR) | 2.4 (0.1–7.2) |
| Ferritine (ng/ml), median (IQR) | 70.9 (10.5–227.5) |
| Faecal calprotectin (μg/g), median (IQR) | 181.0 (40.5–319.0) |
| Medication | |
| Aspirin, n (%) | 12 (10.3) |
| NSAID, n (%) | 8 (6.8) |
| Oral anticoagulants, n (%) | 12 (10.3) |
| Proton pump inhibitor, n (%) | 49 (41.9) |
IQR: interquartile range; NSAID: non-steroidal anti-inflammatory drug
Of the 117 patients included in the study, the indication for esophagogastroduodenoscopy was obscure gastrointestinal bleeding for 57 (48.7%), abdominal discomfort/pain for 36 (30.8%), chronic diarrhoea for 17 (14.5%), suspected malignancy for six (5.1%), and other indications (intestinal tuberculosis) for one (0.9%). Colonoscopy was performed for obscure gastrointestinal bleeding in 59 patients (50.4%), suspected IBD in 42 patients (35.9%), chronic diarrhoea in nine patients (7.7%), and suspected malignancy in six patients (5.1%). The indication for colonoscopy was not available for one patient. Esophagogastroduodenoscopy and colonoscopy provided clinically significant findings in 31 (26.5%) and 23 (19.7%) of the 117 patients, respectively (table 2), all of which were judged to be minimal lesions not relevant for diagnosis.
Table 2Esophagogastroduodenoscopy and colonoscopy endoscopic findings.
| Esophagogastroduodenoscopy (n = 117) | |
| Normal findings, n (%) | 86 (73.5) |
| Reflux esophagitis, n (%) | 8 (6.8) |
| Gastroduodenal erosion, n (%) | 15 (12.8) |
| Other lesions* | 8 (6.8) |
| Colonoscopy (n = 117) | |
| Normal findings, n (%) | 94 (80.3) |
| Inflammation (colitis, terminal ileitis), n (%) | 10 (8.6) |
| Adenomatous polyps, n (%) | 9 (7.7) |
| Angiodysplasia, n (%) | 4 (3.4) |
* Other lesions included angiodysplasia (n = 2), adenomatous polyps (n = 3), Barrett’s oesophagus (n = 2), and autoimmune gastritis (n = 1).
The indications for small bowel capsule endoscopy were obscure gastrointestinal bleeding in 56 of the 117 patients (47.9%), suspected IBD in 50 patients (42.7%), and suspected neoplasia in 11 patients (9.4%; table 3). Small bowel capsule endoscopy was performed a median of 84 (43.5–208.0) days after esophagogastroduodenoscopy and 89 (45.2–205) days after colonoscopy. The median small bowel passage time was 250 (196.0–315.0) minutes. A complete small bowel inspection was achieved in 88.9% of patients. The prevalence of a clinically significant finding in the small bowel was 59.0% (69 of 117 patients). Of the 117 patients, 84 (71.8%) had positive faecal calprotectin test results with a median faecal calprotectin level of 181 (40.5–319.0) μg/g stool. The positive faecal calprotectin test result rate (89.8%) and median faecal calprotectin levels (268 μg/g; 149.8–462.3) were highest in patients with suspected IBD, who also had the highest prevalence of clinically significant findings in small bowel capsule endoscopy (68.0%; table 3).
Table 3Small bowel capsule endoscopy indications and faecal calprotectin values.
| SBCE indication | n (%) | Positive FC result (%) | Median FC value (IQR; μg/g) | SBCE findings (%) |
| All patients | 117 (100) | 84 (71.8) | 181.0 (40.5–319.3) | 69 (59.0) |
| Obscure gastrointestinal bleeding | 56 (47.9) | 33 (58.9) | 72.5 (25.0–255.0) | 32 (57.1) |
| Suspected IBD | 50 (42.7) | 44 (89.8) | 268.0 (149.8–454.0) | 34 (68.0) |
| Suspected neoplasia | 11 (9.4) | 7 (63.6) | 75.0 (25.0–195.8) | 4 (36.7) |
FC: faecal calprotectin; SBCE: small bowel capsule endoscopy: IQR, interquartile range
Small bowel capsule endoscopy (SBCE) findings and their associated faecal calprotectin values are presented in table 4. In total, there were 69 clinically significant findings during SBCE, of which the most common were ulcers/erosions (60 patients; 87.0% of all clinically significant findings). Fifty-six of 69 patients (81.2%) had positive faecal calprotectin test results. Median faecal calprotectin levels were higher in patients with SBCE clinically significant findings (226.0 μg/g; 75.0–408.3) than in patients with normal findings (126.0 μg/g; 25.0–239.0; p = 0.017). The prevalence of SBCE clinically significant findings increased with increasing faecal calprotectin values. SBCE clinically significant findings were present in 28.2% of patients with faecal calprotectin values of <50 μg/g (n = 13 [28.2%]), 61.0% of patients with faecal calprotectin values of 50–250 μg/g (n = 41 [35.0%]), and 72.1% of patients with faecal calprotectin levels of >250 μg/g (n = 43 [36.8%]) (p = 0.015).
Table 4Small bowel capsule endoscopy findings and faecal calprotectin values.
| SBCE finding | n | Positive FC result (%) | Median FC value (IQR; μg/g) | p-value* |
| Normal | 48 | 28 (58.3) | 126.0 (25.0–239.0) | – |
| All findings | 69 | 56 (81.2) | 226.0 (75.0–408.3) | 0.017 |
| Ulcer/erosion | 60 | 49 (81.7) | 239.5 (92.0–434.5) | 0.007 |
| Angiodysplasia | 7 | 5 (71.4) | 64.0 (50.0–172.3) | 0.990 |
| Neoplasia | 2 | 2 (100) | 63.0 (51.0–75.0) | 0.804 |
* FC (faecal calprotectin) values vs normal findings; SBCE: small bowel capsule endoscopy; IQR: interquartile range.
Patients with positive faecal calprotectin test results (66.7%) were more likely to have SBCE clinically significant findings than those with normal faecal calprotectin test results (39.4%; p = 0.007). A ROC analysis was performed to evaluate the value of faecal calprotectin as a diagnostic test to predict SBCE clinically significant findings. It had an AUC of 0.630 (95% confidence interval: 0.54–0.72) and indicated an optimal faecal calprotectin cut-off value of 63 μg/g (figure 2).
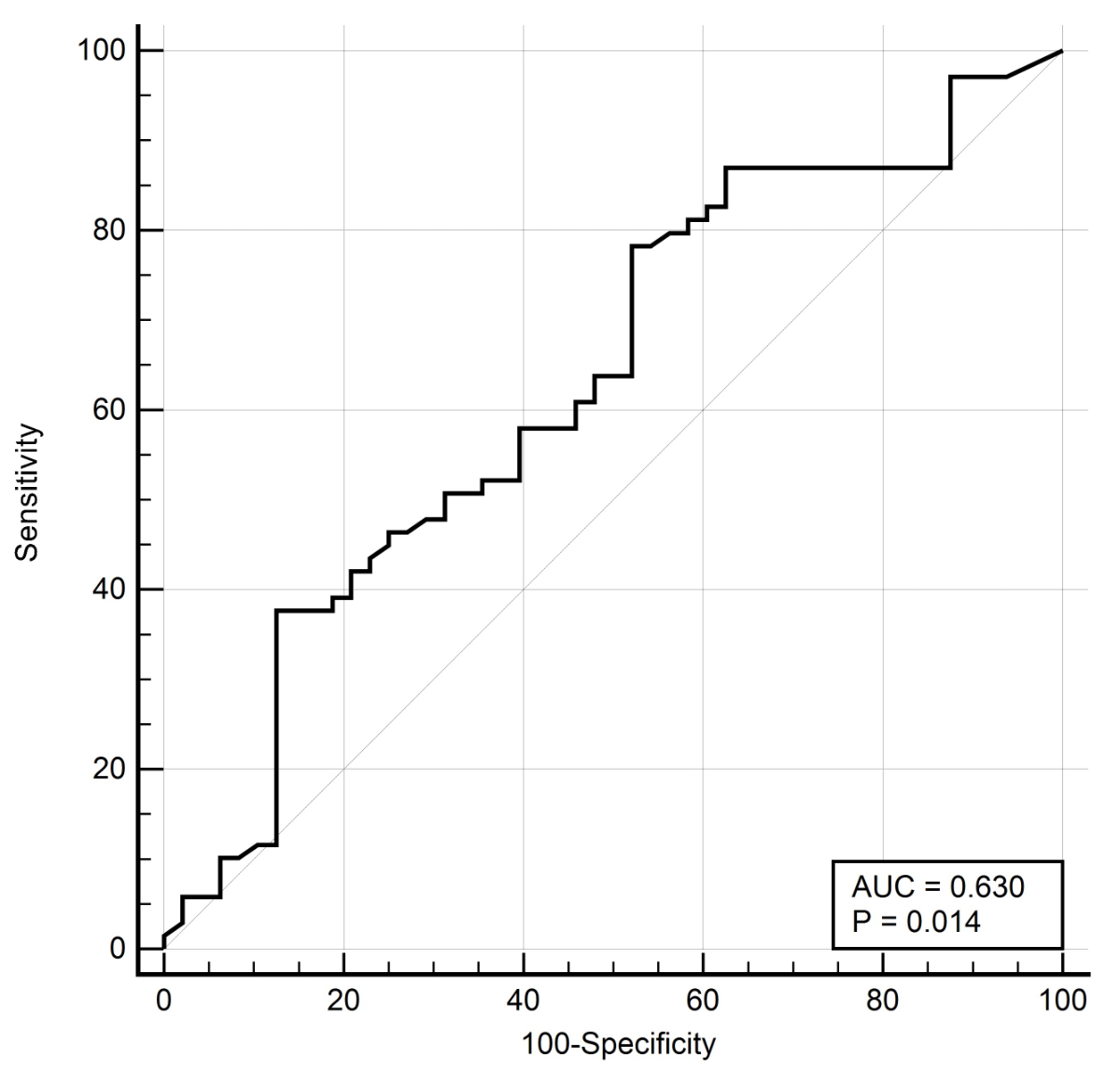
Figure 2 Overall diagnostic value of fecal calprotectin to identify small bowel findings. Receiver operating characteristics (ROC) with area under the curve (AUC).
The identified faecal calprotectin cut-off provided 78.3% sensitivity and 47.9% specificity with an LR+ of 1.50 and an LR− of 0.45 (table 5).
Table 5The faecal calprotectin test characteristics for identifying small bowel lesions.
| AUC | FC cut-off value (μg/g) | Sensitivity (%) | Specificity (%) | LR+ | LR− | PPV | NPV | Accuracy | |
| All indications | 0.630 (0.53–0.72) | 63 | 78.3 (66.7–87.9) | 47.9 (33.3–62.8) | 1.50 (1.10–2.00) | 0.45 (0.30–0.80) | 68.4 (61.6–74.4) | 60.5 (47.3–72.4) | 65.0 |
| Suspected IBD | 0.691 (0.54–0.82) | 236 | 66.7 (48.2–82.0) | 70.6 (44.0–89.7) | 2.27 (1.00–4.90) | 0.47 (0.30–0.80) | 81.5 (67.0–90.5) | 54.5 (39.7–68.6) | 71.4 |
AUC: area under the ROC curve; FC: faecal calprotectin; LR+: positive likelihood ratio; LR−: negative likelihood ratio; PPV: positive predictive value; NPV: negative predictive value
The test’s overall accuracy was 65.0%. Among patients investigated for suspected IBD, the AUC was 0.691 (0.54–0.82) with an optimal faecal calprotectin cut-off value of 236 μg/g (figure 3). Small bowel capsule endoscopy findings were predicted with 66.7% sensitivity and 70.6% specificity with an LR+ of 2.27, an LR− of 0.47, and an overall accuracy of 71.4%.
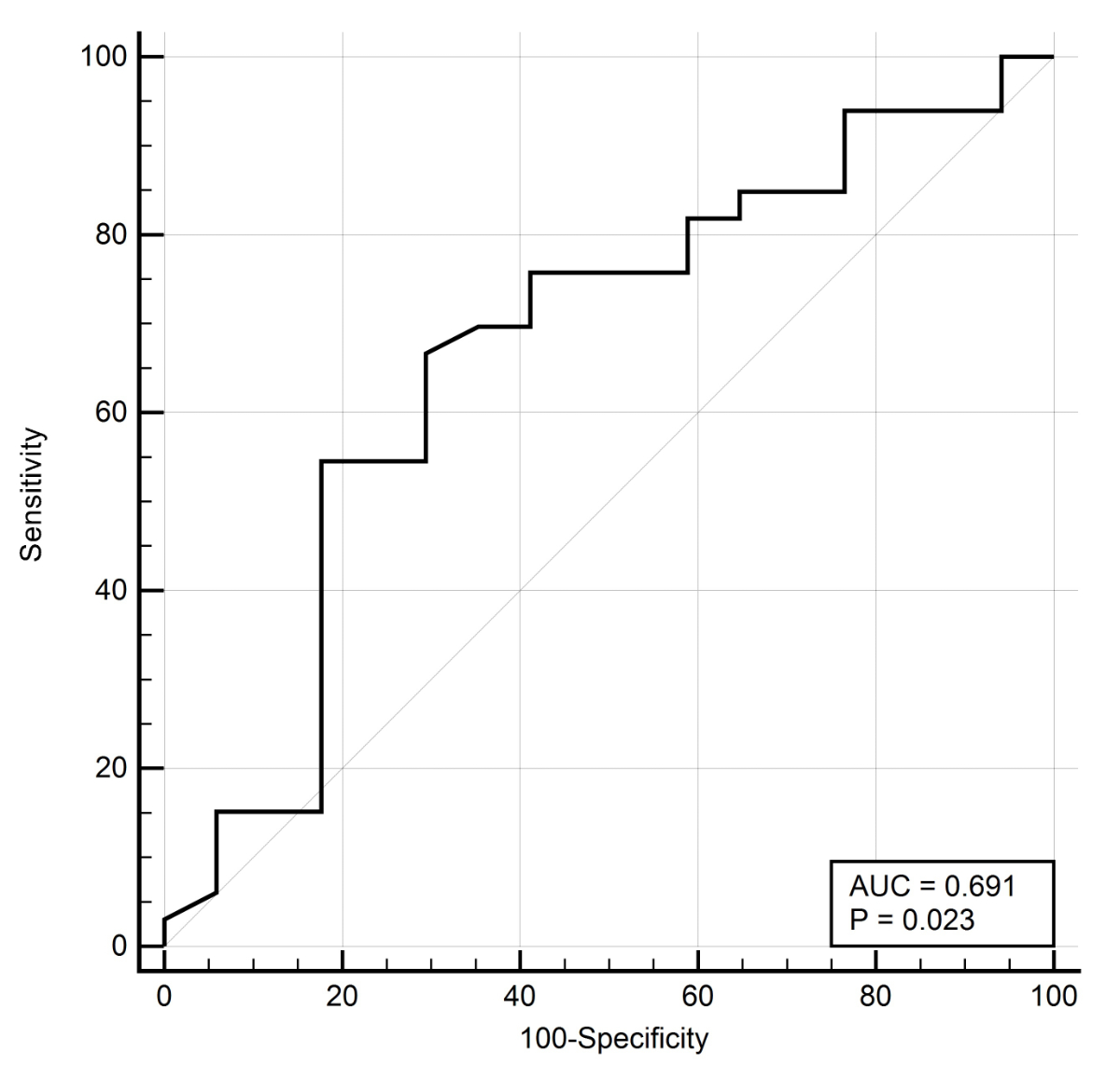
Figure 3 Diagnostic value of fecal calprotectin to identify small bowel findings in patients with suspected Inflammatory bowel disease. Receiver operating characteristics (ROC) with area under the curve (AUC).
This study examined the value of faecal calprotectin testing in predicting small bowel lesions in patients undergoing small bowel capsule endoscopy. Our study provided the following findings. First, patients with small bowel lesions were more likely to have positive faecal calprotectin testing. Second, the prevalence of small bowel clinically significant findings increased with increasing faecal calprotectin values. Third, faecal calprotectin had limited value in identifying patients with significant small bowel lesions. Fourth, the faecal calprotectin testing’s diagnostic accuracy was highest in patients undergoing small bowel capsule endoscopy for suspected IBD.
These findings are of clinical importance since they highlight an unmet need of clinicians to efficiently identify patients for small bowel capsule endoscopy who will benefit most from this laborious and expensive procedure. Triage based on symptoms alone is often complex and may result in late detection of relevant disease. Only 75% of Crohn’s disease patients are diagnosed within two years of disease onset [6]. This diagnostic delay decreases their quality of life and may lead to decreased therapy success, more complications, and higher surgery rates [34]. Faecal calprotectin testing is a well-established diagnostic tool for distinguishing functional gastrointestinal disorders from organic bowel diseases [35, 36]. It accelerates diagnostic investigations and reduces the need for endoscopy [37]. In a primary care setting, referrals to secondary care centres may also be avoided [38, 39].
In our study, most patients with clinically significant findings in small bowel capsule endoscopy also had elevated faecal calprotectin values (81.2%) due to inflammatory lesions in the small bowel in most cases (86.9%). Faecal calprotectin levels were higher in patients with small bowel capsule endoscopy clinically significant findings (226 μg/g) than with normal findings (126.0 μg/g), and patients with positive faecal calprotectin test results were more likely to have small bowel capsule endoscopy clinically significant findings (66.7% vs 39.4%). The overall diagnostic yield was 59.0%, while patients with suspected IBD had the highest yield (68.0%), similar to findings in other studies [10–12, 15, 16].
A recent meta-analysis reported normal faecal calprotectin values to reflect a very low likelihood of small bowel lesions in patients with suspected Crohn’s disease [23]. Others have proposed that in patients with suspected small bowel inflammation and negative bidirectional endoscopy, an faecal calprotectin value of <100 μg/g would not require small bowel capsule endoscopy [24, 31, 40], and patients with faecal calprotectin values >200 μg/g should be referred for small bowel capsule endoscopy with higher priority[24]. In addition, a prospective single-centre study showed no significant association between faecal calprotectin levels >50 μg/g and small bowel capsule endoscopy clinically significant findings [29].
Our study also showed that low faecal calprotectin values <50 μg/g had a significantly lower diagnostic yield (28.2%) than values >250 μg/g (71.2%). However, at the optimal cut-off value of 63 μg/g, faecal calprotectin had limited diagnostic accuracy and modest positive and negative predictive value. Our results are similar to another study in unselected patients undergoing small bowel capsule endoscopy [30], where a positive faecal calprotectin test (>50 μg/g) had 54.2% sensitivity and 69.9% specificity but an overall lower diagnostic small bowel capsule endoscopy yield (37.3%) than in our study (59.0%).
Overall, inflammatory lesions are the most common small bowel capsule endoscopy clinically significant findings. In our study, 86.9% of small bowel clinically significant findings were attributable to inflammatory lesions. Accordingly, the diagnostic performance of faecal calprotectin was greatest in patients with suspected IBD. At the optimal cut-off value of 236 μg/g, the PPV was 81.5%. Our results are comparable to other studies investigating faecal calprotectin in suspected IBD. In a prospective study of 100 patients, an faecal calprotectin value >194 μg/g provided 46.7% sensitivity and 89.8% specificity in identifying small bowel Crohn’s disease [31]. Similarly, a retrospective study of 70 patients reported a PPV for faecal calprotectin values >200 μg/g of 78% [24]. Therefore, faecal calprotectin values >200 μg/g may be useful for prioritising patients for small bowel capsule endoscopy.
Other factors, such as NSAID use, may also increase faecal calprotectin and be potential confounders. A study in healthy subjects showed elevated faecal calprotectin levels in 75% and inflammatory clinically significant findings on small bowel capsule endoscopy in 68% of participants after 150 mg of diclofenac daily for two weeks [18]. However, others could not find an association between NSAIDs and faecal calprotectin levels [30]. Only 8% (8/117) of patients had NSAID treatment in our study.
This study also had several limitations. The retrospective nature of its analysis may lead to a degree of selection bias. Since faecal calprotectin measurement was based on a clinical decision, it favoured the inclusion of patients with higher suspicion of inflammatory lesions in the gastrointestinal tract in this study. This study was performed in a tertiary clinic, and its results may differ in a primary care setting where the prevalence of small bowel pathology might be much lower, affecting the faecal calprotectin test’s performance [41]. However, we believe that our patients are comparable to a real-world setting since all small bowel capsule endoscopies within the region are performed at the participating study centres. Additionally, the pretest conditions for faecal calprotectin measurement were not considered, noting that relevant day-to-day fluctuations in faecal calprotectin levels may occur [33]. Additionally, we anticipated a higher faecal calprotectin diagnostic performance for identifying clinically significant findings during small bowel capsule endoscopy since more participants would increase the robustness of the study’s results.
In summary, this study showed that higher faecal calprotectin levels increase the diagnostic yield of small bowel capsule endoscopy but highlighted the limited diagnostic accuracy of faecal calprotectin. Particularly in patients with suspected IBD, faecal calprotectin testing can prioritise patients requiring small bowel investigations, avoiding diagnostic delays and improving patient management.
AA: data acquisition, manuscript draft and revision. NM: data acquisition. PH: study design, manuscript revision. EB: study design, data acquisition, manuscript draft.
This study was supported in part by unrestricted research grants to D. Burri by the Freiwillige Akademische Gesellschaft (Basel, Switzerland) and the Gottfried und Julia Bangerter-Rhyner-Stiftung (Bern, Switzerland). Bühlmann Laboratories AG provided the ELISA assay to measure faecal calprotectin.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Gerson LB , Fidler JL , Cave DR , Leighton JA . ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol. 2015 Sep;110(9):1265–87. https://doi.org/10.1038/ajg.2015.246
2. Pennazio M , Spada C , Eliakim R , Keuchel M , May A , Mulder CJ , et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015 Apr;47(4):352–76. https://doi.org/10.1055/s-0034-1391855
3. Samuel S , Bruining DH , Loftus EV Jr , Becker B , Fletcher JG , Mandrekar JN , et al. Endoscopic skipping of the distal terminal ileum in Crohn’s disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol. 2012 Nov;10(11):1253–9. https://doi.org/10.1016/j.cgh.2012.03.026
4. Van Assche G , Dignass A , Panes J , Beaugerie L , Karagiannis J , Allez M , et al.; European Crohn’s and Colitis Organisation (ECCO) . The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohn’s Colitis. 2010 Feb;4(1):7–27. https://doi.org/10.1016/j.crohns.2009.12.003
5. Gomollón F , Dignass A , Annese V , Tilg H , Van Assche G , Lindsay JO , et al.; ECCO . 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohn’s Colitis. 2017 Jan;11(1):3–25. https://doi.org/10.1093/ecco-jcc/jjw168
6. Vavricka SR , Spigaglia SM , Rogler G , Pittet V , Michetti P , Felley C , et al.; Swiss IBD Cohort Study Group . Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012 Mar;18(3):496–505. https://doi.org/10.1002/ibd.21719
7. Enns RA , Hookey L , Armstrong D , Bernstein CN , Heitman SJ , Teshima C , et al.; Clinical Practice Guidelines for the Use of Video Capsule Endoscopy . Clinical Practice Guidelines for the Use of Video Capsule Endoscopy. Gastroenterology. 2017 Feb;152(3):497–514. https://doi.org/10.1053/j.gastro.2016.12.032
8. Luján-Sanchis M , Sanchis-Artero L , Larrey-Ruiz L , Peño-Muñoz L , Núñez-Martínez P , Castillo-López G , et al. Current role of capsule endoscopy in Crohn’s disease. World J Gastrointest Endosc. 2016 Sep;8(17):572–83. https://doi.org/10.4253/wjge.v8.i17.572
9. Xue M , Chen X , Shi L , Si J , Wang L , Chen S . Small-bowel capsule endoscopy in patients with unexplained chronic abdominal pain: a systematic review. Gastrointest Endosc. 2015 Jan;81(1):186–93. https://doi.org/10.1016/j.gie.2014.04.062
10. Teshima CW , Kuipers EJ , van Zanten SV , Mensink PB . Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011 May;26(5):796–801. https://doi.org/10.1111/j.1440-1746.2010.06530.x
11. Lim YJ , Lee OY , Jeen YT , Lim CY , Cheung DY , Cheon JH , et al.; Korean Gut Image Study Group . Indications for Detection, Completion, and Retention Rates of Small Bowel Capsule Endoscopy Based on the 10-Year Data from the Korean Capsule Endoscopy Registry. Clin Endosc. 2015 Sep;48(5):399–404. https://doi.org/10.5946/ce.2015.48.5.399
12. Koulaouzidis A , Rondonotti E , Giannakou A , Plevris JN . Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012 Nov;76(5):983–92. https://doi.org/10.1016/j.gie.2012.07.035
13. Yung DE , Rondonotti E , Giannakou A , Avni T , Rosa B , Toth E , et al.; And the Capsule Endoscopy in Young Patients with IDA research group . Capsule endoscopy in young patients with iron deficiency anaemia and negative bidirectional gastrointestinal endoscopy. United European Gastroenterol J. 2017 Nov;5(7):974–81. https://doi.org/10.1177/2050640617692501
14. Olano C , Pazos X , Avendaño K , Calleri A , Ketzoian C . Diagnostic yield and predictive factors of findings in small-bowel capsule endoscopy in the setting of iron-deficiency anemia. Endosc Int Open. 2018 Jun;6(6):E688–93. https://doi.org/10.1055/a-0593-5915
15. Choi M , Lim S , Choi MG , Shim KN , Lee SH . Effectiveness of Capsule Endoscopy Compared with Other Diagnostic Modalities in Patients with Small Bowel Crohn’s Disease: A Meta-Analysis. Gut Liver. 2017 Jan;11(1):62–72. https://doi.org/10.5009/gnl16015
16. Carter D , Katz LH , Bardan E , Salomon E , Goldstein S , Ben Horin S , et al. The accuracy of intestinal ultrasound compared with small bowel capsule endoscopy in assessment of suspected Crohn’s disease in patients with negative ileocolonoscopy. Therap Adv Gastroenterol. 2018 Apr;11:1756284818765908. https://doi.org/10.1177/1756284818765908
17. Goldstein JL , Eisen GM , Lewis B , Gralnek IM , Aisenberg J , Bhadra P , et al. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther. 2007 May;25(10):1211–22. https://doi.org/10.1111/j.1365-2036.2007.03312.x
18. Maiden L , Thjodleifsson B , Theodors A , Gonzalez J , Bjarnason I . A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005 May;128(5):1172–8. https://doi.org/10.1053/j.gastro.2005.03.020
19. Shimoyama T , Yamamoto T , Umegae S , Matsumoto K . Faecal biomarkers for screening small bowel inflammation in patients with Crohn’s disease: a prospective study. Therap Adv Gastroenterol. 2017 Aug;10(8):577–87. https://doi.org/10.1177/1756283X17717683
20. Monteiro S , Barbosa M , Cúrdia Gonçalves T , Boal Carvalho P , Moreira MJ , Rosa B , et al. Fecal Calprotectin as a Selection Tool for Small Bowel Capsule Endoscopy in Suspected Crohn’s Disease. Inflamm Bowel Dis. 2018 Aug;24(9):2033–8. https://doi.org/10.1093/ibd/izy098
21. Egea Valenzuela J , Pereñíguez López A , Pérez Fernández V , Alberca de Las Parras F , Carballo Álvarez F . Fecal calprotectin and C-reactive protein are associated with positive findings in capsule endoscopy in suspected small bowel Crohn’s disease. Rev Esp Enferm Dig. 2016 Jul;108(7):394–400.
22. Jensen MD , Kjeldsen J , Nathan T . Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand J Gastroenterol. 2011 Jun;46(6):694–700. https://doi.org/10.3109/00365521.2011.560680
23. Kopylov U , Yung DE , Engel T , Avni T , Battat R , Ben-Horin S , et al. Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016 Oct;28(10):1137–44. https://doi.org/10.1097/MEG.0000000000000692
24. Koulaouzidis A , Douglas S , Rogers MA , Arnott ID , Plevris JN . Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011 May;46(5):561–6. https://doi.org/10.3109/00365521.2011.551835
25. Yablecovitch D , et al. The Lewis score or the capsule endoscopy Crohn’s disease activity index: which one is better for the assessment of small bowel inflammation in established Crohn’s disease? Therap Adv Gastroenterol, 2018. 11: p. 1756283X17747780.
26. Koulaouzidis A , Sipponen T , Nemeth A , Makins R , Kopylov U , Nadler M , et al. Association Between Fecal Calprotectin Levels and Small-bowel Inflammation Score in Capsule Endoscopy: A Multicenter Retrospective Study. Dig Dis Sci. 2016 Jul;61(7):2033–40. https://doi.org/10.1007/s10620-016-4104-7
27. Koulaouzidis A , Nemeth A , Johansson GW , Toth E . Dissecting Lewis score under the light of fecal calprotectin; an analysis of correlation of score components with calprotectin levels in capsule endoscopy. Ann Gastroenterol. 2015;28(2):259–64.
28. Sipponen T , Haapamäki J , Savilahti E , Alfthan H , Hämäläinen E , Rautiainen H , et al. Fecal calprotectin and S100A12 have low utility in prediction of small bowel Crohn’s disease detected by wireless capsule endoscopy. Scand J Gastroenterol. 2012 Jul;47(7):778–84. https://doi.org/10.3109/00365521.2012.677953
29. Hale MF , Drew K , McAlindon ME , Sidhu R . The diagnostic accuracy of faecal calprotectin and small bowel capsule endoscopy and their correlation in suspected isolated small bowel Crohn’s disease. Eur J Gastroenterol Hepatol. 2016 Oct;28(10):1145–50. https://doi.org/10.1097/MEG.0000000000000696
30. Olsen PA , Fossmark R , Qvigstad G . Fecal calprotectin in patients with suspected small bowel disease—a selection tool for small bowel capsule endoscopy? Scand J Gastroenterol. 2015 Mar;50(3):272–7. https://doi.org/10.3109/00365521.2014.1003395
31. Yousuf H , Aleem U , Egan R , Maheshwari P , Mohamad J , McNamara D . Elevated Faecal Calprotectin Levels are a Reliable Non-Invasive Screening Tool for Small Bowel Crohn’s Disease in Patients Undergoing Capsule Endoscopy. Dig Dis. 2018;36(3):202–8. https://doi.org/10.1159/000485375
32. Zittan E , Kelly OB , Gralnek IM , Silverberg MS , Hillary Steinhart A . Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open. 2018 Jul;2(5):201–6. https://doi.org/10.1002/jgh3.12068
33. Lasson A , Stotzer PO , Öhman L , Isaksson S , Sapnara M , Strid H . The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohn’s Colitis. 2015 Jan;9(1):26–32.
34. Schoepfer AM , Dehlavi MA , Fournier N , Safroneeva E , Straumann A , Pittet V , et al.; IBD Cohort Study Group . Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013 Nov;108(11):1744–53. https://doi.org/10.1038/ajg.2013.248
35. Gisbert JP , McNicholl AG . Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009 Jan;41(1):56–66. https://doi.org/10.1016/j.dld.2008.05.008
36. von Roon AC , Karamountzos L , Purkayastha S , Reese GE , Darzi AW , Teare JP , et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007 Apr;102(4):803–13. https://doi.org/10.1111/j.1572-0241.2007.01126.x
37. van Rheenen PF , Van de Vijver E , Fidler V . Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010 Jul;341 jul15 1:c3369. https://doi.org/10.1136/bmj.c3369
38. D’Angelo F , Felley C , Frossard JL . Calprotectin in Daily Practice: Where Do We Stand in 2017? Digestion. 2017;95(4):293–301. https://doi.org/10.1159/000476062
39. Freeman K , Ryan R , Parsons N , Taylor-Phillips S , Willis BH , Clarke A . Faecal calprotectin testing in UK general practice: a retrospective cohort study using The Health Improvement Network database. Br J Gen Pract. 2021 Oct;71(712):e854–61. https://doi.org/10.3399/BJGP.2021.0125
40. Monteiro S , Barbosa M , Cúrdia Gonçalves T , Boal Carvalho P , Moreira MJ , Rosa B , et al. Fecal Calprotectin as a Selection Tool for Small Bowel Capsule Endoscopy in Suspected Crohn’s Disease. Inflamm Bowel Dis. 2018 Aug;24(9):2033–8. https://doi.org/10.1093/ibd/izy098
41. Conroy S , Hale MF , Cross SS , Swallow K , Sidhu RH , Sargur R , et al. Unrestricted faecal calprotectin testing performs poorly in the diagnosis of inflammatory bowel disease in patients in primary care. J Clin Pathol. 2018 Apr;71(4):316–22. https://doi.org/10.1136/jclinpath-2017-204506