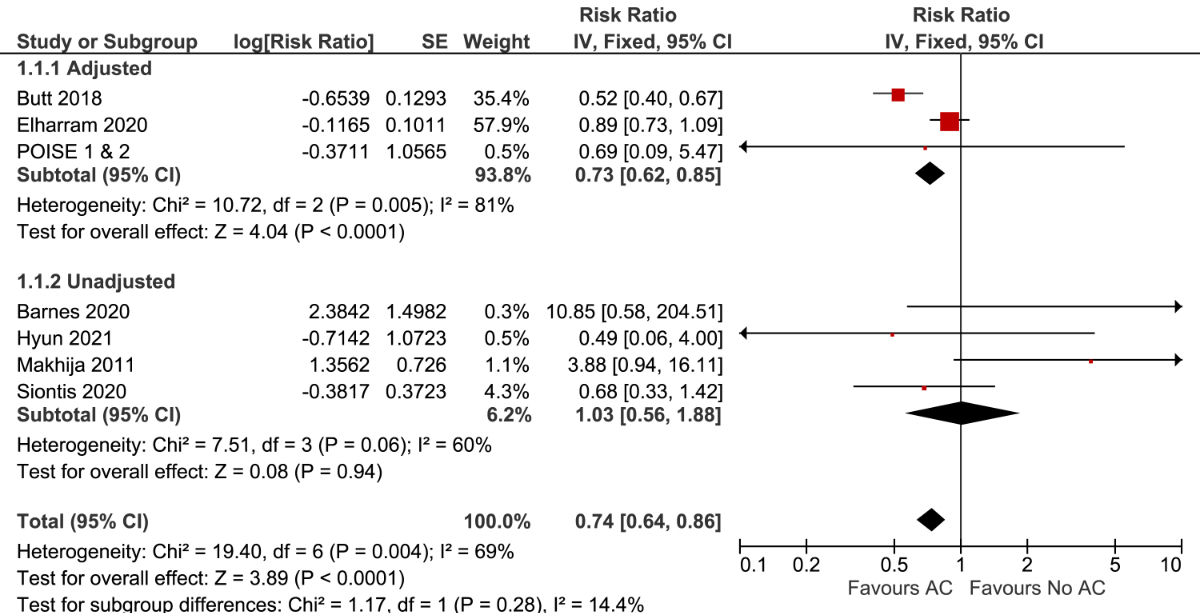
Figure 1 Forest plot for stroke ± systemic embolism stratified by the use of multivariable adjustment. AC: anticoagulation
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40056
Perioperative atrial fibrillation is the most commonly encountered arrhythmia after noncardiac surgery, with its incidence ranging between 5 and 12% [1]. Many clinicians regard perioperative atrial fibrillation as a transient and self-isolated clinical phenomenon [2]. However, recent studies have demonstrated that perioperative atrial fibrillation is associated with an increased risk of stroke, myocardial infarction, and death [3–5].
Oral anticoagulation reduces the long-term risk of ischemic stroke and systemic embolism in patients with atrial fibrillation diagnosed outside of the perioperative setting [6]. In contrast, the efficacy of anticoagulation for preventing stroke and systemic embolism has not been well established for patients with perioperative atrial fibrillation after noncardiac surgery. Oral anticoagulation prevents cardioembolic strokes in patients with clinical atrial fibrillation by inhibiting intracardiac thrombus formation [7]. If perioperative atrial fibrillation represents the first manifestation of clinical atrial fibrillation, oral anticoagulation may reduce the risk of future adverse outcomes. However, it is possible that the increased risks associated with perioperative atrial fibrillation simply reflect a higher burden of cardiovascular disease in this population and that anticoagulation is not expected to be effective in this setting [4]. Even if oral anticoagulation can effectively prevent arterial thromboembolism after perioperative atrial fibrillation, such benefits need to be carefully weighed against any increased risk of bleeding. Clinicians, therefore, remain uncertain about whether these medications should be routinely prescribed in this setting.
To address some of these uncertainties, we conducted a systematic review and meta-analysis assessing the efficacy and safety of anticoagulation use in patients with perioperative atrial fibrillation after noncardiac surgery.
We reported this systematic review and meta-analysis according to the Meta-analyses of Observational Studies in Epidemiology (MOOSE) reporting guidelines [8]. We registered the study protocol with PROSPERO (CRD42021257115).
We identified relevant studies through a systematic literature search of MEDLINE, EMBASE, and CENTRAL from the time of database inception until January 25, 2022. Our search strategy combined keywords and terms related to surgery, atrial fibrillation, and anticoagulation (supplemental methods S1). We identified additional articles by reviewing reference lists from relevant studies and consulting experts in the field.
We considered cohort studies, case-control studies, and randomised controlled trials to be eligible for inclusion. We included studies if they (1) had patients undergoing noncardiac surgery; (2) reported one or more of the prespecified outcomes in patients with a comparison without anticoagulation use after surgery; (3) included ≥100 participants with perioperative atrial fibrillation; and (4) included patients ≥18 years of age. Studies reporting data on patients with perioperative atrial fibrillation after cardiac surgery were included in a separate systematic review [9]. We excluded studies published only as meeting abstracts. We did not exclude studies based on publication language. We conducted screening and full-text reviews independently and in duplicate and resolved discrepancies either through consensus or by consulting with a third independent reviewer. We defined the use of anticoagulation therapy as any anticoagulation drug prescribed at doses considered therapeutic for the treatment of stroke and systemic embolism. We chose to use a broad definition of anticoagulation therapy, as there are multiple options to treat clinical atrial fibrillation, and there is no consensus on the optimal type of drug or drug formulation that should be used to treat perioperative atrial fibrillation. We included therapeutic doses of anticoagulation, as subtherapeutic doses should not be used for stroke prevention in patients with clinical atrial fibrillation.
The main outcomes of interest were stroke, with or without systemic embolism, and bleeding. Acceptable definitions of stroke include any stroke, ischemic stroke, or embolic stroke. We accepted any definition of bleeding used by the individual study authors. Other study outcomes included all-cause mortality, myocardial infarction, and venous thromboembolism.
We performed the data extraction independently and in duplicate using standardised forms. We collected information on the study design, sample size, types of surgical procedures, baseline demographics, study definitions (i.e., perioperative atrial fibrillation, anticoagulation use, and study outcomes), number of perioperative atrial fibrillation patients with and without anticoagulation use, reported associations between anticoagulation use and outcomes, and covariates used for multivariable adjustment. We contacted the study authors to obtain unpublished data and to clarify the number of participants and outcome events.
We used the Risk of Bias in Non-randomized Studies – of Interventions (ROBINS-I) tool to assess the risk of bias for nonrandomised studies [10]. The tool assesses seven bias domains and views each study as a hypothetical randomised controlled trial. We completed risk of bias assessments independently and in duplicate and resolved disagreements either through consensus or by consulting with a third independent reviewer.
We used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework to assess the certainty of the evidence [11]. The tool was used to apply an overall rating to the body of evidence for each outcome of interest. The major domains of GRADE are risk of bias, imprecision, inconsistency, indirectness, and publication bias. Evidence is graded as very low, low, moderate, or high certainty of evidence. By definition, evidence from nonrandomised data starts with low certainty in the GRADE framework.
For our main analyses, we included only observational studies reporting multivariable-adjusted data. We estimated pooled risk ratios (RR) and their corresponding 95% confidence intervals (CIs) using the inverse variance method. Because several of the included studies were large and deemed to be of greater trustworthiness, and only a small number of studies were included in each of the main analyses, we chose to use fixed-effects models [12, 13]. We used tests of interaction to determine whether the summary results differed between studies with and without multivariable adjustments. When no significant differences were found, we additionally reported the pooled RR across both adjusted and unadjusted studies for each outcome. We quantified between-study statistical heterogeneity using the I2 value. Heterogeneity was considered to be important when I2 was >30% [14]. We calculated absolute risk differences (ARDs) and their corresponding 95% CIs for each outcome using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions [15]. We estimated the baseline absolute long-term risk of events using the most representative data available from patients with perioperative atrial fibrillation.
We performed subgroup analyses of studies with multivariable adjustment at low or moderate versus high or critical risk of bias. For the outcome of stroke ± systemic embolism, we performed a sensitivity analysis excluding studies with multivariable adjustments that defined stroke as total stroke (i.e., included non-ischemic strokes). We also performed sensitivity analyses using random-effects models for each outcome. We conducted all analyses using Review Manager (Cochrane Collaboration), version 5.4. Analyses were two-tailed with statistical significance set at P <0.05.
We identified 21,212 unique citations through database searches. After reviewing the full text of 167 articles and undertaking consultations with field experts, 7 nonrandomised studies (including 1 unpublished analysis from the POISE trials) met our eligibility criteria [4, 16–21]. No randomised controlled trials were identified. A flow diagram of the study selection process is shown in supplemental figure S1. Of the 27,822 participants with perioperative atrial fibrillation included, 29.1% reported using anticoagulation after surgery. Three of the included studies provided multivariable adjusted results [4, 17, 18], of which 3 reported stroke ± systemic embolism, 2 reported mortality, 1 reported bleeding, and 1 reported myocardial infarction. No studies reported the risk of venous thromboembolism.
We contacted and received clarifications on the number of participants receiving anticoagulation from 1 study author [21] and the number of events from 1 study author [19]. We obtained the original study data from the POISE-1 and POISE-2 trials and conducted a combined observational analysis consisting of patients with perioperative atrial fibrillation using multivariable Cox regression analyses (supplemental methods S2) [4, 22–24].
The main characteristics of the 7 included studies are outlined in table 1. Additional study characteristics are available in supplemental table S1. The average participant age was 75 years (SD 10.1), and 53.1% were female. Studies included patients undergoing noncardiac surgery (5 studies), thoracic surgery (1 study), and lung transplantation (1 study). All 3 studies reporting multivariable adjusted results reported long-term patient outcomes, with an average follow-up time of 4.1 years (range 0.7 to 4.3). Of the patients who received anticoagulation in these 3 studies, 99.9% were given an oral formulation of anticoagulation.
Table 1Baseline characteristics of the 7 included studies. Baseline characteristics are presented as anticoagulation group / no anticoagulation group where available.
| Author | Year | Country | Type of Surgery | Surgery N | POAF N (%) | POAF Definition | Postoperative medications | CHADS2 Score | CHA₂DS₂-VASc Score | Follow up | |
| AC Use % | ASA Use % | ||||||||||
| Barnes | 2020 | Australia | Lung transplantation | 394 | 100 (25.4%) | Sustained atrial fibrillation confirmed by 12 lead ECG, causing symptoms, and occurring within 30 days of surgery | 39.0 | – | – | – | 30 days |
| Butt | 2018 | Denmark | Noncardiac | 1,520,109 | 3830 (0.3%) | Primary or secondary discharge diagnosis of atrial fibrillation during index hospitalisation by ICD 8 or 10 | – | 23.7 | 1.4 | 3.0 | 3.2 years |
| Elharram | 2020 | Canada | Noncardiac | – | 22,007 | Secondary diagnosis of atrial fibrillation or atrial fibrillation coded as complication of admission by ICD 9 or 10 | 29.4 | 26.2 / 46.2 | – | – | 4.3 years |
| Hyun | 2021 | South Korea | Noncardiac | 322,688 | 315 (0.1%) | Atrial fibrillation diagnosed using ECGs as adjudicated by two authors, occurring before hospital discharge (up to 90 days after admission) | 26.2 | 46.2 | – | 3.0 / 2.1 | Up to 2 years |
| Makhija | 2011 | United States | Thoracic | – | 759 | Atrial fibrillation for >1 hour with ECG documentation; all patients were continuously monitored for 24 hours after surgery within 30 days of surgery or during the index hospitalisation | 30.0 | 30.5 / 17.7 | 1.3 / 1.1 | – | Up to 30 days or during index hospitalisation |
| POISE 1 & 2 | – | International | Noncardiac | 18,361 | 404 (2.2%) | Atrial fibrillation that resulted in angina, congestive heart failure, or symptomatic hypotension, or that required treatment with a rate controlling drug, antiarrhythmic drug, or cardioversion | 14.6 | – | 2.2 / 1.8 | 4.0 / 3.6 | Up to 1 year |
| Siontis | 2020 | United States | Noncardiac | – | 452 | Atrial fibrillation on ECG or rhythm strip during emergency visit, hospitalisation or echocardiogram report within 30 days of surgery | 23.6 | – | 2 | 4 | 5.4 years |
AC: anticoagulation; ASA: acetylsalicylic acid; CABG: coronary artery bypass surgery; CHADS2 score: congestive heart failure, hypertension, age, diabetes, stroke/thromboembolism; CHA₂DS₂-VASc Score: congestive heart failure, hypertension, age, diabetes, stroke/thromboembolism, vascular disease, sex (female); ECG: electrocardiogram; POAF: perioperative atrial fibrillation
The diagnosis of perioperative atrial fibrillation was determined by retrospective chart review in 4 studies, International Classification of Diseases (ICD) codes from administrative databases in 2 studies, and prospective data collection from a randomised trial in 1 study. No studies included patients with preoperative atrial fibrillation. Postoperative anticoagulation status was determined at hospital discharge in 4 studies, during index hospitalisation in 2 studies, and within 30 days after discharge in 1 study. Anticoagulation use after discharge was assessed by 2 of the 5 studies reporting long-term outcomes [25, 26]. For the outcome of stroke ± systemic embolism, 2 studies reported a composite of ischemic stroke and systemic embolism, 2 studies reported stroke but did not provide an outcome definition, 1 study reported a composite of ischemic stroke and transient ischemic attack, and 1 study reported total stroke. For the outcome of bleeding, 1 study included multiple specific types of bleeding (i.e., intracranial, gastrointestinal, intra-ocular, haematuria, haemoptysis, epistaxis) [18], 1 study included events adjudicated as Bleeding Academic Research Consortium type 3 bleeding [19], and 1 study did not specify the definition of bleeding [20].
Six of the seven studies were at a high or critical risk of bias. A summary of the risk of bias assessments is available in supplemental table S2.
Among the studies that reported multivariable-adjusted results, the relative risk of stroke ± systemic embolism was significantly lower in patients using anticoagulation compared to those not using anticoagulation (RR 0.73; 95% CI, 0.62–0.85; p<0.0001; I2 = 81%; 3 studies, n = 26,208). The results remained similar after pooling adjusted and unadjusted studies (RR 0.74; 95% CI, 0.64–0.86, I2 = 69%; 7 studies, n = 27,819) (figure 1). The estimated long-term incidence of stroke ± systemic embolism was 1.2 events per 100 person-years in patients using anticoagulation versus 1.6 events per 100 person-years in patients not using anticoagulation (ARD –0.4; 95% CI, –0.6 to –0.2). The certainty of the evidence was very low and was rated down due to the presence of moderate to high statistical heterogeneity (supplemental table S3). Subgroup analyses demonstrated that a single study at moderate risk of bias had a lower relative risk of thromboembolism compared to two studies at high or critical risk of bias (P for interaction = 0.0001). The subgroup and sensitivity analyses are further detailed in supplemental table S4.

Figure 1 Forest plot for stroke ± systemic embolism stratified by the use of multivariable adjustment. AC: anticoagulation
A single study using multivariable adjustment found that patients using anticoagulation had a higher risk of bleeding than those not using anticoagulation (RR 1.14; 95% CI, 1.04–1.25; n = 22,007). The results remained similar after pooling adjusted and unadjusted studies (RR 1.15; 95% CI, 1.05–1.26; I2 = 0%; 3 studies, n = 23,081) (figure 2). The estimated long-term incidence of bleeding was 3.8 per 100 person-years in patients using anticoagulation versus 3.3 per 100 person-years in patients not using anticoagulation (ARD 0.5; 95% CI, 0.1–0.8). The certainty of the evidence was very low and was rated down due to concerns regarding the risk of bias and the inability to assess consistency of the evidence with only a single study (supplemental table S3).
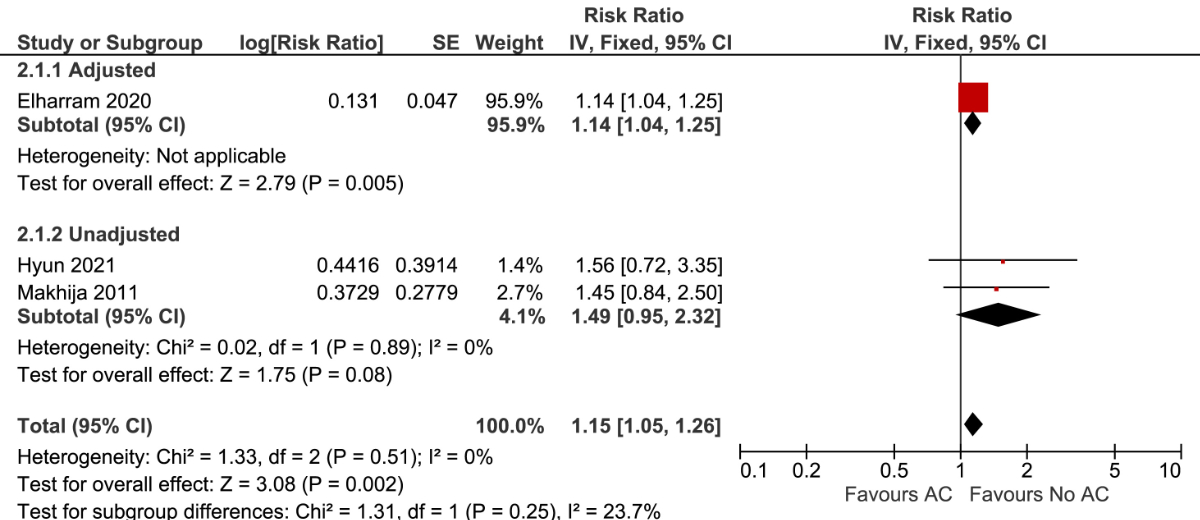
Figure 2 Forest plot for bleeding stratified by the use of multivariable adjustment.
AC: anticoagulation.
The study results for individual outcomes are summarised in table 2. Among studies reporting multivariable adjusted results, there was a statistically significant difference in mortality risk between patients with anticoagulation use compared to those without anticoagulation use (RR 0.45; 95% CI, 0.40–0.51; I2 = 80%; 2 studies, n = 4154; very low certainty) (supplemental figure S2). A single study reporting multivariable-adjusted results found no significant difference in the risk of myocardial infarction (RR 2.19; 95% CI, 0.97–4.96; n = 364; very low certainty) (supplemental figure S3).
Table 2Summary of study results.
| Outcome | N of participants (N of studies) | Relative effect (95% CI) | Anticipated absolute effects in study population (95% CI) (Person-Years) | Certainty of the evidence | ||
| Risk without anticoagulation | Risk with anticoagulation | Difference | ||||
| Stroke ± systemic embolism | 26,208 patients (3) | 0.73 (0.62–0.85) | 1.6 per 100 | 1.2 per 100 (1.0 to 1.4) | 0.4 fewer per 100 (0.6 fewer to 0.2 fewer) | ⊕◯◯◯ Very low |
| Bleeding | 22,007 patients (1) | 1.14 (1.04–1.25) | 3.3 per 100 | 3.8 per 100 (3.4 to 4.1) | 0.5 more per 100 (0.1 more to 0.8 more) | ⊕◯◯◯ Very low |
| Mortality | 4154 patients (2) | 0.45 (0.40–0.51) | 14.8 per 100 | 6.7 per 100 (5.9 to 7.6) | 8.1 fewer per 100 (8.9 fewer to 7.3 fewer) | ⊕◯◯◯ Very low |
| Myocardial Infarction | 364 patients (1) | 2.19 (0.97–4.96) | 12.7 per 100 | 27.7 per 100 (12.3 to 62.7) | 15.1 more per 100 (0.4 fewer to 50.1 more) | ⊕◯◯◯ Very low |
In this systematic review and meta-analysis including over 25,000 participants with perioperative atrial fibrillation after noncardiac surgery, anticoagulation use was associated with a lower risk of stroke ± systemic embolism and death but a higher risk of bleeding. However, no randomised trials were identified in the review, and the overall certainty of the evidence was very low.
Patients with perioperative atrial fibrillation are sometimes prescribed anticoagulation after noncardiac surgery. Studies in our review reported that between 15 and 30% of patients are given anticoagulation shortly after perioperative atrial fibrillation, although it is likely that fewer patients continue to receive treatment during long-term follow-up. The heterogeneous uptake of anticoagulation use in this population likely reflects the fact that physicians hold differing opinions on whether perioperative atrial fibrillation is a transient postoperative phenomenon or the first manifestation of clinical atrial fibrillation requiring anticoagulation based on current risk stratification schemes [27]. In the absence of high-quality evidence, clinicians are faced with a difficult decision when it comes to balancing the potential risks and benefits of using anticoagulation in this population, and it is unclear whether patients are currently being under- or overtreated for this condition. Although some international atrial fibrillation guidelines have suggested the use of anticoagulation in patients with perioperative atrial fibrillation and additional stroke risk factors [28, 29], our review found no high-quality data available to support such recommendations.
One could argue that oral anticoagulation should be prescribed with caution in this population for several reasons. First, we found that the long-term absolute risk of stroke ± systemic embolism in this population was low, with an estimated incidence of 1.2 events and 1.6 events per 100 person-years in patients with and without anticoagulation use, respectively. As the absolute risk difference appears to be relatively small, and bleeding risk is generally higher in elderly populations that are anticoagulated [30], it is possible that the risks could outweigh any potential benefits. However, high-quality data are needed to establish the net benefit-to-risk ratio of anticoagulation in this population. Second, anticoagulation may be less effective in preventing thromboembolism in patients with perioperative atrial fibrillation than in those with non-operative atrial fibrillation. Whereas oral anticoagulation reduces the long-term relative risk of thromboembolism by 62 to 73% in non-operative atrial fibrillation [31], anticoagulation only led to a 27% relative risk reduction in perioperative atrial fibrillation patients. One potential explanation for these differences is that non-cardioembolic strokes may be more common in perioperative atrial fibrillation patients than in non-operative atrial fibrillation patients, and anticoagulation is usually much less effective in these stroke types. This hypothesis is supported by the fact that perioperative atrial fibrillation and atherosclerosis share many of the same risk factors and that a strong association between perioperative atrial fibrillation and myocardial infarction has been previously observed [4]. Third, the risk of clinically important bleeding with anticoagulation use is challenging to estimate for this population due to the heterogeneous bleeding definitions used in previous studies. For example, a retrospective cohort study of more than 20,000 Canadian patients demonstrated an increased bleeding risk with oral anticoagulation use after perioperative atrial fibrillation. However, the definition of bleeding used in this study also included potential minor bleeding events, such as epistaxis. The use of administrative data also limits the interpretation of these results [18, 32]. Of the two other studies included in this review that reported bleeding risk, one included only very serious bleeding events [19], while the other did not provide a definition of bleeding [20]. Large randomised controlled trials, such as the ASPIRE-AF trial, will provide better information on the safety and efficacy of anticoagulation use in this population [33]. Until such data are available, the decision to use anticoagulation in perioperative atrial fibrillation should be carefully considered based on individual thromboembolic and bleeding risks.
Our systematic review has limitations. We identified few studies with multivariable-adjusted data, observed a substantial degree of unexplained heterogeneity, and found that several studies were at increased risk of bias. Consequently, we determined that the certainty of the evidence was very low. Most studies did not account for whether anticoagulation was used beyond the early postoperative period. As it is likely that some patients had their anticoagulation discontinued over time, our risk estimates may have been underestimated. The 2 largest studies we included in our review diagnosed perioperative atrial fibrillation based on ICD codes from administrative databases. As the use of ICD codes has not been validated for the diagnosis of perioperative atrial fibrillation, diagnostic misclassification may have occurred. Patients with perioperative atrial fibrillation are sometimes prescribed single antiplatelet therapy in lieu of anticoagulation. Antiplatelet therapy may have an effect on outcomes similar to those of anticoagulation in this population. Therefore, risk estimates may have been underestimated in studies that did not account for antiplatelet use in their analyses. It is possible that there are other unpublished data apart from the POISE trials that we included. Other unpublished data, apart from the POISE trials from research groups not involved in the current meta-analysis, may exist.
In this systematic review and meta-analysis of patients with perioperative atrial fibrillation after noncardiac surgery, anticoagulation use was associated with a reduced risk of stroke ± systemic embolism and mortality but an increased risk of bleeding. The certainty of the evidence is very low, and the net benefit remains uncertain. Randomised trials are needed to address this frequently encountered problem after noncardiac surgery.
The authors declare no funding sources for this study. Dr. Wang is supported by the Canada Graduate Scholarship – Master’s (Canadian Institutes of Health Research) and the PSI Foundation Research Trainee Award.
Dr. Blum has received grants from the Swiss National Science foundation, the Mach-Gaensslen foundation and the Bangerter-Rhyner foundation outside the submitted work. Dr. McIntyre has received speaking fees from Bayer, Servier, and Boehringer Ingelheim, outside the submitted work. Dr. Healey has received grants and speaking fees from Abbott, BMS/Pfizer, Bayer, Boston Scientific, Medtronic, and Servier, outside the submitted work. Dr. Devereaux has received grants from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, Roche Diagnostics and Siemens, outside the submitted work. Dr. Devereaux has participated in advisory board meetings for Boehringer Ingelheim, Bayer and Quidel Canada, and has attended an expert panel meeting with Boehringer Ingelheim, outside the submitted work. Dr. Conen has received consultancy fees from Roche Diagnostics and Trimedics, and speaker fees from BMS/Pfizer and Servier, outside the submitted work. All other authors have no conflicts of interest to declare.
1. McIntyre WF , Vadakken ME , Rai AS , Thach T , Syed W , Um KJ , et al. Incidence and recurrence of new-onset atrial fibrillation detected during hospitalization for non-cardiac surgery: a systematic review and meta-analysis. Can J Anaesth. 2021 Jul;68(7):1045–56. https://doi.org/10.1007/s12630-021-01944-0
2. Bessissow A , Khan J , Devereaux PJ , Alvarez-Garcia J , Alonso-Coello P . Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost. 2015 Jun;13 Suppl 1:S304–12. https://doi.org/10.1111/jth.12974
3. AlTurki A , Marafi M , Proietti R , Cardinale D , Blackwell R , Dorian P , et al. Major Adverse Cardiovascular Events Associated With Postoperative Atrial Fibrillation After Noncardiac Surgery: A Systematic Review and Meta-Analysis. Circ Arrhythm Electrophysiol. 2020 Jan;13(1):e007437. https://doi.org/10.1161/CIRCEP.119.007437
4. Conen D , Alonso-Coello P , Douketis J , Chan MT , Kurz A , Sigamani A , et al. Risk of stroke and other adverse outcomes in patients with perioperative atrial fibrillation 1 year after non-cardiac surgery. Eur Heart J. 2020 Feb;41(5):645–51. https://doi.org/10.1093/eurheartj/ehz431
5. Huynh JT , Healey JS , Um KJ , Vadakken ME , Rai AS , Conen D , et al. Association Between Perioperative Atrial Fibrillation and Long-term Risks of Stroke and Death in Noncardiac Surgery: Systematic Review and Meta-analysis. CJC Open. 2021 Jan;3(5):666–74. https://doi.org/10.1016/j.cjco.2020.12.025
6. López-López JA , Sterne JA , Thom HH , Higgins JP , Hingorani AD , Okoli GN , et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017 Nov;359:j5058. https://doi.org/10.1136/bmj.j5058
7. Katritsis DG , Gersh BJ , Camm AJ . Anticoagulation in Atrial Fibrillation - Current Concepts. Arrhythm Electrophysiol Rev. 2015 Aug;4(2):100–7. https://doi.org/10.15420/AER.2015.04.02.100
8. Stroup DF , Berlin JA , Morton SC , Olkin I , Williamson GD , Rennie D , et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr;283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008
9. Wang MK , Meyre PB , Heo R , Devereaux PJ , Birchenough L , Whitlock R , et al. Short-term and Long-term Risk of Stroke in Patients With Perioperative Atrial Fibrillation After Cardiac Surgery: Systematic Review and Meta-analysis. CJC Open. 2021 Sep;4(1):85–96. https://doi.org/10.1016/j.cjco.2021.09.011
10. Sterne JA , Hernán MA , Reeves BC , Savović J , Berkman ND , Viswanathan M , et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct;355:i4919. https://doi.org/10.1136/bmj.i4919
11. Guyatt G , Oxman AD , Akl EA , Kunz R , Vist G , Brozek J , et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011 Apr;64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026
12. Tufanaru C , Munn Z , Stephenson M , Aromataris E . Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid-Based Healthc. 2015 Sep;13(3):196–207. https://doi.org/10.1097/XEB.0000000000000065
13. Murad MH , Montori VM , Ioannidis JP , Prasad K , Cook DJ , Guyatt G . Fixed-Effects and Random-Effects Models. In: Guyatt G , Rennie D , Meade MO , Cook DJ , editors . Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 3rd ed. New York (NY): McGraw-Hill Education; 2015.
14. Chapter 10. In: Deeks J , Higgins J , Altman D . Analysing data and undertaking meta-analyses. Cochrane; 2019. Available from https://training.cochrane.org/handbook/current/chapter-10[ [cited 2020 Sep 16]]. https://doi.org/10.1002/9781119536604.ch10
15. Schünemann HJ , Viste GE , Higgings JP , Santesso N , Deesk JJ , Glasziou P , et al. Chapter 15: Interpreting results and drawing conclusions. 2020 [Feb 4, 2021]; Version 6.1
16. Barnes H , Gurry G , McGiffin D , Westall G , Levin K , Paraskeva M , et al. Atrial Flutter and Fibrillation Following Lung Transplantation: Incidence, Associations and a Suggested Therapeutic Algorithm. Heart Lung Circ. 2020 Oct;29(10):1484–92. https://doi.org/10.1016/j.hlc.2019.10.011
17. Butt JH , Olesen JB , Havers-Borgersen E , Gundlund A , Andersson C , Gislason GH , et al. Risk of Thromboembolism Associated With Atrial Fibrillation Following Noncardiac Surgery. J Am Coll Cardiol. 2018 Oct;72(17):2027–36. https://doi.org/10.1016/j.jacc.2018.07.088
18. Elharram M , Samuel M , AlTurki A , Quon M , Behlouli H , Bessissow A , et al. Anticoagulant Use and the Risk of Thromboembolism and Bleeding in Postoperative Atrial Fibrillation After Noncardiac Surgery. Can J Cardiol. 2021 Mar;37(3):391–9. https://doi.org/10.1016/j.cjca.2020.08.023
19. Hyun J , Cho MS , Nam GB , Kim M , Do U , Kim J , et al. Natural Course of New-Onset Postoperative Atrial Fibrillation after Noncardiac Surgery. J Am Heart Assoc. 2021 Apr;10(7):e018548. https://doi.org/10.1161/JAHA.120.018548
20. Makhija Z , Allen MS , Wigle DA , Shen KR , Cassivi SD , Nichols FC 3rd , et al. Routine anticoagulation is not indicated for postoperative general thoracic surgical patients with new-onset atrial fibrillation. Ann Thorac Surg. 2011 Aug;92(2):421–6. https://doi.org/10.1016/j.athoracsur.2011.04.066
21. Siontis KC , Gersh BJ , Weston SA , Jiang R , Kashou AH , Roger VL , et al. Association of New-Onset Atrial Fibrillation After Noncardiac Surgery With Subsequent Stroke and Transient Ischemic Attack. JAMA. 2020 Sep;324(9):871–8. https://doi.org/10.1001/jama.2020.12518
22. Devereaux PJ , Mrkobrada M , Sessler DI , Leslie K , Alonso-Coello P , Kurz A , et al.; POISE-2 Investigators . Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014 Apr;370(16):1494–503. https://doi.org/10.1056/NEJMoa1401105
23. Devereaux PJ , Sessler DI , Leslie K , Kurz A , Mrkobrada M , Alonso-Coello P , et al.; POISE-2 Investigators . Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014 Apr;370(16):1504–13. https://doi.org/10.1056/NEJMoa1401106
24. Devereaux PJ , Yang H , Yusuf S , Guyatt G , Leslie K , Villar JC , et al.; POISE Study Group . Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008 May;371(9627):1839–47. https://doi.org/10.1016/S0140-6736(08)60601-7
25. Butt JH , Olesen JB , Gundlund A , Kümler T , Olsen PS , Havers-Borgersen E , et al. Long-term Thromboembolic Risk in Patients With Postoperative Atrial Fibrillation After Left-Sided Heart Valve Surgery. JAMA Cardiol. 2019 Nov;4(11):1139–47. https://doi.org/10.1001/jamacardio.2019.3649
26. Butt JH , Xian Y , Peterson ED , Olsen PS , Rørth R , Gundlund A , et al. Long-term Thromboembolic Risk in Patients With Postoperative Atrial Fibrillation After Coronary Artery Bypass Graft Surgery and Patients With Nonvalvular Atrial Fibrillation. JAMA Cardiol. 2018 May;3(5):417–24. https://doi.org/10.1001/jamacardio.2018.0405
27. Wang MK , Douketis J . Postoperative atrial fibrillation after non-cardiac surgery: how important is it and what do we do about it? Eur J Intern Med. 2021 Mar;85:25–6. https://doi.org/10.1016/j.ejim.2021.01.024
28. January CT , Wann LS , Alpert JS , Calkins H , Cigarroa JE , Cleveland JC Jr , et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014 Dec;64(21):e1–76. https://doi.org/10.1016/j.jacc.2014.03.022
29. Hindricks G , Potpara T , Dagres N , Arbelo E , Bax JJ , Blomström-Lundqvist C , et al.; ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021 Feb;42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612
30. Torn M , Bollen WL , van der Meer FJ , van der Wall EE , Rosendaal FR . Risks of oral anticoagulant therapy with increasing age. Arch Intern Med. 2005 Jul;165(13):1527–32. https://doi.org/10.1001/archinte.165.13.1527
31. Tereshchenko LG , Henrikson CA , Cigarroa J , Steinberg JS . Comparative Effectiveness of Interventions for Stroke Prevention in Atrial Fibrillation: A Network Meta-Analysis. J Am Heart Assoc. 2016 May;5(5):e003206. https://doi.org/10.1161/JAHA.116.003206
32. McIntyre WF , Wang MK , Conen D . Balancing the Risks and Benefits of Oral Anticoagulant Use in Patients With Postoperative Atrial Fibrillation. Can J Cardiol. 2021 Jun;37(6):938.e11. https://doi.org/10.1016/j.cjca.2020.10.011
33. National Institutes of Health - United States National Library of Medicine . Anticoagulation for stroke prevention in patients with recent episodes of perioperative atrial fibrillation after noncardiac surgery (ASPIRE-AF). 2022 [April 7, 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03968393
The appendix is available in the pdf version of the article.