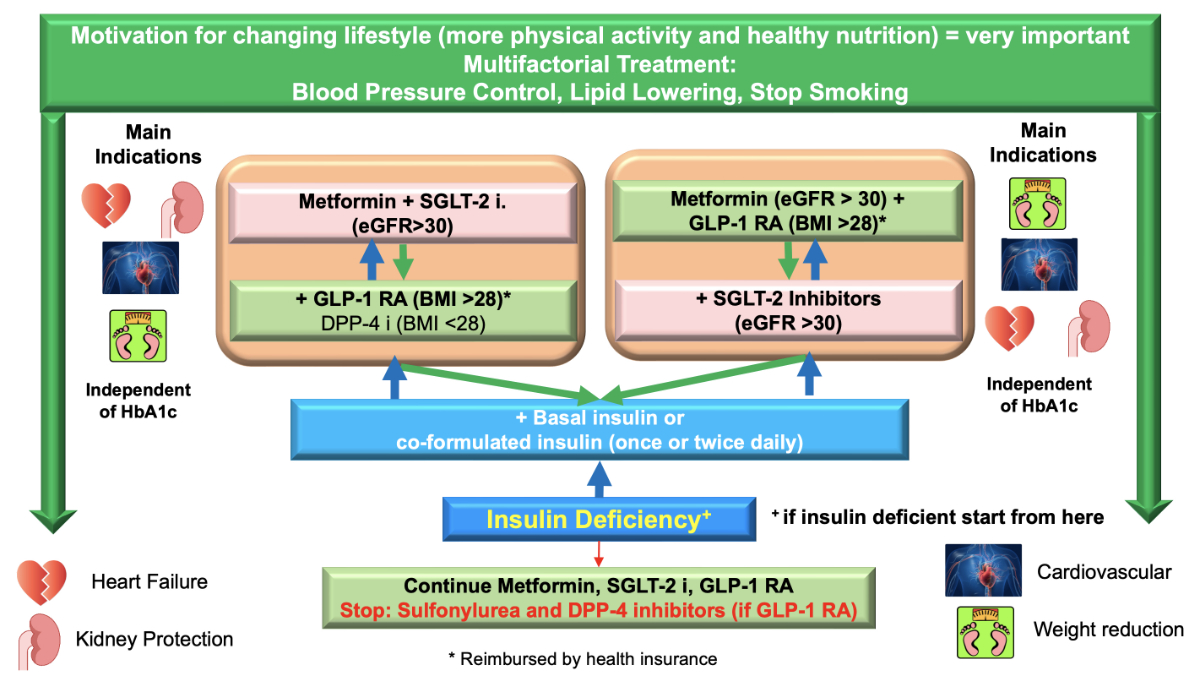
Figure 1 Flow chart of the updated 2023 recommendations at a glance. Co-formulated insulin: Ultra-long-acting insulin Degludec (70%) and short-acting insulin Aspart (30%).
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40060
dipeptidyl-peptidase 4 inhibitors
glucagon-like 1 peptide
glucose-dependent insulinotropic polypeptid
sodium-glucose transporter 2 inhibitors
With the announcement in the year 2008 by the Federal Drug Administration (FDA) in the USA that each new antidiabetic drug has to prove its cardiovascular safety in a cardiovascular outcome trial, a revolution in diabetes treatment took place. The initial trials with DPP-4 inhibitors proved their cardiovascular safety, but no additional short-term benefits were seen in these trials [1–4].
In 2015, the EMPA-REG trial demonstrated that empagliflozin, an SGLT-2 inhibitor, was able to reduce 3-point major adverse cardiovascular effects (MACE), cardiovascular death, hospitalisation for heart failure, combined kidney outcome, and even mortality. The LEADER trial with liraglutide, a GLP-1 receptor agonist, was able to achieve significant results in 3-point MACE, cardiovascular death, combined kidney outcomes, and mortality as well. These two trials were the beginning of a success story of all SGLT-2 inhibitors and GLP-1 receptor agonists. What was newly discovered by these trials was the benefit of all SGLT-2 inhibitors for a reduction in hospitalisation due to heart failure (both reduced and preserved ejection fraction) [5] and the reduction of stroke in all GLP-1 receptor agonist trials [6]. Taking all these trials into account, we call this a diabetes revolution [7–15], which resulted in the updated Swiss Society for Endocrinology and Diabetes (SGED/SSED) recommendations illustrated in figure 1. A summary of all these trials with the major outcomes is shown in figure 2.

Figure 1 Flow chart of the updated 2023 recommendations at a glance. Co-formulated insulin: Ultra-long-acting insulin Degludec (70%) and short-acting insulin Aspart (30%).
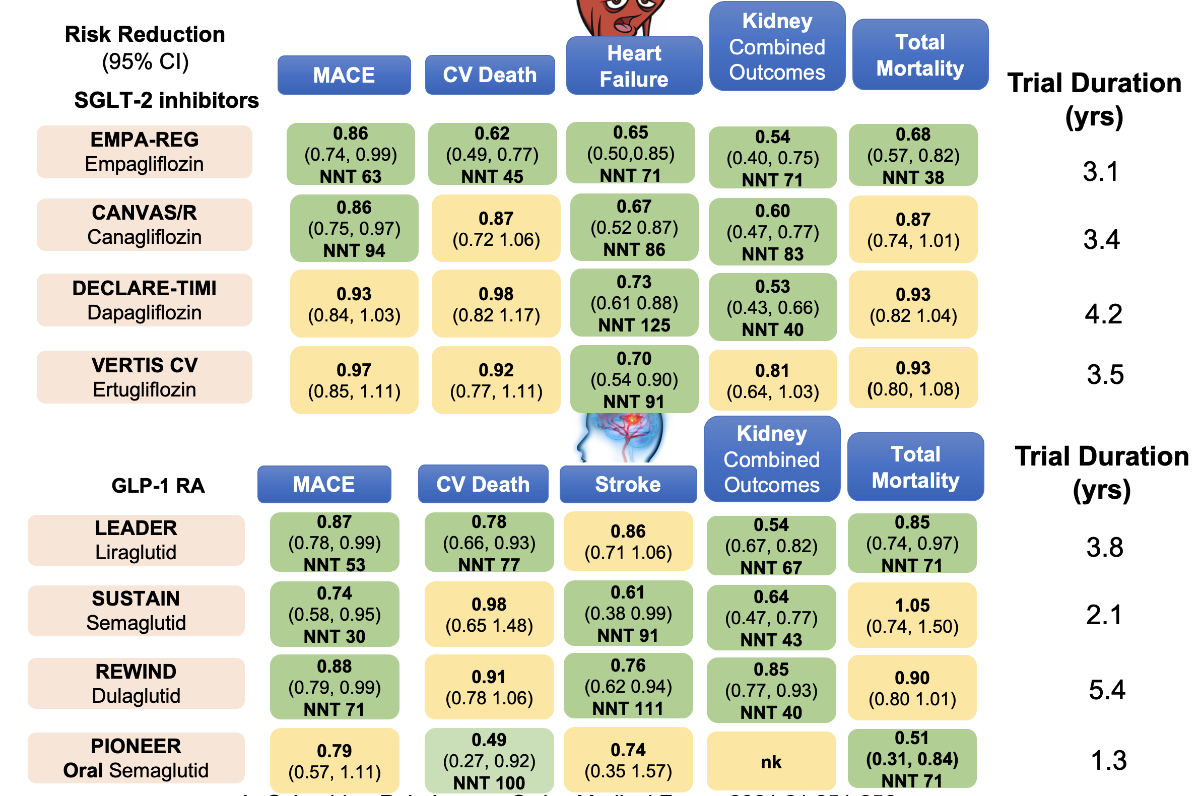
Figure 2 Summary of cardiovascular outcome trials with SGLT-2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus [62].
The new European Association for the Study of Diabetes (EASD) / American Diabetes Association (ADA) guidelines of diabetes [16] have carefully incorporated these previously mentioned trial outcomes, and they put emphasis on cardiorenal protection, prevention, or treatment of heart failure on one side and achieving weight reduction and reducing glycaemia on the other side. However, the focus of the Swiss recommendations lies on the general practitioners that take care of most people with type 2 diabetes and, therefore, the EASD/ADA guidelines are still too detailed. Because basically all patients with type 2 diabetes belong to the high or very high cardiovascular risk group, as defined by the European Cardiology Society (table 1) [17], the recommendations for cardiorenal protection or prevention or treatment of heart failure apply to all patients with type 2 diabetes, which simplifies treatment recommendations considerably.
Table 1Cardiovascular risks in diabetes (ESC 2021) [17].
| Patient category | Subgroups | Risk categories | CVD risk and therapy benefits estimation | |
| Patients with type 2 diabetes mellitus; patients with type 1 diabetes >40 years of age may be classified according to these criteria | Patients with well conrolled short-standing diabetes mellitus (<10 years), no evidence of target organ damage and no additional atherosclerotic cardiovascular disease risk factors | Moderate risk | N/A | |
| Patients with diabetes mellitus without atherosclerotic cardiovascular disease and/or severe target organ damage, and not fulfilling the moderate risk criteria | High risk | Residual 10-year cardiovascular disease risk estimation after general prevention goals (e.g., with the ADVANCE risk score or DIAL model). Consider lifetime cardiovascular disease risk and benefit estimation of risk factor treatment (e.g., DIAL model) | ||
| Patients with diabetes mellitus with established atherosclerotic cardiovascular diseaseand/or severe target organ damage | eGFR <45 ml/min irrespective of albuminuria | Very high risk | Residual 10-year cardiovascular disease risk estimation after general prevention goals (e.g., with the SMART risk score for established cardiovascular disease or ADVANCE risk score or DIAL model). Consider lifetime cardiovascular disease risk and benefit estimation of risk factor treatment (e.g., DIAL model) | |
| eGFR 45–60 ml/min and microalbuminuria (albumin-creatinine ratio 3–30 mg/mmol) | ||||
| Proteinuria (albumin-creatinine ratio >30 mg/mmol) | ||||
| Presence of microvascular disease in at least 3 different sites (retinopathy plus microalbluminuria plus neuropathy) | ||||
Figure 1 summarizes the new Swiss recommendations for the treatment of type 2 diabetes mellitus. As a first step, we emphasize lifestyle changes (increased physical activity and healthy nutrition) and a multifactorial treatment (blood pressure control and lowering of lipids), as detailed in the next chapter. The initial medical treatment should always be a combination treatment with metformin and an SGLT-2 inhibitor or metformin and a GLP-1 receptor agonist. Metformin is maintained as a first-line treatment because all cardiovascular outcome trials were performed on the basis of metformin treatment and because no other antidiabetic drug has an explicit effect of reducing hepatic glucose production. In persons with type 2 diabetes, if the initial double combination is not sufficient, a triple combination (SGLT-2 inhibitor, GLP-1 receptor agonist, and metformin) is recommended. This triple combination has not been officially tested in the above-mentioned cardiovascular outcome trials, but there is more and more real-world experience in Europe and in the USA [18, 19] that proves that the triple combination with metformin, SGLT-2 inhibitor, and GLP-1 receptor agonist is the best treatment to reduce 3-point major adverse cardiovascular effects, total mortality, and heart failure as compared to other combinations. The combination of metformin and sulfonylurea that was practised for more than 60 years shows more than 10 times higher mortality and more than 7 times higher 3-point MACE occurrence than the modern triple combination [18].
If the triple combination is not sufficient to reduce the HbA1c to the desired target, insulin treatment is necessary. It is important to keep in mind that a quarter of all patients with type 2 diabetes (sometimes misdiagnosed) require insulin treatment. If insulin deficiency is the predominant factor at the outset of type 2 diabetes, the order of medications has to be reversed (figure 1: arrows in blue). Insulin first and then cardio-renal protective medications (SGLT-2 inhibitors and/or GLP-1 receptor agonists) (figure 1).
Lifestyle intervention is recommended as the first-line treatment of pre-diabetes and diabetes at all ages. Healthy nutrition, weight control, and physical activity are essential. Ideally, they should be carried out concomitantly (figure 3).
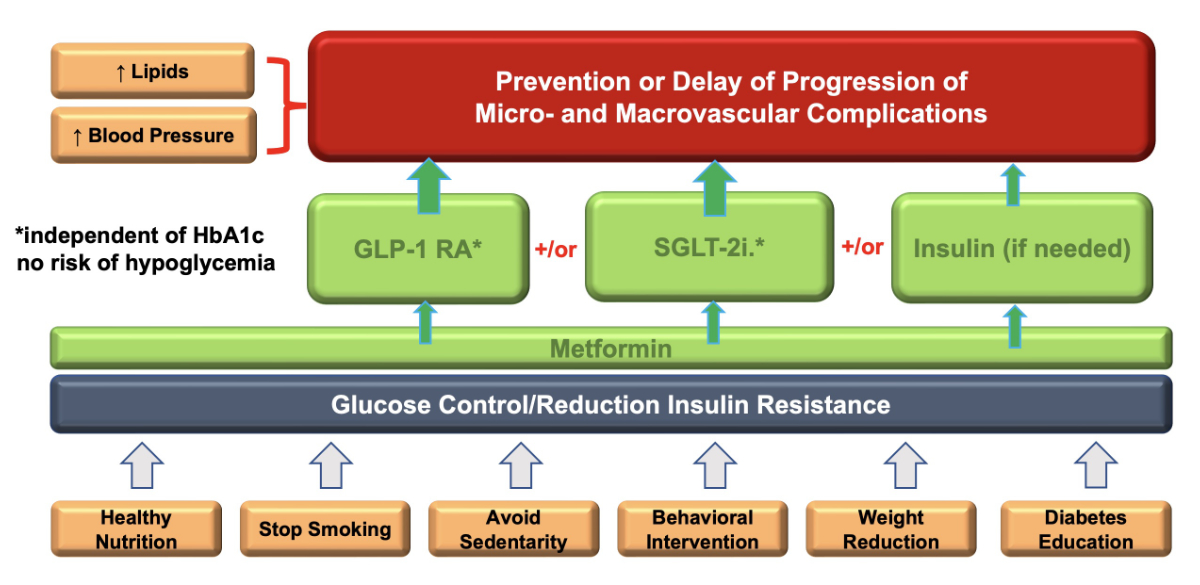
Figure 3 Important factors in diabetes treatment.
The main targets are to improve the following:
With a weight loss of >15% in the DIRECT trial, a diabetes prevention rate of 86% could be achieved [20]. When comparing all diets with each other, the adherence rate to a particular diet is the most important predictor for weight loss [21]. In persons with pre-diabetes and obesity, trials with high-dose GLP-1 receptor agonists or GLP-1/GIP receptor agonists could achieve normalization of glucose levels in 83 to 95% of the cases [22, 23]. A meta-analysis demonstrated the reduction of new-onset type 2 diabetes mellitus in people with prediabetes with SGLT-2 inhibitors [24].
The Steno-2 trial [25] has well demonstrated the role of a multifactorial treatment in the care of type 2 diabetes mellitus, including hyperglycaemia management, blood pressure control, lowering LDL-cholesterol, and stop smoking. It is the task of the treating physician to make a reasonable choice of interventions and the order in which these interventions are introduced in the individual patient. It is crucial to take patients’ preferences into consideration and to prioritize with the patient the impact of body weight, glucose control, and cardio-renal protection in his/her individual condition.
For the control of high LDL cholesterol, a high-potency statin (rosuvastatin, atorvastatin) is the first choice. If the targets cannot be achieved, ezetimibe is added, and if still not at target, pro-protein-C subtilisin kexin type 9 (PCSK-9) inhibitors might be given [17], depending on the respective limitations in a specific country.
The target for blood pressure is also individualised [17] and should be 130/70–79 mm Hg. In a younger patient, the systolic blood pressure can be lowered below 130 mm Hg but should not be below 120 mm Hg, whereas in a person above the age of 65 years, the recommended systolic blood pressure is between 130–139 mm Hg. The choice of drugs is usually an early combination of ACE-inhibitor and a calcium antagonist. If the ACE-inhibitor is not tolerated, an ARB (angiotensin II receptor blocker or sartan) can be given [17]. People with diabetes have a higher platelet reactivity and turnover, resulting in a pro-thrombotic status [17]. Platelet aggregation inhibition by aspirin or by other drugs is accepted in patients with established cardiovascular disease, but it is generally not recommended in primary prevention.
Considering that non-adherence to diabetes medical standards (<2 × HbA1c, Lipid profile at target, nephropathy status and ophthalmologist visit per year) in diabetes care is associated with an increased probability of future hospitalization among patients with diabetes [26, 27] (table 2). The suggested target in practice is ≥70 out of 100 points among all patients with type 2 diabetes mellitus [27].
Table 2SGED Score [26, 27]: suggested target: ≥70/100 points.
| Criteria | Intervention | Aim (on a yearly basis) | Points |
| Regular visits | General diabetes control | <80% of patients with ≥3 visits | 10 |
| Lifestyle measures | BMI >25, physical exercise, and nutrition counselling | <80% of patients have BMI ≤25 or if BMI >25 received counselling ≥1/year | 5 |
| Nicotin abuse: smoking cessation counselling | >80% patients non-smokers or if active smokers received counselling ≥1/year | 5 | |
| Glycaemic control | HbA1c measurement (DCCT traceable) | Annual mean at least 2 measurements: | |
| ≥85% of patients <9.0% | 12 | ||
| ≥60% of patients <8.0% | +8 | ||
| ≥40% of patients <7.0% | +5 | ||
| Blood pressure | Blood pressure measurement (mm Hg) | Annual mean at least 2 measurements: ≥65% of patients <140/90 mmHg | 15 |
| LDL-C if <75 years of age | LDL-Cholesterol measurement | Annual mean: ≥63% of patients <2.6 mmol/l | 10 |
| Nephropathy screening | Measurement of serum creatinine + microalbuminuria | ≥80% of patients screened | 10 |
| Retinopathy screening | Ophthalmological consultation | ≥80% of patients screened at least every second year | 10 |
| Foot examination | Pulses (Arteria dorsalis pedis, Arteria tibialis posterior), monofilament and vibration sense | ≥80% of patients screened | 10 |
BMI: Body Mass Index; DCCT: Diabetes Control and Complications Trial; HbA1c: haemoglobin A1c; LDL: low-density lipoprotein cholesterol; SGED/SSED: Swiss Society for Endocrinology and Diabetes
In patients with chronic kidney disease (impaired GFR and/or albuminuria), antidiabetic treatment should include SGLT-2 inhibitors independent of glucose control because SGLT-2 inhibitors have shown particularly beneficial cardiorenal effects in patients with and even without diabetes [28]. SGLT-2 inhibitors reduce not only renal and cardiovascular endpoints but also mortality in patients with chronic kidney disease [28]. Although the glucose-lowering efficacy of SGLT-2 inhibitors is reduced or even absent when GFR is markedly decreased, the nephroprotective effects remain preserved, and, therefore, we recommend continuing SGLT-2 inhibitors, even if the GFR falls below 30 ml/min. However, due to limited experience with SGLT-2 inhibitors in patients with severely decreased GFR, we do not recommend starting SGLT-2 inhibitors in patients with GFR <30 ml/min. Usually, SGLT-2 inhibitors are used in combination with metformin. Due to the risk of lactic acidosis, metformin must be discontinued when GFR drops <30 ml/min. With a GFR 30–45 ml/min, the maximum daily dose of metformin is 2 × 500 mg or 1000 mg in retarded form. Also, GLP-1 receptor agonists do have nephroprotective effects, although not to the same extent as SGLT-2 inhibitors. GLP-1 receptor agonists (in patients with BMI >28 kg/m2) can be used without dose adjustment, even in patients with severely decreased GFR or dialysis. DPP-4 inhibitors doe not have short-term nephroprotective effects [1–4], but they can be used as an alternative to GLP-1 receptor agonists (e.g., in patients with BMI <28 kg/m2 or intolerance of GLP-1 receptor agonists). DPP-4 inhibitors are safe to use in patients with decreased GFR, but the dose needs adjustment to kidney function (except linagliptin). Sulfonylureas, including gliclazide, should not be used in patients with eGFR <30 ml/min because of the increased risk of hypoglycaemia. In patients treated with insulin, insulin requirement is reduced, and the risk of hypoglycaemia is increased when kidney function declines. Therefore, insulin regimens and insulin preparations with the lowest risk for hypoglycaemia are preferred in patients with a decreased GFR. A non-steroidal mineralocorticoid receptor antagonist, finerenone, has been shown to decrease the decline in chronic kidney disease in patients with type 2 diabetes mellitus by 22% and reduce the combined cardiovascular outcome by 14% [29–31].
Heart failure is a common complication of diabetes, with a prevalence of up to 30% in individuals with diabetes above the age of 65 years, even in patients without other cardiovascular risk factors [32, 33]. Typical symptoms of heart failure are breathlessness, orthopnea, reduced exercise tolerance, fatigue, tiredness, and ankle swelling. A normal ECG makes the diagnosis of heart failure unlikely. The ECG may reveal abnormalities such as atrial fibrillation, Q waves, LV hypertrophy (LVH), and a widened QRS complex that increase the likelihood of a diagnosis of heart failure and also may guide therapy [34]. If clinical suspicion exists and ECG abnormalities are present, measurement of the following markers is recommended: natriuretic peptides (B-type natriuretic peptide [BNP] or N-terminal pro-BNP [NT-proBNP]) on at least a yearly basis [35]. If NT-proBNP is >125 pg/ml or BNP >35 pg/ml, transthoracic echocardiography will result in the diagnosis of heart failure. It is, however, not recommended as routine screening for all patients with diabetes [34].
SGLT-2 inhibitors are beneficial for the prevention or treatment of all forms of heart failure (HFpEF, HFmEF, HFrEF) with and without diabetes mellitus [36–39].
Regarding metformin treatment, no randomised controlled trials relative to heart failure risk have been performed, but a meta-analysis of nine cohort studies of nearly 34,000 individuals suggests that metformin was associated with a 20% reduced mortality risk and a smaller but significant reduction in all-cause hospitalization in individuals with heart failure compared with control subjects [40].
A meta-analysis with GLP-1 receptor agonists suggests that this group does not only decrease stroke, 3-point major adverse cardiovascular effects, and mortality but also significantly improves the heart failure outcome [41].
Several observational studies that compared sulfonylurea therapy with metformin or with other agents suggested that sulfonylurea may be associated with an increased risk of heart failure events [42]. In meta-analyses and randomised trials, thiazolidinediones showed an increased risk of heart failure, heart failure hospitalization or death, weight gain, lower extremity oedema, and increased cardiovascular risk [43]. Therefore, thiazolidinediones are not recommended in patients with heart failure. DPP-4 inhibitors (especially saxagliptin and alogliptin) are not recommended for patients with diabetes with heart failure, but linagliptin or sitagliptin can be used to lower glucose levels if a GLP-1 receptor agonist is not indicated (BMI <28) or not tolerated [1–4].
Taking all cardiovascular outcome trials together, SGLT2 inhibitors have been clinically proven to be an effective treatment for heart failure, independent of the HbA1c value whether diabetes is present or not. Therefore, they should be introduced as soon as possible for the treatment or prevention of heart failure. If additional glycaemic control is needed, the use of a GLP-1 receptor agonist and metformin is recommended. Insulin should be added if a triple treatment of SGLT-2 inhibitors, GLP-1 receptor agonist, and metformin is not sufficient to reach individual glycaemic targets. Insulin can negatively affect heart failure due to known side effects like fluid retention, weight gain, and hypoglycaemia.
Sixty to 90 percent of all people with type 2 diabetes mellitus are obese [44]. Besides the prevention of micro- and macrovascular complications, the main target of diabetes treatment is, therefore, to reduce weight [16]. Obesity, in combination with a sedentary lifestyle, is the main risk factor associated with type 2 diabetes mellitus and increased insulin resistance. Therefore, losing weight and keeping an active lifestyle with physical activity and resistance training are of utmost importance. With a BMI of >28 kg/m² the use of GLP-1 receptor agonists, in combination with metformin or as monotherapy in the case of metformin intolerance, in the therapy of type 2 diabetes mellitus is reimbursed by health insurance. It has to be mentioned, however, that this group of medications reduces glucose even if the BMI is below 28, but in Switzerland, it will not be reimbursed by health insurance.
The potency of GLP-1 receptor agonists and GLP-1/GIP receptor agonists varies between different GLP-1 receptor agonists and dosages with regard to weight loss. GLP-1 receptor agonists with a high potency are semaglutide or, in higher dosages, liraglutide and dulaglutide. So far, the best results with weight loss have been achieved with 2.4 mg semaglutide and the new GLP-1/GIP receptor agonist tirzepatide. However, the cardiovascular outcome trials of these treatments are still ongoing [16, 22, 23, 45]. GLP-1 receptor agonists in lower dosages with proven evidence for reducing cardiovascular outcomes (semaglutide, liraglutide, or dulaglutide) are, therefore, at present, the preferred treatment option in the vast majority of type 2 diabetes mellitus cases.
In contrast to SGLT-2 inhibitors, GLP-1 receptor agonists lead to a more substantial weight loss and should therefore be given priority over SGLT-2 inhibitors in obese patients with type 2 diabetes [7–15]. The extent of weight loss increases with dose escalation. Currently, high-dose semaglutide administration (2.4 mg semaglutide) has been shown to be the most effective GLP-1 receptor agonist in terms of weight loss, and it is the second GLP-1 receptor agonist with approval for weight loss treatment together with high dose liraglutide (3.0 mg). Close to market approval is tirzepatide, a dual GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist with high efficacy in glycaemic control and weight-loss effect when compared to semaglutide administration. The main limitations of GLP-1 receptor agonists are their side effects, such as nausea and vomiting. These side effects occur mainly in the first days to weeks of therapy. A correlation between the occurrence of nausea as a side effect and the effect on weight loss could not be proven. Conscious nutrition and the avoidance of large portions can sometimes positively influence the symptoms of nausea. Although GLP-1 receptor agonists can also be used in higher-grade renal failure, increased nausea is sometimes a limitation of use, particularly in end-stage renal failure. SGLT-2 inhibitors also have a weight-reducing effect but to a lesser extent. The extended indication of SGLT-2 inhibitors in heart failure and nephroprotection also allows the combination of these drugs in different indications in patients with and without type 2 diabetes [28, 38, 39]. GLP-1 receptor agonists have the greatest effect on weight reduction, and they are given to reduce cardiovascular complications (including stroke), renal complications, and mortality.
In addition to drug therapy, bariatric surgery should be evaluated, and it is considered to be therapeutically effective in difficult-to-control type 2 diabetes with HbA1c >8% and a BMI of >30 kg/m². However, the gap with regard to weight loss between bariatric surgery and high-dose semaglutide or tirzepatide is shrinking [16, 22, 23, 45].
The management of type 2 diabetes in people with NAFLD/NASH should include lifestyle modification with a goal of weight loss, including strong consideration of medical and/or surgical approaches to weight loss in those at higher risk of hepatic fibrosis. GLP-1 receptor agonists have evidence of a benefit. SGLT2 inhibitors have been shown to reduce elevated levels of liver enzymes and hepatic fat content in people with NAFLD, but currently, there is less evidence for SGLT-2 inhibitors as treatment for NASH. NAFLD and, in particular, NASH are also associated with an increased risk of cardiovascular complications (figure 4) [16].
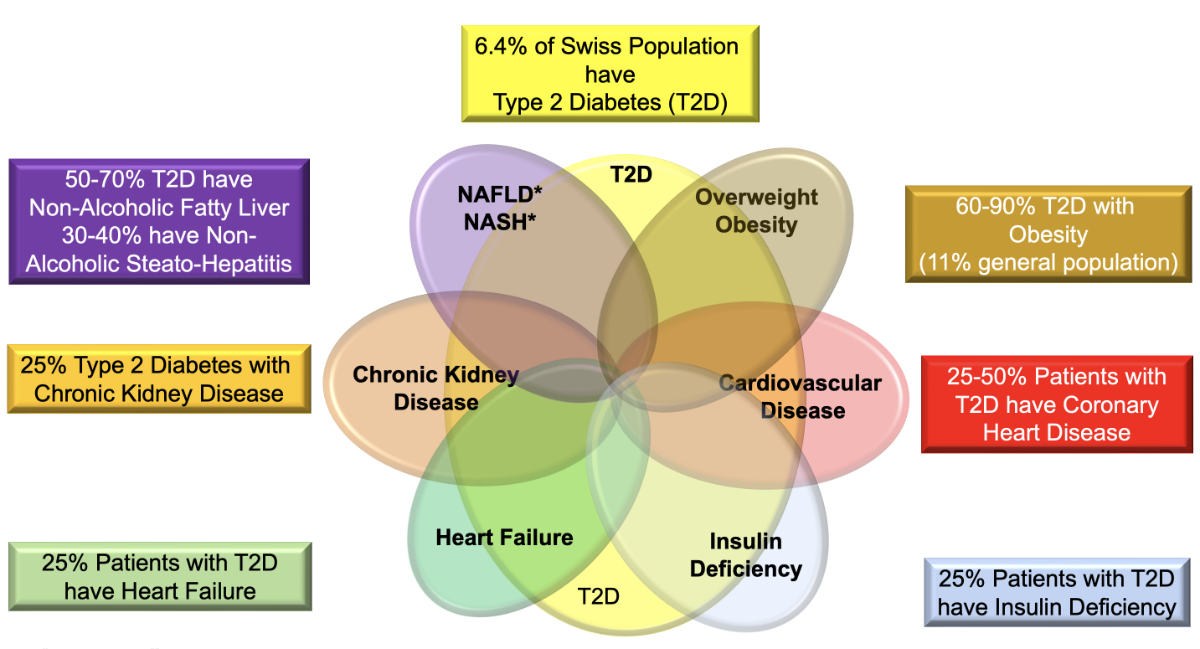
Figure 4 Comorbidities in type 2 diabetes mellitus.
In specific circumstances, insulin may be the preferred agent for lowering glucose, specifically in the setting of severe hyperglycaemia (HbA1c >10%), particularly when associated with the typical signs of insulin deficiency like weight loss or ketonuria/ketosis and with acute glycaemic dysregulation (e.g., during hospitalization, surgery, or acute illness), in normal or underweight people or when the diagnosis of type 1 diabetes is suspected [16]. In these circumstances, giving insulin is never wrong, and after euglycaemia is restored, it might be possible to stop insulin treatment for certain people with type 2 diabetes. Type 2 diabetes is not a uniform disease. The general rule is that two pathogenetic factors are prominent such as insulin resistance and relative insulin deficiency. Either of the two can be dominant and appear before the other. Insulin resistance is generally linked to visceral obesity and physical inactivity. In the face of extreme insulin resistance, even if insulin and C-peptide are in the normal range, the insulin produced may not be sufficient to achieve normal glucose homeostasis.
Attempts to describe subtypes of type 2 diabetes have clearly demonstrated five subtypes of diabetes. In about 6% of the cases, type 1 diabetes was not diagnosed, and an additional 18% of patients show a clear insulin deficiency in the absence of typical antibodies for type 1 diabetes mellitus (anti-GAD, anti-IA2 or anti-ZnT8). A third form in about 15% of patients is characterised by very high insulin resistance and high insulin levels. These patients have more NAFLD/NASH and more renal complications. The remaining two forms are well known: diabetes associated with obesity (20%) and diabetes associated with increasing age (40%) [46].
Whenever treating a patient with type 2 diabetes, the physician should be aware that 25% of patients have an insulin deficiency and sometimes are wrongly diagnosed as type 2 diabetes (type 1 diabetes, monogenic form of diabetes and mitochondrial diabetes, or pancreatic diabetes [chronic pancreatitis]). The contribution of type 1 diabetes and specific forms of diabetes is about 5% each. The pathogenetic mechanism of most specific diabetes forms (except Maturity Onset Diabetes of the Young [MODY] 2, glucokinase mutations) is an insulin deficiency, which is treated in most instances with insulin first (except MODY 1 and 3, which are treated with sulfonylureas), and subsequently considering cardiorenal protective effects with SGLT-2 inhibitors and or GLP-1 receptor agonists (figure 1).
The ADA/EASD developed an algorithm based on age, specific antibodies, family history of diabetes and determination of C-peptide levels to diagnose the correct diabetes type and insulin deficiency [47]. If C-peptide is >200 and <600 pmol/l, specific antibodies are negative, and a strong family history of diabetes is missing, insulin deficiency can be diagnosed. Type 1 diabetes is still possible in the early stages [48], and C-peptide measurement has to be repeated three years later. In this situation, treatment with co-formulated insulin at the main meal or at the two main meals might be preferable to ultra-long-acting insulin alone because postprandial glucose levels are also lowered, and it is an easier treatment than a basal-bolus system with the same HbA1c levels but less hypoglycaemia [49] (figure 1).
The main goal of diabetes control is to maintain the HbA1c as close to normal as possible with avoidance of hypoglycaemia. In most patients, this level will be an HbA1c of 7.0%. In younger people with a short history of diabetes and/or patients with microvascular complications, this goal should be reduced to 6.5 % if this can be reached without significant and repetitive hypoglycaemia. An HbA1c level <6.5% is not dangerous regarding hypoglycaemia or cardiovascular complications if no insulin or no sulfonylurea are used.
For older patients, patients with a history of severe hypoglycaemia, patients with co-morbidities (vision trouble, osteoporosis, neurologic disease such as autonomic neuropathy) or patients with restricted life expectancy, a higher HbA1c target of 7–8% is reasonable. In all instances, an HbA1c level >8.0% should be avoided because the associated complications outweigh the possible benefits of a higher HbA1c. In some cases, avoiding hypoglycaemia and hyperglycaemia may be the only goal and a reduction in the number of medications may be necessary. The physician and the patient will decide together on an individual Hb1Ac target, which could change over time. For an HbA1c target of 7.0 %, most of the patients will need a blood sugar pre-prandial <7 mmol/l and post prandial <10 mmol/l. In most cases, a satisfactory HbA1c will be reached when the patient achieves a time in a range of 3.9 to 10 mmol/l in ≥70%.
It has been shown that hypoglycaemia is associated with worse outcomes and higher mortality. As GLP-1 receptor agonists and SGLT-2 inhibitors are not associated with a risk of hypoglycaemia and are efficient in reducing blood glucose, they represent the first choice of medication with concomitant metformin use. Intensive insulin treatment (basal-bolus insulin), with or without the other medications, has the highest rate of hypoglycaemia. The use of an ultra-long-acting basal insulin, however, has quite a low rate of hypoglycaemia, even lower than the use of sulfonylurea [18]. The highest hypoglycaemia rates are produced with the combined use of insulin and sulfonylurea. Therefore, this combination should always be avoided.
While the use of sulfonylureas – which are associated with hypoglycaemia – has dropped dramatically in the last years in favour the newer medications, they are still used in selected cases (e.g., maturity-onset diabetes of the young [MODY] 1 and 3). The highest risk exists in long-acting sulfonylureas with active metabolites (glibenclamide, glimepiride). At the present time, we recommend only gliclazide as the risk of hypoglycaemia with this specific molecule is very low due to the shorter half-life and no active metabolites.
We prefer a basal insulin over a sulfonylurea when the HbA1 target is not reached after GLP-1 receptor agonists, SGLT-2 inhibitors and metformin. A basal Insulin is simple to use, efficient in lowering blood glucose, and is cardiovascular safe, as shown in the ORIGIN study for glargine and in the DEVOTE Study for insulin degludec. The increased risk of hypoglycaemia with the newer ultra-long-acting insulins (degludec and glargine 300) is low if used in monotherapy. The risk of hypoglycaemia will increase if an intensive therapy (basal -bolus regimen) is used. Under these circumstances, the prescription of the nasal spray of glucagon (Baqsimi ® ) is recommended and might help to apply glucagon much easier to a patient with severe hypoglycaemia. It has been shown that the use of twice-daily co-formulated insulin for the main meals achieves the same HbA1c as a basal-bolus regimen but with much lower hypoglycaemia rates (daytime and during the night) [49]. If insulin is started, the concomitant use of SGLT-2, GLP-1 receptor agonists, and metformin should be continued (figure 1).
The ADA cut-off to define older adults with diabetes has been set at 65 years [50]. Older adults with diabetes represent nearly half of all individuals with diabetes mellitus worldwide, and the prevalence of diabetes above 65 years in western countries varies between 16 to 30% [50, 51]. Longer life expectancy and lifelong exposure to cardiometabolic risk factors are the main factors explaining this increase in diabetes prevalence among the elderly [52]. Older patients with diabetes have a higher risk of common geriatric syndromes, including frailty, cognitive impairment and dementia, urinary incontinence, traumatic falls and fractures, disability, and side effects of polypharmacy, which have an important impact on the quality of life and may interfere with anti-diabetic treatment. Malnutrition is a common symptom, even if the patient is obese. Because of all these factors, clinical management of type 2 diabetes in elderly patients currently represents a real challenge for the physician [53].
If elderly people have no appetite, medications with minimal side effects (avoid loss of appetite and hypoglycaemia) and maximal benefit are preferred. SGLT-2 inhibitors for cardio-renal protection are undisputed. Particularly, the prevention or treatment of all forms of heart failure, which is becoming more frequent with advancing age and comorbidities (>25% in the age group above 65 years) [32] and carries a high mortality rate, is extremely important. In some elderly men with hypertrophy of the prostrate, SGLT-2 inhibitors cause some more nycturia and are, therefore, not appreciated by the patients. If there is insulin deficiency, an ultra-long-acting basal insulin or a co-formulated insulin is required before the use of SGLT-2 inhibitors. In malnourished patients, GLP-1 receptor agonists are not the preferred group because one would like to prevent loss of appetite. The alternative would be DPP-4 inhibitors since they lower HbA1c in each category of chronic kidney disease and have no side effects. The preferred drug is linagliptin because it does not have to adapt to eGFR (in contrast to sitagliptin).
Metformin should be used as well if the eGFR is >30 ml/min. In case of nausea, the dose should be reduced to 1000 mg per day, even if the eGFR is >45 ml/min. In this case, a retarded formulation of metformin once a day might be used.
Insulin secretagogue agents have to be used with caution because of their significant hypoglycaemic risk in this age group with reduced kidney function; short-acting sulfonylureas, such as gliclazide or the glinide, repaglinide, are preferred [53]. The glycaemic target depends on the use of medications that might cause hypoglycaemia (insulin and sulfonylurea). If none of these agents is used, the HbA1c should be 6.5–7.0% for patients in the age range of 65–80 years. In the age group >80 years, an HbA1c target of <7.5–8.0% is sufficient. If insulin or sulfonylurea are used, the HbA1c target should always be <8.0% in all age groups.
The use of different preparations from the same class of drugs (e.g., two different SGLT inhibitors or two different DPP-4 inhibitors) is not reasonable and is, therefore, a forbidden combination. GLP-1 receptor agonists do not need cost approval before treatment is started, but GLP-1 receptor agonists are reimbursed only in patients with BMI >28 kg/m2 at the start of the therapy. If the BMI falls below 28 kg/m2 during therapy with GLP-1 receptor agonists, GLP-1 receptor agonists can be continued. The combination of GLP-1 receptor agonist and DPP-4 inhibitor is not indicated, due to overlapping mechanisms of action, and is not cost-effective. If a GLP-1 receptor agonist is used, it is necessary to stop the DPP-4 inhibitor beforehand. Unfortunately, the combination of GLP-1 receptor agonist and SGLT-2 inhibitor is still not always reimbursed by general health insurance. For the favourable combination of GLP-1 receptor agonist and SGLT-2 inhibitor, a cost approval is still required. Because of the increased risk for hypoglycaemia, insulin and sulfonylureas should not be combined whenever possible.
In situations resulting in dehydration (diarrhoea, fever, vomiting) or if food intake is not guaranteed (nausea, vomiting, perioperatively), some antidiabetic medications must be temporarily stopped. It is important to inform patients which medications need to be stopped in these situations (table 3). Metformin needs to be temporarily stopped in all situations leading to relevant dehydration, acute kidney injury or hypoxemia because of the risk of lactic acidosis. SGLT-2 inhibitors should be temporarily stopped in situations when intake of carbohydrates is not possible (vomiting, prolonged fasting, perioperatively, before gastric or colon endoscopy) due to the risk of ketoacidosis. Medications with a risk for hypoglycaemia (insulin and sulfonylureas) need to be temporarily stopped or adjusted in dose in all situations in which intake of carbohydrates is not guaranteed. Insulin therapy needs dose adjustment during acute illness but should never be stopped completely.
Table 3When to discontinue or change antidiabetic drugs.
| Medication | When to temporarily stop medication |
| Metformin | Dehydration |
| Acute kidney injury | |
| Hypoxemia | |
| SGLT-2 inhibitor | Dehydration |
| Prolonged fasting | |
| Perioperatively (2 days before) | |
| Before endoscopy (2 days before) | |
| Sulfonylurea (Gliclazide) | Stop when fasting |
| Acute kidney injury | |
| Insulin | Reduce dose when fasting |
SGLT-2: Sodium-Glucose Transporter 2
Antidiabetic medication costs vary over a broad range. The older antidiabetic drugs (e.g., metformin, gliclazide) are cheap, whereas DPP-4i, SGLT-2 inhibitors, and insulin are costlier, and GLP1-receptor agonists are the most expensive drugs. Clinical outcomes studies have demonstrated the effectiveness and benefits of the new drugs, especially of SGLT2i and GLP1-receptor agonists, in populations with cardiovascular disease or with high risk for cardiovascular disease, heart failure, and chronic kidney disease. However, it is important to assess whether these additional clinical benefits offset the relatively high cost of these drugs.
As first-line agents, SGLT-2 inhibitors and GLP-1 receptor agonists would improve type 2 diabetes outcomes, but they are probably not cost-effective compared to metformin due to their high medication costs [54]. However, several studies showed that SGLT-2 inhibitors and GLP-1 receptor agonists as an add-on therapy to metformin are cost-effective and maybe cost-saving compared to other antidiabetic drugs [55]. In particular, in combination with metformin, SGLT-2 inhibitor treatment is cost-effective or even cost-saving compared to DPP-4i, sulfonylurea, or glitazone medication [55]. Using diabetes model-based simulations, SGLT2i is a cost-saving treatment in patients with chronic kidney disease and a cost-effective treatment in patients with HFrEF compared to the standard of care [56, 57]. Similarly, add-on therapy of GLP1 receptor agonists to metformin was found to be cost-effective compared to insulin or DPP-4i therapy [55, 58]. In addition, diabetes model-based simulations showed that GLP1 receptor agonists might be cost-effective in patients with or at high cardiovascular risk compared to the standard care [59, 60]. It is important to mention that current cost-effectiveness analyses for these drugs for Switzerland are not available. However, the above-stated study results originating from other European countries (e.g., Netherlands, Sweden, England) are probably also applicable to the Swiss setting.
The view on how to treat type 2 diabetes has completely changed over the last few years. Cardiovascular outcome trials, however, proved that GLP-1 receptor agonists and SGLT-2 inhibitors have some direct effects on cardio-renal protection independent of glucose control [7–15]. This led to a change in paradigm that in persons with type 2 diabetes and a high to very high cardiovascular risk (basically all patients with type 2 diabetes) [17], the primary choice of treatment is either an SGLT-2 inhibitor or a GLP-1 receptor agonist. In order to facilitate the use of antidiabetic treatment and combinations, we summarised in tables 4 and 5 the current available medications with generic and trade names. The cumulative glycaemic exposure is tightly linked to the development of microvascular complications. Metformin is used in early combination treatment with GLP-1 receptor agonists or an SGLT-2 inhibitor to reduce hepatic glucose production because it was the basic treatment to which SGLT-2 inhibitors and GLP-1 receptor agonists were added [7–15]. If this initial dual treatment regimen does not lower HbA1c to the individual desired level, then the third medication is added, either GLP-1 receptor agonists or SGLT-2 inhibitors (figure 1). As seen in figure 3, the multifactorial approach is essential to reduce all cardiovascular risk factors, and permanent lifestyle changes contribute markedly to reducing all complications of diabetes mellitus. However, It is obvious that the adherence level of general practitioners to monitor these risk factors and complications is very low and has to be improved in order to reduce hospitalizations for complications [53]. When applying these new, updated recommendations, some caution must be applied when applying the sick day rules and treating elderly people with many comorbidities (figure 5).

Figure 5 Sick day rules [63-65].
If physicians have a focus on weight management, particularly in younger patients, to reduce obesity early, many diseases associated with obesity, including diabetes, could be prevented [22, 23, 28]. For weight management, treatments with high potency are recommended, such as GLP-1 receptor agonists in higher dosages and the new dual GLP-1/GIP agonists. The costs of new medications are the topic of many deliberations in modern health care. Therefore, a cost-effectiveness analysis of these updated recommendations was added because, nowadays, it is very important to know if a treatment is cost-saving or cost-effective. In most of these analyses, only the direct costs are evaluated and not the sum of direct and indirect costs, which would make almost all our current treatment recommendations cost-saving. The newest trials with SGLT-2 inhibitors, that also included patients without diabetes [36–39, 61], led to new indications to treat chronic kidney disease and heart failure without concomitant diabetes mellitus.
Table 4Oral antidiabetic medications with/without cardiovascular outcome trials.
| Class and substance | Cardiovascular outcome trials | Trade name | Combinations with metformin and others | |
| Biguanide | Metformin | + | Gluophage ® or Generics | |
| SGLT-2 inhibitors | Canagliflozin | + | Invokana® | Vokanamet® |
| Dapagliflozin | + | Forxiga ® | Xigduo ® XR, Qtern® (+ saxagliptin) | |
| Empagliflozin | + | Jardiance® | Jardiance Met®, Glixambi® (+ linagliptin) | |
| Ertugliflozin | + | Steglatro® | Segluuromet®, Steglujan® (+ sitagliptin) | |
| GLP-1 receptor agonists | Semaglutide | + | Rybelsus® | |
| DPP-4 inhibitors | Alogliptin | + | Vipidia® (heart failure possible) | Vipdamet® |
| Linagliptin | + | Trajenta® | Jentadueto® | |
| Saxagliptin | + | Onglyza® (not in heart failure) | Kombiglyze XR® | |
| Sitagliptin | + | Januvia® | Janumet®, Janumet XR® | |
| Vildagliptin | – | Galvus® | Galvumet® | |
| Sulfonylurea | Gliclazide | + | Diamicron® or Generics | |
| Glibenclamid | – | Daonil®/Semi-Daonil® | Glucovance®/Glucovance mite® | |
| Glimpepirid | + | Amaryl® or Generics |
GLP-1 RA: glucagon-like 1 peptide receptor agonists; DPP-4 i: dipeptidyl-peptidase 4 inhibitors; SGLT-2 i: sodium-glucose transporter 2 inhibitors
Table 5Injectable antidiabetic medications with/without cardiovascular outcome trials.
| Class and substance | Cardiovascular outcome trials | Trade name | Combinations | |
| GLP-1 receptor agonists and GLP-1/GIP receptor agonists | Exenatide long-acting | + | Bydureon® (once weekly) | |
| Liraglutide (1.8/3.0 mg) | + | Victoza® daily, Saxenda® daily | ||
| Lixisenatide | + | Lyxumia® | Suliqua® 100/50; 100/33 (+ glargine) | |
| Semaglutide (1.0/2.4 mg) | + | Ozempic®, Wegovy® once weekly | ||
| Dulaglutide (1.5, 3.0, 4.5 mg) | + | Trulicity® once weekly | ||
| Tirzepatide* (GLP-1/GIP receptor agonist; 5, 10, 15 mg) | – | Mounjaro® once weekly | ||
| Insulin analogues, long-acting | Degludec | + | Tresiba® | |
| Detemir | – | Levemir® | ||
| Glargin 100 | Lantus® | |||
| Glargin 300 | – | Toujeo® | ||
| Glargin 100 biosimilar | – | Abasaglar® | ||
| Human insulin intermediate action | Neutral Protamin Hagedorn (NPH) | – | Insulatard®, Huminsulin® | |
| Insulin analogues, fast-acting | Aspart | – | NovoRapid®, Fiasp® (ultra-fast) | |
| Glulisin | – | Apidra® | ||
| Lispro | – | Humalog®, Lyumjev® (ultra-fast) | ||
| Premixed or co-formulated insulins | Aspart/NPH | – | NovoMix® (not available in Switzerland any more) | |
| Lispro/NPH | – | HumalogMix® 25/75, 50/50 | ||
| Aspart/degludec (co-formulated) | + | Ryzodeg® 30/70 |
GIP: glucose-dependent insulinotropic polypeptide; GLP-1: glucagon-like 1 peptide
The fact that SGLT-2 inhibitors are indicated for people with chronic kidney disease and heart failure, with and without diabetes, and that GLP-1 receptor agonists and GLP-1/GIP receptor agonists are given in obesity represents, in our opinion, the second revolution in medical treatment.
Giacomo Gastaldi: honoraria or payment for lectures, manuscript writing, and educational events from Novo Nordisk, Medtronic, Insulet Corporation, Eli Lillly Diabetes, Sanofi Diabetes, Dexcom, Roche, OM-Pharma and support for travel and attending meetings from Eli Lilly Diabetes and Sanofi Diabetes; participation on Advisory Boards for NovoNordisk, OM-pharma and Ascencia.
Barbara Lucchini: Advisory Boards and Lectures for Abbott, Astra Zeneca, Bayer, Boehringer Ingelheim, Medtronic, Novo-Nordisk and Sanofi.
Sebastien Thalmann: financial support Novo Nordisk and E. Lilly.
Stephanie Alder: no conflict of interest.
Markus Laimer: no conflict of interest.
Michael Brändle: Advisory Boards and Lectures for Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, E. Lilly, Novartis, and Novo-Nordisk. Research conflicts: none.
Peter Wiesli: Advisory Boards and Lectures for Abbott, Amgen, Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, E. Lilly, Mundipharma, Medtronic, Novo-Nordisk, Roche, and Sanofi, Research conflicts: none.
Roger Lehmann: Advisory Boards and Lectures for Abbott, Amgen, Astra Zeneca, Boehringer Ingelheim, Daiichi Sankyo, E. Lilly, Mundipharma, Medtronic, Novo-Nordisk, Roche, and Sanofi, Research conflicts: none.
1. Green JB , Bethel MA , Armstrong PW , Buse JB , Engel SS , Garg J , et al.; TECOS Study Group . Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015 Jul;373(3):232–42. https://doi.org/10.1056/NEJMoa1501352
2. Rosenstock J , Kahn SE , Johansen OE , Zinman B , Espeland MA , Woerle HJ , et al.; CAROLINA Investigators . Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA. 2019 Sep;322(12):1155–66. https://doi.org/10.1001/jama.2019.13772
3. Rosenstock J , Perkovic V , Johansen OE , Cooper ME , Kahn SE , Marx N , et al.; CARMELINA Investigators . Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019 Jan;321(1):69–79. https://doi.org/10.1001/jama.2018.18269
4. Scirica BM , Bhatt DL , Braunwald E , Steg PG , Davidson J , Hirshberg B , et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013 Oct;369(14):1317–26. https://doi.org/10.1056/NEJMoa1307684
5. Jhund PS , Kondo T , Butt JH , Docherty KF , Claggett BL , Desai AS , et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022 Sep;28(9):1956–64. https://doi.org/10.1038/s41591-022-01971-4
6. Bellastella G , Maiorino MI , Longo M , Scappaticcio L , Chiodini P , Esposito K , et al. Glucagon-Like Peptide-1 Receptor Agonists and Prevention of Stroke Systematic Review of Cardiovascular Outcome Trials With Meta-Analysis. Stroke. 2020 Feb;51(2):666–9. https://doi.org/10.1161/STROKEAHA.119.027557
7. Husain M , Birkenfeld AL , Donsmark M , Dungan K , Eliaschewitz FG , Franco DR , et al.; PIONEER 6 Investigators . Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019 Aug;381(9):841–51. https://doi.org/10.1056/NEJMoa1901118
8. Mann JF , Ørsted DD , Brown-Frandsen K , Marso SP , Poulter NR , Rasmussen S , et al.; LEADER Steering Committee and Investigators . Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017 Aug;377(9):839–48. https://doi.org/10.1056/NEJMoa1616011
9. Marso SP , Bain SC , Consoli A , Eliaschewitz FG , Jódar E , Leiter LA , et al.; SUSTAIN-6 Investigators . Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016 Nov;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141
10. Marso SP , Daniels GH , Brown-Frandsen K , Kristensen P , Mann JF , Nauck MA , et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016 Jul;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827
11. Neal B , Perkovic V , Mahaffey KW , de Zeeuw D , Fulcher G , Erondu N , et al.; CANVAS Program Collaborative Group . Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925
12. Cannon CP , Pratley R , Dagogo-Jack S , Mancuso J , Huyck S , Masiukiewicz U , et al.; VERTIS CV Investigators . Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med. 2020 Oct;383(15):1425–35. https://doi.org/10.1056/NEJMoa2004967
13. Gerstein HC , Colhoun HM , Dagenais GR , Diaz R , Lakshmanan M , Pais P , et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019 Jul;394(10193):121–30. https://doi.org/10.1016/S0140-6736(19)31149-3
14. Gerstein HC , Sattar N , Rosenstock J , Ramasundarahettige C , Pratley R , Lopes RD , et al.; AMPLITUDE-O Trial Investigators . Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N Engl J Med. 2021 Sep;385(10):896–907. https://doi.org/10.1056/NEJMoa2108269
15. Zinman B , Wanner C , Lachin JM , Fitchett D , Bluhmki E , Hantel S , et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720
16. Davies MJ , Aroda VR , Collins BS , Gabbay RA , Green J , Maruthur NM , et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov;45(11):2753–86. https://doi.org/10.2337/dci22-0034
17. Visseren FL , Mach F , Smulders YM , Carballo D , Koskinas KC , Bäck M , et al.; ESC National Cardiac Societies; ESC Scientific Document Group . 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Sep;42(34):3227–337. https://doi.org/10.1093/eurheartj/ehab484
18. Jensen MH , Kjolby M , Hejlesen O , Jakobsen PE , Vestergaard P . Risk of Major Adverse Cardiovascular Events, Severe Hypoglycemia, and All-Cause Mortality for Widely Used Antihyperglycemic Dual and Triple Therapies for Type 2 Diabetes Management: A Cohort Study of All Danish Users. Diabetes Care. 2020 Jun;43(6):1209–18. https://doi.org/10.2337/dc19-2535
19. Dave CV , Kim SC , Goldfine AB , Glynn RJ , Tong A , Patorno E . Risk of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Addition of SGLT2 Inhibitors Versus Sulfonylureas to Baseline GLP-1RA Therapy. Circulation. 2021 Feb;143(8):770–9. https://doi.org/10.1161/CIRCULATIONAHA.120.047965
20. Lean ME , Leslie WS , Barnes AC , Brosnahan N , Thom G , McCombie L , et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018 Feb;391(10120):541–51. https://doi.org/10.1016/S0140-6736(17)33102-1
21. Dansinger ML , Gleason JA , Griffith JL , Selker HP , Schaefer EJ . Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005 Jan;293(1):43–53. https://doi.org/10.1001/jama.293.1.43
22. Wilding JP , Batterham RL , Calanna S , Davies M , Van Gaal LF , Lingvay I , et al.; STEP 1 Study Group . Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Mar;384(11):989–1002. https://doi.org/10.1056/NEJMoa2032183
23. Jastreboff AM , Aronne LJ , Ahmad NN , Wharton S , Connery L , Alves B , et al.; SURMOUNT-1 Investigators . Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jul;387(3):205–16. https://doi.org/10.1056/NEJMoa2206038
24. Mori Y , Duru OK , Tuttle KR , Fukuma S , Taura D , Harada N , et al. Sodium-Glucose Cotransporter 2 Inhibitors and New-onset Type 2 Diabetes in Adults with Prediabetes: systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2022 Dec;108(1):221–31. https://doi.org/10.1210/clinem/dgac591
25. Gæde P , Oellgaard J , Carstensen B , Rossing P , Lund-Andersen H , Parving HH , et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016 Nov;59(11):2298–307. https://doi.org/10.1007/s00125-016-4065-6
26. Huber CA , Reich O , Früh M , Rosemann T . Effects of Integrated Care on Disease-Related Hospitalisation and Healthcare Costs in Patients with Diabetes, Cardiovascular Diseases and Respiratory Illnesses: A Propensity-Matched Cohort Study in Switzerland. Int J Integr Care. 2016 Apr;16(1):11. https://doi.org/10.5334/ijic.2455
27. Christ E , Czock A , Renström F , Ammeter T , Ebrahimi F , Zechmann S , et al. Evaluation of type 2 diabetes care management in nine primary care practices before and after implementation of the Criteria of Good Disease Management of Diabetes established by the Swiss Society of Endocrinology and Diabetology. Swiss Med Wkly. 2022 Jul;152(2930):w30197. https://doi.org/10.4414/SMW.2022.w30197
28. Heerspink HJ , Langkilde AM , Wheeler DC . Dapagliflozin in Patients with Chronic Kidney Disease. Reply [Reply]. N Engl J Med. 2021 Jan;384(4):389–90.
29. Agarwal R , Kolkhof P , Bakris G , Bauersachs J , Haller H , Wada T , et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021 Jan;42(2):152–61. https://doi.org/10.1093/eurheartj/ehaa736
30. Pitt B , Filippatos G , Agarwal R , Anker SD , Bakris GL , Rossing P , et al.; FIGARO-DKD Investigators . Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021 Dec;385(24):2252–63. https://doi.org/10.1056/NEJMoa2110956
31. Bakris GL , Agarwal R , Anker SD , Pitt B , Ruilope LM , Rossing P , et al.; FIDELIO-DKD Investigators . Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020 Dec;383(23):2219–29. https://doi.org/10.1056/NEJMoa2025845
32. Boonman-de Winter LJ , Rutten FH , Cramer MJ , Landman MJ , Liem AH , Rutten GE , et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012 Aug;55(8):2154–62. https://doi.org/10.1007/s00125-012-2579-0
33. Pop-Busui R , Januzzi JL , Bruemmer D , Butalia S , Green JB , Horton WB , et al. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care. 2022 Jul;45(7):1670–90. https://doi.org/10.2337/dci22-0014
34. McDonagh TA , Metra M , Adamo M , Gardner RS , Baumbach A , Böhm M , et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368
35. Pandey A , Vaduganathan M , Patel KV , Ayers C , Ballantyne CM , Kosiborod MN , et al. Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes. JACC Heart Fail. 2021 Mar;9(3):215–23. https://doi.org/10.1016/j.jchf.2020.10.013
36. McMurray JJ , Solomon SD , Inzucchi SE , Køber L , Kosiborod MN , Martinez FA , et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019 Nov;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303
37. Petrie MC , Verma S , Docherty KF , Inzucchi SE , Anand I , Belohlávek J , et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA. 2020 Apr;323(14):1353–68. https://doi.org/10.1001/jama.2020.1906
38. Solomon SD , McMurray JJ , Claggett B , de Boer RA , DeMets D , Hernandez AF , et al.; DELIVER Trial Committees and Investigators . Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022 Sep;387(12):1089–98. https://doi.org/10.1056/NEJMoa2206286
39. Anker SD , Butler J , Filippatos G , Ferreira JP , Bocchi E , Böhm M , et al.; EMPEROR-Preserved Trial Investigators . Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021 Oct;385(16):1451–61. https://doi.org/10.1056/NEJMoa2107038
40. Eurich DT , Weir DL , Majumdar SR , Tsuyuki RT , Johnson JA , Tjosvold L , et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013 May;6(3):395–402. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000162
41. Sattar N , Lee MM , Kristensen SL , Branch KR , Del Prato S , Khurmi NS , et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021 Oct;9(10):653–62. https://doi.org/10.1016/S2213-8587(21)00203-5
42. Richardson TL Jr , Hackstadt AJ , Hung AM , Greevy RA , Grijalva CG , Griffin MR , et al. Hospitalization for Heart Failure Among Patients With Diabetes Mellitus and Reduced Kidney Function Treated With Metformin Versus Sulfonylureas: A Retrospective Cohort Study. J Am Heart Assoc. 2021 Apr;10(8):e019211. https://doi.org/10.1161/JAHA.120.019211
43. Nissen SE , Wolski K . Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007 Jun;356(24):2457–71. https://doi.org/10.1056/NEJMoa072761
44. Stumvoll M , Goldstein BJ , van Haeften TW . Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005 Apr;365(9467):1333–46. https://doi.org/10.1016/S0140-6736(05)61032-X
45. Rubino D , Abrahamsson N , Davies M , Hesse D , Greenway FL , Jensen C , et al.; STEP 4 Investigators . Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021 Apr;325(14):1414–25. https://doi.org/10.1001/jama.2021.3224
46. Ahlqvist E , Prasad RB , Groop L . Subtypes of Type 2 Diabetes Determined From Clinical Parameters. Diabetes. 2020 Oct;69(10):2086–93. https://doi.org/10.2337/dbi20-0001
47. Holt RI , DeVries JH , Hess-Fischl A , Hirsch IB , Kirkman MS , Klupa T , et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021 Dec;64(12):2609–52. https://doi.org/10.1007/s00125-021-05568-3
48. Davis AK , DuBose SN , Haller MJ , Miller KM , DiMeglio LA , Bethin KE , et al.; T1D Exchange Clinic Network . Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015 Mar;38(3):476–81. https://doi.org/10.2337/dc14-1952
49. Philis-Tsimikas A , Astamirova K , Gupta Y , Haggag A , Roula D , Bak BA , et al. Similar glycaemic control with less nocturnal hypoglycaemia in a 38-week trial comparing the IDegAsp co-formulation with insulin glargine U100 and insulin aspart in basal insulin-treated subjects with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019 Jan;147:157–65. https://doi.org/10.1016/j.diabres.2018.10.024
50. American Diabetes Association Professional Practice Committee . 13. Older Adults: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022 Jan;45 Suppl 1:S195–207. https://doi.org/10.2337/dc22-S013
51. Sinclair AJ , Rodriguez-Mañas L . Diabetes and Frailty: Two Converging Conditions? Can J Diabetes. 2016 Feb;40(1):77–83. https://doi.org/10.1016/j.jcjd.2015.09.004
52. Cowie CC , Rust KF , Ford ES , Eberhardt MS , Byrd-Holt DD , Li C , et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009 Feb;32(2):287–94. https://doi.org/10.2337/dc08-1296
53. Longo M , Bellastella G , Maiorino MI , Meier JJ , Esposito K , Giugliano D . Diabetes and Aging: From Treatment Goals to Pharmacologic Therapy. Front Endocrinol (Lausanne). 2019 Feb;10:45. https://doi.org/10.3389/fendo.2019.00045
54. Choi JG , Winn AN , Skandari MR , Franco MI , Staab EM , Alexander J , et al. First-Line Therapy for Type 2 Diabetes With Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists : A Cost-Effectiveness Study. Ann Intern Med. 2022 Oct;175(10):1392–400. https://doi.org/10.7326/M21-2941
55. Zozaya N , Capel M , Simon S , Soto-Gonzales A . A systematic review of economic evaluations in non-insulin antidiabetic treatments for patients with type 2 diabetes mellitus. Glob Reg Health Technol Assess. 2019;2019:1–26. https://doi.org/10.1177/2284240319876574
56. Willis M , Nilsson A , Kellerborg K , Ball P , Roe R , Traina S , et al. Cost-Effectiveness of Canagliflozin Added to Standard of Care for Treating Diabetic Kidney Disease (DKD) in Patients with Type 2 Diabetes Mellitus (T2DM) in England: Estimates Using the CREDEM-DKD Model. Diabetes Ther. 2021 Jan;12(1):313–28. https://doi.org/10.1007/s13300-020-00968-x
57. McEwan P , Darlington O , McMurray JJ , Jhund PS , Docherty KF , Böhm M , et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail. 2020 Nov;22(11):2147–56. https://doi.org/10.1002/ejhf.1978
58. Hunt B , Malkin SJ , Moes RG , Huisman EL , Vandebrouck T , Wolffenbuttel BH . Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. BMJ Open Diabetes Res Care. 2019 Oct;7(1):e000705. https://doi.org/10.1136/bmjdrc-2019-000705
59. Guzauskas GF , Rind DM , Fazioli K , Chapman RH , Pearson SD , Hansen RN . Cost-effectiveness of oral semaglutide added to current antihyperglycemic treatment for type 2 diabetes. J Manag Care Spec Pharm. 2021 Apr;27(4):455–68. https://doi.org/10.18553/jmcp.2021.27.4.455
60. Shah D , Risebrough NA , Perdrizet J , Iyer NN , Gamble C , Dang-Tan T . Cost-effectiveness and budget impact of liraglutide in type 2 diabetes patients with elevated cardiovascular risk: a US-managed care perspective. Clinicoecon Outcomes Res. 2018 Nov;10:791–803. https://doi.org/10.2147/CEOR.S180067
61. Aeberli I , Molinari L , Spinas G , Lehmann R , l’Allemand D , Zimmermann MB . Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am J Clin Nutr. 2006 Oct;84(4):748–55. https://doi.org/10.1093/ajcn/84.4.748
62. Schneider L , Lehmann R . Swiss Diabetes Guide: Entscheidungshilfe für eine personalisierte Therapie beim Typ 2 Diabetes mellitus (SGED 2020). Swiss Medical Forum. 2021;21:251–6.
63. van Berlo-van de Laar IR , Vermeij CG , Doorenbos CJ . Metformin associated lactic acidosis: incidence and clinical correlation with metformin serum concentration measurements. J Clin Pharm Ther. 2011 Jun;36(3):376–82. https://doi.org/10.1111/j.1365-2710.2010.01192.x
64. Liu J , Li L , Li S , Wang Y , Qin X , Deng K , et al. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020 Sep;22(9):1619–27. https://doi.org/10.1111/dom.14075
65. Colacci M , Fralick M . Response: Sodium-Glucose Cotransporter-2 Inhibitors and Risk of Diabetic Ketoacidosis Among Adults With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Can J Diabetes. 2022 Mar;46(2):110. https://doi.org/10.1016/j.jcjd.2021.10.003