Management of giant-cell arteritis in Switzerland: an online national survey
DOI: https://doi.org/10.57187/smw.2023.40051
Michele
Iudicia, Andrea Katharina
Hemmigb, Mihaela
Stegertb, Delphine Sophie
Courvoisiera, Sabine
Adlerc, d, Mike Oliver
Beckere, Christoph T.
Bergerf, g, Diana
Danh, Axel
Finckha, Alfred
Mahri, Thomas
Neumanni, Stephan
Reichenbachd, Camillo
Ribij, Luca
Seitzd, Peter
Villigerk, Lukas
Wildil, Thomas
Daikelerb, on behalf of Giant Cell
Arteritis SCQM Study Group
a Division of Rheumatology, Department of Medicine, Faculty of Medicine, Geneva
University Hospitals, Geneva, Switzerland
b Department
of Rheumatology, University Hospital Basel, Basel, Switzerland
c Department
of Rheumatology and Immunology, Kantonsspital Aarau, Aarau, Switzerland
d Department
of Rheumatology, Immunology and Allergology, University Hospital, University of
Bern, Bern, Switzerland
e Department
of Rheumatology, University Hospital Zurich, University of Zurich, Zurich,
Switzerland
f
University Center for Immunology, University Hospital Basel, Basel, Switzerland
g Department
Biomedicine, Translational Immunology, University of Basel, Basel, Switzerland
h Department
of Rheumatology, Lausanne University Hospital (CHUV) and University of
Lausanne, Lausanne, Switzerland
i Cantonal
Hospital St. Gallen, St. Gallen, Switzerland
j Service
of Immunology and Allergy, Lausanne University Hospital (CHUV), Lausanne,
Switzerland
k
University of Bern and Medical Center Monbijou, Bern, Switzerland
lDepartment
of Rheumatology, Cantonal Hospital Winterthur, Winterthur, Switzerland
Summary
AIMS OF THE STUDY: To assess current practices in diagnosing, treating,
and following-up giant-cell arteritis by specialists in Switzerland and to
identify the main barriers to using diagnostic tools.
METHODS: We performed a national survey of specialists potentially caring
for patients with giant-cell arteritis. The survey was sent by email to all
members of the Swiss Societies of Rheumatology and for Allergy and Immunology. A reminder was
sent to nonresponders after 4 and 12 weeks. Its questions covered the
following dimensions: respondents’ main characteristics, diagnosis, treatment,
and imaging’s role during follow-up. The main study results were summarized
using descriptive statistics.
RESULTS: Ninety-one specialists, primarily aged 46–65 years (n = 53/89; 59%),
working in academic or nonacademic hospitals or private practice, and treating
a median of 7.5 (interquartile range [IQR]: 3–12) patients with giant-cell
arteritis per year participated in this survey. Ultrasound of temporal
arteries/large vessels (n = 75/90; 83%) and positron-emission-tomography-computed
tomography (n = 52/91; 57%) or magnetic resonance imaging (n = 46/90; 51%) of
the aorta/extracranial arteries were the most common techniques used to
diagnose giant-cell arteritis with cranial or large vessel involvement,
respectively. Most participants reported a short time to obtain imaging tests
or arterial biopsy. The glucocorticoid tapering scheme, glucocorticoid-sparing
agent, and glucocorticoid-sparing treatment duration varied among the
participants. Most physicians did not follow a predefined repeat imaging scheme
for follow-up and mainly relied on structural changes (vascular thickening,
stenosis, or dilatation) to drive treatment choice.
CONCLUSIONS: This survey indicates that imaging and temporal biopsy are
rapidly accessible for diagnosing giant-cell arteritis in Switzerland but
highlights heterogeneous practice in many disease management areas.
Introduction
Giant cell arteritis is large vessel
vasculitis usually occurring in those aged over 50 years [1]. In addition to
the classical cranial phenotype, giant-cell arteritis can affect large vessels
in isolation or combination with cranial arteries and is frequently associated
with polymyalgia rheumatica [1].
Giant-cell arteritis can cause severe
complications such as vision loss, critical vascular stenosis, ischemic stroke,
or life-threatening haemorrhage secondary to aneurysmal rupture [1]. A prompt
diagnosis, balanced treatment, and careful follow-up are necessary to preserve
and restore a patient’s health and minimize the risks of damage accrual [2, 3]
and treatment-related side effects.
Our knowledge about giant-cell arteritis
has steadily improved in recent years. The increasing availability of advanced
imaging techniques has favoured a more accurate giant-cell arteritis diagnosis
and characterization [4, 5]. Randomized controlled trials have shown the
utility of methotrexate and tocilizumab (anti-interleukin 6 receptor [IL6R]) as
glucocorticoid sparing agents [6–8], with other molecules currently being
tested [9, 10]. However, many questions about giant-cell arteritis diagnosis,
treatment, and optimal patient follow-up remain unanswered. International
guidelines [2, 3] exist, but some of their aspects are based mainly on
low-quality data or driven by expert opinion. Additionally, the management of giant-cell
arteritis patients can be influenced by other factors, such as the care setting,
physician’s experience, or resource availability. All these aspects and the
coexistence of different giant-cell arteritis phenotypes likely contribute to
heterogeneity in management.
As a first step in developing guidance for
physicians caring for giant-cell arteritis patients, we conducted this study to assess current practices in diagnosing,
treating, and following-up giant-cell arteritis by specialists in Switzerland
and to identify the main barriers to using diagnostic tools.
Methods
Data collection and procedure
Data were collected between March and June
2021 using an online survey. The study data were collected and managed using research
electronic data capture (REDCap) tools hosted at Geneva University Hospitals [11,
12]. This study used a cross-sectional online observational anonymous survey of
members of the Swiss Society of Rheumatology (SSR) and The Swiss Society for
Allergology and Immunology (SSAI; table 1).
Table 1Participants’ characteristics and diagnostic
approaches.
|
Question
|
Participants, n (%)
|
Missing (%)
|
| Age (years) |
25–35 |
7 (8) |
2 |
| 36–45 |
23 (26) |
| 46–55 |
26 (29) |
| 56–65 |
27 (30) |
| >65 |
6 (7) |
| Male, n (%) |
|
58 (64) |
2 |
| Years of medical practice |
0–5 |
0 (0) |
2 |
| 6–10 |
10 (11) |
| 10–15 |
14 (15.5) |
| 16–20 |
14 (15.5) |
| 21–30 |
38 (42) |
| >30 |
13 (14) |
| Setting* |
Private practice |
44 (49) |
1 |
| Nonacademic hospital |
46 (52) |
| Academic hospital |
14 (17) |
| Other |
2 (2) |
| Speciality* |
Rheumatology |
72 (79) |
1 |
| Immunology |
17 (19) |
| Internal medicine |
26 (29) |
| Other |
2 (2) |
| Number of patients with giant-cell arteritis seen
per year, median (IQR) |
7.5 (3–12) |
1 |
| When suspecting giant-cell arteritis with cranial
symptoms, which diagnostic tests do you prescribe in daily practice?* |
Ultrasound of temporal arteries |
75 (83) |
1 |
| MRI of temporal or other cranial arteries |
30 (33) |
| PET-CT of the aorta/extracranial arteries |
29 (32) |
| MRI of the aorta/extracranial arteries |
29 (32) |
| Contrast-enhanced angiography |
5 (5) |
| Temporal artery biopsy |
46 (51) |
| Temporal artery biopsy only if signs of vasculitis
are absent at imaging |
38 (42) |
| When suspecting giant-cell arteritis without cranial
symptoms, which diagnostic tests do you prescribe in daily practice?* |
Ultrasound of temporal artery |
47 (52) |
1 |
| MRI of temporal or other cranial arteries |
14 (15) |
| PET-CT of the aorta/extracranial arteries |
52 (57) |
| MRI of the aorta/extracranial arteries |
46 (51) |
| Contrast-enhanced CT |
6 (7) |
| Temporal artery biopsy |
22 (24) |
| Temporal artery biopsy only if signs of vasculitis
are absent at imaging |
19 (21) |
| Temporal artery biopsy in my hospital/clinics:* |
Is usually performed in <3 working days |
57 (63) |
1 |
| Can usually only be performed after ≥3 working
days |
23 (25) |
| Is correctly performed (temporal artery specimen
>1 cm) |
59 (65) |
| The pathologist describes histology in detail
(inflammation, fibrosis, and vessel occlusion) |
55 (60) |
| The pathologist’s comment (vasculitis vs other) is
based on the description |
28 (31) |
| The histology is performed at my institution |
33 (36) |
| The histology is performed by a specialized lab |
28 (31) |
| The ultrasound of temporal arteries in my
hospital/clinics is:* |
Performed by myself or my colleagues in the
rheumatology/immunology/internal medicine unit |
18 (20) |
1 |
| Performed by angiologists |
54 (59) |
| Performed by neurologists |
22 (24) |
| The ultrasound of temporal arteries in my
hospital/clinics is usually available: |
On the day of presentation |
21 (23) |
9 |
| Within one working day |
15 (16) |
| Within two working days |
27 (30) |
| Within four working days |
12 (13) |
| Within six working days |
8 (9) |
| The person performing the ultrasound routinely
examines:* |
The branches of the temporal artery |
74 (81) |
1 |
| The carotid arteries |
61 (67) |
| The axillary arteries |
45 (49) |
| The vertebral arteries |
37 (41) |
| The subclavian arteries |
45 (49) |
| The iliac arteries |
8 (9) |
| The femoral arteries |
13 (14) |
| The person performing the ultrasound:* |
Describes the ‘halo’ sign for the temporal artery |
78 (86) |
1 |
| Describes the compression sign for the temporal
artery |
57 (63) |
| Has great experience with diagnosing giant-cell
arteritis |
25 (27) |
| Has moderate experience with diagnosing giant-cell
arteritis |
37 (41) |
| Has only limited experience with diagnosing giant-cell
arteritis |
10 (11) |
| The MRI of temporal arteries or other cranial
arteries in my hospital/clinics is usually available: |
Within two working days |
26 (29) |
14 |
| Within four working days |
30 (33) |
| Within six working days |
16 (18) |
| After >6 working days |
6 (7) |
| The MRI of the aorta/extracranial arteries in my
hospital/clinics is usually available: |
Within two working day |
27 (30) |
13 |
| Within four working days |
30 (33) |
| Within six working days |
12 (13) |
| After >6 working days |
11 (11) |
| The PET/CT of the aorta/extracranial arteries in my
hospital/clinics is usually available: |
Within two working days |
10 (11) |
14 |
| Within four working days |
28 (31) |
| Within six working days |
25 (27) |
| After >6 working days |
15 (16) |
Structure of the survey
A 29-question online survey (tables 1–3)
was developed to investigate differences in giant-cell arteritis diagnosis,
treatment, and follow-up practices in Switzerland. It was designed by a board
of Swiss experts in the field (MI, AKM, MS, and TD) following recommendations
from the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) [13].
It included a combination of closed- and open-ended questions and
multiple-choice questions. It was developed in English to account for language differences
across Swiss Cantons.
The
questions covered the following dimensions: respondents’ main characteristics
(age, place of residence, speciality, work setting, and years of medical
practice), diagnosis (diagnostic tools used in patients with cranial
involvement and noncranial, large-vessel giant-cell arteritis involvement; the average
time to obtain specific diagnostic tests; specialists performing temporal
artery biopsy; and perceived quality), treatment (tapering glucocorticoid scheme,
the extent of glucocorticoid-sparing agent use, glucocorticoid-sparing agent
choice and treatment duration, and supportive therapy), and imaging’s role during
follow-up (which imaging technique, when and for how long after remission, and treatment
decisions). The complete survey is reported in tables 1–3. The survey was
pretested for its feasibility and understandability by all authors of this
study. No significant adjustments were needed.
Table 2Main online survey results: treatment.
|
Question
|
Participants, n (%)
|
Missing (%)
|
| What predefined glucocorticoid tapering scheme do
you follow after giant-cell arteritis diagnosis? |
Slow: e.g. taper glucocorticoids to 15–20 mg/day
within 2–3 months and then to <5 mg/day after one year (EULAR
recommendations, 2020) |
42 (46) |
4 |
| Fast: e.g. 26-week taper protocol (GiACTA) |
10 (11) |
| Fast: 26-week taper protocol (GiACTA) combined with tocilizumab |
21 (23) |
| I do not follow a predefined glucocorticoid tapering
scheme |
11 (12) |
| Other |
3 (3) |
| Which glucocorticoid-sparing agent do you
prescribe?* |
Methotrexate |
65 (71) |
1 |
| Tocilizumab |
87 (96) |
| I do not prescribe glucocorticoid-sparing agents |
1 (1) |
| Other |
4 (4) |
| When do you prescribe a glucocorticoid-sparing agent
(methotrexate/tocilizumab)?* |
In cases with relapsing disease |
58 (64) |
1 |
| To every patient with giant-cell arteritis who has
already developed or is at increased risk of developing glucocorticoid-related
side effects or complications (osteoporosis, glaucoma, diabetes, and cardiovascular
disease) |
62 (68) |
| Never |
1 (1) |
| In every patient, regardless of newly
diagnosed/relapsing disease or glucocorticoid-related adverse events |
17 (19) |
| Other |
3 (3) |
| When do you discontinue glucocorticoid monotherapy once
you have achieved disease remission? |
6 months after diagnosis |
11 (12) |
5 |
| 12 months after diagnosis |
28 (31) |
| 24 months after diagnosis |
21 (23) |
| It depends on vascular complications |
20 (22) |
| Other |
6 (7) |
| When do you discontinue a glucocorticoid-sparing
agent once you have achieved disease remission? |
12 months after the start of glucocorticoid-sparing
agent use |
23 (25) |
2 |
| 18 months after the start of glucocorticoid-sparing
agent use |
7 (8) |
| 24 months after the start of glucocorticoid-sparing
agent use |
11 (12) |
| It depends on vascular complications |
16 (18) |
| I do not follow a predefined scheme |
29 (32) |
| Other |
3 (3) |
| Do you prescribe antiplatelet agents to giant-cell
arteritis patients? |
Always |
13 (14) |
2 |
| Only in cases with ocular giant-cell arteritis-related
ischemia |
15 (16) |
| In cases with cranial giant-cell arteritis symptoms |
16 (18) |
| Only when indicated for other ‘non-vasculitic’
reasons (e.g. coronary heart disease) |
40 (44) |
| Other |
5 (5) |
| Which supportive therapy do you prescribe in
addition to glucocorticoid?* |
Vitamin D |
87 (96) |
1 |
| Calcium |
77 (85) |
| Trimethoprim/sulfamethoxazole |
26 (29) |
| Trimethoprim/sulfamethoxazole only when combined
with methotrexate or tocilizumab |
7 (8) |
| Other |
7 (8) |
| Do you routinely perform a DEXA scan for
osteoporosis?* |
Before the start of glucocorticoid therapy or soon
afterwards |
71 (78) |
1 |
| After 12 months of glucocorticoid therapy |
11 (12) |
| Never |
1 (1) |
| Other |
9 (10) |
Table 3Main online survey results: imaging after
diagnosis.
|
Question
|
Participants, n (%)
|
Missing (%)
|
| After diagnosis, how often do you perform imaging to
monitor structural damage (vascular thickening, stenosis, or dilatation)? |
After 3, 6, 9, and 12 months, then yearly if in remission |
1 (1) |
3 |
| After 6 and 12 months, then yearly if in remission |
13 (14) |
| After 12 months, then yearly if in remission |
13 (14) |
| Every two years, if in remission |
8 (9) |
| I do not follow a predefined follow-up scheme |
50 (55) |
| None of the above; please specify |
3 (3) |
| Which imaging technique(s) do you mainly use to
monitor vascular structural damage over time (vascular thickening, stenosis,
or dilatation)?* |
PET-CT of the aorta/extracranial arteries |
9 (10) |
1 |
| MRI of the aorta/extracranial arteries |
52 (57) |
| Contrast-enhanced CT |
10 (11) |
| Ultrasound for supra-aortic arteries and abdominal
aorta and CT for the thoracic aorta |
22 (24) |
| Other |
7 (8) |
| Do you routinely use imaging to guide your treatment
strategy/Do you base your treatment strategy on imaging findings? |
In patients treated with tocilizumab |
8 (9) |
4 |
| In all patients with large-vessel involvement before
discontinuing treatment |
17 (19) |
| I perform ultrasound or MRI before discontinuing treatment
only in patients with temporal artery
-giant-cell arteritis |
3 (3) |
| No |
34 (37) |
| Only in cases with suspected relapse |
24 (26) |
| Other |
1 (1) |
| Apart from detecting structural vascular damage,
does any other imaging finding drive your treatment decisions during
follow-up?* |
I plan a treatment escalation in cases with
increased fluorodeoxyglucose uptake in the arterial wall at PET-CT |
24 (26) |
1 |
| I plan a treatment escalation in cases with
inflammatory signs in the arterial wall at MRI |
31 (34) |
| I plan a treatment escalation in cases with
inflammatory signs in the arterial wall at CT |
11 (12) |
| No, I only modify treatment in cases with the appearance/progression
of signs of structural vascular damage |
41 (45) |
| Other |
3 (4) |
Participants
Specialists were eligible to participate if they were
practising in Switzerland and could understand English. The invitation link to participate in the REDCap survey was sent by
the Swiss Society of Rheumatology and the Swiss Society for Allergology and
Immunology to all their members via email. A reminder was sent after 4 and
12 weeks.Participants were
invited to share the survey link with colleagues from other specialities
involved in managing giant-cell arteritis (snowball sampling technique).
The survey was accompanied by a cover letter explaining
the study’s purposes. Respondents were not compensated for their participation,
which was voluntary and implied consent. All responses were anonymous, and no
identifying information was collected. Participants could quit the survey
anytime and use a back function to change their answers.
Statistical analysis
Categorical variables are presented as
frequencies and percentages. Continuous variables are presented as medians (interquartile
ranges). Categorical variables were compared using Chi-square or Fisher’s exact
tests, as appropriate. We used subgroup analyses to investigate differences
according to participants’ work settings (private practice, nonacademic
hospital, or academic hospital) and experience, defined based on the median
number of individual giant-cell arteritis patients seen per year: ≤7, less experienced; >7, experienced. A P-value <0.05 was
considered statistically significant. Analyses were performed using the R
statistical software (v.4.1;
R Development Core Team, Vienna, Austria).
Ethics approval
This study did not require ethical approval since it did not
involve human participants.
Results
Participant’s main characteristics
Due to the snowball sampling technique, in
which Swiss Society of Rheumatology and Swiss Society for Allergology and
Immunology members could send the survey link to other specialists who may
treat giant-cell arteritis, a participation rate could not be calculated.
Ninety-one specialists, mostly rheumatologists (n = 72; 79%), working in
nonacademic hospitals (n = 46; 52%), private practice (n = 44; 49%), or
academic hospitals (n = 14; 17%) and treating a median of 7.5 (interquartile
range [IQR] 3–12) patients per year participated in this survey. Detailed
information on participants is shown in table 1. Forty-three (47%) participants
were considered ‘experienced’
according to the above definition. Twenty-six participants (27%) declared more
than one specialization.
Diagnosis
Diagnostic tests planned in patients with
suspected giant-cell arteritis with cranial or large vessel involvement
When giant-cell arteritis with cranial
involvement was suspected, the participants reported ordering a median of 2 (IQR
2–3) diagnostic tests. Those most commonly prescribed were ultrasound of the
temporal and/or axillary arteries (83%) and temporal artery biopsy (51%; table
1). About 42% stated performing temporal artery biopsy only when imaging was
negative. In contrast, the imaging techniques most commonly used to confirm large
vessel involvement (median of 2 [IQR 1–3] modalities) were fluorine-18
fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT;
57%), ultrasound of the temporal and/or axillary arteries (52%), and magnetic
resonance imaging (MRI) of the aorta/extracranial arteries (51%). Twenty
participants reported planning a temporal artery biopsy in cases with normal
findings at imaging, even in the absence of cranial symptoms (table 1).
We found no difference in the number of
requested diagnostic modalities between physicians with more or less expertise
for patients with cranial (median = 2 [IQR 2–3] vs 2 [IQR 2–3]; p = 0.11) or large
vessel (median = 2 [IQR 1–3] vs 2 [IQR 1–3]; p = 0.37) involvement.
Ultrasound of the temporal and/or axillary
arteries
Most physicians (64/84; 76%) reported that ultrasound
of the temporal and/or axillary arteries was accessible within 1–2 working
days. Ultrasound of the temporal and/or axillary arteries was typically
performed by angiologists (59%) or neurologists (24%), with some participants
(20%) stating they performed the ultrasound of the temporal and/or axillary
arteries themselves. The expertise of the individual performing the ultrasound
of the temporal and/or axillary arteries was rated as moderate (41%), great
(27%), or limited (11%). In a few cases, iliac (9%) and femoral (14%) arteries
were also studied (table 1).
Temporal artery biopsy
Most participants (63%) stated that temporal
artery biopsy was typically available in 1–2 working days. Satisfaction with
the quality of the arterial sample (‘correctly
done, sample length >1 cm’; 65%) and pathology reports (60%) was high (table
1).
Time to obtain diagnostic tests: MRI and fluorine-18
fluorodeoxyglucose PET-CT
MRI of the aorta/extracranial arteries and fluorine-18
fluorodeoxyglucose PET-CT were reported to be available in ≤4 working days in
about 70% and 50% of cases, respectively (table 1).
Treatment
Glucocorticoid tapering schemes and sparing
agents
Glucocorticoid monotherapy was the most
common remission induction option, with different approaches in its
discontinuation. Only 46% of participants followed the European League Against
Rheumatism (EULAR) recommendations, with a higher guideline adherence among ‘experienced’ physicians (58% vs 37%; p = 0.07; figure 1a), but without
a difference between participants working inside or outside academic settings (figure
1a). Notably, 12% of respondents did not follow a predefined glucocorticoid
tapering scheme (table 2).
A glucocorticoid-sparing agent was
considered mainly in cases with relapsing diseases (64%) or in giant-cell
arteritis patients who had developed or were at increased risk of developing glucocorticoid-related
adverse effects or complications (68%; table 2). Fewer than 20% of participants
(26% of ‘experienced’ vs 12% of ‘less experienced’;
p = 0.08; figure 1b) stated they prescribed glucocorticoid-sparing agents to
every giant-cell arteritis patient. Even if not statistically significant, the
absolute proportion of physicians always prescribing glucocorticoid-sparing
agents was higher for those working in private practice (22.0%) and nonacademic
hospitals (13.0%) than in academic hospitals (5.5%; p = 0.19; figure 1b).
Tocilizumab was the most commonly used glucocorticoid-sparing agent (96%), with
71% also reporting using methotrexate. The glucocorticoid-sparing agent treatment
duration varied widely, with a third of participants (32%) not following a
predefined protocol regardless of their expertise or setting (figure 1c). The
remaining participants indicated planning discontinuation after at least 12 (25%),
18 (8%), or 24 (12%) months of therapy.
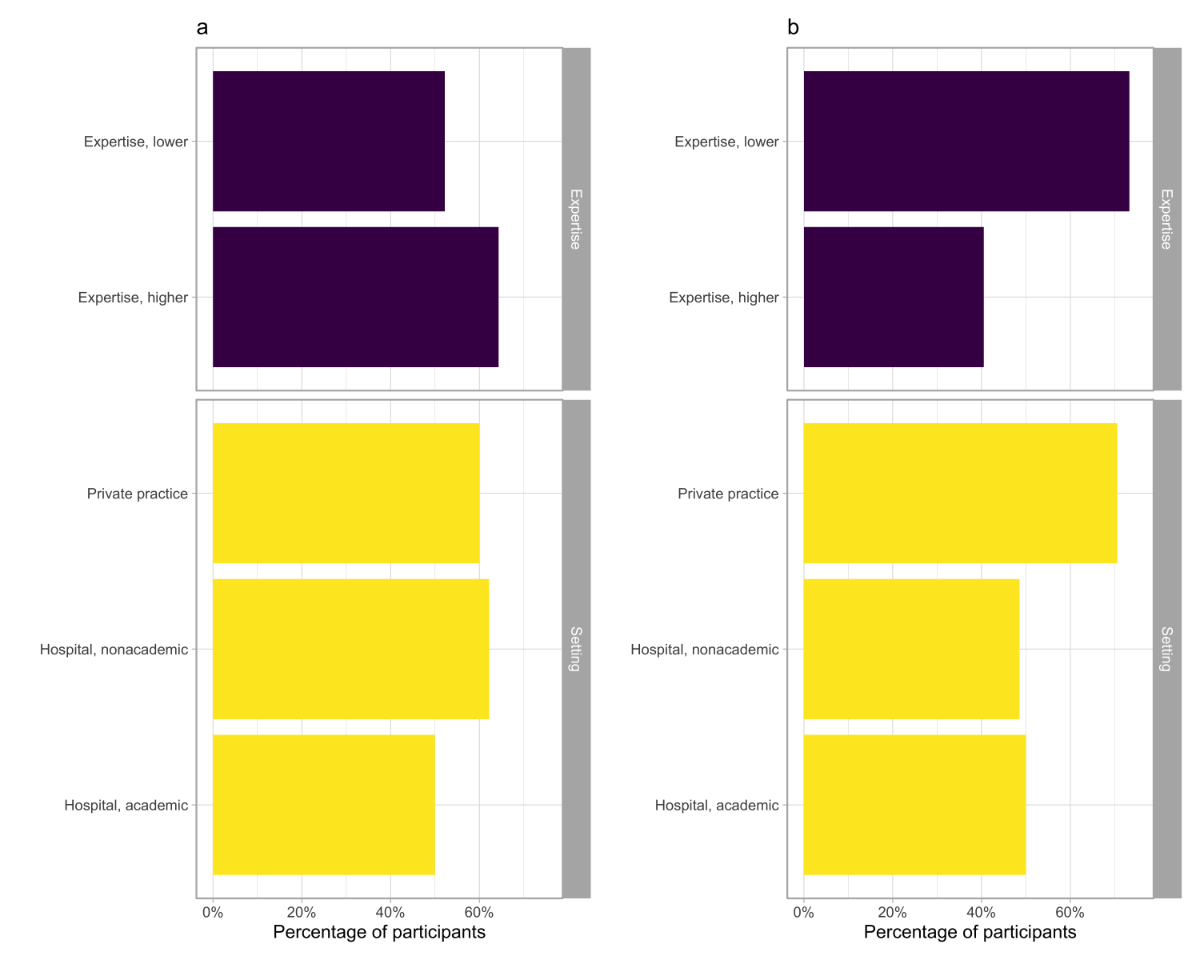
Figure 1 The
proportions of participants (a) tapering glucocorticoids according to EULAR
recommendations [2], (b) prescribing a glucocorticoid-sparing agent to every giant-cell
arteritis patient, (c) not following any prespecified tapering scheme for glucocorticoid-sparing
agents, or (d) not prescribing antiplatelet agents for giant-cell arteritis. Results
are shown according to the participants’ expertise (purple bars) or setting
(academic, nonacademic hospital, private practice; yellow bars).
Supportive treatment
About half of the participants reported
using antiplatelet agents for giant-cell arteritis as follows: always (14%),
only in cases with ocular involvement (16%), or in cases with symptoms suggestive
of cranial involvement (18%). Less than half of the participants (44%) reported
never prescribing antiplatelet agents for giant-cell arteritis (figure 1d). The
proportion of participants using vitamin D, calcium, and
trimethoprim/sulfamethoxazole prophylaxis with glucocorticoid is reported in table
2.
Imaging use during follow-up
Imaging for monitoring structural damage
(vascular thickening, stenosis, or dilatation)
The frequency of screening for vascular
complications by imaging was heterogeneous (table 3). The most frequently used
techniques were MRI of the aorta/extracranial arteries (57%; figure 2a), ultrasound
of supra-aortic arteries and abdominal aorta with computed tomography of the
thoracic aorta (24%), or fluorine-18 fluorodeoxyglucose PET-CT (10%; table 3).
The failure to follow any predefined scheme to monitor vascular complications
was higher among ‘less experienced’ participants
(71% vs 41%; p <0.001; figure 2b).
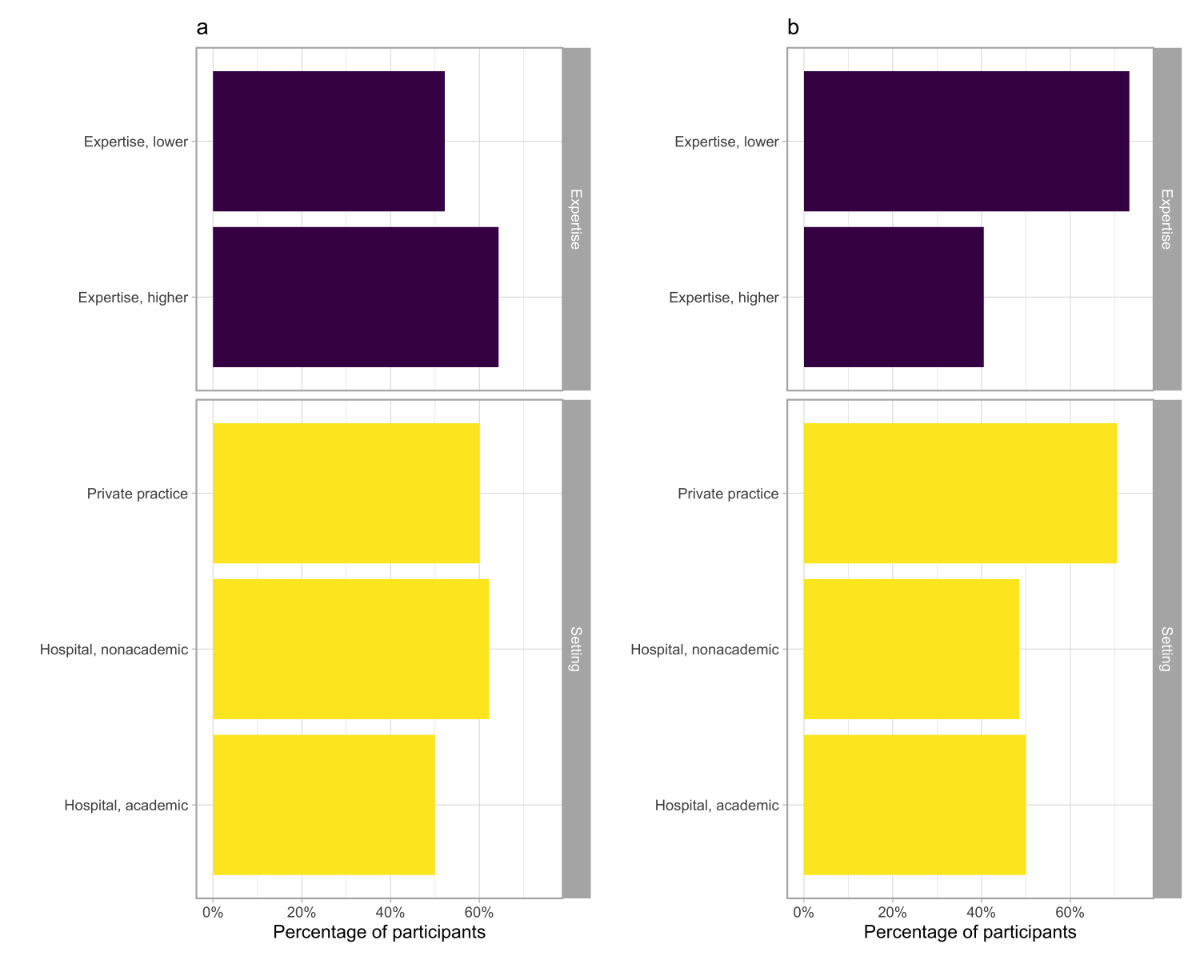
Figure 2 The
proportions of participants (a) using MRI to monitor vascular structural damage
or (b) not following any prespecified scheme to perform imaging after
diagnosis.
Imaging driving treatment choices (beyond
structural damage)
Thirty-seven per cent of participants
reported that follow-up imaging did not affect treatment, and 26% would only
consider imaging results in patients with suspected relapse (table 3).
Inflammatory signs on MRI (34%) or an increased fluorine-18 fluorodeoxyglucose
uptake on PET-CT (24%) were reported as imaging findings more often supporting
treatment escalation decisions.
Discussion
We investigated how specialists manage
patients with giant-cell arteritis in Switzerland to identify differences in
diagnostic, treatment, and follow-up strategies that could be addressed to
harmonize management and improve patient outcomes.
A key finding was that physicians in
Switzerland have rapid and broad access to the diagnostic tools typically used
to diagnose giant-cell arteritis. These include imaging modalities (MRI, ultrasound,
and PET-CT) and temporal artery biopsy. Access to imaging for diagnosing giant-cell
arteritis did not represent a barrier for most survey participants. In line
with the EULAR recommendations, the ultrasound of the temporal and/or axillary
arteries [3] is the most reported tool used to confirm a diagnosis of giant-cell
arteritis with cranial involvement. About 8 in 10 participants reported planning
this imaging technique in cases with suspected giant-cell arteritis with
cranial involvement compared to half considering temporal artery biopsy. While ultrasound
(musculoskeletal) is a key rheumatological competence, most specialists do not
perform it themselves. Half of the participants stated they performed temporal
artery biopsy only in cases with negative imaging results. This diagnostic
approach contrasts with recent data from two French studies where temporal
artery biopsy was performed in about 85%–90% of cases and ultrasound of
temporal arteries in only one-third of cases [14, 15]. Ultrasound is possibly not
as readily available in France. Spanish specialists involved in a
cross-sectional survey in 2020 also considered temporal artery biopsy as the
reference diagnostic test [16]. Interestingly, ultrasound of the temporal
arteries was also the Swiss participants’ second most frequent imaging
technique after fluorine-18 fluorodeoxyglucose PET-CT of aorta/extracranial
arteries in cases with suspected large vessel involvement. The prompt
availability and absence of patient contraindications or potential risks make
this diagnostic tool appealing. Indeed, ultrasound may be diagnostic in giant-cell
arteritis patients even without typical cranial vasculitis signs [17].
Most participants reported using glucocorticoid
monotherapy as induction-remission treatment, but only about half stated
following the EULAR guideline’s tapering scheme. Glucocorticoid-sparing agents
(tocilizumab more often than methotrexate) are primarily prescribed to patients
with relapsing disease or an increased risk of developing glucocorticoid-related
side effects. Notably, most participants still used methotrexate as the glucocorticoid-sparing
agent despite the availability of tocilizumab. This preference could be
explained by rheumatologists’ good knowledge of this drug, its ease of
prescription, and its low cost. The observed predominant use of glucocorticoid
monotherapy to induce disease remission and the willingness to limit the
prescription of glucocorticoid-sparing agents to patients with a higher glucocorticoid
exposure risk or experiencing a flare is consistent with other studies [14, 16].
After diagnosis, imaging was considered a
tool to support treatment decisions mainly when structural damage (vascular
thickening, stenosis, or dilatation) occurs or there is active vascular
inflammation when a relapse is suspected. Which imaging technique should be
used and how often it should be repeated over the giant-cell arteritis course
is still being debated [3]. The absence of robust data explains the differing
use of imaging during follow-up. Current evidence on the roles of imaging
modalities for monitoring disease activity or outcome prediction is scarce [18].
Neither imaging findings at diagnosis nor over the disease course were found to
predict disease relapse across published studies [18]. However, potential
vascular complications, especially in patients with large vessel involvement,
favour regular aortal imaging [19]. Research in this field is a critical unmet
need that should be investigated with targeted studies.
Our study was not powered to detect
significant differences between participants with different expertise in
managing giant-cell arteritis patients. However, our findings suggest that ‘more experienced’ physicians more often follow the EULAR
recommendations for glucocorticoid tapering and are less prone to
systematically prescribe a glucocorticoid-sparing agent. The unavailability of
long-term studies demonstrating the best scheme of glucocorticoid-sparing
agents likely explained why one-third of participants, regardless of their
patient volume and working setting, do not follow any predefined tapering
scheme.
This study had some limitations. First, the
inclusion of participants from other specialties could have led to different and
more generalizable results. Second, the definition of ‘expertise’ based on the number of patients with giant-cell arteritis
treated per year does not fully capture the participants’ experience or
knowledge about the best disease management approach. Furthermore, using the snowball
technique prevented us from calculating the participation rate, and
heterogeneity within centres could not be explored due to the small sample size.
Finally, we did not explore the prescription of bone protection medications in
patients chronically treated with steroids. Readers should be aware of such
limitations.
In conclusion, our survey allowed us to
characterize better the current approaches in diagnosing and treating giant-cell
arteritis patients in Switzerland. Regarding diagnosis, the main points of
interest are the ease of obtaining imaging for giant-cell arteritis patients
with both cranial and large vessel involvement, general satisfaction with the
way temporal artery biopsy is performed, and the wide use of ultrasound on
temporal arteries. Regarding treatment, most participants use glucocorticoid
monotherapy as induction-remission treatment, with glucocorticoid-sparing
agents, mostly tocilizumab, prescribed in cases with relapsing disease or to minimize
steroid exposure in patients with contraindications to glucocorticoid or at
higher risk of corticosteroid-related complications. We have highlighted a
relatively high variability in how glucocorticoid and glucocorticoid-sparing
agents have been used over time. Regarding imaging, identifying and monitoring
structural damage (vascular thickening, stenosis, or dilatation) and detecting
active inflammation signs (in relapsing patients) are the leading drivers of
treatment decisions. However, there remains very poor agreement about how
imaging should be planned over the giant-cell arteritis course.
The heterogeneity in managing giant-cell
arteritis reflects existing gaps in research and, to some extent, different
physician experiences. Regular updated national recommendations may be helpful in
broadly disseminating recent developments and their implications on daily
practice. Treatment intensity, prognostic factors, the value of imaging for
defining active vs inactive disease, and the way patients should be followed
for structural vascular complications must be studied in prospective cohorts.
One such longitudinal cohort is the Giant-Cell Arteritis and Polymyalgia Rheumatica
Module of the Swiss Cohort Quality Management, established in 2020. Finally, a
Swiss association for the study of vasculitides (VASAS, vasas.ch), has been
recently created to foster research in this field and promote knowledge of
these life-threatening diseases among patients and physicians.
Data availability statement
The data supporting this study’s findings
are available upon reasonable request.
Acknowledgements
The data supporting this study’s findings
are available upon reasonable request.
We thank all participants for
completing the online survey.
Authors
contribution: All the
authors were involved in drafting the article or revising it critically for
important intellectual content, and all authors approved the final published version.
MI and TD had full access to all study data and take responsibility for its integrity
and the accuracy of its analysis.
Study conception and design: MI, AKH, MS, and TD.
Data acquisition: MI, AKH, MS, DSC, SA, MOB, CTB, DD, AF, AM,
TN, SR, CR, LS, PV, LW, and TD.
Data analysis and interpretation: MI, AKH, MS, DSC, SA, MOB, CTB, DD,
AF, AM, TN, SR, CR, LS, PV, LW, and TD.
Dr Michele Iudici
Division of Rheumatology
Geneva University Hospitals
CH-1205 Geneva
Switzerland
michele.iudici[at]hcuge.ch
References
1.
Dejaco C
,
Brouwer E
,
Mason JC
,
Buttgereit F
,
Matteson EL
,
Dasgupta B
. Giant cell arteritis and polymyalgia rheumatica: current challenges and opportunities. Nat Rev Rheumatol. 2017 Oct;13(10):578–92. https://doi.org/10.1038/nrrheum.2017.142
2.
Hellmich B
,
Agueda A
,
Monti S
,
Buttgereit F
,
de Boysson H
,
Brouwer E
, et al.
2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020 Jan;79(1):19–30. https://doi.org/10.1136/annrheumdis-2019-215672
3.
Dejaco C
,
Ramiro S
,
Duftner C
,
Besson FL
,
Bley TA
,
Blockmans D
, et al.
EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018 May;77(5):636–43. https://doi.org/10.1136/annrheumdis-2017-212649
4.
Camellino D
,
Matteson EL
,
Buttgereit F
,
Dejaco C
. Monitoring and long-term management of giant cell arteritis and polymyalgia rheumatica. Nat Rev Rheumatol. 2020 Sep;16(9):481–95. https://doi.org/10.1038/s41584-020-0458-5
5.
Koster MJ
,
Matteson EL
,
Warrington KJ
. Large-vessel giant cell arteritis: diagnosis, monitoring and management. Rheumatology (Oxford). 2018 Feb;57 suppl_2:ii32–42. https://doi.org/10.1093/rheumatology/kex424
6.
Mahr AD
,
Jover JA
,
Spiera RF
,
Hernández-García C
,
Fernández-Gutiérrez B
,
Lavalley MP
, et al.
Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007 Aug;56(8):2789–97. https://doi.org/10.1002/art.22754
7.
Stone JH
,
Tuckwell K
,
Dimonaco S
,
Klearman M
,
Aringer M
,
Blockmans D
, et al.
Trial of Tocilizumab in Giant-Cell Arteritis. N Engl J Med. 2017 Jul;377(4):317–28. https://doi.org/10.1056/NEJMoa1613849
8.
Villiger PM
,
Adler S
,
Kuchen S
,
Wermelinger F
,
Dan D
,
Fiege V
, et al.
Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016 May;387(10031):1921–7. https://doi.org/10.1016/S0140-6736(16)00560-2
9.
Venhoff N
,
Schmidt WA
,
Lamprecht P
,
Tony HP
,
App C
,
Sieder C
, et al.
Efficacy and safety of secukinumab in patients with giant cell arteritis: study protocol for a randomized, parallel group, double-blind, placebo-controlled phase II trial. Trials. 2021 Aug;22(1):543. https://doi.org/10.1186/s13063-021-05520-1
10.
Harkins P
,
Conway R
. Giant cell arteritis: what is new in the preclinical and early clinical development pipeline? Expert Opin Investig Drugs. 2021;1–12.
11.
Harris PA
,
Taylor R
,
Thielke R
,
Payne J
,
Gonzalez N
,
Conde JG
. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010
12.
Harris PA
,
Taylor R
,
Minor BL
,
Elliott V
,
Fernandez M
,
O’Neal L
, et al.; REDCap Consortium
. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019 Jul;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
13.
Eysenbach G
. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004 Sep;6(3):e34. https://doi.org/10.2196/jmir.6.3.e34
14.
Mahr A
,
Hachulla E
,
de Boysson H
,
Guerroui N
,
Héron E
,
Vinzio S
, et al.
Presentation and Real-World Management of Giant Cell Arteritis (Artemis Study). Front Med (Lausanne). 2021 Nov;8:732934. https://doi.org/10.3389/fmed.2021.732934
15.
de Boysson H
,
Liozon E
,
Ly KH
,
Dumont A
,
Delmas C
,
Aouba A
. The different clinical patterns of giant cell arteritis. Clin Exp Rheumatol. 2019;37 Suppl 117(2):57-60.
16.
González-Gay MA
,
Ortego-Centeno N
,
Ercole L
. Clinical practice in giant cell arteritis based on a survey of specialists. Rev Clin Esp (Barc). 2022 May;222(5):266–71. https://doi.org/10.1016/j.rceng.2021.02.002
17.
de Miguel EM
,
Macchioni P
,
Conticini E
,
Campochiaro C
,
Karalilova R
,
Monti S
, et al.
OP0184 Prevalence of subclinical giant cell arteritis in patients with Polymyalgia rheumatica. Ann Rheum Dis. 2022;81 Suppl 1:122–3. https://doi.org/10.1136/annrheumdis-2022-eular.1696
18.
Duftner C
,
Dejaco C
,
Sepriano A
,
Falzon L
,
Schmidt WA
,
Ramiro S
. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018 Feb;4(1):e000612. https://doi.org/10.1136/rmdopen-2017-000612
19.
Berger CT
,
Sommer G
,
Aschwanden M
,
Staub D
,
Rottenburger C
,
Daikeler T
. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018 Aug;148:w14661. https://doi.org/10.4414/smw.2018.14661

