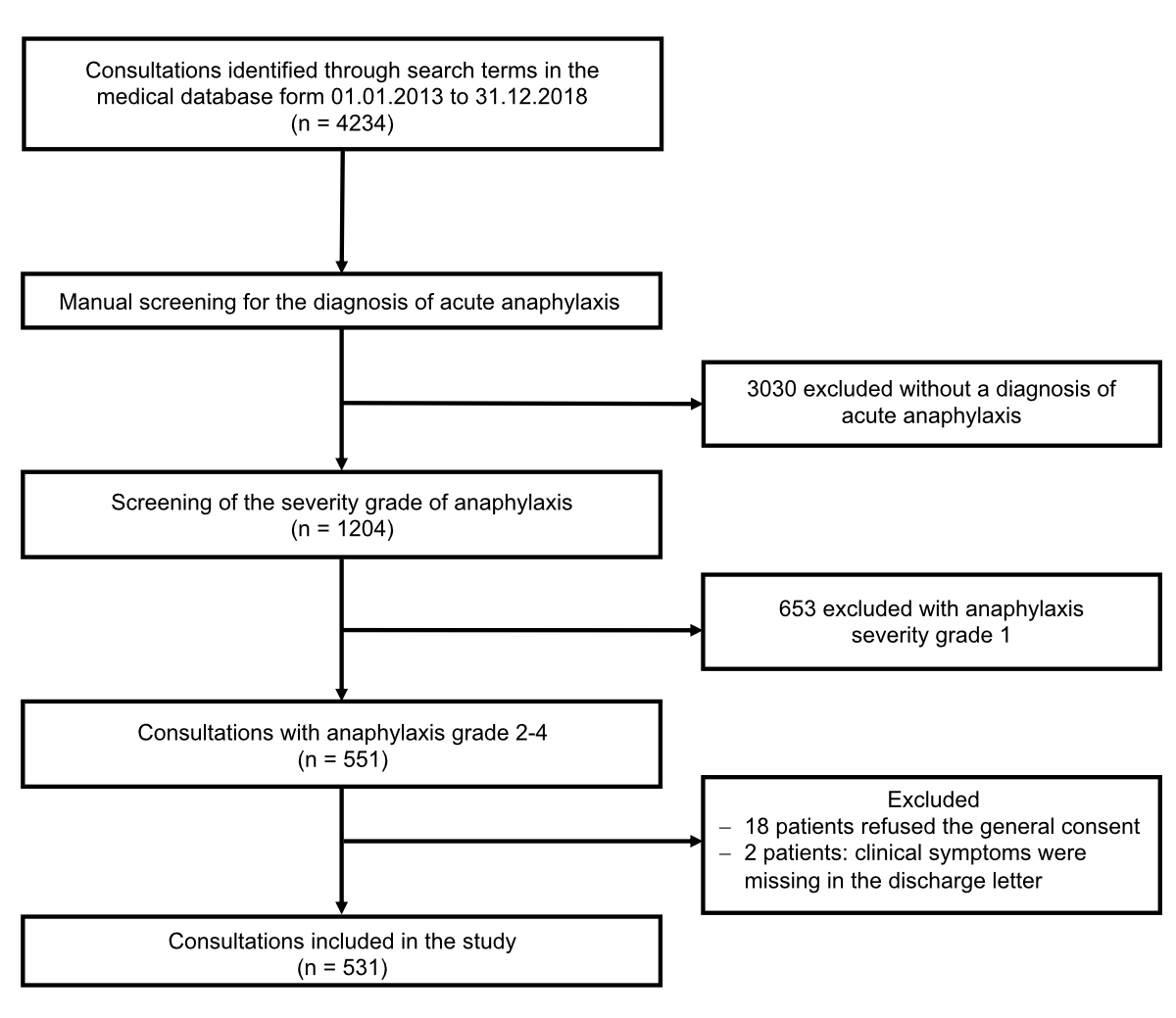
Figure 1 Flowchart of the study.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40065
Anaphylaxis is the most severe form of systemic reaction caused by acute mast cell activation due to an IgE or non-IgE mediated mechanism with the release of potent mediators [1]. The dynamics and severity of an anaphylactic reaction are unpredictable. Symptoms usually occur acutely within minutes up to two, seldom more hours of exposure to a potentially triggering source. Anaphylaxis often begins with skin symptoms, further gastrointestinal symptoms may occur, and it can rapidly progress to a potentially life-threatening medical emergency due to respiratory and cardiovascular problems [2, 3].
The diagnosis of anaphylaxis is made clinically based on signs and symptoms [2]. Several international guidelines have been established to define criteria for the diagnosis of anaphylaxis [4–7]. Nevertheless, diagnosis and determination of the severity of anaphylaxis remain difficult, especially when skin symptoms are absent [4, 8]. Because of its potentially dangerous dynamic, emergency medical services and primary care physicians have a crucial role in promptly recognising and treating patients with anaphylaxis [8].
According to current international treatment guidelines, intramuscular epinephrine is the only first-line medication to manage moderate and severe anaphylaxis [5, 9–12]. The intramuscular application is safe and effective within minutes [13]. Antihistamines and corticosteroids are considered second-line therapy, as a life-saving effect in the acute treatment of anaphylaxis has not been proven [14, 15]. Contrary to these international treatment guidelines, several studies have reported a low injection rate of epinephrine in patients with anaphylaxis [9]: half of the patients presenting to an emergency department with anaphylaxis never receive epinephrine [16]. This undersupply may increase morbidity and mortality among patients with anaphylaxis [17].
The aim of this retrospective study was, first, to analyse whether patients with moderate and severe anaphylaxis were treated with epinephrine in the prehospital setting and during their stay in our emergency department according to current guidelines and, second, to determine what factors influence the practice of administering epinephrine to patients with anaphylaxis.
This study was conducted at the Department of Emergency Medicine for Adults of the Inselspital, Bern University Hospital, Switzerland. This is a single-centre retrospective cohort study with a study period from 1 January 2013 to 31 December 2018. All patients aged ≥16 years treated for anaphylaxis in the University Hospital Bern emergency department were included.
A full-text keyword search was performed in the diagnosis list (first or second diagnosis) of the medical reports of all patients admitted to our emergency department within the given period using the following defined keyword list combined with the Boolean operator “OR“: allergic reaction, allergic shock, anaphylaxis and anaphylactic shock. Second, one experienced physician (VE) and one advanced pre-graduated medical student (DG) screened (one person per patient) the full-text fields “diagnoses”, “history”, and “clinical assessment” for characteristics of acute anaphylaxis and classified the severity of anaphylaxis for each patient according to the criteria of Ring and Messmer [18]. Because epinephrine particularly is recommended for anaphylaxis severity grade ≥2, patients with anaphylaxis severity grade 1 were excluded after the above-mentioned grading process. Patients without acute anaphylaxis as the reason for admission and those who refused or withdrew their general consent to use their anonymised data were excluded from the study.
The data were extracted from the database of the patient management system of the Department of Emergency Medicine for Adults of the Inselspital, Bern University Hospital, Switzerland (Ecare, Turnhout, Belgium):
All analyses were conducted in the R Language for Statistical Computing REF (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).We employed the base functions in R to calculate descriptive statistics. The glm function with a logit link was used to estimate logistic regressions. The dependent variable was whether epinephrine was administered or not. For this purpose, we combined the different times and contexts of epinephrine administration (self-administration; by emergency medical services; at the emergency department) into a dichotomous variable (administered/not administered; 1/0). Furthermore, we employed a stepwise procedure for estimating the association of the respective predictors on epinephrine administration. In the first step, we estimated four logistic regressions for the four organ systems. All models included age and degree of severity as a control variable. We entered age as a linear term in the regression models but transformed the variable into seven age intervals, beginning with 25 years or younger, then in ten-year intervals (26 to 35; 36 to 34 etc.) and up to 75 years or older. In the second step, we fitted a model that combined all predictors from the first step: age group and degree of severity as well as four dummy-variables for the four organ systems. We predicted the probability of receiving epinephrine for combinations of symptoms using R’s prediction function and the fitted models.
The regional ethics committee of the Canton of Bern, Switzerland, approved the study (KEK: 2019-02349).
From 1 January 2013 to 31 December 2018, 4234 out of 260’485 patients referred to our emergency department were identified in the medical database using the above search terms. Of those, 3683 patients were excluded: 3030 without an acute diagnosis of anaphylaxis (e.g., history of anaphylaxis only) and 653 with anaphylaxis severity grade 1. Of the remaining 551 patients with an anaphylaxis severity grade ≥2, 18 refused general consent, and no detailed information about clinical symptoms was available for two patients. Finally, 531 patients met all requirements and were included in the study analysis (figure 1). For these 531 patients, no missing data were observed on the variables analysed in this study.

Figure 1 Flowchart of the study.
The annual incidence of anaphylaxis in general (including grade 1 reaction) was 20.07/100’000 inhabitants, and for moderate and severe anaphylaxis was 9.18/100’000 inhabitants.
Half of the patients with anaphylaxis were male (n = 248, 46.7%) and the majority of patients were ≤ 55 years old (n = 432, 81.4%). More than half of the patients had a history of known allergy (n = 314, 59.1%), and one-third had a past anaphylaxis (n = 179, 33.7%). Patients with symptoms of anaphylaxis were mostly triaged as category 2 (n = 307, 57.8%) according to the Swiss Triage System. Ninety-one (17.1%) patients were evaluated in the rescue bay of the emergency department. The most frequent suspected triggers were drugs (n = 169, 31.8%), foods (n = 136, 25.6%) and insect stings (n = 94, 17.7%) (table 1).
Table 1Demographic data, n = 531.
| n | % | ||
| Sex | Female | 283 | 53.3% |
| Male | 248 | 46.7% | |
| Age group (years) | 16-25 | 128 | 24.1% |
| 26-35 | 115 | 21.7% | |
| 36-45 | 98 | 18.5% | |
| 46-55 | 91 | 17.1% | |
| 56-65 | 53 | 10.0% | |
| 66-75 | 26 | 4.9% | |
| ≥ 76 | 20 | 3.8% | |
| History / comorbidities | Allergy known* | 314 | 59.1% |
| Prior anaphylactic episode | 179 | 33.7% | |
| Asthma | 58 | 10.9% | |
| Triage category** | Category 1 | 85 | 16.0% |
| Category 2 | 307 | 57.8% | |
| Category 3 | 131 | 24.7% | |
| Category 4 | 2 | 0.4% | |
| Unknown | 6 | 1.1% | |
| Place of treatment at the emergency department | Rescue Bay | 91 | 17.1% |
| Suspected triggers | Drugs | 169 | 31.8% |
| Food | 136 | 25.6% | |
| Insect venoms | 94 | 17.7% | |
| Aeroallergens*** | 6 | 1.1% | |
| Contact allergens | 3 | 0.6% | |
| Multiple triggers suspected | 64 | 12.1% | |
| Unknown | 125 | 23.5% | |
* Many patients had more than one known allergy.
** Swiss Triage System (STS), categories: 1 = acutely life-threatening: treatment immediately by a physician, 2 = high urgency: treatment within 20 minutes by a physician, 3 = urgency: treatment within 120 minutes by a physician, 4 = less urgency: no urgent treatment situation.
*** Aeroallergens e.g., pollen, house dust mites, animal epithelia, fungal spores.
Most patients evaluated at our emergency department with anaphylaxis had a moderate grade 2 reaction (n = 364, 68.5%), 162 (30.5%) patients had a grade 3, and only 5 (0.9%) patients had a grade 4 reaction (figure 2).
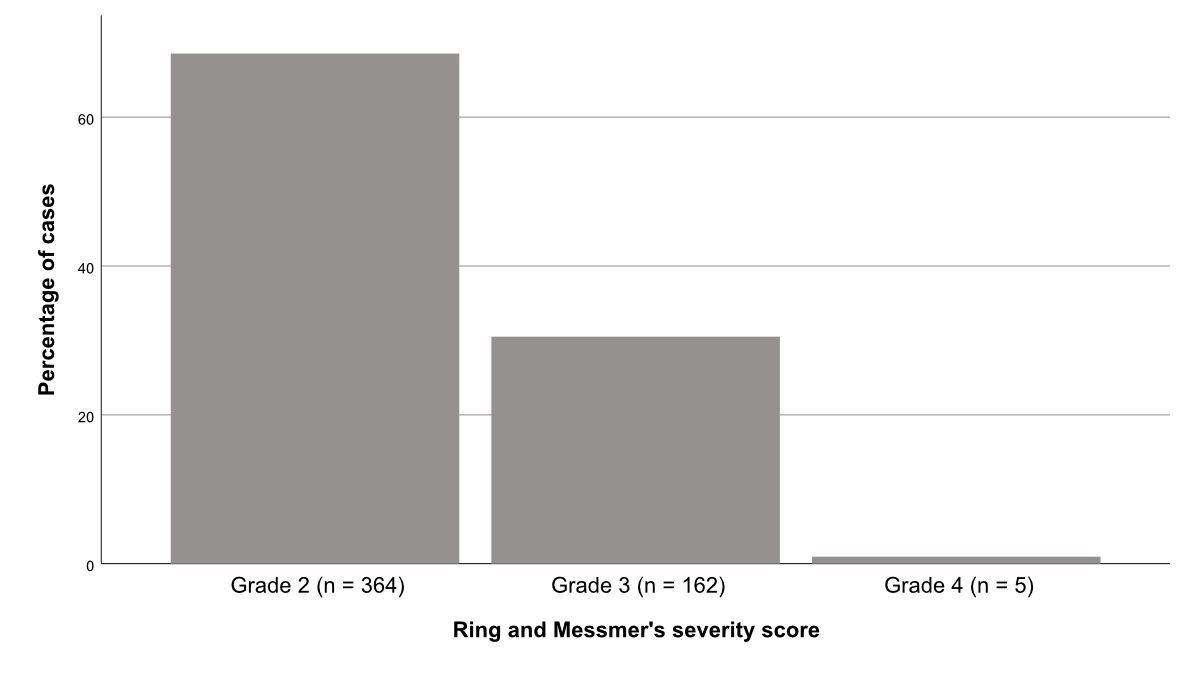
Figure 2 Severity grade according to Ring and Messmer’s classification of the patients with anaphylaxis presenting at the emergency department.
Prehospital, 237 (44.6%) patients received antihistamines, 208 (39.2%) patients received corticosteroids, and epinephrine was administered in 109 (20.5%) patients (either by self-medication or emergency medical services, respectively). At the emergency department, 499 (94.0%) patients received antihistamines, and 491 (92.5%) patients received corticosteroids. Epinephrine was administered in 143 (26.8%) patients. In total (prehospital and at the emergency department), 252 (47.3%) patients were treated with epinephrine at least once. Epinephrine was administered intramuscularly at a dose of either 0.3 mg (administration using an epinephrine autoinjector in the prehospital setting) or 0.5 mg in 144 (27.1%) patients and subcutaneously in 8 (1.5%) patients; 113 patients (21.2%) received epinephrine by inhalation, 39 (7.3%) intravenously, and 1 (0.2%) via the intraosseous route. Patients with grade 2 reactions received epinephrine intramuscularly in 21.4% (n = 78), patients with grade 3 reaction in 38.9% (n = 63), and patients with grade 4 reaction in 60% (n = 3). Inhaled epinephrine was administered in 32.1% (n = 52) of patients with grade 3 anaphylaxis. Intravenous epinephrine was used in 60% (n = 3) of patients with grade 4 anaphylaxis. Subcutaneous epinephrine was administered in 4 (1.1%) patients with grade 2 and in 4 (2.5%) patients with grade 3 anaphylaxis. Intraosseous epinephrine was given in 1 (20%) patient with grade 4 anaphylaxis.
Most emergency department-treated patients (n = 464, 87.1%) were discharged home. In 311 (58.6%) cases, an allergy work-up was recommended. Emergency kits were given to 381 (71.8 %) patients before emergency department discharge. An 0.3 mg epinephrine autoinjector was provided to 205 (38.5%) patients (table 2).
Table 2Drug administration prehospital and at the emergency department and follow-up recommendations, n = 531.
| Total | Anaphylaxis grade 2 | Anaphylaxis grade 3 | Anaphylaxis grade 4 | ||||||
| n = 531 | % | n = 364 | % | n = 162 | % | n = 5 | % | ||
| Self-treatment by patient (prehospital) | Antihistamines | 97 | 18.3% | 63 | 17.3% | 34 | 21.0% | 0 | 0 |
| Corticosteroids | 72 | 13.6% | 47 | 12.9% | 25 | 15.4% | 0 | 0 | |
| Epinephrine autoinjector | 24 | 4.5% | 13 | 3.6% | 9 | 5.6% | 2 | 40% | |
| Treatment by emergency medical services (prehospital) | Antihistamines | 140 | 26.4% | 82 | 22.5% | 55 | 34.0% | 3 | 60% |
| Corticosteroids | 136 | 25.6% | 79 | 21.7% | 53 | 32.7% | 4 | 80% | |
| Epinephrine | 85 | 16.0% | 36 | 9.9% | 44 | 27.2% | 5 | 100% | |
| Treatment in the emergency department | Antihistamines | 499 | 94.0% | 344 | 94.5% | 152 | 93.8% | 3 | 60.0% |
| Corticosteroids | 491 | 92.5% | 336 | 92.3% | 151 | 93.2% | 4 | 80.0% | |
| Epinephrine | 143 | 26.8% | 82 | 22.5% | 60 | 37.0% | 1 | 20.0% | |
| Route of epinephrine administration* | Intramuscular | 144 | 27.1% | 78 | 21.4% | 63 | 38.9% | 3 | 60.0% |
| Inhalative | 113 | 21.2% | 60 | 16.5% | 52 | 32.1% | 1 | 20.0% | |
| Intravenous | 39 | 7.3% | 7 | 1.9% | 29 | 17.9% | 3 | 60.0% | |
| Subcutaneous | 8 | 1.5% | 4 | 1.1% | 4 | 2.5% | 0 | 0 | |
| Intraosseous | 1 | 0.2% | 0 | 0 | 0 | 0 | 1 | 20.0% | |
| Airway management | Intubation | 6 | 1.1% | 0 | 0 | 2 | 1.2% | 4 | 80.0% |
| Tracheotomy/coniotomy | 1 | 0.2% | 0 | 0 | 0 | 0 | 1 | 20.0% | |
| Disposition | Discharge from the emergency department | 464 | 87.1% | 338 | 92.6% | 125 | 77.2% | 1 | 20.0% |
| Admitted to the hospital | 65 | 12.2% | 25 | 6.9% | 36 | 22.2% | 4 | 80.0% | |
| Follow up | Referral to an allergist | 311 | 58.6% | 218 | 59.9% | 93 | 57.4% | 0 | 0 |
| Delivery of an emergency kit** | 381 | 71.8% | 265 | 72.8% | 114 | 70.4% | 2 | 40.0% | |
| Delivery of an epinephrine autoinjector | 205 | 38.6% | 126 | 34.6% | 77 | 47.5% | 2 | 40.0% | |
* The route of epinephrine administration contains the administration in the prehospital setting and at the emergency department.
** Emergency kit contains 2 tablets of antihistamines and corticosteroids each.
We used logistic regression to determine predictors of epinephrine application. The analysis was conducted in two steps. In the first step, we fitted four independent regression models for integumentary, gastrointestinal, respiratory, or cardiovascular symptoms. We found Odds Ratios of OR = 0.83 (CI 0.47–1.47) for the integumentary symptoms, which was neither a significant increase nor a decrease in the odds for receiving epinephrine. A presentation with gastrointestinal symptoms was associated with decreased odds of applying epinephrine (OR = 0.40; CI 0.26–0.62). For respiratory symptoms, we found more than a doubling in the odds for epinephrine administration (OR = 2.59; CI 1.71–3.99). A doubling in odds was found when cardiac symptoms were presented (OR = 2.20; CI 1.53–3.18). In general, both degrees of severity and patient age were associated with an increased likelihood of epinephrine administration, irrespective of the organs involved. Odds Ratios ranged between OR = 2.91 and OR = 4.00 for degree of severity and OR = 1.11 and OR = 1.16 for age group across the four models. All details are given in table 3.
Table 3Predictors of epinephrine administration.
| Predictors | Integumentary symptoms model | Gastrointestinal symptoms model | Respiratory symptoms model | Cardiovascular symptoms model | Combined model | |||||
| Odds Ratios | CI | Odds Ratios | CI | Odds Ratios | CI | Odds Ratios | CI | Odds Ratios | CI | |
| Severity | 3.04 | 2.07–4.47 | 4.00 | 2.65–6.12 | 3.15 | 2.14–4.68 | 2.91 | 1.98–4.31 | 3.35 | 2.17–5.23 |
| Age | 1.14 | 1.03–1.27 | 1.11 | 1.00–1.24 | 1.16 | 1.04–1.29 | 1.12 | 1.01–1.25 | 1.11 | 0.99–1.25 |
| Symptoms | ||||||||||
| Integumentary | 0.83 | 0.47–1.47 | 0.98 | 0.54–1.81 | ||||||
| Gastrointestinal | 0.40 | 0.26–0.62 | 0.62 | 0.39–1.00 | ||||||
| Respiratory | 2.59 | 1.71–3.99 | 3.14 | 1.95–5.14 | ||||||
| Cardiovascular | 2.20 | 1.53–3.18 | 2.94 | 1.96–4.46 | ||||||
In a second step, all four predictors for symptoms in the respective organ systems were included in one multivariate logistic regression. The correlations between symptoms were low, as indicated by a mean for Kendall’s Tau for each bi-variate correlation of Tau = -0.13. The severity of symptoms was tripling the odds for an epinephrine application (OR = 3.35, CI 2.17–5.23), while the effect for the age group was negligible (OR = 1.11, CI 0.99–1.25). As in the previous analyses, respiratory (OR = 3.14, CI 1.95–5.14) or cardiovascular symptoms (OR = 2.94, CI 1.96–4.46) were associated with epinephrine administration. Again, odds ratio estimates were similar to the separated models from step one for integumentary and gastrointestinal symptoms. For integumentary symptoms, we found an Odds Ratio of OR = 0.98 (CI 0.54–1.81). For gastrointestinal symptoms, the estimate was OR = 0.62 (CI 0.39–1.00). Importantly, this second, combined model also allows for predicting the likelihood of epinephrine administration given certain combinations of symptoms. Using R’s predict function, we estimated that for patients displaying both integumentary and gastrointestinal symptoms, the probability of epinephrine administration was 14.5%. In contrast, the probability of patients displaying both cardiovascular and respiratory symptoms was 72.3%. Finally, as a robustness check, we estimated the models using either the untransformed age variable or the seven age intervals. Results only differed marginally.
In this study, the annual incidence of anaphylaxis was comparable with other studies [3, 20].
Consistent with the literature, the most common suspected causes of anaphylaxis in adults were drugs (31.8%), food (25.6%), and insect stings (17.7%), and many cases were caused by exposure to an unknown trigger (23.5%) [8, 21-23].
Epinephrine is the drug of first choice in the treatment of anaphylaxis, as it is the only drug that eliminates all symptoms of anaphylaxis and saves lives [7, 24]. Despite this knowledge, epinephrine administration in anaphylaxis varies from 25% to 70% [21, 25–27]. In this study, approximately half of the patients (47.3%) with moderate and severe anaphylaxis were treated with epinephrine. Although one-third of all patients (33.7%) had a history of anaphylaxis, only 4.5% of patients self-administered epinephrine prehospital. One reason for this may be that many patients prescribed epinephrine do not have their own epinephrine device available in an emergency [28, 29]. In the prehospital setting, emergency medical services treated 16% of patients with epinephrine, and in the emergency department, another 26.8% of patients received epinephrine. Two-thirds of the patients received epinephrine intramuscularly. Because of the severity of anaphylaxis, epinephrine was administered intravenously in 7.3%. A minority, however, got epinephrine intentionally subcutaneously. Even though epinephrine administered subcutaneously has been shown to be as effective as intramuscularly injected adrenaline, this does not conform to international recommendations [30]. Nonetheless, it underscores the importance of education for those working in emergency medicine. In this, as in previous studies, administering antihistamines (94% in the emergency department) and steroids (92.5% in the emergency department) was much more common than using epinephrine. Current guidelines recommend the administration of antihistamines as optional and only for the symptomatic relief of pruritus and rash [20]. In addition, a meta-analysis found that commonly used corticosteroids do not reduce the likelihood of a biphasic course with a late reaction [13, 31]. Nevertheless, antihistamines and corticosteroids are almost routinely administered by physicians as the first medications for patients with anaphylaxis [16, 25]. This may be a dangerous mechanism, as healthcare providers cite antihistamine use as the most common reason for not using epinephrine [14, 32].
Our results show that the degree of anaphylaxis severity was a significant predictor of epinephrine administration. Respiratory and cardiovascular symptoms increased the likelihood of epinephrine administration. In contrast, cardiorespiratory stable patients with combined skin and gastrointestinal symptoms rarely received epinephrine. Abdominal pain, vomiting, and diarrhoea are probably often interpreted as not being serious symptoms of anaphylaxis that would warrant treatment with epinephrine. Some team members of emergency medical services/physicians still believe that “shock” must be present to diagnose anaphylaxis and indicate epinephrine administration [33]. As the diagnosis of acute anaphylaxis is based on symptoms without a confirmatory diagnostic test, it reflects the interpretation by the emergency medical service team/physician in charge. On the one hand, misconception or incorrect interpretation of severity may lead to inadequate recognition of anaphylaxis and suboptimal treatment. On the other hand, rapid stabilisation of a patient until arrival at the emergency department with improvement of symptoms (spontaneously, by removal of the trigger, e.g., by vomiting, or by medication) is probably another reason for the lack of epinephrine administration despite a formal grade 2 reaction; because in a stable patient in the emergency department whose symptoms are resolving, administration of epinephrine is no longer necessary [14].
In addition, fear of side effects, particularly in patients with preexisting cardiovascular disease, may account for physicians’ reluctance to prescribe epinephrine [9, 25, 34]. However, plasma concentrations of epinephrine return to adequate levels very rapidly after intramuscular application [35]; adverse effects occurred almost exclusively in adult patients who received inappropriate intramuscular or intravenous doses [36].
After successful treatment of an anaphylactic event, a management plan should be in place before patient discharge. This is important because one-third of all patients will have a repeat event after an anaphylaxis [37, 38]. In this study, an emergency kit with antihistamines and corticosteroids was provided at discharge from the emergency department in 71.8%, and 38.6% of patients received an epinephrine autoinjector. Follow-up with an allergist was recommended in 58.6% of cases. Prescription and application training of an epinephrine autoinjector and referral to an allergist to properly identify relevant allergens are paramount to successful patient care [10, 16, 21, 39]. A previous study has shown that confirmed triggers are often different from the triggers suspected by the patient or physician in the emergency department [8]. Nevertheless, in general, many patients diagnosed with anaphylaxis in the emergency departments are never evaluated by an allergist [16, 21].
In this study, there was no accumulation of life-threatening incidents. A total of 10 patients were hospitalised in the intensive care unit (e.g., for need of ventilation, circulatory support, progressive or two-stage progression), there were no deaths within the study period. Despite the underuse of epinephrine as first-line treatment, the fatality rate is extremely low with a mortality at <0.5% per episode of anaphylaxis [23, 45].
In summary, there are no absolute contraindications for administering epinephrine in anaphylaxis [9, 20], and it is even recommended if the diagnosis is uncertain [40].Importantly, the administration of antihistamines and corticosteroids should never delay the administration of epinephrine and fluid resuscitation during patient stabilisation in anaphylaxis [41].
This study has several limitations. As with all retrospective data analyses, we cannot rule out documentation bias or missed patients, despite careful data extraction and analysis. No follow-up after discharge from hospital was possible. Furthermore, there is a potential for misclassification bias as the severity grade of anaphylaxis was done retrospectively due to symptoms noted in the patient electronic data record.
Epinephrine is the drug of choice when anaphylaxis is suspected. However, only half of the patients with anaphylaxis grade ≥2, according to Ring & Messmer criteria, received epinephrine based on national and international guidelines. Respiratory and cardiovascular symptoms are key symptoms for epinephrine administration, whereas gastrointestinal symptoms seem to be interpreted as non-severe symptoms of anaphylaxis. The main reasons for restrictive administration of epinephrine both in the prehospital setting and in the emergency department include misinterpretation of anaphylaxis, fear of the adverse effects of epinephrine, or that clinical improvement has occurred in the course to emergency department admission after initial treatment. Nevertheless, fatal outcomes in patients with anaphylaxis are rare. Altogether, the education of medical staff is crucial for excellent management of anaphylaxis and to increase the administration rate of epinephrine. Further research concerning epinephrine administration in anaphylaxis is an important issue.
We would like to thank Brigitta Gahl, PhD, from the Clinical Trial Unit of the University of Bern for initial statistical support.
Authors’ contributions: SE, DG, VE, AH and JK contributed to the conception and design of the study. DG and VE did the manual coding of the data. SKS and SE performed the analysis and interpretation of the data supported by AH and MR. SE drafted the manuscript and AH, MR, DG, VE, JK, SKS and AE revised it critically. All authors approved the final version to be published.
This research received no external funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Fischer D , Vander Leek TK , Ellis AK , Kim H . Anaphylaxis. Allergy Asthma Clin Immunol. 2018 Sep;14(S2 Suppl 2):54. https://doi.org/10.1186/s13223-018-0283-4
2. Muraro A , Werfel T , Hoffmann-Sommergruber K , Roberts G , Beyer K , Bindslev-Jensen C , et al.; EAACI Food Allergy and Anaphylaxis Guidelines Group . EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014 Aug;69(8):1008–25. https://doi.org/10.1111/all.12429
3. Ring J , Beyer K , Biedermann T , Bircher A , Fischer M , Fuchs T , et al. Guideline (S2k) on acute therapy and management of anaphylaxis: 2021 update: S2k-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Medical Association of German Allergologists (AeDA), the Society of Pediatric Allergology and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Society for Neonatology and Pediatric Intensive Care (GNPI), the German Society of Dermatology (DDG), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Respiratory Society (DGP), the patient organization German Allergy and Asthma Association (DAAB), the German Working Group of Anaphylaxis Training and Education (AGATE). Allergo J Int. 2021;30(1):1–25. https://doi.org/10.1007/s40629-020-00158-y
4. Sampson HA , Muñoz-Furlong A , Campbell RL , Adkinson NF Jr , Bock SA , Branum A , et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006 Feb;117(2):391–7. https://doi.org/10.1016/j.jaci.2005.12.1303
5. Simons FE , Ardusso LR , Bilò MB , Cardona V , Ebisawa M , El-Gamal YM , et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014 May;7(1):9. https://doi.org/10.1186/1939-4551-7-9
6. Lieberman P , Nicklas RA , Oppenheimer J , Kemp SF , Lang DM , Bernstein DI , et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477-80.e1-42.
7. Muraro A , Roberts G , Worm M , Bilò MB , Brockow K , Fernández Rivas M , et al.; EAACI Food Allergy and Anaphylaxis Guidelines Group . Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014 Aug;69(8):1026–45. https://doi.org/10.1111/all.12437
8. Alvarez-Perea A , Tanno LK , Baeza ML . How to manage anaphylaxis in primary care. Clin Transl Allergy. 2017 Dec;7(1):45. https://doi.org/10.1186/s13601-017-0182-7
9. Choi YJ , Kim J , Jung JY , Kwon H , Park JW . Underuse of Epinephrine for Pediatric Anaphylaxis Victims in the Emergency Department: A Population-based Study. Allergy Asthma Immunol Res. 2019 Jul;11(4):529–37. https://doi.org/10.4168/aair.2019.11.4.529
10. Simons FE , Ardusso LR , Bilò MB , El-Gamal YM , Ledford DK , Ring J , et al.; World Allergy Organization . World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011 Feb;4(2):13–37. https://doi.org/10.1097/WOX.0b013e318211496c
11. Brown JC , Simons E , Rudders SA . Epinephrine in the Management of Anaphylaxis. J Allergy Clin Immunol Pract. 2020 Apr;8(4):1186–95. https://doi.org/10.1016/j.jaip.2019.12.015
12. Lieberman P , Nicklas RA , Randolph C , Oppenheimer J , Bernstein D , Bernstein J , et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015 Nov;115(5):341–84. https://doi.org/10.1016/j.anai.2015.07.019
13. Anagnostou K , Turner PJ . Myths, facts and controversies in the diagnosis and management of anaphylaxis. Arch Dis Child. 2019 Jan;104(1):83–90. https://doi.org/10.1136/archdischild-2018-314867
14. Shaker MS , Wallace DV , Golden DB , Oppenheimer J , Bernstein JA , Campbell RL , et al.; Collaborators; Chief Editors; Workgroup Contributors; Joint Task Force on Practice Parameters Reviewers . Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020 Apr;145(4):1082–123. https://doi.org/10.1016/j.jaci.2020.01.017
15. Wood RA , Camargo CA Jr , Lieberman P , Sampson HA , Schwartz LB , Zitt M , et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014 Feb;133(2):461–7. https://doi.org/10.1016/j.jaci.2013.08.016
16. Prince BT , Mikhail I , Stukus DR . Underuse of epinephrine for the treatment of anaphylaxis: missed opportunities. J Asthma Allergy. 2018 Jun;11:143–51. https://doi.org/10.2147/JAA.S159400
17. Sheikh A , Shehata YA , Brown SG , Simons FE . Adrenaline for the treatment of anaphylaxis: cochrane systematic review. Allergy. 2009 Feb;64(2):204–12. https://doi.org/10.1111/j.1398-9995.2008.01926.x
18. Ring J , Messmer K . Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977 Feb;1(8009):466–9. https://doi.org/10.1016/S0140-6736(77)91953-5
19. Rutschmann OT , Hugli OW , Marti C , Grosgurin O , Geissbuhler A , Kossovsky M , et al. Reliability of the revised Swiss Emergency Triage Scale: a computer simulation study. Eur J Emerg Med. 2018 Aug;25(4):264–9. https://doi.org/10.1097/MEJ.0000000000000449
20. Dudley LS , Mansour MI , Merlin MA . Epinephrine for anaphylaxis: underutilized and unavailable. West J Emerg Med. 2015 May;16(3):385–7. https://doi.org/10.5811/westjem.2015.3.25337
21. De Vera MJ , Tagaro IC . Anaphylaxis diagnosis and management in the Emergency Department of a tertiary hospital in the Philippines. Asia Pac Allergy. 2020 Jan;10(1):e1. https://doi.org/10.5415/apallergy.2020.10.e1
22. Lippner E , Dinakar C. Increasing Emergency Department Visits for Anaphylaxis, 2005-2014. Pediatrics. 2017;140(Supplement 3):S188.1-S.
23. Turner PJ , Campbell DE , Motosue MS , Campbell RL . Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J Allergy Clin Immunol Pract. 2020 Apr;8(4):1169–76. https://doi.org/10.1016/j.jaip.2019.11.027
24. Simons KJ , Simons FE . Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol. 2010 Aug;10(4):354–61. https://doi.org/10.1097/ACI.0b013e32833bc670
25. Ruiz Oropeza A , Lassen A , Halken S , Bindslev-Jensen C , Mortz CG . Anaphylaxis in an emergency care setting: a one year prospective study in children and adults. Scand J Trauma Resusc Emerg Med. 2017 Nov;25(1):111. https://doi.org/10.1186/s13049-017-0402-0
26. Fleischer DM , Perry TT , Atkins D , Wood RA , Burks AW , Jones SM , et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012 Jul;130(1):e25–32. https://doi.org/10.1542/peds.2011-1762
27. Robinson M , Greenhawt M , Stukus DR . Factors associated with epinephrine administration for anaphylaxis in children before arrival to the emergency department. Ann Allergy Asthma Immunol. 2017 Aug;119(2):164–9. https://doi.org/10.1016/j.anai.2017.06.001
28. DeSantiago-Cardenas L , Rivkina V , Whyte SA , Harvey-Gintoft BC , Bunning BJ , Gupta RS . Emergency epinephrine use for food allergy reactions in Chicago Public Schools. Am J Prev Med. 2015 Feb;48(2):170–3. https://doi.org/10.1016/j.amepre.2014.09.005
29. Curtis C , Stukus D , Scherzer R . Epinephrine preparedness in pediatric patients with food allergy: an ideal time for change. Ann Allergy Asthma Immunol. 2014 Jun;112(6):560–2. https://doi.org/10.1016/j.anai.2014.04.009
30. Duvauchelle T , Robert P , Donazzolo Y , Loyau S , Orlandini B , Lehert P , et al. Bioavailability and Cardiovascular Effects of Adrenaline Administered by Anapen Autoinjector in Healthy Volunteers. J Allergy Clin Immunol Pract. 2018;6(4):1257–63. https://doi.org/10.1016/j.jaip.2017.09.021
31. Lee S , Bellolio MF , Hess EP , Erwin P , Murad MH , Campbell RL . Time of Onset and Predictors of Biphasic Anaphylactic Reactions: A Systematic Review and Meta-analysis. J Allergy Clin Immunol Pract. 2015;3(3):408-16.e1-2.
32. Simons FE , Clark S , Camargo CA Jr . Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol. 2009 Aug;124(2):301–6. https://doi.org/10.1016/j.jaci.2009.03.050
33. Kastner M , Harada L , Waserman S . Gaps in anaphylaxis management at the level of physicians, patients, and the community: a systematic review of the literature. Allergy. 2010 Apr;65(4):435–44. https://doi.org/10.1111/j.1398-9995.2009.02294.x
34. Ko BS , Kim JY , Seo DW , Kim WY , Lee JH , Sheikh A , et al. Should adrenaline be used in patients with hemodynamically stable anaphylaxis? Incident case control study nested within a retrospective cohort study. Sci Rep. 2016 Feb;6(1):20168. https://doi.org/10.1038/srep20168
35. Ring J , Klimek L , Worm M . Adrenaline in the Acute Treatment of Anaphylaxis. Dtsch Arztebl Int. 2018 Aug;115(31-32):528–34. https://doi.org/10.3238/arztebl.2018.0528
36. Kawano T , Scheuermeyer FX , Stenstrom R , Rowe BH , Grafstein E , Grunau B . Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation. 2017 Mar;112:53–8. https://doi.org/10.1016/j.resuscitation.2016.12.020
37. Tejedor-Alonso MA , Pérez-Codesido S , Nieto-Nieto A , Gonzalez-Moreno A , Rosado Ingelmo A , Laiseca García J , et al. Recurrence of anaphylaxis: A systematic review of observational studies. Allergy. 2021.
38. Worm M , Moneret-Vautrin A , Scherer K , Lang R , Fernandez-Rivas M , Cardona V , et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014 Oct;69(10):1397–404. https://doi.org/10.1111/all.12475
39. Kemp SF , Lockey RF , Simons FE ; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis . Epinephrine: the drug of choice for anaphylaxis-a statement of the world allergy organization. World Allergy Organ J. 2008 Jul;1(7 Suppl):S18–26. https://doi.org/10.1097/WOX.0b013e31817c9338
40. Waserman S , Chad Z , Francoeur MJ , Small P , Stark D , Vander Leek TK , et al. Management of anaphylaxis in primary care: canadian expert consensus recommendations. Allergy. 2010 Sep;65(9):1082–92. https://doi.org/10.1111/j.1398-9995.2010.02418.x
41. Muraro A , Halken S , Arshad SH , Beyer K , Dubois AE , Du Toit G , et al.; EAACI Food Allergy and Anaphylaxis Guidelines Group . EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. 2014 May;69(5):590–601. https://doi.org/10.1111/all.12398