Detection of mostly
viral pathogens and high proportion of antibiotic treatment initiation in
hospitalised children with community-acquired pneumonia in Switzerland – baseline
findings from the first two years of the KIDS-STEP trial
DOI: https://doi.org/10.57187/smw.2023.40040
Malte
Kohns Vasconcelosabc, Patrick M.
Meyer Sauteurd, Kristina
Keitelef, Regina
Santorog, Adrian
Eglih, Michael
Coslovskyi, Michelle
Seilerj, Marco
Luràk, Henrik
Köhlerl, Natasha
Loevym, Christian R.
Kahlertn, Ulrich
Heiningera, Johannes
van den Ankerb, Julia A.
Bielickiab
a Department of Infectious Diseases
and Vaccinology, University of Basel Children’s Hospital (UKBB), Switzerland
b Department of Paediatric Pharmacology,
University of Basel Children’s Hospital (UKBB), Basel, Switzerland
c Institute for Medical Microbiology and Hospital
Hygiene, Heinrich Heine University Düsseldorf, Germany
d Division of Infectious Diseases and Hospital
Epidemiology, University Children’s Hospital Zurich, Switzerland
e Department of Paediatric Emergency Medicine,
Department of Paediatrics, University Children's Hospital, Inselspital,
University of Bern, Switzerland
f Swiss Tropical and Public Health Institute,
University of Basel, Switzerland
g Paediatric Research Centre, University of Basel
Children’s Hospital (UKBB), Basel, Switzerland
h Clinical Bacteriology and Mycology, University
Hospital Basel, and Applied Microbiology Research, University of Basel,
Switzerland
i Clinical Trial Unit, University of Basel,
Switzerland
j Paediatric Emergency Department, University
Children’s Hospital Zurich, Switzerland
k Division of Paediatric Pulmonology, Children's
Hospital Lucerne, Switzerland
l Paediatric Emergency Unit, Children's Hospital
Aarau (KSA), Switzerland
m Paediatric Platform for Clinical Research,
Department of Woman, Child and Adolescent Medicine, Geneva University
Hospitals, Geneva, Switzerland
n Infectious Diseases and Hospital Epidemiology,
Children's Hospital of Eastern Switzerland, St Gallen, Switzerland
Summary
AIMS OF THE STUDY: Globally, since the introduction of conjugate-vaccines
against encapsulated bacteria, respiratory viruses have caused most hospitalisations
for community-acquired pneumonia. The aim of this study was to describe pathogens
detected and their association with clinical findings in Switzerland.
METHODS: Baseline data were analysed for all trial participants enrolled
between September 2018 and September 2020 into the KIDS-STEP Trial, a
randomised controlled superiority trial on the effect of betamethasone on
clinical stabilisation of children admitted with community-acquired pneumonia.
Data included clinical presentation, antibiotic use and results of pathogen
detection. In addition to routine sampling, nasopharyngeal specimens were
analysed for respiratory pathogens using a panel polymerase chain reaction test
covering 18 viral and 4 bacterial pathogens.
RESULTS: 138 children with a median age of 3 years were enrolled at the
eight trial sites. Fever (obligatory for enrolment) had been present for
median 5 days before admission. Most common symptoms were reduced activity
(129, 93.5%) and reduced oral intake (108, 78.3%). Oxygen saturation <92%
was found in 43 (31.2%). Forty-three participants (29.0%) were already on
antibiotic treatment prior to admission and 104 participants (75.4%) received
antibiotic treatment on admission. Pathogen testing results were available from
132 children: 31 (23.5%) had respiratory syncytial virus detected, 21 (15.9%)
human metapneumovirus. The pathogens detected showed expected seasonal and age
preponderance and were not associated with chest X-ray findings.
CONCLUSIONS: In the
context of the predominantly viral pathogens detected, the majority of
antibiotic treatment is probably unnecessary. The ongoing trial, as well as
other studies, will be able to provide comparative pathogen detection data to
compare pre- and post-COVID-19-pandemic settings.
This trial is registered on https://clinicaltrials.gov (NCT03474991) and
on the Swiss National Clinical Trials Portal (SNCTP000002864).
Introduction
In Europe, community-acquired pneumonia accounts for 10 to 15% of
paediatric hospital admissions [1].
Studies conducted in different settings since the introduction of conjugate
vaccines for Streptococcus pneumoniae and Haemophilus influenzae
type b consistently found the majority of admissions due to acute respiratory
infections and more specifically for community-acquired pneumonia to be caused
by respiratory viruses, most prominently respiratory syncytial virus (RSV) and
influenza viruses [2–4]. Detection of
individual respiratory viruses is not associated with increased severity or
extended length of hospital stay [5],
although some studies have shown increased length of stay for combinations of
RSV and influenza or rhinoviruses [6, 7].
Current clinical guidelines for children hospitalised with CAP uniformly
recommend antibiotic treatment in the presence of World Health Organization (WHO)
danger signs but are incongruent regarding antibiotics in other children.
The KIDS-STEP trial enrols children at eight sites providing medical
care to a large proportion of Switzerland’s paediatric population [8]. The ancillary microbiology study has the
aim to provide data for pathogen subgroup analyses of the effect of
betamethasone for community-acquired pneumonia and to differentiate
pathogen-driven effects on length of hospital stay from medication effects. The
COVID-19 pandemic has disrupted the seasonality of acute respiratory infections
in Switzerland and may result in changed patterns of aetiology of community-acquired
pneumonia in the coming years [9]. We
therefore conducted a preliminary pathogen analysis for the trial participants
enrolled up to autumn 2020 to provide representative, high-quality and mostly
prepandemic data on pathogen detection in children in Switzerland hospitalised
for community-acquired pneumonia. The aim of this study was to describe the pathogens
detected and their association with clinical findings at baseline in
Switzerland.
Methods
The KIDS-STEP Trial is a randomised controlled superiority trial on the
effect of betamethasone on clinical stabilisation of children hospitalised with
community-acquired pneumonia. The full trial protocol has been published [8]. In brief, children are screened following
a clinical diagnosis or differential diagnosis of community-acquired pneumonia
and the decision to admit as an inpatient and are eligible if they are between
6 months and 14 years of age and fulfil a clinical case definition of community-acquired
pneumonia at eight paediatric emergency departments across Switzerland. The
clinical case definition is a temperature ≥38°C and at
least two from a list of signs and symptoms of a lower respiratory tract
infection. Detailed eligibility criteria are presented in table 1. Co-primary
outcomes of the main trial are (1) clinical stabilisation defined as
normalisation of initially deranged vital signs or discharge from hospital and
(2) re-admission to hospital within 4 weeks from randomisation. Enrolment into
the main trial has been delayed owing to the COVID-19 pandemic and is currently
ongoing [9].
All parents or legal guardians gave written informed consent including
the ancillary study presented here. The trial and ancillary study were approved
by the local ethics committee of the trial centre (Ethikkommission Nordwest-
und Zentralschweiz (EKNZ), study no. 2018-00563), other local ethics committees
in Switzerland for participating sites and the regulatory authority Swissmedic
(2018 DR 3070). The trial is registered on https://clinicaltrials.gov
(NCT03474991) and on the Swiss National Clinical Trials
Portal (SNCTP000002864).
Table 1Eligibility criteria for the KIDS-STEP trial
[8].
|
Inclusion criteria (all must be fulfilled)
|
| At least 6 months of age and less
than 14 years of age |
| Body weight between 5 kg and 45 kg |
| Admission to hospital (i.e.,
assignment of an inpatient case number or receipt of in-hospital treatment in
a designated short stay unit) |
| Clinical diagnosis of CAP |
A. Temperature
≥
38 °C measured by any method or history of fever in last 48 hours
reported by parents
|
| AND |
|
B. at least two of the following
signs and/or symptoms:
|
| Presence of cough (observed or
reported in last 72 to 96 hours) |
| Increased age-specific respiratory
rate as defined by American Heart Association Accredited Pediatric Advance
Life Support guidelines during assessment in the paediatric emergency
department (first or second triage or clinical examination) |
| Hypoxaemia (<92% arterial
oxygen saturation) in room air as measured by pulse oximetry (SpO2)
[10, 11] |
| Signs of laboured/difficult
breathing, including nasal flaring, chest retractions, grunting, abdominal
breathing and shortness of breath |
| Clinical signs of lobar pneumonia
including focal dullness to percussion, focal reduced breath sounds, crackles
with asymmetry |
| Parent and/or child (as
age-appropriate) willing to accept all possible randomised allocations and to
be contacted for three telephone follow-up visits up to and including at 4 weeks after randomisation |
| Informed consent form for trial
participation signed by participants and/or caregivers |
|
Exclusion criteria (excluded if
of the following are present)
|
| Presence of local complications
(empyema or pleural effusion with clinically identified need for drainage,
pneumothorax and pulmonary abscess). |
| Chronic underlying disease
associated with an increased risk of very severe CAP or CAP of unusual
aetiology, such as sickle cell disease, primary or secondary
immunodeficiency, chronic lung disease and cystic fibrosis. |
| Bilateral wheezing without focal
chest signs AND clinical indication for primary administration of steroids
(most likely to represent respiratory tract infection affecting the medium
airways, i.e., not pneumonia). |
| Admission to hospital with a
primary clinical diagnosis of bronchiolitis. |
| Inability to tolerate oral
medication. |
| Documented allergy or any other
known contraindication to any trial medication. |
| Subacute or chronic conditions
requiring higher betamethasone equivalent or known primary or secondary
adrenal insufficiency. |
| Known diabetes mellitus (type 1). |
| Hospitalisation within the last
two weeks preceding current admission with the possibility that pneumonia
could be hospital-acquired or healthcare-associated. |
| Completion of a course of systemic
corticosteroids within 2 weeks from enrolment for courses of >5 days. |
| Transfer for any reason to a
non-participating hospital directly from the paediatric emergency department. |
| Parents are unlikely to be able to
reliably participate in telephone follow-up because of significant language
barriers. |
| Participation in another study
with an investigational drug within the 30 days preceding and during the
present study. |
| Previous enrolment into the
current study. |
| Enrolment of the investigator,
his/her family members, and other dependent persons. |
For this interim baseline analysis, we included participants enrolled over
the first two years since opening of the trial, i.e., from September 2018 until
calendar week 36 in 2020. Clinical data at presentation, management during the
first 24 hours after admission and pathogen detection results from standard of care samples
taken on the day of admission were collected
prospectively for the main trial and were extracted from the trial database
held at the University of Basel’s clinical trials unit. Positive pathogen detection
results from standard of care testing were collected through the trial’s case
report forms with specific fields for RSV, influenza viruses, S. pneumoniae,
H. influenzae and Mycoplasma pneumoniae and free text for other
respiratory pathogens. SARS-CoV-2 test results were collected in amended case
report forms from 19 May 2020 onwards. Standard of care samples were obtained according
to routine procedures at the trial sites, local materials were used and the
analyses performed in local laboratories.
Randomised treatment allocation to betamethasone or placebo remained concealed.
The interim analysis was exploratory and sample size and endpoints were not
pre-specified. For the current report, use and release of clinical follow-up
data from the main trial needed to be limited in order to prevent jeopardising
the integrity of the trial.
As an ancillary study offered to all trial participants, nasopharyngeal
swabs are taken on the day of admission. Nasopharyngeal swabs were collected on
the day of randomisation using swabs (FLOQSwab®) and transferred to 3 ml
universal transport medium (UTM®) by Copan (Brescia, Italy). The suspended
swabs were processed within 48 hours. After vortexing for 30 seconds, UTM was
transferred to sterile storage tubes in aliquots of 900 µl. Aliquots were
stored at −80°C until analysis. One aliquot per
patient was used for pathogen detection with Filmarray BIOFIRE® Respiratory
Panel 2.1 plus (bioMérieux,
Marcy-l'Étoile, France). The panel detects the following targets: adenovirus,
coronavirus 229E, coronavirus HKU1, coronavirus OC43, coronavirus NL63, Middle
East respiratory syndrome coronavirus (Mers-CoV), human metapneumovirus, human
rhinovirus/enterovirus, influenza A, influenza A/H1, influenza A/H1-2009,
influenza A/H3, influenza B, parainfluenza 1, parainfluenza 2, parainfluenza 3,
parainfluenza 4, RSV, Bordetella
pertussis, Bordetella parapertussis,
Chlamydophila pneumoniae and M. pneumoniae. The analyses were done
before addition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
to an updated version of the panel.
Data management and statistical operations were performed in Stata 15
(College Station, Texas). C-reactive protein values were grouped into lower
than 80 mg/l and equal to or higher than 80 mg/l [12]. For comparisons between groups Wilcoxon
rank-sum test or Kruskal-Wallis tests were used for continuous variables and
chi-square tests for categorical variables. The alluvial plot for figure 1
was drawn using the online tool RawGraphs 2.0 beta.
Ethics
The trial and ancillary study were approved by the local ethics
committee of the trial centre (Ethikkommission Nordwest- und Zentralschweiz
(EKNZ), study no. 2018-00563), other local ethics committees in Switzerland for
participating sites and the regulatory authority Swissmedic (2018 DR 3070).
Results
The first 138 children enrolled in the trial were included in the
current analysis. The majority of participants were enrolled in autumn and
winter 2019/20 (detailed in supplementary figure S1 in the appendix). Thirty
(21.7%) were enrolled after detection of the first case of COVID-19 in
Switzerland, mostly in March 2020 and only 6 were enrolled between April and
September 2020.
Table 2Clinical characteristics at presentation and
antibiotic treatment.
|
All
|
138 (100%)
|
|
Demographics
|
| Sex, n (%) |
Female |
66 (47.8) |
| Male |
72 (52.2) |
| Median age in years (IQR) |
3.04 (1.67–4.67) |
|
Season of inclusion n (%)
|
| Season |
April to September |
29 (21.0) |
| October to March |
109 (79.0) |
|
Signs at presentation n (%)
|
| Wheeze |
Present |
29 (21.0) |
| Not present |
104 (75.4) |
| Unknown |
5 (3.6) |
| Crackles |
Present |
88 (63.8) |
| Not present |
48 (34.8) |
| Unknown |
2 (1.5) |
| Retractions |
Present |
90 (65.2) |
| Not present |
46 (33.3) |
| Unknown |
2 (1.5) |
| Reduced oral intake |
Present |
108 (78.3) |
| Not present |
27 (19.6) |
| Unknown |
3 (2.2) |
| Reduced activity |
Present |
129 (93.5) |
| Not present |
7 (5.1) |
| Unknown |
2 (1.5) |
| Oxygen saturation <92% |
Present |
43 (31.2) |
| Not present |
95 (68.8) |
| Unknown |
0 (0.0) |
| C-reactive protein >80 mg/l |
Present |
32 (23.2) |
| Not present |
71 (51.5) |
| Unknown |
35 (25.4) |
| Chest X-ray |
Lobar consolidation and patchy
infiltrates |
7 (5.1) |
| Lobar consolidation only |
46 (33.3) |
| Patchy infiltrates only |
47 (34.1) |
| Inconclusive infiltrates |
8 (5.8) |
| Not suggestive of pneumonia |
4 (2.9) |
| Not done |
26 (18.8) |
| Chest sonography |
Done |
14 (10.1) |
| Not done or not documented |
124 (89.9) |
| Days coughing before presentation |
Median (IQR) |
5 (3–8) |
| Unknown |
0 (0.0) |
| Days of fever before presentation |
Median (IQR) |
5 (2–6) |
| Unknown |
0 (0.0) |
| Antibiotic
treatment n (%) |
| Decision to treat with antibiotics on admission |
Yes |
104 (75.4) |
| AP only |
61 (44.2) |
| AP + BLI |
27 (19.6) |
| Ceph |
9 (6.5) |
| BL + M |
4 (2.9) |
| Other |
3 (2.2) |
Clinical characteristics of the participants at trial entry are
presented in table 2. The median age was 3 years with an even distribution
between sexes. A high proportion of participants had signs of more severe
disease, including 65.2% with chest retractions and 31.2% with an oxygen
saturation below 92%.
Forty-three participants (29.0%) were already on antibiotic treatment
prior to admission and 104 participants (75.4%) received antibiotic treatment
on admission. Figure 1 shows antibiotic treatment of trial participants before
and after admission. The most common antibiotics used were aminopenicillins (58.7%
of those receiving antibiotic treatment after admission). When antibiotics were
used, 85.6% were Access group antibiotics according to the WHO’s Essential
Medicines List AWaRe classification [13].
Six of the 27 participants (22.2%) initially treated with an aminopenicillin
plus a beta-lactamase inhibitor were switched to an aminopenicillin only within
the first 24 hours after admission.
Within the first 24 hours, 91 participants (65.9%)
received supplemental oxygen and four (2.9%) were placed on some form of
respiratory support.
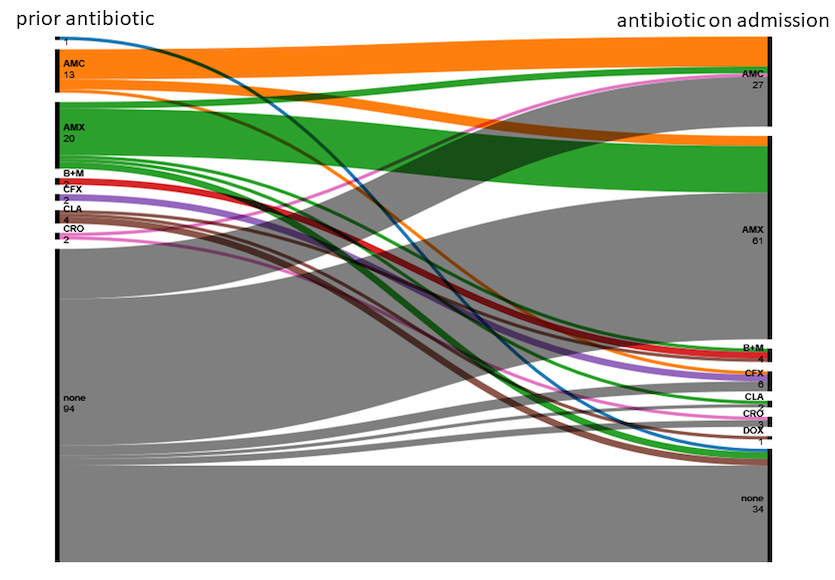
Figure 1 Alluvial plot of
antibiotic treatment prior to admission and on admission. No label: unknown;
AMC: amoxicillin + clavulanic acid; AMX: amoxicillin; BL + M: beta-lactam +
macrolide; CFX: cefuroxime; CLA: clarithromycin; CRO: ceftriaxone; DOX:
doxycycline
Respiratory specimens for pathogen testing were obtained from 132 of 138
participants (95.7%). These were either part of the standard of care or nasopharyngeal
swabs obtained as study samples. Eighty-four participants (60.9%) had both
kinds of sample taken, 25 (18.1%) only standard of care samples, 23 (16.7%) only
study samples, and 6 (4.3%) had no sample taken.
Standard of care samples were obtained from 109 participants (79.0%).
Except for one tracheal aspirate, all other respiratory standard of care
samples were nasopharyngeal swabs or nasopharyngeal aspirates. Seventy-two
(66.1%) were tested for viruses and bacteria, 16 (14.7%) for viruses and 21
(19.3%) for bacteria only. Testing for bacteria was most commonly done by
bacterial culture. The respiratory pathogens detected on standard of care samples
are listed in table 3. No positive SARS-CoV-2 tests were reported. Blood
cultures were obtained in 86 patients but yielded no positive results.
Study nasopharyngeal swabs at trial entry were obtained from 107
participants (77.5%). Table 3 shows the respiratory pathogens detected on nasopharyngeal
swabs in these participants. The most frequently detected pathogen was human
rhinovirus/enterovirus, followed by RSV and hMPV.
Twenty-one of the 24 participants where multiple pathogens were detected
in study samples carried multiple (up to four) different viruses. In the other
three participants, M. pneumoniae was
found in combination with a virus (one adenovirus, two rhinovirus).
When information from standard of care and study samples was combined, 5
(3.8%) participants had co-detection of bacteria and viruses. Ninety-seven
participants (73.5%) had at least one respiratory virus detected in any sample
and 92 (69.7%) had one or more viruses but no bacteria detected. All
co-detections of different pathogens including study and standard of care samples are
detailed in supplementary table S1 in the appendix.
Table 3Pathogens detected in study participants. S. pneumoniae and H. influenzae were not tested for on study samples; data collected
on standard of care samples and pathogens other than RSV, influenza viruses, S. pneumoniae, H. influenzae and M. pneumoniae
was collected as free text and may be incomplete – numbers detected should be
interpreted with care.
|
Pathogen
|
Participants tested
|
|
Standard of care samples
|
Study samples
|
On any sample
|
| All samples |
109 (100%) |
107 (100%) |
132 (100%) |
| Adenovirus |
1 (0.9) |
11 (10.3) |
12 (9.1) |
| RSV |
19 (17.4) |
29 (27.1) |
31 (23.5) |
| hMPV |
4 (3.7) |
19 (17.8) |
21 (15.9) |
| Influenza viruses |
12 (11.0) |
11 (10.3) |
15 (11.4) |
| PIV |
3 (2.8) |
8 (7.5) |
11 (8.3) |
| Human rhinovirus/enterovirus |
11 (10.0) |
32 (29.9) |
35 (26.5) |
| Endemic coronaviruses |
4 (3.7) |
7 (6.5) |
10 (7.6) |
| S. pneumoniae |
2 (1.8) |
- |
2 (1.5) |
|
H. influenzae
|
1 (0.9) |
- |
1 (0.8) |
|
B. pertussis
|
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
B. parapertussis
|
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
C. pneumoniae
|
0 (0.0) |
1 (0.9) |
1 (0.8) |
|
M. pneumoniae
|
5 (4.6) |
6 (5.6) |
10 (7.6) |
| Number detected |
0 |
49 (45.0) |
13 (12.2) |
26 (19.7) |
| 1 |
58 (53.2) |
70 (65.4) |
78 (59.1) |
| >1 |
2 (1.8) |
24 (22.4) |
28 (21.2) |
Detection of respiratory pathogens showed age- and season-related
patterns (table 4). RSV was more commonly found in younger children and during
the peak acute respiratory infection season from October to March. Influenza
virus was equally more common during the acute respiratory infection season,
and M. pneumoniae was more commonly
detected in older children.
Patchy changes on chest X-ray were found in a higher proportion of
children than lobar consolidations with any of the listed pathogens detected,
but this finding was not supported by statistical evidence.
Table 4Age, season and chest X-ray findings by
detection of respiratory pathogens.
| |
Not detected
|
Detected
|
p-value
|
|
RSV
|
| Age, median (IQR) |
3.33 (1.75–5.08) |
2.42 (1.42–3.17) |
0.015 |
| Season, n (%) |
Apr to Sep |
28 (96.6) |
1 (3.5) |
0.006 |
| Oct to Mar |
79 (72.5) |
30 (27.5) |
| CXR, n (%) |
Consolidation |
47 (88.7) |
6 (11.3) |
0.068 |
| Patchy |
33 (70.2) |
14 (29.8) |
| Not suggestive |
9 (75.0) |
3 (25.0) |
|
Influenza virus
|
| Age, median (IQR) |
2.92 (1.58–4.5) |
4.08 (2.33–5.75) |
0.100 |
| Season, n (%) |
Apr to Sep |
29 (100.0) |
0 (0.0) |
0.034 |
| Oct to Mar |
94 (86.2) |
15 (13.8) |
| CXR, n (%) |
Consolidation |
49 (92.5) |
4 (7.6) |
0.211 |
| Patchy |
42 (89.4) |
5 (10.6) |
| Not suggestive |
9 (75.0) |
3 (25.0) |
|
hMPV
|
| Age, median (IQR) |
3.17 (1.92–4.75) |
2.17 (1.58–3.17) |
0.066 |
| Season, n (%) |
Apr to Sep |
25 (86.2) |
4 (13.8) |
0.810 |
| Oct to Mar |
92 (84.4) |
17 (15.6) |
| CXR, n (%) |
Consolidation |
46 (86.8) |
7 (13.2) |
0.280 |
| Patchy |
36 (76.6) |
11 (23.4) |
| Not suggestive |
11 (91.7) |
1 (8.3) |
|
Seasonal (endemic) coronaviruses
|
| Age, median (IQR) |
3.13 (1.67–4.75) |
2.33 (1.25–3.17) |
0.123 |
| Season, n (%) |
Apr to Sep |
29 (100.0) |
0 (0.0) |
0.090 |
| Oct to Mar |
99 (90.8) |
10 (9.2) |
| CXR, n (%) |
Consolidation |
49 (92.5) |
4 (7.6) |
0.341 |
| Patchy |
41 (87.2) |
6 (12.8) |
| Not suggestive |
12 (100.0) |
0 (0.0) |
|
M. pneumoniae
|
| Age, median (IQR) |
2.92 (1.58–4.50) |
6.75 (4.50–8.83) |
<0.001 |
| Season, n (%) |
Apr to Sep |
26 (89.7) |
3 (10.3) |
0.469 |
| Oct to Mar |
102 (93.6) |
7 (6.4) |
| CXR, n (%) |
Consolidation |
51 (96.2) |
2 (3.8) |
0.131 |
| Patchy |
41 (87.2) |
6 (12.8) |
| Not suggestive |
12 (100.0) |
0 (0.0) |
Discussion
In line with international data, RSV is the most commonly detected
pathogen that has been found to be strongly associated with hospital admission [2, 4]. Compared with other European settings,
hMPV was detected more frequently in the Swiss population. This may either
reflect differences in local aetiology or the winter season 2019/20 may have
seen uncommonly large numbers of hMPV infections. Both RSV and influenza showed
the expected seasonality. Since very few participants were enrolled after the
onset of the COVID-19 pandemic, we were unable to capture possible changes in
seasonality of pathogens. As expected, M. pneumoniae was more commonly
detected in older children [14]. Human
rhinovirus was overall the most frequently detected pathogen, but previous
studies have shown that detection of human rhinovirus in upper respiratory
tract samples is only very weakly associated with pneumonia or hospital
admission due to acute respiratory infection [2,
4].
Of the children admitted with community-acquired pneumonia, 75.4% received
antibiotic treatment. Antibiotics were selected mostly in accordance with international
treatment guidelines and from the AWaRe classification’s Access group [11, 13]. Assessed against the pathogen testing
results, it is likely that a large proportion of these antibiotic prescriptions
were unnecessary. German guidelines advise withholding antibiotics in children
without WHO danger signs, whereas current UK guidelines advise treating and
reviewing in due course, especially after receiving pathogen testing results.
The proportion of children receiving antibiotics on admission in our study is
broadly comparable to recent studies from the USA and Europe [4, 15, 16]. Future analyses of the trial will
show if antibiotics were stopped during the participants’ hospitalisation.
Earlier availability of results from rapid syndromic pathogen testing may
prevent antibiotic prescriptions in children with acute respiratory infections
including pneumonia, but results from single-centre or retrospective studies
have so far been disappointing [17, 18].
Judicious prescribing can be aided by the implementation of Antimicrobial
Stewardship programmes, which have so far not been extended to Swiss paediatric
emergency departments [19].
Overall, 81.2% of patients had a chest X-ray.
This is surprising because German language guidelines that were written with
participation of Swiss members do not routinely recommend a chest X-ray in
children without WHO danger signs [11].
The PERCH study demonstrated that abnormal chest X-ray findings were
associated with severe or very severe pneumonia and slightly longer duration of
symptoms [20]. However, 46% of children
with clinically severe or very severe pneumonia had a normal chest X-ray,
demonstrating that radiology alone is insufficient to identify children with
more severe disease [20]. In a
high-resource setting, the decision on antibiotic treatment taken before chest X-ray was altered in less than half of cases where radiological findings
were discordant (i.e., not suggestive of pneumonia in children with a pre-X-ray
plan for antibiotics and vice versa), indicating that care providers are aware
of the limited predictive value of chest X-ray [21]. Pleura and lung sonography have demonstrated a high negative
predictive value for pathological findings on chest X-ray in paediatric community-acquired
pneumonia and can help to avoid unnecessary irradiation in settings where
sufficient resources for ultrasound are available [22].
The presented study has some important limitations. Pathogen testing
both on study and routine samples was mostly limited to upper respiratory tract
samples. A strong association of detection of pathogens in upper respiratory
tract samples with hospitalisation for acute respiratory infection is only seen
with some respiratory viruses (RSV, influenza virus, hMPV and parainfluenza
viruses) but not in bacteria or human rhinovirus/enterovirus [4]. Lower respiratory tract samples may be
better suited for detection of aetiologically relevant bacteria [23, 24]. Additionally, for standard of care
samples only positive findings were collected. However, in paediatric clinical
routine, sampling is most commonly limited to upper respiratory tract samples [25]. Because more than a quarter of children
had already received antibiotic treatment prior to inclusion in the trial, a
higher proportion of detection of typical bacteria may have been missed [26]. The test method applied to study samples
did not include S. pneumoniae or H. influenzae. It is likely that
S. pneumoniae would otherwise have been detected in a substantial
proportion of participants, but the relevance of this finding to establish a
causal agent for the community-acquired pneumonia episode would have been
questionable [2, 4, 27].
A second important limitation is the small sample size. Although we were
able to sample more than 10% of children admitted for community-acquired
pneumonia at the participating centres during the study period, we were not
able to assess pathogen interactions or seasonality, age preponderance or
association with length of hospital stay for rarer pathogens.
The trial was designed to capture a real-world patient population and is
thus using a pragmatic clinical case definition [28].
Patients were selected for screening for the trial based on the clinician’s
diagnosis of community-acquired pneumonia.
All
patients admitted for community-acquired pneumonia at the participating sites
were recorded as pre-screened patients and more than a third of these fulfilled
the eligibility criteria. Although this proportion is arguably higher than in
most medication trials, it is still likely that some patient groups are
disproportionately affected by the exclusion criteria. These may likely include
children with M. pneumoniae infection, who often present without fever,
and older children who would often only be hospitalised in the presence of
respiratory or immunological comorbidities that would result in exclusion from
the trial. The demographics of included children nonetheless closely resemble
those of all children admitted for community-acquired pneumonia in similar
settings [1]. We therefore believe that
the data we present are generalisable to children admitted with community-acquired
pneumonia in Switzerland and largely transferable to similar European settings.
Most participants were enrolled in the winter season before the start of the
COVID-19 pandemic. In this way, the findings can provide information for
pre-post pandemic comparisons on pathogen detection and management of
paediatric community-acquired pneumonia.
Data availability statement
Raw data can be obtained from the sponsor (UKBB) upon request to the
Trial Steering Committee via the corresponding author once the trial has been
completed. Any requests for access to raw data will be welcomed as long as they
are scientifically valid, appropriate consent for the requested level of data
sharing has been obtained from participants and as long as the planned data use
does not conflict with ongoing analyses by the trial team.
Acknowledgements
The authors thank the families participating in
the KIDS-STEP trial and the contributing staff at all trial sites.
The authors thank Angela Huttner and Theoklis
Zaoutis (Independent Data Monitoring Committee and the Trial Steering
Committee) for advice on focus and delimitation of the current report against
the planned final report on the clinical trial.
KIDS-STEP Trial Group:
Trial Management Group: Johannes van den Anker
(Principal Investigator), Julia A. Bielicki (Co-Principal Investigator), Regina
Santoro (Trial Manager), Malte Kohns Vasconcelos (Trial Physician), Michael
Coslovsky (Trial Statistician)
Trial Steering Committee: Diana Gibb (chair),
Theoklis Zaoutis, Olaf Neth, Henri van Werkhoven
Independent Data Manitoring Committee: Angelika
Huttner (chair), Fiona Vanobhergen, Wolfgang Stöhr
University of Basel Clinical Trials Unit:
Madeleine Vollmer, Patricia Arnaiz, Patrick Simon
Research Pharmacy, University Hospital Basel:
Stefanie Deuster, Anne Henn
Microbiology Laboratory, University Hospital
Basel: Adrian Egli
Trial Sites:
Aarau – Henrik Köhler (PI), Patrick Haberstich,
Rachel Kusche, Dominik Müller-Suter
Basel – Ulrich Heininger (PI), Barbara Kern,
Svetlana Beglinger, Michel Ramser, Claudia Werner, Linda Stamm, Aurora Frei
Bern – Kristina Keitel (PI), Daniel Garcia,
Verena Wyss, PedNet Bern
Geneve – Anne Mornand (PI), Constance
Barazzone, Klara Posfay Barbe, Natasha Loevy, Alban Glangetas, Sébastien Papis
Lausanne – Jean-Yves Pauchard (PI), Linda
Guihard, Raquel Marques, Danielle Bally
Luzern – Marco Lurà (PI), Alex Donas, Michael
Büttcher, Leopold Simma, Martina Bieri, Susanne Krieg, Diana Schirmann, Xenia
Mandanis, Katja Hrup, Janine Stritt
St. Gallen – Christian Kahlert (PI), Konstanze
Zöhrer, Anita Niederer-Loher, Tanja Wachinger
Zürich – Christoph Berger (PI), Patrick M. Meyer Sauteur, Michelle
Seiler, Elena Pànisovà
Correspondence:
Malte Kohns Vasconcelos
Department of Paediatric Pharmacology
University of Basel Children’s Hospital (UKBB)
Spitalstrasse 33
CH-4056 Basel
malte.kohns[at]ukbb.ch
References
1. Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997-2008. Thorax. 2010 Sep;65(9):770–4. https://doi.org/10.1136/thx.2010.137802
2. O’Brien KL, Levine OS, Knoll MD, Feikin DR, DeLuca AN, Driscoll AJ, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet (London, England). 2019;394(10200):757-79. Epub 2019/07/02. doi: https://doi.org/10.1016/s0140-6736(19)30721-4. PubMed PMID: 31257127; PubMed Central PMCID: PMCPMC6727070. https://doi.org/10.1016/S0140-6736(19)30721-4
3. Jain S, Finelli L; CDC EPIC Study Team. Community-acquired pneumonia among U.S. children. N Engl J Med. 2015 May;372(22):2167–8. https://doi.org/10.1056/NEJMc1504028
4. Kohns Vasconcelos M, Loens K, Sigfrid L, Iosifidis E, Epalza C, Donà D, et al. Aetiology of acute respiratory infection in preschool children requiring hospitalisation in Europe-results from the PED-MERMAIDS multicentre case-control study. BMJ Open Respir Res. 2021;8(1). Epub 2021/07/31. doi: https://doi.org/10.1136/bmjresp-2021-000887. PubMed PMID: 34326154; PubMed Central PMCID: PMCPMC8323363.
5. Williams DJ, Zhu Y, Grijalva CG, Self WH, Harrell FE, Jr., Reed C, et al. Predicting Severe Pneumonia Outcomes in Children. Pediatrics. 2016;138(4). Epub 2016/10/01. doi: https://doi.org/10.1542/peds.2016-1019. PubMed PMID: 27688362; PubMed Central PMCID: PMCPMC5051209 conflicts of interest to disclose.
6. Mazur NI, Bont L, Cohen AL, Cohen C, von Gottberg A, Groome MJ, et al.; South African Severe Acute Respiratory Illness (SARI) Surveillance Group. Severity of Respiratory Syncytial Virus Lower Respiratory Tract Infection With Viral Coinfection in HIV-Uninfected Children. Clin Infect Dis. 2017 Feb;64(4):443–50. https://doi.org/10.1093/cid/ciw756
7. Calvo C, García-García ML, Pozo F, Paula G, Molinero M, Calderón A, et al. Respiratory Syncytial Virus Coinfections With Rhinovirus and Human Bocavirus in Hospitalized Children. Medicine (Baltimore). 2015 Oct;94(42):e1788. https://doi.org/10.1097/md.0000000000001788 https://doi.org/10.1097/MD.0000000000001788
8. Kohns Vasconcelos M, Meyer Sauteur PM, Santoro R, Coslovsky M, Lurà M, Keitel K, et al. Randomised placebo-controlled multicentre effectiveness trial of adjunct betamethasone therapy in hospitalised children with community-acquired pneumonia: a trial protocol for the KIDS-STEP trial. BMJ open. 2020;10(12):e041937. Epub 2020/12/31. doi: https://doi.org/10.1136/bmjopen-2020-041937. PubMed PMID: 33376176; PubMed Central PMCID: PMCPMC7778765.
9. Kohns Vasconcelos M, Meyer Sauteur PM, Keitel K, Santoro R, Heininger U, van den Anker J, et al. Strikingly Decreased Community-acquired Pneumonia Admissions in Children Despite Open Schools and Day-care Facilities in Switzerland. Pediatr Infect Dis J. 2021 Apr;40(4):e171–2. https://doi.org/10.1097/inf.0000000000003026 https://doi.org/10.1097/INF.0000000000003026
10. Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al.; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1–23. https://doi.org/10.1136/thoraxjnl-2011-200598
11. Rose MA, Barker M, Liese J, Adams O, Ankermann T, Baumann U, et al. S2k-Leitlinie Management der ambulant erworbenen Pneumonie bei Kindern und Jugendlichen (pädiatrische ambulant erworbene Pneumonie, pCAP). Pneumologie. 2020 Aug;74(8):515–44. https://doi.org/10.1055/a-1139-5132
12. Keitel K, Samaka J, Masimba J, Temba H, Said Z, Kagoro F, et al. Safety and Efficacy of C-reactive Protein-guided Antibiotic Use to Treat Acute Respiratory Infections in Tanzanian Children: A Planned Subgroup Analysis of a Randomized Controlled Noninferiority Trial Evaluating a Novel Electronic Clinical Decision Algorithm (ePOCT). Clin Infect Dis. 2019 Nov;69(11):1926–34. https://doi.org/10.1093/cid/ciz080
13. Hsia Y, Lee BR, Versporten A, Yang Y, Bielicki J, Jackson C, et al.; GARPEC and Global-PPS networks. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health. 2019 Jul;7(7):e861–71. https://doi.org/10.1016/s2214-109x(19)30071-3 https://doi.org/10.1016/S2214-109X(19)30071-3
14. Dierig A, Hirsch HH, Decker ML, Bielicki JA, Heininger U, Ritz N. Mycoplasma pneumoniae detection in children with respiratory tract infections and influence on management - a retrospective cohort study in Switzerland. Acta Paediatr. 2020;109(2):375-80. Epub 2019/06/07. doi: https://doi.org/10.1111/apa.14891. PubMed PMID: 31168877; PubMed Central PMCID: PMCPMC7159768.
15. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. The New England journal of medicine. 2015;372(9):835-45. Epub 2015/02/26. doi: https://doi.org/10.1056/NEJMoa1405870. PubMed PMID: 25714161; PubMed Central PMCID: PMCPMC4697461.
16. Williams DJ, Hall M, Gerber JS, Neuman MI, Hersh AL, Brogan TV, et al.; Pediatric Research in Inpatient Settings Network. Impact of a National Guideline on Antibiotic Selection for Hospitalized Pneumonia. Pediatrics. 2017 Apr;139(4):e20163231. https://doi.org/10.1542/peds.2016-3231
17. Mattila S, Paalanne N, Honkila M, Pokka T, Tapiainen T. Effect of Point-of-Care Testing for Respiratory Pathogens on Antibiotic Use in Children: A Randomized Clinical Trial. JAMA Network Open. 2022;5(6):e2216162-e. doi: https://doi.org/10.1001/jamanetworkopen.2022.16162.
18. Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database of Systematic Reviews. 2014(9). doi: https://doi.org/10.1002/14651858.CD006452.pub4. PubMed PMID: CD006452.
19. Donà D, Barbieri E, Daverio M, Lundin R, Giaquinto C, Zaoutis T, et al. Implementation and impact of pediatric antimicrobial stewardship programs: a systematic scoping review. Antimicrobial resistance and infection control. 2020;9:3. Epub 2020/01/09. doi: https://doi.org/10.1186/s13756-019-0659-3. PubMed PMID: 31911831; PubMed Central PMCID: PMCPMC6942341.
20. Fancourt N, Deloria Knoll M, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, et al. Chest Radiograph Findings in Childhood Pneumonia Cases From the Multisite PERCH Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64(suppl_3):S262-s70. Epub 2017/06/03. doi: https://doi.org/10.1093/cid/cix089. PubMed PMID: 28575361; PubMed Central PMCID: PMCPMC5447837.
21. Nelson KA, Morrow C, Wingerter SL, Bachur RG, Neuman MI. Impact of Chest Radiography on Antibiotic Treatment for Children With Suspected Pneumonia. Pediatr Emerg Care. 2016 Aug;32(8):514–9. https://doi.org/10.1097/pec.0000000000000868 https://doi.org/10.1097/PEC.0000000000000868
22. Claes AS, Clapuyt P, Menten R, Michoux N, Dumitriu D. Performance of chest ultrasound in pediatric pneumonia. Eur J Radiol. 2017 Mar;88:82–7. https://doi.org/10.1016/j.ejrad.2016.12.032
23. Loens K, Van Heirstraeten L, Malhotra-Kumar S, Goossens H, Ieven M. Optimal sampling sites and methods for detection of pathogens possibly causing community-acquired lower respiratory tract infections. Journal of clinical microbiology. 2009;47(1):21-31. Epub 2008/11/21. doi: https://doi.org/10.1128/jcm.02037-08. PubMed PMID: 19020070; PubMed Central PMCID: PMCPMC2620840. https://doi.org/10.1128/JCM.02037-08
24. Tschiedel E, Goralski A, Steinmann J, Rath PM, Olivier M, Mellies U, et al. Multiplex PCR of bronchoalveolar lavage fluid in children enhances the rate of pathogen detection. BMC pulmonary medicine. 2019;19(1):132. Epub 2019/07/20. doi: https://doi.org/10.1186/s12890-019-0894-7. PubMed PMID: 31319825; PubMed Central PMCID: PMCPMC6639929.
25. Kohns Vasconcelos M, Renk H, Popielska J, Nyirenda Nyang'wa M, Burokiene S, Gkentzi D, et al. SARS-CoV-2 testing and infection control strategies in European paediatric emergency departments during the first wave of the pandemic. Eur J Pediatr. 2020:1-7. Epub 2020/10/15. doi: https://doi.org/10.1007/s00431-020-03843-w. PubMed PMID: 33051714; PubMed Central PMCID: PMCPMC7553380.
26. Forster J, Piazza G, Goettler D, Kemmling D, Schoen C, Rose M, et al. Effect of Prehospital Antibiotic Therapy on Clinical Outcome and Pathogen Detection in Children With Parapneumonic Pleural Effusion/Pleural Empyema. Pediatr Infect Dis J. 2021 Jun;40(6):544–9. https://doi.org/10.1097/inf.0000000000003036 https://doi.org/10.1097/INF.0000000000003036
27. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004 Mar;4(3):144–54. https://doi.org/10.1016/s1473-3099(04)00938-7 https://doi.org/10.1016/S1473-3099(04)00938-7
28. Dal-Ré R, Janiaud P, Ioannidis JP. Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic? BMC Med. 2018 Apr;16(1):49. https://doi.org/10.1186/s12916-018-1038-2
Appendix: Supplementary
data

Figure S1 Inclusion over time.
Table S1Pathogen combinations.
|
Infl
|
hMPV
|
CoV
|
Mp
|
Sp
|
Hi
|
PIV
|
AdV
|
R/EV
|
Cpn
|
|
Count
co-detections
|
| 1 |
2 |
3 |
0 |
0 |
0 |
0 |
1 |
5 |
0 |
RSV
|
12 |
| – |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
0 |
Infl
|
4 |
| – |
– |
6 |
0 |
1 |
0 |
1 |
0 |
2 |
0 |
hMPV
|
12 |
| – |
– |
– |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
CoV
|
5 |
| – |
– |
– |
– |
0 |
0 |
0 |
1 |
2 |
1 |
Mp
|
4 |
| – |
– |
– |
– |
– |
0 |
0 |
0 |
0 |
0 |
Sp
|
1 |
| – |
– |
– |
– |
– |
– |
0 |
0 |
0 |
0 |
Hi
|
0 |
| – |
– |
– |
– |
– |
– |
– |
0 |
3 |
0 |
PIV
|
5 |
| – |
– |
– |
– |
– |
– |
– |
– |
3 |
0 |
AdV
|
5 |
| – |
– |
– |
– |
– |
– |
– |
– |
– |
0 |
R/EV
|
19 |
| – |
– |
– |
– |
– |
– |
– |
– |
– |
– |
Cpn
|
1 |

