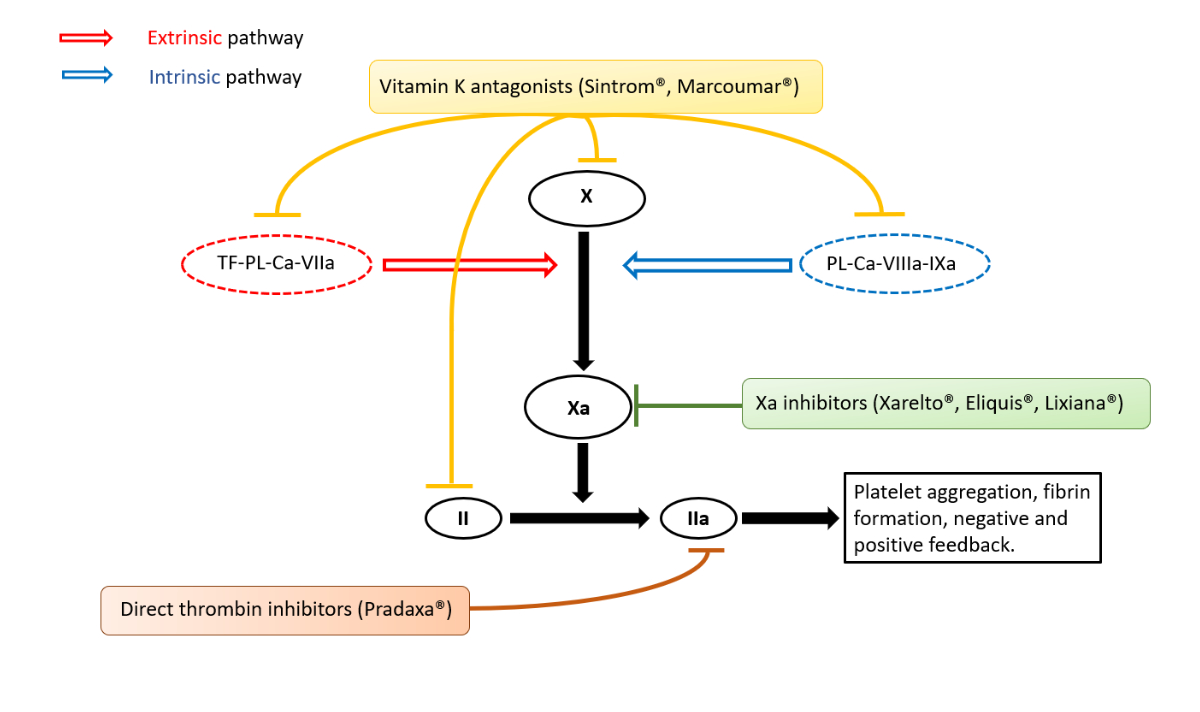
Figure 1 Simplified coagulation cascade (extrinsic and intrinsic) with targets of different anticoagulants.
DOI: https://doi.org/10.57187/smw.2023.40036
Since their introduction more than 10 years ago, direct oral anticoagulants (DOACs) are now routinely prescribed in Switzerland. Rivaroxaban (Xarelto®, Bayer Vital GmbH Deutschland), a coagulation factor Xa inhibitor, was introduced in 2009 and approved in 2012 [1], followed a few years later by apixaban (Eliquis®, Bristol-Myers Squibb SA) and edoxaban (Lixiana®, Daiichi Sankyo), also factor Xa inhibitors (fig. 1).

Figure 1 Simplified coagulation cascade (extrinsic and intrinsic) with targets of different anticoagulants.
Dabigatran (Pradaxa®, Boehringer Ingelheim Pharmaceuticals), a thrombin inhibitor (or direct factor IIa inhibitor) (fig. 1), also appeared in 2012 and completes the therapeutic range for stroke prevention in the context of atrial fibrillation, treatment of pulmonary embolism or deep vein thrombosis. Due to ease of prescribing and a better safety profile than vitamin K antagonists [2], the prescription of DOACs is becoming more important every year. However, major haemorrhage under anticoagulant therapy remains a dreaded complication for physicians faced with emergencies. The aim of this article is to provide an overview of the measures to be adopted by any physician confronted with these situations, based on current recommendations and the published literature.
The Medline database was searched for articles published since 1998 in English and dealing with topics in haematology, and Google Scholar was searched for the literature in French language. The terms used were “major bleeding”, “anticoagulation reversal”, “laboratory measurement of anticoagulation” and “direct oral anticoagulants”.
In an emergency, two situations must prompt rapid and effective neutralisation of anticoagulation: a major haemorrhage or surgery that cannot be delayed.
Severity of bleeding is defined according to the criteria of the International Society of Thrombosis and Hemostasis, published in 2005 and valid today [3, 4]. Major haemorrhage is defined as follows: fatal haemorrhage; haemorrhage in a critical area or organ; and haemorrhage that has resulted in a fall in haemoglobin of at least 20 g/l or has required the transfusion of more than two units of packed red blood cells in less than 1 hour [3, 4] (table 1).
Table 1Criteria for major bleeding as defined by the Control of Anticoagulation Subcommittee of the International Society on Thrombosis and Hemostasis.
| Fatal haemorrhage |
| Symptomatic bleeding into a critical organ or area such as the intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, or pericardial space or intramuscular space with the development of compartment syndrome |
| Haemorrhage with a decrease in haemoglobin of at least 20 g/l (1.24 mmol/l) or requiring the transfusion of at least two units of packed red blood cells |
It is important to recall that gastrointestinal haemorrhage should not be considered as a critical organ haemorrhage and it is the combination of the other criteria for severity of bleeding that should prompt coagulation correction. The anticoagulation control does not replace, but rather complements, the usual measures for treating haemorrhagic shock. Examples include external compression manoeuvres if the haemorrhage is external, tourniquet placement for major limb bleeding, pelvic girdling in the case of an unstable pelvic fracture, prevention of hypothermia, oxygen therapy, controlled and effective volume resuscitation and early administration of tranexamic acid if indicated [5]. In the case of surgery for certain conditions that cannot be deferred (e.g., open fracture, intracranial bleeding, amputation, intestinal perforation) [6], neutralisation of the anticoagulant must be discussed on a case-by-case basis by a multidisciplinary team (emergency doctor, surgeon, anaesthetist, other specialist), depending on the balance between the risks of bleeding and of thrombosis.
Conventional coagulation tests are used to determine the patient's degree of anticoagulation and to monitor the effectiveness of the treatment. Various tests (prothrombin time [PT], international normalised ratio [INR], partial thromboplastin time [PTT] or anti-Xa) should be considered, depending on the anticoagulant prescribed and the analytical resources available to the hospital (table 2).
Table 2Coagulation tests.
| Coagulation tests | Abbreviations | Normal values |
| International normalised ratio | INR | 0.8–1.2 |
| Prothrombin time | PT | 11 to 13.5 seconds |
| Activated partial thromboplastin time | aPTT | 26–37 seconds |
| Diluted thrombin time | dTT | <20 seconds |
| Ecarin clotting time | ECT | 22.6-29 seconds |
| Thrombin time | TT | 14–19 seconds |
It takes some time to obtain the results of these classical coagulation tests. Rotational thromboelastometry (ROTEM®, Rotem Medical) or thromboelastography (TEG®, Haemonetics) are whole blood analysis techniques that can be monitored in real time at the patient's bedside or at the point of care and thus allow a rapid assessment of the coagulation status and guidance for therapy based on the results. Of note, although they are now routinely used in the operating theatre, few emergency departments have access to them.
In cases of major bleeding (table 1) in patients on anticoagulants, rapid neutralisation of the anticoagulant effect without waiting for laboratory results is essential, regardless of the indication for treatment. It is important to emphasise that if a haemostatic procedure is required to control the bleeding, priority should be given to organising and performing the procedure [7]. Recognition of the severity of the bleeding and its origin is therefore essential. In a patient with a mechanical heart valve and a major haemorrhage, neutralisation of the anticoagulant should also be carried out while assessing the thrombotic risk associated with the delay before reintroduction of the anticoagulant [8]. Although activated charcoal is effective in reducing plasma concentrations of anticoagulants, its administration is not recommended in international guidelines in cases of major bleeding.
With treatment with acenocoumarol (Sintrom®, Merus Labs International, plasma elimination half-life 8–11 hours), warfarin (Coumandine®, Bristol-Myers Squibb, plasma elimination half-life 35–45 hours) or phenprocoumone (Marcoumar®, Meda, plasma elimination half-life 160 hours), the degree of anticoagulation is quantitatively reflected by prolongation of the PT (in seconds), most often reported as the prothrombin rate expressed as a percentage (PT%), or the INR, which increases when the PT% is lowered [8] . Many protocols rely on INR results to guide anticoagulation neutralisation. Biologically, neutralisation of anticoagulation with vitamin K antagonists is considered effective when the INR is less than 1.3 [9, 10]. It is advisable to check the INR 30 minutes after a reversal and periodically thereafter, with the frequency determined by the severity of bleeding.
For patients on DOACs, conventional tests only randomly reflect effective anticoagulation. Specific tests have been developed and can be incorporated into routine practice (table 3) [9, 11, 12].
Table 3Laboratory results indicating the presence of an anticoagulant effect according to laboratory results and type of anticoagulation.
| INR | aPTT | anti-Xa activity | dTT or ECT | TT | |
| Anti-vitamin K (Marcoumar®, Sintrom®) | >1.8, correlated with concentration | >37 s | – | – | – |
| Anti-Xa (Xarelto®, Eliquis®, Lixiana®) | Normal or increased | Normal or increased | >30–50 ng/ml, correlated with concentration | – | – |
| Anti-IIa (Pradaxa®) | Normal | Normal or increased | – | >50 ng/ml, correlated with concentration | Increased, but not quantitative |
PT: prothrombin time; INR: International normalised ratio; aPTT: activated partial thromboplastin time; dTT: diluted thrombin time; ECT: ecarin clotting time; TT: thrombin time
For rivaroxaban (Xarelto®), apixaban (Eliquis®) or edoxaban (Lixiana®), the reference for assessing anticoagulation is the measurement of anti-Xa activity expressed in ng/ml and calibrated to the anticoagulant (table 3) [11, 13]. This allows an anticoagulant to be detected and its concentration to be determined. To achieve what is considered effective neutralisation, a result of less than 30 ng/ml in the case of invasive procedures or 50 ng/ml in the case of major bleeding is required [10, 14]. Although these tests can be performed in almost all Swiss hospitals, the time taken to obtain the results sometimes leads to the use of conventional tests. For example, a decrease in PT% or an increase in INR may indicate the presence of the molecule without giving any indication of its concentration. It should be recalled that normal results for these tests do not exclude the presence of effective anticoagulation [4, 11]. Direct determination by liquid chromatography-mass spectrometry or mass spectrometry is an alternative [14, 15].
For dabigatran (Pradaxa®), two assays are used to measure its concentration and correlate it with the level of anticoagulation: the diluted thrombin time (dTT) or the ecarin clotting time (ECT) expressed in seconds and converted in ng/ml. A result of less than 30 ng/ml in the case of invasive procedures or 50 ng/ml in the case of major bleeding is considered satisfactory for neutralising the anticoagulant effect of dabigatran [10, 11, 14]. Other less specific tests may reflect the presence of the drug in the blood without determining its concentration. A prolongation of the activated PTT (aPTT) expressed in seconds indicates significant anticoagulation but it is not excluded in the case of a normal result. As the TT is extremely sensitive to dabigatran, a normal value excludes the presence of the anticoagulant in the blood (table 3) [11, 12].
Although bleeding on anticoagulants is a known problem, few randomised studies have been carried out on large populations [13, 16] and even fewer with a control arm [17, 18], mainly because of the difficulty of carrying out such studies in extreme emergencies. Therefore, strategies for anticoagulation neutralisation are based mainly on expert consensus [4, 6]. Regardless of the strategy chosen (table 4), it is usually recommended that coagulation tests be repeated after administration of the various therapies, with the timing depending on the type of antidote administered [19], and that the efficacy of the treatment instituted be reassessed at short intervals (table 5). However, the clinician must be careful with specific antidotes. For example commercial anti-FXa-activity assays are unsuitable for measuring anti-FXa activity following administration of andexanet alfa as these assays result in erroneously elevated anti-FXa activity levels, thereby causing a substantial underestimation of the reversal activity of andexanet alfa.
Table 4Management strategy for major bleeding according to anticoagulant.
| Molecules | Strategies | ||
| 1 | 2 | ||
| Anti-vitamin K | Acenocoumarol (Sintrom®) | Prothrombin complex concentrate + vitamin K | Fresh frozen plasma + vitamin K |
| Warfarin (Coumadine®) | |||
| Phenprocoumone (Marcoumar®) | |||
| Direct factor IIa inhibitor | Dabigatran (Pradaxa®) | Idarucizumab | Prothrombin complex concentrate |
| Direct factor Xa inhibitors | Rivaroxaban (Xarelto®) | Andexanet alfa (Rivaroxaban, Apixaban): if critical and available! | Prothrombin complex concentrate |
| Apixaban (Eliquis®) | |||
| Edoxaban (Lixiana®) | |||
Table 5Drugs administered to neutralise anticoagulation of different anticoagulants and the effective cost of drugs administered for a 70 kg adult patient.
| Indication | Dosage | Major contraindications | Major side effects | Pharmacology | Cost for reversal | |
| Vitamin K (Phytomenadione, Konakion®) | Anticoagulation with VKA | 10 mg IV over 20 minutes | Component-related anaphylactic reaction | Anaphylactic reaction associated with a component | Synthesis of factors II, VII, IX and X | Approx. CHF 3 |
| No effective anticoagulation for the next 2 weeks | Onset usually 1 to 2 hours after medication, peak after 4 to 6 hours | |||||
| Hepatic absorption | ||||||
| PCC (Beriplex®, Octaplex®, Prothromplex®) | Anticoagulation with VKA, anti-Xa or anti-IIa | If VKA: According to the formula Dose (IU) = body weight x 40 x [Targeted - measured prothrombin rate]/100 -> if no INR available: 1750 IU (25 IU/kg) | Component-related anaphylactic reaction | Increased risk of thromboembolic events | Supplementation of human factors II, VII, IX and X | Approx. CHF 1180 for 1750 IU |
| If anti-Xa: 2100–3500 IU (30 or 50 IU/kg depending on clinical context) | Heparin-induced thrombocytopenia | Headache | Half-lives vary from 4 hours (factor VII) to 60 hours (factor II) | Approx. CHF 1420 for 2100 IU | ||
| If anti-IIa: 2100–3500 IU (30 or 50 IU/kg depending on clinical context) | Caution in patients with a history of disseminated intravascular coagulopathy, pulmonary embolism or myocardial infarction | Approx. CHF 2360 for 3500 IU | ||||
| FFP | VKA anticoagulation in the absence of PCC | 700–1050 ml total (10–15 ml/kg) | Patients with circulating anti-IgA antibodies | Hypervolaemia associated with transfusion | Supplementation of plasma proteins and factors | Approx. CHF 400 for 700 ml |
| Cardiac decompensation in heart disease | Half-lives vary according to different coagulation factors | Approx. CHF 570 for 1050 ml | ||||
| Post-transfusion respiratory distress | ||||||
| Andexanet alfa (Ondexxya®) | Anticoagulation with rivaroxaban (Xarelto®), apixaban (Eliquis®) | Low dose: Rivaroxaban ≤10 mg, apixaban ≤5 mg, Elapsed time >8 h regardless of dose: bolus 400 mg at 30 mg/min, then infusion 480 mg at 4 mg/min | Component-related anaphylactic reaction | Increased risk of thromboembolic events | Reversible binding of factor Xa | Approx. CHF 12,300 for low-dose therapy |
| High dose: Rivaroxaban >10 mg; apixaban >5 mg with elapsed time <8 h: bolus 800 mg at 30 mg/min, then infusion 960 mg at 8 mg/min | Common infusion reactions | Half-life of 4–7 h | Approx. CHF 22,200 for high-dose therapy | |||
| Low renal elimination | ||||||
| Degraded by endogenous proteases | ||||||
| Idarucizumab (Praxbind®) | Anticoagulation with dabigatran (Pradaxa®) | 2 boluses of 2.5 mg at 15 min interval | Component-related anaphylactic reaction | Increased risk of thromboembolic events | Irreversible binding of dabigatran | Approx. CHF 4000 |
| Half-life of 9.5–10.8 h | ||||||
| Hepatic metabolism and renal elimination | ||||||
| rFVIIa (Novoseven®) | Failed first-line therapies | 6300 μg administered over 5 min (90 μg/kg) | Component-related anaphylactic reaction | Increased risk of thromboembolic events | Activated factor VII | Approx. CHF 5270 |
| Half-life approx. 2.5 h | ||||||
| FXIII (Fibrogammin®) | Failed first-line therapies | 1050–1400 IU maximum dose 250 IU/min | Component associated anaphylactic reaction | Increased risk of thromboembolic events | Factor XIII | Approx. CHF 910 for 1050 IU |
| Half-life approx. 6–8 days | Approx. CHF 1130 for 1400 IU |
VKA: vitamin K antagonist; PT: prothrombin time; INR: International normalised ratio; FFP: fresh frozen plasma; aPTT: activated partial thromboplastin time; dTT: diluted thrombin time; ECT: ecarin clotting time; TT: thrombin time; PCC: prothrombin complex concentrate
In the case of vitamin K antagonist anticoagulation, neutralisation consists of the administration of 10 mg oral or intravenous vitamin K1 (phytomenadione; Konakion®, Neon Healthcare Ltd.) (table 5). However, the peak effect of vitamin K1 has a delay of 4-6 hours after administration, with maximum efficacy at 24–48 hours [20]. Thus, it is recommended to administer prothrombin complex concentrate (PCC) (Beriplex® CSL Behring AGP, Octaplex® Octapharma AG or Prothromplex® Takeda Pharma AG) at the same time in order to supplement coagulation factors during the first 6–10 hours, with a dosage adapted to the initial measured prothrombin rate, the target prothrombin rate after administration of the product, and the patient's weight (according to the formula for PCC: dose (IU) = body weight x 40 x [targeted − measured prothrombin rate]/100). An alternative strategy is to adjust the dose of PCCs solely to the baseline INR value (table 6) [21].
Table 6Administration of prothrombin complex concentrate (PCC) according to baseline international normalised ratio (INR) value in major bleeding on vitamin K antagonists.
| Baseline INR | Value PCC dose in IU/kg |
| 2–4 | 25 |
| 4–6 | 35 |
| >6 | 50 |
If an INR is not available at the start of management, a dose of 25 IU/kg is recommended. The maximum dose is set at 3000 IU intravenously but will usually vary between 1000 and 2500 IU intravenously, administered at a rate of 8 ml/min – 200 IU/min (Beriplex®), 3 ml/min – 75 IU/min (Octaplex®) or 1 ml/min – 30 IU/min (Prothromplex®) [19].
Fresh frozen plasma (FFP) is no longer used as a first-line treatment for anticoagulation neutralisation because of the risk of adverse effects related to the amount of fluid administered, delay in delivery, risks of transfusion and its lack of efficacy in restoring normal haemostasis, as well as the risk of thromboembolic events [20, 22]. Thus, if PCC is available, the use of FFP is not recommended. In the absence of available PCCs, however, 10–15 ml/kg of FFP can be administered (i.e., for a 70 kg patient, usually 3–4 units) [20] (table 4).
Several antidotes to oral anticoagulants are now available. Other promising therapies are currently under development, such as ciraparantag, a synthetic molecule that reverses all DOACs. In the absence of sufficient data, they are not developed here.
Since the end of 2020, andexanet alfa (Ondexxya®, AstraZeneca AG) has been approved in Switzerland as an antidote to rivaroxaban (Xarelto®, Bayer (Schweiz) AG) and apixaban (Eliquis®, Bristol-Myers Squibb SA), following the results of the ANNEXA-4 study. This drug is an inactive recombinant of coagulation factor Xa that neutralises the activity of factor Xa inhibitors. This study showed a significant decrease in anti-Xa activity and excellent haemostasis in patients receiving andexanet alfa [18], but further studies are needed owing to the small number of patients enrolled.
The dosage of the antidote varies according to the dose and the time since the last anticoagulant dose [6, 23] (table 5). If the last dose is low (rivaroxaban ≤10 mg or apixaban ≤5 mg) or the time is >8 hours regardless of the dose, andexanet alfa can be given at a low dosage (400 mg bolus at a rate of 30 mg/min, followed by a 480 mg infusion at a rate of 4 mg/min). If the last dose of anticoagulant is high (rivaroxaban >10 mg or apixaban >5 mg) with a delay of <8 hours, the dosage of andexanet alfa will be high (bolus of 800 mg at a rate of 30 mg/min, followed by an infusion of 960 mg at a rate of 8 mg/min). Some hospitals also adjust the regimen according to specific anti-Xa activity (low dose if anti-Xa 100–200 ng/ml; high dose if anti-Xa >200 ng/ml). It is important to note that the measurement of anti-Xa activity after administration of andexanet alfa is not reliable [24].
However, this antidote is extremely expensive and carries a significant thromboembolic risk, and it is not yet widely used in all Swiss hospitals. The indications are strictly limited to severe bleeding and defined by each institution. In cases where the antidote is not available or where bleeding is excluded from the restrictive criteria, first-line administration of PCC is recommended at a dosage of 30–50 IU/kg [4, 6, 23] (table 5). In Switzerland, Swissmedic has not yet given its approval for the use of this antidote in patients treated with edoxaban (Lixiana®) in the absence of formal studies, despite the fact that its action profile is similar to that of other molecules [25].
An antidote to dabigatran (Pradaxa®, Boehringer Ingelheim GmbH) has been available in Switzerland since 2016. Idarucizumab (Praxbind®, Boehringer Ingelheim Pharmaceuticals) is a humanised monoclonal antibody fragment that binds to dabigatran with high affinity and rapidly reverses its anticoagulant effect (nonrandomised prospective REVERSE-AD study) [17]. Idarucizumab should be administered in two boluses of 2.5 mg intravenously at an interval of no more than 15 minutes [17, 20] (table 5). In the absence of idarucizumab, the administration of PCC at a dose of 30–50 IU/kg is recommended [20] (table 6). Despite a good safety profile, the cost of this treatment is not negligible and it seems reasonable to use Idarucizumab in life-threatening situations [25]. Note that dabigatran is predominantly eliminated by the kidneys. However, haemodialysis is no longer a first-line option for neutralising since the development of the specific antidote [26].
Recombinant activated factor VII (rFVIIa; eptacog alfa, Novoseven®, Novo Nordisk Inc.) can be considered as an option when a first neutralisation strategy has not improved haemostasis. Only a few studies have evaluated the use of off-label prescription of rFVIIa for anticoagulation neutralisation. Despite good results in rapidly improving INR and haemostasis, the high risk of thrombotic events and the lack of data make rFVIIa a drug of last resort [26]. If necessary, rFVIIa at a dose of 90 μg/kg over 5 minutes is proposed, with the possibility of repeat dosing (table 5).
Human recombinant factor XIII (FXIII, Fibrogammin®, CSL Behring AG) can also be offered as an off-label treatment in situations where the first lines of neutralisation have failed. A dose of 15–20 IU/kg is recommended with intravenous administration and a maximum dose per minute of 250 IU (table 5).
Anticoagulation neutralisation for DOACs has made significant progress in recent years. The development of specific tests and the introduction of various antidotes now make it possible to optimise and codify the management of patients requiring urgent neutralisation. The formal indications and strategies for emergency reversal according to the molecules (and their pharmacokinetics) must be known. Neutralisation of the anticoagulant effect must always be weighed against the risk/benefit balance, bearing in mind the undesirable effects, particularly thrombo-embolic, and the considerable costs of these antidotes. It is certain that these recommendations will evolve over time. They will have to be adapted with the marketing of new molecules and future studies.
No financial support was reported.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Groupe de travail RivaMoS Suisse. Questions et réponses sur l’utilisation du rivaroxaban (Xarelto) dans la pratique. Rev Med Suisse. 2013;9:1375–85.
2. Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014 Oct;124(15):2450–8. https://doi.org/10.1182/blood-2014-07-590323
3. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005 Apr;3(4):692–4. https://doi.org/10.1111/j.1538-7836.2005.01204.x
4. Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017 Dec;70(24):3042–67. https://doi.org/10.1016/j.jacc.2017.09.1085
5. Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit Care. 2019;23:98.
6. Kluger Y, Ben-Ishay O, Sartelli M, Ansaloni L, Abbas AE, Agresta F, et al. World society of emergency surgery study group initiative on Timing of Acute Care Surgery classification (TACS). World J Emerg Surg. 2013 May;8(1):17. https://doi.org/10.1186/1749-7922-8-17
7. Haute Autorité de Santé. Prise en charge des surdosages en antivitamines K, des situations à risque hémorragique et des accidents hémorragiques chez les patients traités par antivitamines K en ville et en milieu hospitalier—avril 2008 [French.]. J Mal Vasc. 2008 Dec;33(4-5):202–13. https://doi.org/10.1016/j.jmv.2008.09.002
8. Huda SA, Kahlown S, Jilani MH, Chaudhuri D. Management of life-threatening bleeding in patients with mechanical heart valves. Cureus. 2021 Jun;13(6):e15619. https://doi.org/10.7759/cureus.15619
9. Pollack CV Jr. Coagulation assessment with the new generation of oral anticoagulants. Emerg Med J. 2016 Jun;33(6):423–30. https://doi.org/10.1136/emermed-2015-204891
10. Sauter TC, Eberle B, Wuillemin WA, Thiele T, Angelillo-Scherrer A, Exadaktylos AK, et al. How I manage patients with anticoagulation-associated bleeding or urgent surgery. Swiss Med Wkly. 2018 Mar;148:w14598.
11. Cuker A, Siegal DM, Crowther MA, et al. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128-39
12. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants. Chest. 2017 Jan;151(1):127–38. https://doi.org/10.1016/j.chest.2016.08.1462
13. DeAngelo J, Jarrell D, Cosgrove R, Camamo J, Edwards C, Patanwala AE. Comparison of 3-factor versus 4-factor prothrombin complex concentrate with regard to warfarin reversal, blood product use, and costs. Am J Ther. 2018;25(3):e326–32. https://doi.org/10.1097/MJT.0000000000000643
14. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI; Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016 Mar;14(3):623–7. https://doi.org/10.1111/jth.13227
15. Jayaraman S, DeAntonio JH, Leichtle SW, Han J, Liebrecht L, Contaifer D, et al. Detecting direct oral anticoagulants in trauma patients using liquid chromatography-mass spectrometry: A novel approach to medication reconciliation. J Trauma Acute Care Surg. 2020 Apr;88(4):508–14. https://doi.org/10.1097/TA.0000000000002527
16. Hickey M, Gatien M, Taljaard M, Aujnarain A, Giulivi A, Perry JJ. Outcomes of urgent warfarin reversal with frozen plasma versus prothrombin complex concentrate in the emergency department. Circulation. 2013 Jul;128(4):360–4. https://doi.org/10.1161/CIRCULATIONAHA.113.001875
17. Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal — full cohort analysis. N Engl J Med. 2017 Aug;377(5):431–41. https://doi.org/10.1056/NEJMoa1707278
18. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al.; ANNEXA-4 Investigators. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019 Apr;380(14):1326–35. https://doi.org/10.1056/NEJMoa1814051
19. Moia M, Squizzato A. Reversal agents for oral anticoagulant-associated major or life-threatening bleeding. Intern Emerg Med. 2019 Nov;14(8):1233–9. https://doi.org/10.1007/s11739-019-02177-2
20. Simon EM, Streitz MJ, Sessions DJ, Kaide CG. Anticoagulation Reversal. Emerg Med Clin North Am. 2018 Aug;36(3):585–601. https://doi.org/10.1016/j.emc.2018.04.014
21. Sarode R, Milling TJ Jr, Refaai MA, Mangione A, Schneider A, Durn BL, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013 Sep;128(11):1234–43. https://doi.org/10.1161/CIRCULATIONAHA.113.002283
22. Goldstein JN, Refaai MA, Milling TJ Jr, Lewis B, Goldberg-Alberts R, Hug BA, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015 May;385(9982):2077–87. https://doi.org/10.1016/S0140-6736(14)61685-8
23. Cuker A, Burnett A, Triller D, Crowther M, Ansell J, Van Cott EM, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. 2019 Jun;94(6):697–709. https://doi.org/10.1002/ajh.25475
24. Swissmedic. OndexxyaTM, poudre pour solution pour perfusion (andexanet alfa). [Internet]. [cited 2021 Oct 1]. Available from: https://www.swissmedic.ch/swissmedic/fr/home/humanarzneimittel/authorisations/new-medicines/ondexxyatm-pulver-andexanet-alfa.html, accessed 23 May 2022).
25. Volpe M, Jenny E, Guillermin A, Bloch J. Réversibilité des anticoagulants oraux directs : quid des nouveaux traitements disponibles? [Reversal of direct oral anticoagulants: what about new treatments available?]. Rev Med Suisse. 2022 Sep;18(794):1638–43. https://doi.org/10.53738/REVMED.2022.18.794.1638
26. Getta B, Muller N, Motum P, Hsu D, Zebeljan D, Rosenfeld D. Intermittent haemodialysis and continuous veno-venous dialysis are effective in mitigating major bleeding due to dabigatran. Br J Haematol. 2015 May;169(4):603–4. https://doi.org/10.1111/bjh.13236