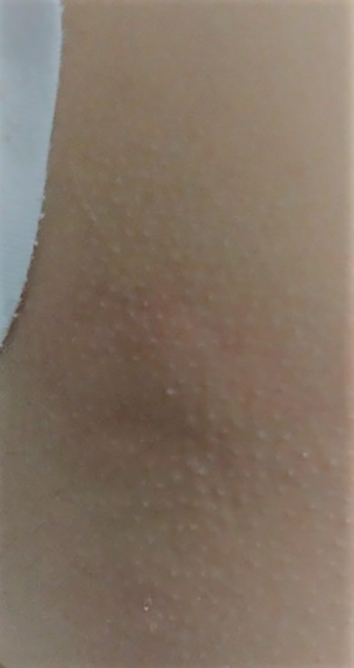
Figure 1 Urticarial rash typical of an immediate hypersensitivity reaction few minutes after intravenous administration of hydrocortisone 50 mg.
DOI: https://doi.org/10.4414/smw.2023.40025
Corticosteroids have been widely used for many years for the treatment of various diseases such as asthma, allergic disorders, autoimmune disorders, after transplantation (solid organ as well as bone marrow / stem cell transplantations), nephrological and dermatological disorders, owing to their anti-inflammatory effects and immunosuppressive properties [1, 2]. Corticosteroids can be administered via different routes: orally, intravenously, intraarticular, intramuscularly, sub-cutaneously, topically (drops/creams) and by inhalation (nose/lungs) [1, 2]. As discussed below, corticosteroid agents can be classified into several classes according to their chemical compositions [3–5].
In spite of their beneficial effects, corticosteroids can cause significant adverse effects such as immunosuppression, hypertension, diabetes, osteoporosis, avascular necrosis and growth retardation in children [6]. Although corticosteroids are commonly used for the treatment of various allergic disorders [7], they may cause, though quite uncommonly, allergic hypersensitivity reactions. Because of the wide usage of corticosteroids, even uncommon allergic hypersensitivity reactions are clinically important, thus physicians (general practitioners as well as allergy and dermatology specialists) should be aware of such reactions. The allergic hypersensitivity reactions can be immediate (usually up to 1 hour after corticosteroid administration) or delayed (appearing after more than 1 hour up to several days after corticosteroid administration) [8–12]. Klim Gittelman et al. and Borja et al. reported immediate hypersensitivity reactions in 0.1–0.3% of patients under systemic corticosteroid treatment [13, 14]. However, the real prevalence of such reactions is unknown since large control studies are not available and most of the relevant data are based on case reports. Delayed hypersensitivity reactions, which are more common than immediate reactions, were reported in 0.5–5% of patients under corticosteroid treatment (especially topical) [6, 15].
Several risk factors have been reported to be associated with both immediate and delayed corticosteroids allergic hypersensitivity. Cutaneous alkalisation (e.g., in sweat areas, skin infections, atopic dermatitis, venous stasis) may lead to a high cortisol degradation rate with high arginine binding, which increases cortisol antigenicity [8, 16]. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) and prolonged treatment with high-dose corticosteroids were also reported to be risk factors for corticosteroid hypersensitivity reactions [12, 17–19]. Immediate allergic hypersensitivity reactions to corticosteroids are mediated, at least in part, by specific Ig-E antibodies (type I response). However, non-IgE-mediated immediate allergic reactions involving direct activation of mast cell, basophils or the complement system, as well as inhibition of the cyclooxygenase pathways, were also reported in the pathogenesis of immediate corticosteroids hypersensitivity reactions [8, 12, 18]. Delayed allergic hypersensitivity reactions to corticosteroids are mostly mediated by T cells (type IV response) [8, 19]. It appears that binding of the corticosteroids (or their degradations products) to cutaneous or serum proteins generate stable antigens (allergens), which stimulate the immune system towards an allergic (type I immediate and type IV delayed hypersensitivity reactions) response [8, 9]. As discussed below, the allergic response may be directed not only against the culprit corticosteroid agent, but also against other corticosteroids of the same class, although cross-reactivity between different corticosteroid classes was also reported (see table 1) [4–6]. Class cross-reactivity (for allergic hypersensitivity reactions) was mainly reported for patients with delayed hypersensitivity reactions to corticosteroids, whereas such cross-reactivity for patients with immediate reactions is not yet established [11, 12, 18, 20, 21].
Corticosteroid agents are divided into five classes according to their chemical characteristics (e.g., side chains, hydrogenations, methylations) (table 1) [4–6]. This classification is important for the evaluation and management of hypersensitivity allergic reactions (mainly delayed type).
Table 1Classes of corticosteroid medications [4, 5, 8, 9].
| Class A |
| Hydrocortisone |
| Prednisone |
| Prednisolone |
| Methylprednisolone |
| Cortisone acetate |
| Fludrocortisone |
| Tixocortol pivalate*/** |
| Class B |
| Triamcinolone acetonide |
| Fluocinolone acetonide |
| Desonide |
| Fluocinonide |
| Halcinonide |
| Flunisolide |
| Budesonide*/** |
| Class C |
| Betamethasone* |
| Dexamethasone |
| Fluocortolone |
| Desoximetasone |
| Paramethasone |
| Class D1 |
| Beclomethasone* |
| Beclomethasone dipropionate |
| Beclomethasone valerate |
| Betamethasone dipropionate |
| Betamethasone valerate |
| Clobetasol-17-proprionate |
| Clobetasone-17-butyrate |
| Mometasone furoate |
| Fluticasone |
| Class D2 |
| Methylprednisolone aceponate* |
| Prednicarbate |
| Difluprednate |
| Hydrocortisone-17-butyrate |
| Hydrocortisone-17-propionate |
* Preferred agents (from each class) for patch test
** Included in the ESCD (European Society of Contact Dermatitis) recommended standard for patch test [46]
Glucocorticoids are classified according to chemical structure and side chains. Delayed hypersensitivity to one class member usually indicates cross reactivity to all class members. Data regarding cross reactivity between class members in immediate type hypersensitivity is not yet established.
Class C and D1 cause very few allergic reactions. Most allergic reactions were observed following treatment with class A (mainly hydrocortisone, methylprednisolone and prednisone) corticosteroid agents followed by class B (mainly triamcinolone) and class D2 corticosteroids (table 1). Because of cross-reactivity with co-sensitisation between agents of the same class, corticosteroid allergic hypersensitivity is usually not drug specific. D2 corticosteroids also share co-sensitivity with classes A and B [5, 22]. Recently, Beack et al. suggested a new classification with division into three classes of corticosteroids. Class 1 includes classes A and D2, class 2 includes class B and class 3 includes classes C and D1 [23]. Because of the chemical similarities within corticosteroids classes (table 1), allergic reactions to one class member may occur following treatment with another member of the same class. Thus, an agent of different corticosteroid class should be considered as an alternative agent.
Immediate allergic hypersensitivity reactions (presenting usually within 1 hour of corticosteroid administration) have been reported with almost all corticosteroid agents [11] and following all routes of administration (oral, intravenous, intramuscular, subcutaneous, intra-articular, inhalation, nasal and topical) [18, 24]. The most prevalent culprit agents are hydrocortisone, prednisone, methylprednisolone and triamcinolone acetate (class A agents) [20, 25–29]. Immediate allergic hypersensitivity reactions include anaphylaxis, urticaria, angioedema, flushing and presyncope (fig. 1) [8, 11, 20, 24]. Baker et al. reported that the most common routes of corticosteroid administration causing immediate allergic reactions were intraarticular followed by oral and intravenous treatment [20]. Other reports point to the intravenous and oral administration routes [18, 24, 26, 30]. Corticosteroids were reported to induce bronchospasm in asthmatic patients who are allergic to aspirin [31, 32]. Thus, such allergic hypersensitivity reactions should be considered in asthmatic patients with clinical deterioration and bronchospasm despite corticosteroid treatment [31, 32].

Figure 1 Urticarial rash typical of an immediate hypersensitivity reaction few minutes after intravenous administration of hydrocortisone 50 mg.
The diagnosis of an immediate hypersensitivity reaction to corticosteroids is based on clinical history and physical examination at the time of the suspicious event. The signs and symptoms of the allergic reaction may resemble those of the basic disorder of the patient (e.g., urticaria), thus the diagnosis is quite challenging. Skin testing with the culprit agent and with possible alternative corticosteroids is recommended (fig. 2).
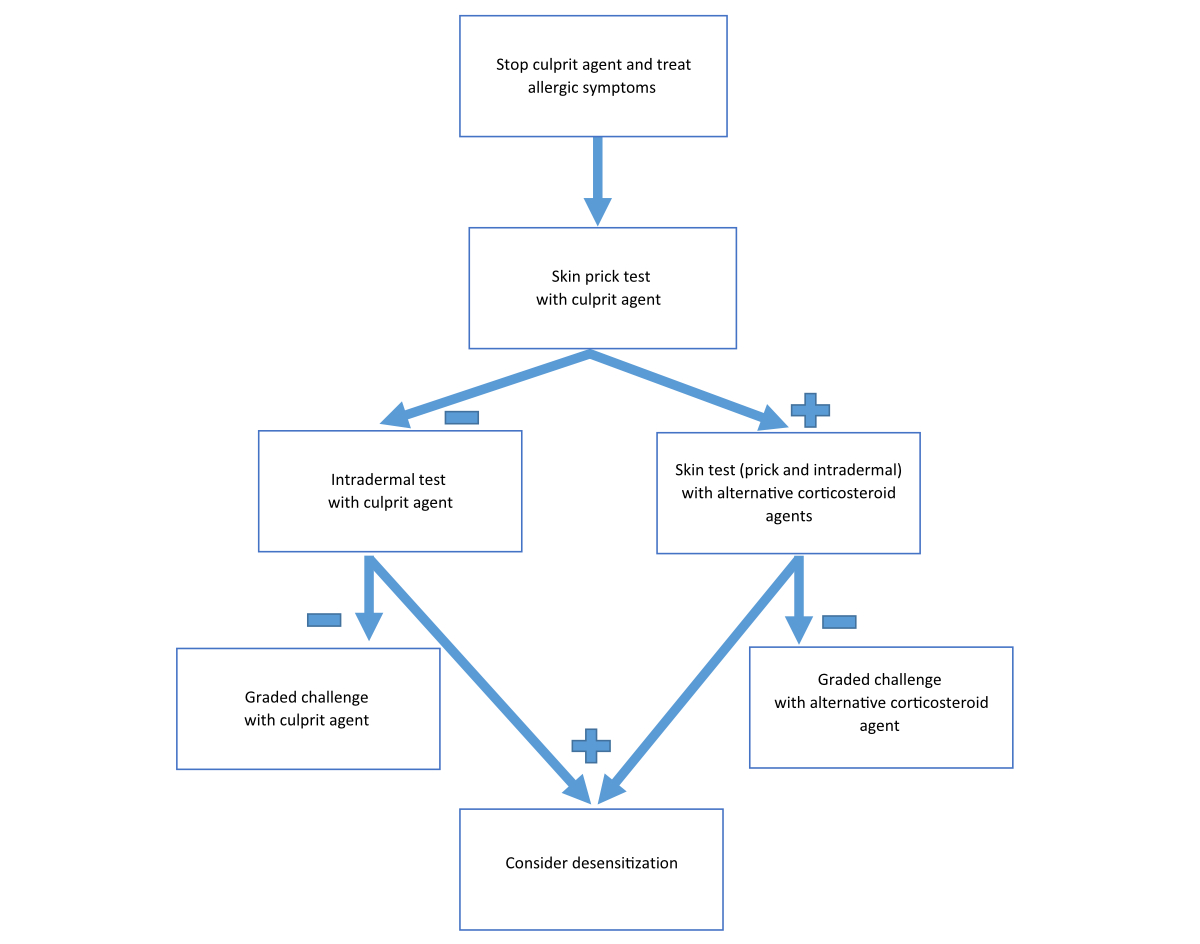
Figure 2 Procedure in the case of clinical suspicion of immediate hypersensitivity reaction to a corticosteroid.
Skin testing should be started with skin prick test and if the results are negative an intradermal test is indicated. All skin tests must include positive (histamine) and negative (saline) controls as well as the preservatives and excipients (e.g., E466 carboxymethyl cellulose [CMC], polysorbate, polyethylene glycol) or local anaesthetic agents that were given at the time of the allergic event, concomitantly with the suspicious culprit agent. Skin tests (prick and intradermal) for corticosteroids were reported to be safe, without systemic allergic reactions [20] (fig. 3).
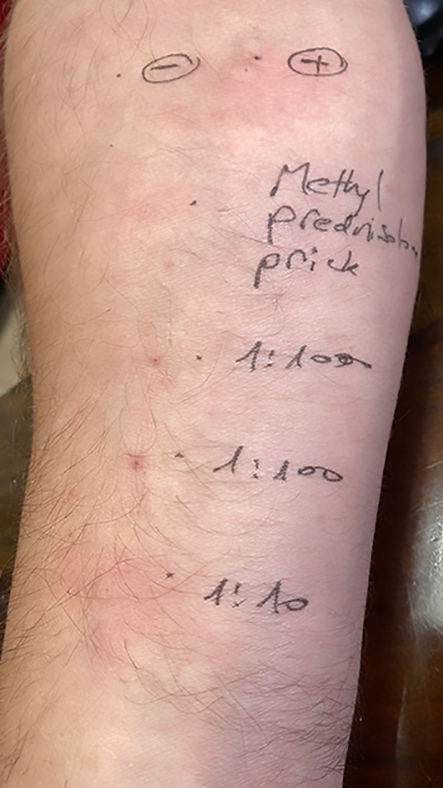
Figure 3 Positive intradermal test for methylprednisolone as part of evaluation of immediate hypersensitivity reaction. Positive and negative controls are included in the test.
The specificity and sensitivity of skin tests for immediate corticosteroid allergic hypersensitivity is not established. Moreover, as discussed above, some of the immediate allergic hypersensitivity reactions to corticosteroids are not IgE-mediated or caused by a corticosteroid hapten (binding to cutaneous or serum proteins to form the allergic antigen), but not by the corticosteroid itself [8]. Thus, the skin test maybe negative in spite of the hypersensitivity. Therefore, a negative skin test (prick and intradermal) does not exclude corticosteroid allergic hypersensitivity. Thus, following negative skin tests (prick and intradermal), graded challenge, under close observation, is the gold standard for confirming or refuting immediate hypersensitivity to corticosteroids.
In-vitro tests for the diagnosis of immediate corticosteroids hypersensitivity are not available for routine clinical usage. However, the basophil activation test (CD203c expression following in-vitro exposure of basophils to the culprit corticosteroid) may be helpful in the diagnosis of corticosteroid immediate hypersensitivity [33].
Venturini et al. [18] reported positive skin tests (prick and intradermal) in six out of seven patients with immediate reactions to corticosteroids. Four patients reacted with the culprit agent and the other two with the additive carboxymethyl cellulose. All three patients with anaphylaxis were found to have a positive skin test [18]. Sousa et al. [24] reported four children with corticosteroid-induced anaphylaxis. All patients had positive skin tests (two by prick and two by intradermal tests) to the culprit corticosteroid. A more recent study demonstrated positive skin tests in seven out of eight patients with corticosteroid induced anaphylaxis and in one out of four patients with corticosteroid-induced urticaria. Skin tests of patients who presented with presyncope or flushing were all negative. In the latter study, one case with a false positive and another case with false negative skin tests were reported [20]. Taken together, it appears that skin tests are valuable in the diagnosis of corticosteroid-induced anaphylaxis. The efficacy of skin tests in the diagnosis of other forms of immediate hypersensitivity to corticosteroids is not yet defined.
Treatment of immediate allergic reactions to corticosteroids is based on administration of intravenous fluids, adrenaline and antihistamines. When indicated, oxygen with or without airway opening should also be given [34]. Exposure to the culprit corticosteroid (as defined by the clinical history, skin test or graded challenge) must be avoided. Alternative, safe, corticosteroid agents should be used for future treatment. As discussed above, the cross-reactivity between different corticosteroid classes (or within the same class) for immediate hypersensitivity reaction is not established [8, 10, 11, 21]. Thus, the safety of the alternative agent should be defined by negative skin tests and a negative graded challenge test. Corticosteroids such as betamethasone, dexamethasone or the new steroid agent deflazacort, that were shown to be less allergenic can be considered as alternative agents (following negative skin tests and challenge) [28]. In patients without an alternative safe corticosteroid agent or when challenge is avoided owing to history of severe corticosteroid-induced anaphylaxis, desensitisation to the culprit corticosteroid can be considered (see fig. 3). This procedure should be under close clinical observation and it is only temporarily effective (as long as the patient is receiving the drug). Thus, it must be repeated every time that the patient needs corticosteroid treatment [28, 35, 36].
The most common delayed hypersensitivity reaction to corticosteroids is allergic contact dermatitis, which appears following topical corticosteroid treatment [8, 12] (fig. 4). It presents more than 1 hour and up to a few days following corticosteroid exposure as local or systemic erythema/eczema [10, 12, 21, 37–39].
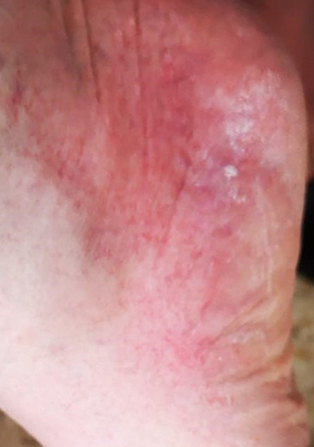
Figure 4 Contact dermatitis of the foot – delayed-type hypersensitivity few days after treatment with prednisolone acetate 0.5%.
Less often, exfoliative dermatitis, erythema multiforme, acute generalised exanthematous pustulosis (AGEP) or postural reactions have been reported [8, 10, 11]. Chronic dermatitis, atopic dermatitis, leg stasis and ulcers were shown to be risk factors for topical corticosteroid-induced contact dermatitis. This is, most probably, due to chronic long-term corticosteroid usage in these situations, and to the high penetration and degradation (leading to a higher protein binding rate) of the corticosteroid via the inflamed skin [8, 19]. Delayed hypersensitivity reactions were also reported in patients following inhaled, intranasal or ophthalmic corticosteroids, presenting as facial, perinasal or periorbital erythema with oedema, respectively [40, 41]. Generalised contact dermatitis was also reported following oral or intraarticular corticosteroid treatment [42].
The diagnosis of corticosteroid-induced delayed hypersensitivity, especially following topical use, is quite difficult since the allergic contact dermatitis may be similar to the basic skin disease of the patients. Thus, in all patients with worsening of their skin disease following the initiation of corticosteroid treatment or in patients who recovered rapidly after corticosteroids withdrawal, allergic contact dermatitis due to corticosteroids should be considered [10, 19, 43]. Indeed, Gonul et al. reported that 22% of patients with contact dermatitis who did not respond to topical corticosteroids had a positive corticosteroid patch test [44]. Occasionally, the local allergic contact dermatitis is more prominent at the edge of the topical corticosteroid application areas (“edge effect”). This may result from the high concentration of the culprit agent at the centre of the area (more anti-inflammatory effect), whereas at the edge where its concentration is lower, the allergic reaction can be manifested. Such phenomena can also be observed in early reading (24–48 hours) of a corticosteroids patch test [10]. Upon clinical suspicion, discontinuation of the culprit corticosteroid followed by diagnostic procedures are indicated (fig. 5). In vitro diagnostic tests, such as lymphocyte transformation or interferon-gamma (IFN-γ) assays, are not validated and are not available for clinical practice [9]. Skin prick tests (always negative) are useless for the diagnosis of delayed hypersensitivity to corticosteroids [38]. Intradermal tests should be avoided since they may cause skin atrophy [38].
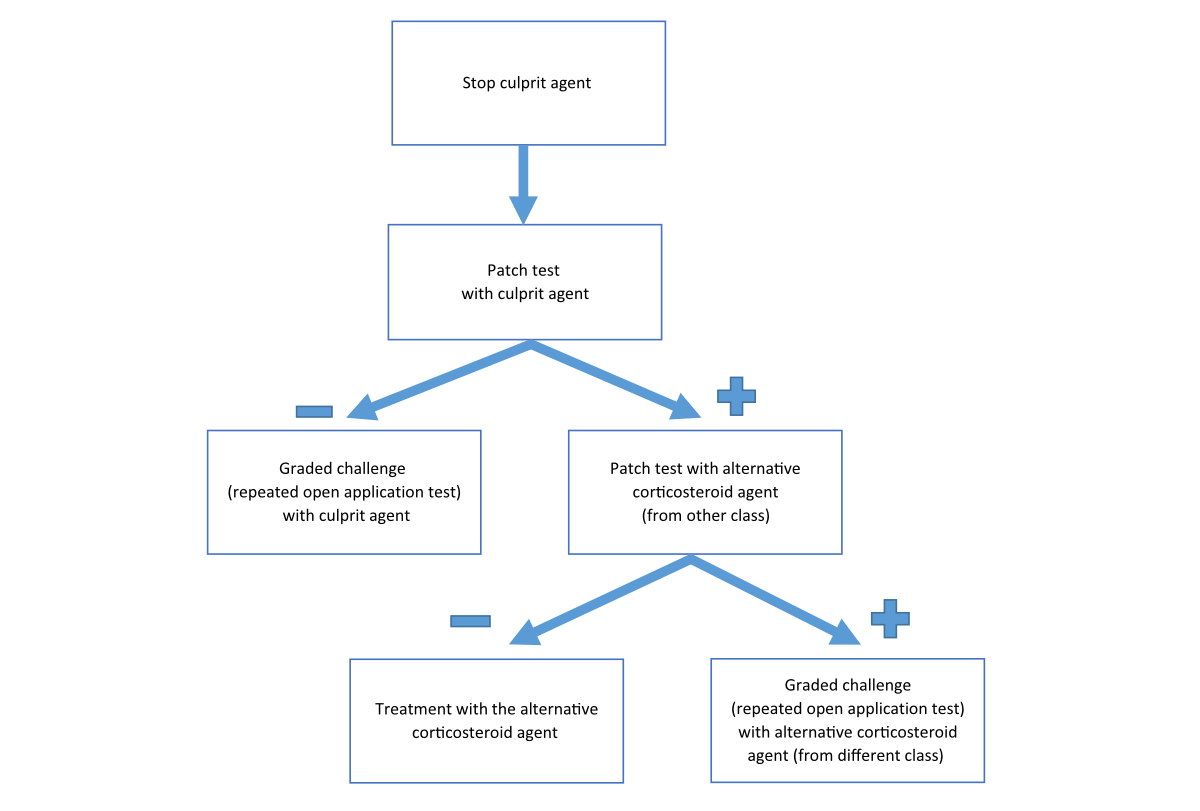
Figure 5 Procedure in the case of clinical suspicion of delayed hypersensitivity reaction to a corticosteroid.
Patch testing (a commercial or ethanolic self-made preparation is the standard method for the detection and diagnosis of delayed hypersensitivity to corticosteroids (fig. 6) [6, 10, 11, 19, 38, 43 ]. It should be noted that the delayed hypersensitivity reaction to a corticosteroid agent can be due to additives (vehicles) such as lactose, carboxymethyl cellulose, polyethylene glycol, parabens, fragrance and sorbitan sesquioleate within the corticosteroid preparation [11]. Thus, the appropriate vehicle should be included in the patch test, especially in patients with convincing history of corticosteroid-induced delayed hypersensitivity but a negative patch test to the pure culprit agent [11]. Patch tests should obviously include the suspected culprit corticosteroid as well as agents from the same and other corticosteroid classes (table 1). Patch test should be removed after 48 hours and read on days 2, 4 and 7 [10, 11, 21, 43, 45].
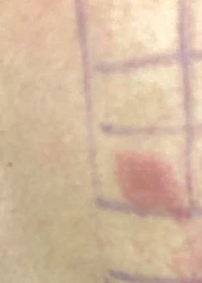
Figure 6 Positive European standard patch test in a patient with a delayed hypersensitivity reaction to budesonide
Delayed hypersensitivity to corticosteroids is usually not drug specific, thus, co-sensitisation or cross-reactivity between agents from the same class or even from different classes is common (table1). Some patients with delayed hypersensitivity to corticosteroids are sensitised to the lateral chain(s) of the culprit corticosteroid molecule. Therefore, those patients are also sensitised to other corticosteroids (with similar structure) of the same class (table 1) [19, 21]. In these cases, following a negative patch test with corticosteroid agent(s) from other classes, alternative corticosteroid(s) can be offered (see fig. 5). Other patients are sensitised to the skeletal structure of the corticosteroids. These patients demonstrate a high rate of cross reactivity within and between the corticosteroid classes (table 1) [21]. Thus, extensive patch test workup with different corticosteroid preparations from different classes are needed in order to find a safe alternative corticosteroid medication [6, 21]. According to patch test results, a graded challenge (including repeated open application tests) to the suggested alternative corticosteroid agent is mandatory in order to avoid hypersensitivity reactions.
Baeck et al. reported 315 patients with delayed hypersensitivity to corticosteroids [19]. The higher rate of positive patch tests was found for class A, followed by class B and then class D1 corticosteroid medications. Similar results were observed by Pratt et al., reporting patch tests of 741 patients with corticosteroid-induced delayed hypersensitivity reactions [45]. It should be noted that about 40% of patients with corticosteroid-induced delayed hypersensitivity reactions had a positive patch test to more than one class of corticosteroids, 6% of them reacted to corticosteroids from all five classes, demonstrating a high rate of cross-reactivity [19].
The North American Contact Dermatitis Group reported that 741 out of 17,987 (4.12%) patients who underwent patch tests were positive for corticosteroid medications [45]. Accordingly, the European Society of Contact Dermatitis (ECDS) recommend the use of tixocortol pivalate 0.1% (class A) and budesonide 0.01% (class B) in all standard patch test series [6, 46].
To conclude, physicians of all medical disciplines should be aware that corticosteroids can cause (“paradoxically”) immediate or delayed allergic hypersensitivity reactions. The diagnosis of such allergic reactions is challenging since it is often difficult to distinguish between hypersensitivity reactions and the deterioration of the basic inflammatory disease (e.g. worsening of asthma or dermatitis). Thus, a high index of suspicion is needed in order to identify the culprit corticosteroid and to find an alternative safe corticosteroid medication.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005 Oct;353(16):1711–23. https://doi.org/10.1056/NEJMra050541
2. Strehl C, Ehlers L, Gaber T, Buttgereit F. Glucocorticoids-All-Rounders Tackling the Versatile Players of the Immune System. Front Immunol. 2019 Jul;10:1744. https://doi.org/10.3389/fimmu.2019.01744
3. Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989 Jul;121(1):27–34. https://doi.org/10.1111/j.1365-2133.1989.tb01396.x
4. Goossens A, Matura M, Degreef H. Reactions to corticosteroids: some new aspects regarding cross-sensitivity. Cutis. 2000 Jan;65(1):43–5.
5. Matura M, Goossens A, Matura M. Contact allergy to corticosteroids. Allergy. 2000 Aug;55(8):698–704. https://doi.org/10.1034/j.1398-9995.2000.00121.x
6. Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013 Aug;9(1):30. https://doi.org/10.1186/1710-1492-9-30
7. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J. 2021 Dec;59(1):59.
8. Vatti RR, Ali F, Teuber S, Chang C, Gershwin ME. Hypersensitivity reactions to corticosteroids. Clin Rev Allergy Immunol. 2014 Aug;47(1):26–37. https://doi.org/10.1007/s12016-013-8365-z
9. Rodrigo C, Alejandro C, Javier H, et al. Hypersensivity Reactions to Steroids [Review]. Glob Vaccines Immunol. 2018;3.
10. Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermat. 2012 Jan;66(1):38–45. https://doi.org/10.1111/j.1600-0536.2011.01967.x
11. Otani IM, Banerji A. Immediate and Delayed Hypersensitivity Reactions to Corticosteroids: evaluation and Management. Curr Allergy Asthma Rep. 2016 Mar;16(3):18. https://doi.org/10.1007/s11882-016-0596-7
12. Baeck M, Marot L, Nicolas JF, Pilette C, Tennstedt D, Goossens A. Allergic hypersensitivity to topical and systemic corticosteroids: a review. Allergy. 2009 Jul;64(7):978–94. https://doi.org/10.1111/j.1398-9995.2009.02038.x
13. Klein-Gitelman MS, Pachman LM. Intravenous corticosteroids: adverse reactions are more variable than expected in children. J Rheumatol. 1998 Oct;25(10):1995–2002.
14. Borja JM, Galindo PA, Feo F, Gomez E. Urticaria to methylprednisolone sodium hemisuccinate. Allergy. 2001 Aug;56(8):791. https://doi.org/10.1034/j.1398-9995.2001.056008791.x
15. Isaksson M, Bruze M. Corticosteroids. Dermatitis. 2005 Mar;16(1):3–5.
16. Ventura MT, Calogiuri GF, Muratore L, Di Leo E, Buquicchio R, Ferrannini A, et al. Cross-reactivity in cell-mediated and IgE-mediated hypersensitivity to glucocorticoids. Curr Pharm Des. 2006;12(26):3383–91. https://doi.org/10.2174/138161206778194006
17. Vind-Kezunovic D, Johansen JD, Carlsen BC. Prevalence of and factors influencing sensitization to corticosteroids in a Danish patch test population. Contact Dermat. 2011 Jun;64(6):325–9. https://doi.org/10.1111/j.1600-0536.2011.01898.x
18. Venturini M, Lobera T, del Pozo MD, González I, Blasco A. Immediate hypersensitivity to corticosteroids. J Investig Allergol Clin Immunol. 2006;16(1):51–6.
19. Baeck M, Chemelle JA, Terreux R, Drieghe J, Goossens A. Delayed hypersensitivity to corticosteroids in a series of 315 patients: clinical data and patch test results. Contact Dermat. 2009 Sep;61(3):163–75. https://doi.org/10.1111/j.1600-0536.2009.01602.x
20. Baker A, Empson M, The R, Fitzharris P. Skin testing for immediate hypersensitivity to corticosteroids: a case series and literature review. Clin Exp Allergy. 2015 Mar;45(3):669–76. https://doi.org/10.1111/cea.12441
21. Voltolini S, Fumagalli F. Delayed corticosteroid hypersensitivity: a clinical management proposal. Eur Ann Allergy Clin Immunol. 2021 Jul;53(4):171–6. https://doi.org/10.23822/EurAnnACI.1764-1489.164
22. Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol. 2014 Jul;14(1):33. https://doi.org/10.1186/1472-6890-14-33
23. Baeck M, Chemelle JA, Goossens A, Nicolas JF, Terreux R. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011 Oct;66(10):1367–74. https://doi.org/10.1111/j.1398-9995.2011.02666.x
24. de Sousa NG, Santa-Marta C, Morais-Almeida M. Systemic corticosteroid hypersensitivity in children. J Investig Allergol Clin Immunol. 2010;20(6):529–32.
25. Cheam H, Butani L. Immunoglobulin E-mediated reactions to corticosteroids. Curr Allergy Asthma Rep. 2005 Jan;5(1):22–7. https://doi.org/10.1007/s11882-005-0050-8
26. Kamm GL, Hagmeyer KO. Allergic-type reactions to corticosteroids. Ann Pharmacother. 1999 Apr;33(4):451–60. https://doi.org/10.1345/aph.18276
27. Rachid R, Leslie D, Schneider L, Twarog F. Hypersensitivity to systemic corticosteroids: an infrequent but potentially life-threatening condition. J Allergy Clin Immunol. 2011 Feb;127(2):524–8. https://doi.org/10.1016/j.jaci.2010.09.030
28. Ventura MT, Calogiuri GF, Matino MG, Dagnello M, Buquicchio R, Foti C, et al. Alternative glucocorticoids for use in cases of adverse reaction to systemic glucocorticoids: a study on 10 patients. Br J Dermatol. 2003 Jan;148(1):139–41. https://doi.org/10.1046/j.1365-2133.2003.05061.x
29. Patel A, Bahna SL. Immediate hypersensitivity reactions to corticosteroids. Ann Allergy Asthma Immunol. 2015 Sep;115(3):178–182.e3. https://doi.org/10.1016/j.anai.2015.06.022
30. Burgdorff T, Venemalm L, Vogt T, Landthaler M, Stolz W. IgE-mediated anaphylactic reaction induced by succinate ester of methylprednisolone. Ann Allergy Asthma Immunol. 2002 Oct;89(4):425–8. https://doi.org/10.1016/S1081-1206(10)62046-7
31. Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermat. 2002 Aug;47(2):123–4. https://doi.org/10.1034/j.1600-0536.2002.470210_16.x
32. Lauerma AI. Screening for corticosteroid contact sensitivity. Comparison of tixocortol pivalate, hydrocortisone-17-butyrate and hydrocortisone. Contact Dermat. 1991 Feb;24(2):123–30. https://doi.org/10.1111/j.1600-0536.1991.tb01664.x
33. Ben Said B, Leray V, Nicolas JF, Rozieres A, Berard F. Methylprednisolone-induced anaphylaxis: diagnosis by skin test and basophil activation test. Allergy. 2010 Apr;65(4):531–2. https://doi.org/10.1111/j.1398-9995.2009.02199.x
34. Muraro A, Worm M, Alviani C, Cardona V, DunnGalvin A, Garvey LH, et al.; European Academy of Allergy and Clinical Immunology, Food Allergy, Anaphylaxis Guidelines Group. EAACI guidelines: Anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357–77. https://doi.org/10.1111/all.15032
35. Solensky R. Drug desensitization [vi.]. Immunol Allergy Clin North Am. 2004 Aug;24(3):425–43. https://doi.org/10.1016/j.iac.2004.03.008
36. Angel-Pereira D, Berges-Gimeno MP, Madrigal-Burgaleta R, Ureña-Tavera MA, Zamora-Verduga M, Alvarez-Cuesta E. Successful rapid desensitization to methylprednisolone sodium hemisuccinate: a case report. J Allergy Clin Immunol Pract. 2014;2(3):346–8. https://doi.org/10.1016/j.jaip.2013.12.011
37. Burden AD, Beck MH. Contact hypersensitivity to topical corticosteroids. Br J Dermatol. 1992 Nov;127(5):497–500. https://doi.org/10.1111/j.1365-2133.1992.tb14847.x
38. Soria A, Baeck M, Goossens A, Marot L, Duveille V, Derouaux AS, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids? Contact Dermat. 2011 Jun;64(6):313–24. https://doi.org/10.1111/j.1600-0536.2011.01888.x
39. Baeck M, Goossens A. Systemic contact dermatitis to corticosteroids. Allergy. 2012 Dec;67(12):1580–5.
40. Baeck M, Pilette C, Drieghe J, Goossens A. Allergic contact dermatitis to inhalation corticosteroids. Eur J Dermatol. 2010;20(1):102–8. https://doi.org/10.1684/ejd.2010.0826
41. Cadinha S, Malheiro D, Rodrigues J, Castro E, Castel-Branco MG. Delayed hypersensitivity reactions to corticosteroids. Allergol Immunopathol (Madr). 2005;33(6):329–32. https://doi.org/10.1016/S0301-0546(05)73252-3
42. Santos-Alarcón S, Benavente-Villegas FC, Farzanegan-Miñano R, Pérez-Francés C, Sánchez-Motilla JM, Mateu-Puchades A. Delayed hypersensitivity to topical and systemic corticosteroids. Contact Dermat. 2018 Jan;78(1):86–8. https://doi.org/10.1111/cod.12841
43. Reitamo S, Lauerma AI, Stubb S, Käyhkö K, Visa K, Förström L. Delayed hypersensitivity to topical corticosteroids. J Am Acad Dermatol. 1986 Apr;14(4):582–9. https://doi.org/10.1016/S0190-9622(86)70073-X
44. Gönül M, Gül U. Detection of contact hypersensitivity to corticosteroids in allergic contact dermatitis patients who do not respond to topical corticosteroids. Contact Dermat. 2005 Aug;53(2):67–70. https://doi.org/10.1111/j.0105-1873.2005.00638.x
45. Pratt MD, Mufti A, Lipson J, Warshaw EM, Maibach HI, Taylor JS, et al. Patch Test Reactions to Corticosteroids: Retrospective Analysis From the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28(1):58–63. https://doi.org/10.1097/DER.0000000000000251
46. Isaksson M, Brandão FM, Bruze M, Goossens A; European Society of Contact Dermatitis. Recommendation to include budesonide and tixocortol pivalate in the European standard series. ESCD and EECDRG. Contact Dermat. 2000 Jul;43(1):41–2.