Acute flaccid myelitis in Switzerland – association with enterovirus D68
DOI: https://doi.org/10.57187/smw.2023.40045
Sandra
Bigiab, Alban
Ramettec, Maria
Teresa Barbanic,
Andreas Bierid, Angelika
Hoffmanne, Christoph
Aebif
a Institute
of Social and Preventive Medicine, University of Bern Switzerland
b Department
of Neurology, Bern University Hospital, Inselspital, University of Bern, Switzerland
c Institute
for Infectious Diseases, University of Bern, Switzerland
d Department
of Paediatrics, Cantonal Hospital Aarau, Switzerland
e University
Institute of Diagnostic and Interventional Neuroradiology, University Hospital
Bern, Inselspital, University of Bern, Switzerland
f Division
of Paediatric Infectious Diseases, Department of Paediatrics, Bern University
Hospital, Inselspital, University of Bern, Switzerland
Summary
Poliomyelitis-like acute flaccid myelitis associated
with enterovirus D68 (EV-D68) has emerged globally during the past decade. Here
we describe the first documented case reported from Switzerland, and a second,
suspected case occurring in temporal association. AFM occurs primarily in
children, is usually heralded by a febrile, respiratory prodrome followed by
acute-onset, usually asymmetrical, limb weakness with some predilection for the
upper extremities, and respiratory muscle compromise in one third of reported cases.
There is no specific therapy and the majority of cases result in permanent
neurological sequelae. A comprehensive diagnostic workup and timely reporting
to the health authorities are essential. Surveillance of respiratory and stool samples
for EV-D68 and other neurotropic enteroviruses is in place in several European
countries and warrants consideration in Switzerland. This could entail the
extension of the poliomyelitis surveillance program of the Federal Office of
Public Health by monitoring and enteroviral typing of respiratory samples from patients
with acute flaccid paralysis.
Background
The term acute flaccid myelitis was created by the US Centers
for Disease Control and Prevention (CDC) in 2014 to describe patients, usually
children, with acute onset of acute flaccid limb weakness of unknown aetiology
without and with lesions in grey matter of the spinal cord [1]. Since then, enterovirus
type D68 (EV-D68) has emerged as the major driver of this poliomyelitis-like
disease in many regions of the world [2, 3]
including Europe, where circulation was first described in detail in 2014 [4]. Several hundred cases have now been
reported worldwide [2].
First
isolated in 1962 in the USA from respiratory specimens [5], EV-D68 enters the body by way of the respiratory tract, which
is unusual for an enterovirus and resembles the rhinoviruses [6]. EV-D68 was later found to cause epidemics
of acute respiratory disease, usually in a biennial pattern with peak
activities in the late summer [7] and autumn
of even-numbered years [8, 9]. The first
cases of acute flaccid myelitis were reported from California in 2012 [10]. An outbreak in Colorado in 2014 established
a spaciotemporal connection between cases of acute flaccid
myelitis and
the circulation of EV-D68[11].
Subsequent studies established a firm link between EV-D68 and acute
flaccid myelitis.
This clinical manifestation is thus typically heralded by a prodromal illness
of the respiratory tract [12], followed
by acute-onset, severe weakness of one or more extremities with some predilection
for the upper limbs [2, 13]. Weakness is
usually asymmetrical, but symmetrical disease also occurs. These features
reflect spinal disease of the anterior horn or, less frequently, of the brainstem
motor nuclei and usually result in persistent motor disability similar to
poliomyelitis [2, 13].
Some
European countries saw an out-of-phase activity of EV-D68 in the autumn of 2019
[14]. Most unusual, however, was the
complete absence of the expected wave in 2020. This was attributed at least in
part to the population-wide non-pharmaceutical interventions implemented to
curb the COVID-19 pandemic, which largely suppressed the circulation of enteroviruses
in general [15, 16]. However, a new wave
of EV-D68 infections was reported in many European countries in the autumn of
2021. Although cases of acute flaccid myelitis were not observed in one survey [17], we report two cases, with EV-D68
recovered from one patient, and a non-speciated enterovirus in the other, occurring
in the late autumn of 2021. To our knowledge, this is the first report of EV-D68-associated
acute flaccid myelitis in Switzerland.
Case reports
The air distance between the two patients’ places of residence was 90 km. They or
their close family members did not knowingly spend time with each other or in
the same geographic vicinity.
Patient 1
Patient
1 was a 9-year-old, previously healthy, male child with his immunisations up to
date including the vaccines against poliomyelitis and tick-borne encephalitis.
He came down with low-grade fever, rhinorrhoea and a sore throat in early
November 2021. No other family member was ill, but he mentioned a similar illness
among some of his classmates. Two days later he noticed complete inability to
move his right arm and complained of orthostatic dizziness. On admission to the
hospital, he was fully alert. His body temperature was 38.0°C. Heart rate,
respiratory rate and transcutaneous O2 saturation were 92–145 bpm, 28/minute
and 90–92%, respectively. General physical examination revealed mild pharyngeal
redness, productive cough and mild jugular retractions, but was otherwise
normal. Neurologically, he showed a near total flaccid palsy of his right arm
with no ability to execute visible movements in his right shoulder and upper
arm (muscle strength M0–1 on a conventional five-point scale [18]) and with limited function in his right
hand and wrist (M2–3). There was no neck stiffness, no sensory impairment and
no bladder dysfunction. Magnetic resonance imaging (MRI) revealed a contiguous
T2-hyperintense lesion predominantly within the grey matter of the spinal cord
reaching from the craniocervical junction to thoracic vertebra 1, indicative of
myelitis (fig. 1, panel a).
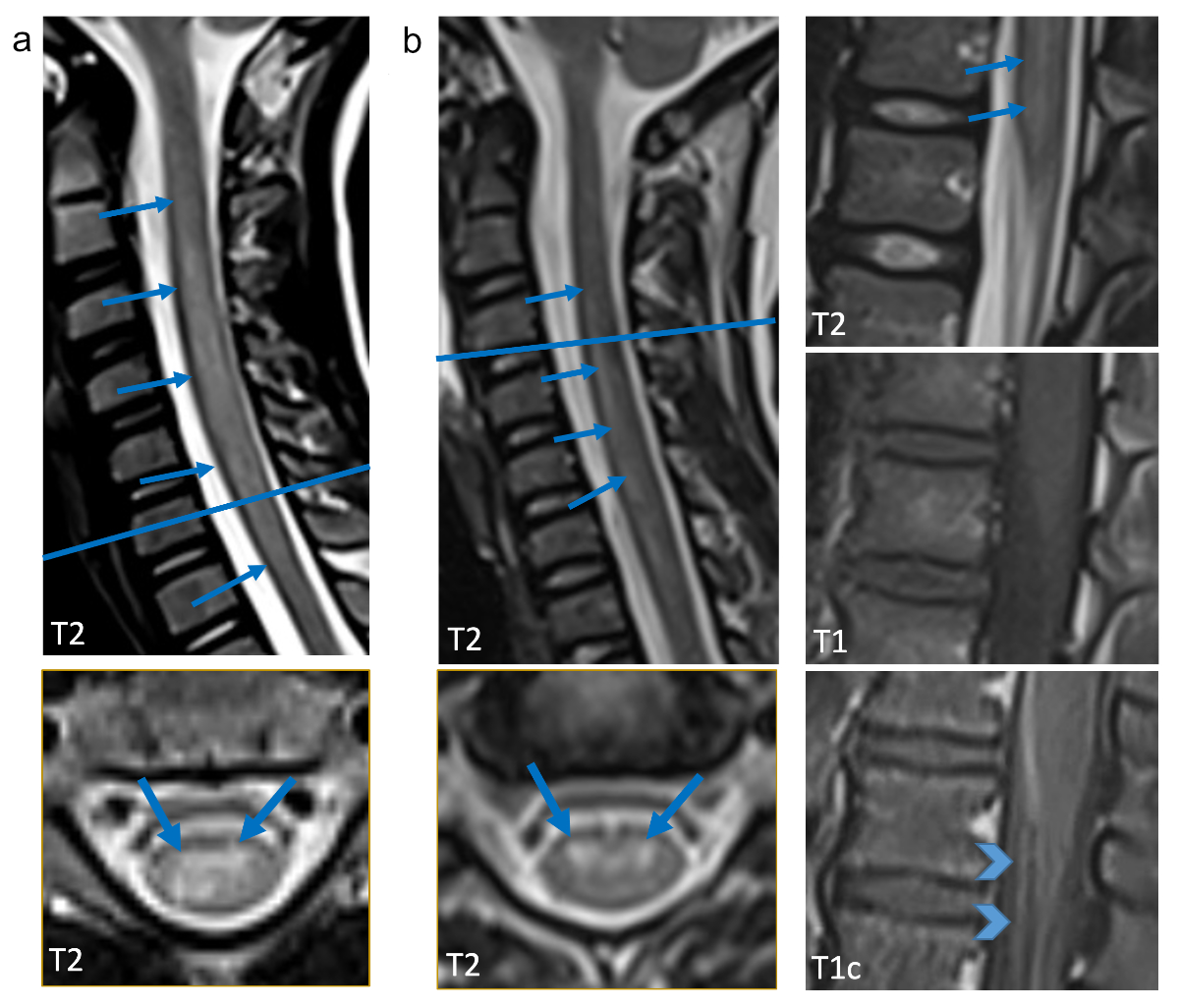
Figure 1 Representative sagittal and axial T2w images
are shown (a: patient 1, 9.3-year-old old boy; b: patient 2, 7.1-year-old girl)
as well as T1w images with and without contrast agent (b). Cervical myelitis
with increased T2 signal is present in both children (arrows), in the boy only
in the cervical spinal cord (a), in the girl additionally in the lumbar spinal
cord (b). On the axial slice predominant involvement of the grey matter is
evident (arrows point to anterior horns). In b (second column) sagittal T1w
images show enhancement of the cauda equina and anterior nerve root enhancement
(arrow heads).Representative sagittal and axial T2w images
are shown (a: patient 1, 9.3-year-old old boy; b: patient 2, 7.1-year-old girl)
as well as T1w images with and without contrast agent (b). Cervical myelitis
with increased T2 signal is present in both children (arrows), in the boy only
in the cervical spinal cord (a), in the girl additionally in the lumbar spinal
cord (b). On the axial slice predominant involvement of the grey matter is
evident (arrows point to anterior horns). In b (second column) sagittal T1w
images show enhancement of the cauda equina and anterior nerve root enhancement
(arrow heads).
The
cervical spinal cord was swollen but did not show contrast enhancement. His
laboratory data are listed in table 1. Enterovirus polymerase chain reaction (PCR)
testing from both cerebrospinal fluid (CSF) and stool were negative, but enterovirus
PCR on a nasopharyngeal swab was positive. Sanger sequencing following reverse
RNA transcription of a viral genomic region located between the VP4 and the VP2
capsid proteins [19] later identified
EV-D68. Shotgun metatranscriptomic sequencing (Illumina® MiSeq, Nextera Flex, 2
× 150 bp, 300 cycles) [20] of the total extracted RNA revealed the
presence of few reads (5/4,528,656 reads), which nonetheless unambiguously
identified EV-D68 (bootstrap 100%) [21]
in two recovered regions (316 bases of 5'UTR, and 132 bases of the capsid
protein 1A of VP4; data not shown), covering in total 6.1% of the EV-D68
reference genome sequence AY426531.1. Based on the initial assumption of an
immune-mediated inflammatory process, he received high-dose intravenous methylprednisolone,
followed by plasmapheresis and intravenous immunoglobulin replacement (table 1).
There was no neurological improvement. He was otherwise stable except for
episodes of mild blood pressure instability and was discharged home after 18
days. Six months later and after intense outpatient rehabilitation he regained some
function in his right hand and wrist (M3–4), but the near complete palsy of his
upper right arm persisted.
Patient 2
Patient
2 was a 7-year-old, previously healthy girl, whose illness started with fever
above 39.0°C, rhinorrhoea and cough. No other family members had similar
symptoms. Five days later and after the fever had subsided, her parents noted an
abnormal gait, weakness of her left arm and speech difficulties. On admission,
her temperature was 36.9°C. Heart rate, respiratory rate and transcutaneous O2
saturation were 102 bpm, 29/minute and 100%, respectively. Her general physical
examination was normal. Neurologically, she showed bilateral paralytic disease.
Assessment of muscle weakness revealed a patchy distribution with marked
weakness in her left shoulder (M3) and mild weakness in her legs (M4, right
proximal and left distal leg). There was no neck stiffness, no sensory
impairment and no bladder dysfunction. MR1 revealed multilevel T2-hyperintense myelitis,
affecting the grey matter of the spinal cord with contrast enhancement in the
anterior horns of the grey matter. Additionally, there was leptomeningeal
enhancement (fig. 1, panel B). Her laboratory data are listed in table 1.
Microbiological tests were negative for enterovirus by PCR from both CSF and
stool, but enterovirus PCR was positive in a nasopharyngeal swab. Both partial
sequencing of the enteroviral VP4/VP2 region [19]
and shotgun metatranscriptomic sequencing were unsuccessful in identifying EV-D68
or another enterovirus type molecularly. Her treatment consisted of intravenous
high-dose methylprednisolone, followed by intravenous immunoglobulin (table 1).
Her gait abnormality and speech disturbance disappeared within several days,
but her left shoulder weakness persisted for several weeks. On her last
follow-up visit 6 months after the onset of her illness, she had completely
recovered.
Table 1Clinical, laboratory, radiology findings and management of two
cases with acute flaccid myelitis. Clinical, laboratory, radiology findings and management of two
cases with acute flaccid myelitis.
|
Finding
|
Case 1
|
Case 2
|
|
Clinical symptoms
and signs
|
|
|
| Gender |
M |
F |
| Age |
9 years, 3 months |
7 years, 1 month |
| Comorbidity |
None |
None |
| Vaccines received |
Up to date including, polio and TBE |
Up to date, including polio (not TBE) |
| Prodromal illness |
Upper respiratory tract infection |
Upper respiratory tract infection |
| Onset of paralysis |
5 November 2021 |
17 December 2021 |
| General manifestations on admission |
Fever, pharyngitis, cough, jugular retractions |
None |
| Neurological examination |
Near total right arm palsy |
Left shoulder and bilateral leg weakness |
|
Haematology,
chemistry, immunology, CSF [reference range]
|
| White blood cell count (G/l) [3.0–12.5] |
8.34 |
9.65 |
| Lymphocyte count (G/l) [1.00–8.00] |
1.56 |
2.65 |
| C-reactive protein (mg/l) [<5] |
6 |
<3 |
| ALT, AST,
LDH, creatine kinase |
Within normal limits |
Within normal limits |
| Vitamin B12 (pmol/l) [145–569] |
530 |
554 |
| Serum IgG (g/l) [5.0–11.7] |
11.7 |
7.37 |
| Serum autoantibodies1 |
Negative |
Negative |
| CSF white
blood cell count (×106/l) |
186 |
46 |
| – Mononuclear cells |
148 |
45 |
| –Polymorphonuclear cells |
38 |
1 |
| CSF glucose (mmol/l) [3.33–4.44] |
3.36 |
3.1 |
| CSF protein (g/l) [0.20–0.40] |
0.36 |
0.63 |
| CSF lactate (mmol/l) [1.20–2.10] |
1.89 |
2.80 |
| CSF IgG intrathecal synthesis (%) [<10] |
15 |
0 |
| CSF IgA intrathecal synthesis (%) [<10] |
0 |
0 |
| CSF IgM intrathecal synthesis (%) [<10] |
20 |
21 |
| CSF autoantibodies (aquaporin-4; MOG) |
Negative |
Negative |
|
Microbiology
|
| Nasopharyngeal swab direct immunofluorescence
panel2 |
Negative |
Negative |
| Nasopharyngeal swab for SARS-CoV-2 PCR |
Negative |
Negativ |
| Nasopharyngeal swab for Mycoplasma pneumoniae, Chlamydia
pneumoniae and Bordetella pertussis
PCR |
Negative |
Not done |
| Nasopharyngeal swab for enteroviruses PCR |
Positive |
Positive |
| VP4/VP2 Sanger sequencing |
EV D68 |
Unsuccessful |
| Shotgun metatranscriptomics |
D68 |
Unsuccessful |
| CSF Biofire® multiplex PCR |
Negative |
Not done |
| CSF specific Enterovirus PCR |
Negative |
Negative |
| CSF specific HSV1/2 PCR |
Not done |
Negative |
| CSF Epstein-Bar virus PCR |
Not done |
Negative |
| CSF M. pneumoniae PCR |
Not done |
Negative |
| Serology panel I3 |
Negative |
Negative |
| Serology panel II4 |
Positive |
Positive |
| Stool molecular bacterial enteritis panel5 |
Negative |
Not done |
| Stool enterovirus PCR and culture |
Negative |
Negative |
|
Radiology
|
| Magnetic resonance imaging |
see figure 1 |
|
Therapy
|
|
|
| Methylprednisolone IV 30 mg/kg (days) |
5 |
5 |
| Plasmapheresis (days) |
5 |
– |
| IV immunoglobulin 0.4 g/kg per day (days) |
5 |
2 |
|
Outcome
|
|
|
| Neurological findings |
Persistent proximal: right arm palsy |
Complete recovery |
Discussion
The
clinical manifestations in our two patients were prototypical for EV-D68-associated
acute flaccid myelitis. They consisted of a febrile respiratory prodrome, followed by
rapid-onset asymmetric motor weakness, moderate CSF pleocytosis and
T2-hyperintense, longitudinally extensive spinal cord grey matter disease on MRI.
Major differential diagnoses, such as Guillain-Barré syndrome, demyelinating
disease, other infectious causes or spinal stroke were ruled out using
appropriate laboratory (table 1) and neuroimaging studies (fig. 1) and by the
subsequent clinical course. Although the differentiation between inflammation
and stroke by MRI may be difficult, CSF pleocytosis in both cases clearly
established an inflammatory process.
EV-D68-associated
acute flaccid myelitis occurs mainly in children. EV-D68 circulates throughout all regions
of the world [22] and most individuals
encounter it at some point in their lifetime. In Europe, recent seroprevalence
studies from the Netherlands [23] and the
UK [24] indicate that primary infection
with this pathogen occurs early in life. Reinfections by different strains may
be common later in life [25]. Data from
Switzerland are scanty, but the isolation of EV-D68 from Swiss patients has
recently been reported [25, 26]. Most
infections solely induce an acute respiratory syndrome, but EV-D68 is – or has
recently become [27] – neurotropic and has
clearly been linked to acute flaccid myelitis epidemiologically [10], virologically [28], immunologically [29] and
in animal models [30]. This is
corroborated by evidence from autopsy material from a
deceased child with acute flaccid myelitis, showing EV-D68 RNA and protein
in anterior horn motor neurones of the spinal cord [31].
The predilection for the paediatric age group may indicate that neurological
disease preferentially complicates primary infection rather than reinfection in
later life.
We
failed to find reports on EV-D68-associated acute flaccid
myelitis from
Switzerland. Indeed, it is a rare clinical entity and may not have occurred previously
in this country as the role of EV-D68 in its pathogenesis has only emerged during
the last decade. Alternatively, cases did occur, but were not reported or were
missed because of lack of awareness or negative test results. In fact, searching
for EV-D68 is quite challenging both in epidemiological surveillance and in
clinical practice. First, EV-D68 replicates preferentially in the respiratory
rather than the gastrointestinal epithelium. Stool PCR and culture are mostly negative.
Routine surveillance of poliomyelitis eradication, which has been in place in
Switzerland for many years in accordance with WHO recommendations, focuses on
syndromic surveillance of acute flaccid paralysis and laboratory testing of
stool specimens (https://www.spsu.ch/en/home). It is thus likely that EV-D68 is
missed. Second, clinicians may tend to focus their search for a causative
pathogen in cases of acute flaccid myelitis on CSF studies. Unfortunately, CSF
enteroviral PCR and CSF metagenomic next-generation sequencing are virtually
always negative in acute flaccid myelitis [12, 32, 33], which may have hampered
its association with EV-D68 for many years. Rather, the odds of detecting
EV-D68 are best in nasopharyngeal material collected during the first few days
of illness [2]. Third, routine virological testing in clinical practice does
not identify specific enterovirus types. Nasopharyngeal PCR or
immunofluorescence [34] testing usually consists of combined
detection of enterovirus and rhinovirus species. Single or multiplex CSF PCR may
detect enterovirus spp., but do not identify the enterovirus types involved.
Thus, a clinical suspicion of AFM should be communicated to the clinical virologist
to ensure appropriate testing using type-specific PCR or sequencing for
identification of EV-D68. This should be done in a timely manner, because knowledge
of the presence of EV-D68 may obviate therapeutic interventions that are expensive,
ineffective and potentially harmful.
There is currently no established treatment
for acute
flaccid myelitis and the majority of patients suffer from persistent
motor sequelae [13]. Corticosteroids and
plasmapheresis administered to our two patients have no role in acute flaccid
myelitis and were given based on the hypothesis of a non-infectious
inflammatory process before EV-D68 could be identified. Also, there is
currently no evidence for a beneficial effect of intravenous immunoglobulins,
although recent evidence from a mouse acute flaccid myelitis model demonstrated
that early administration of a neutralising monoclonal antibody may improve
paralytic outcome [35].
Because of its recent emergence as a
neurotropic agent, monitoring of the clinical burden of EV-D68-associated acute flaccid
myelitis in the population is paramount. This can be achieved by epidemiological
surveillance, either specifically for EV-D68 or, preferentially, by general
genomic surveillance of neurotropic enterovirus types circulating in the
community, such as enterovirus A71. Such programmes have been implemented in
several countries and provide us with what we currently know about EV-D68 in
Europe [17, 36]. Enhanced efforts to generate genomic viral surveillance data should
not be limited to influenza and SARS-CoV-2 but be extended to other
microorganisms that pose emergent public health threats. This report
illustrates that EV-D68 is one of them.
Conclusion
EV-D68-associated
acute flaccid myelitis is an emerging health threat primarily for children. No host predisposing
factors are known. This case report illustrates the classic clinical course of acute flaccid myelitis and the diagnostic approach needed to
maximise the yield of EV-D68 in clinical specimens. This process must be
initiated by the clinician who first sees a patient with acute flaccid
paralysis of any possible cause. This report should also serve as a call to
action for expanding genomic enterovirus surveillance in Switzerland.
Acknowledgment and ethics statement
The
authors thank the patients and their families for their cooperation.
Bern
University Hospital General Consent (GC) was granted by the patients’ legal
guardians for the use of routine clinical data in the medical record for
research purposes and scientific publications. In addition, oral informed consent
was obtained from the legal guardians as required by the University Hospital
for case reports.
Christoph Aebi, MD
Division of Paediatric Infectious Disease
Department of Paediatrics
Bern University Hospital (Inselspital)
University of Bern
Freiburgstrasse 15
CH-3010 Bern
christoph.aebi[at]insel.ch
References
1. U.S. Centers for Disese Control and Prevention Acute neurologic illness with focal limb weakness of unknown etiology in children. CDC Stacks 2014. Available at: https://stacks.cdc.gov/view/cdc/25378
2. Murphy OC, Messacar K, Benson L, Bove R, Carpenter JL, Crawford T, et al.; AFM working group. Acute flaccid myelitis: cause, diagnosis, and management. Lancet. 2021 Jan;397(10271):334–46. https://doi.org/10.1016/S0140-6736(20)32723-9
3. Messacar K, Asturias EJ, Hixon AM, Van Leer-Buter C, Niesters HG, Tyler KL, et al. Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality. Lancet Infect Dis. 2018 Aug;18(8):e239–47. https://doi.org/10.1016/S1473-3099(18)30094-X
4. Poelman R, Schuffenecker I, Van Leer-Buter C, Josset L, Niesters HG, Lina B; ESCV-ECDC EV-D68 study group. European surveillance for enterovirus D68 during the emerging North-American outbreak in 2014. J Clin Virol. 2015 Oct;71:1–9. https://doi.org/10.1016/j.jcv.2015.07.296
5. Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967 Mar;85(2):297–310. https://doi.org/10.1093/oxfordjournals.aje.a120693
6. Blomqvist S, Savolainen C, Råman L, Roivainen M, Hovi T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J Clin Microbiol. 2002 Nov;40(11):4218–23. https://doi.org/10.1128/JCM.40.11.4218-4223.2002
7. Duval M, Mirand A, Lesens O, Bay JO, Caillaud D, Gallot D, et al. Retrospective Study of the Upsurge of Enterovirus D68 Clade D1 among Adults (2014-2018). Viruses. 2021 Aug;13(8):1607. https://doi.org/10.3390/v13081607
8. Kidd S, Yee E, English R, Rogers S, Emery B, Getachew H, et al. National Surveillance for Acute Flaccid Myelitis - United States, 2018-2020. MMWR Morb Mortal Wkly Rep. 2021 Nov;70(44):1534–8. https://doi.org/10.15585/mmwr.mm7044a2
9. Kramer R, Sabatier M, Wirth T, Pichon M, Lina B, Schuffenecker I, et al. Molecular diversity and biennial circulation of enterovirus D68: a systematic screening study in Lyon, France, 2010 to 2016. Euro Surveill. 2018 Sep;23(37):1700711. https://doi.org/10.2807/1560-7917.ES.2018.23.37.1700711
10. Van Haren K, Ayscue P, Waubant E, Clayton A, Sheriff H, Yagi S, et al. Acute Flaccid Myelitis of Unknown Etiology in California, 2012-2015. JAMA. 2015 Dec;314(24):2663–71. https://doi.org/10.1001/jama.2015.17275
11. Messacar K, Schreiner TL, Maloney JA, Wallace A, Ludke J, Oberste MS, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015 Apr;385(9978):1662–71. https://doi.org/10.1016/S0140-6736(14)62457-0
12. Messacar K, Schreiner TL, Van Haren K, Yang M, Glaser CA, Tyler KL, et al. Acute flaccid myelitis: A clinical review of US cases 2012-2015. Ann Neurol. 2016 Sep;80(3):326–38. https://doi.org/10.1002/ana.24730
13. Knoester M, Helfferich J, Poelman R, Van Leer-Buter C, Brouwer OF, Niesters HG; 2016 EV-D68 AFM Working Group. Twenty-nine Cases of Enterovirus-D68-associated Acute Flaccid Myelitis in Europe 2016: A Case Series and Epidemiologic Overview. Pediatr Infect Dis J. 2019 Jan;38(1):16–21. https://doi.org/10.1097/INF.0000000000002188
14. Midgley SE, Benschop K, Dyrdak R, Mirand A, Bailly JL, Bierbaum S, et al. Co-circulation of multiple enterovirus D68 subclades, including a novel B3 cluster, across Europe in a season of expected low prevalence, 2019/20. Euro Surveill. 2020 Jan;25(2):1900749. https://doi.org/10.2807/1560-7917.ES.2020.25.2.1900749
15. Stoffel L, Agyeman PK, Keitel K, Barbani MT, Duppenthaler A, Kopp MV, et al. Striking Decrease of Enteroviral Meningitis in Children During the COVID-19 Pandemic. Open Forum Infect Dis. 2021 Apr;8(6):ofab115. https://doi.org/10.1093/ofid/ofab115
16. Kuo SC, Tsou HH, Wu HY, Hsu YT, Lee FJ, Shih SM, et al. Nonpolio Enterovirus Activity during the COVID-19 Pandemic, Taiwan, 2020. Emerg Infect Dis. 2021 Jan;27(1):306–8. https://doi.org/10.3201/eid2701.203394
17. Benschop KS, Broberg EK, Hodcroft E, Schmitz D, Albert J, Baicus A, et al. Molecular Epidemiology and Evolutionary Trajectory of Emerging Echovirus 30, Europe. Emerg Infect Dis. 2021 Jun;27(6):1616–26. https://doi.org/10.3201/eid2706.203096
18. Medical. Research Council. Aids to the examination of the peripheral nervous system, Memorandum No. 45, London.: Her Majesty's Stationery Office, 1981.
19. Linsuwanon P, Payungporn S, Samransamruajkit R, Posuwan N, Makkoch J, Theanboonlers A, et al. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect. 2009 Aug;59(2):115–21. https://doi.org/10.1016/j.jinf.2009.05.009
20. Grädel C, Terrazos Miani MA, Baumann C, Barbani MT, Neuenschwander S, Leib SL, et al. Whole-Genome Sequencing of Human Enteroviruses from Clinical Samples by Nanopore Direct RNA Sequencing. Viruses. 2020 Jul;12(8):841. https://doi.org/10.3390/v12080841
21. Kroneman A, Vennema H, Deforche K, v d Avoort H, Peñaranda S, Oberste MS, et al. An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol. 2011 Jun;51(2):121–5. https://doi.org/10.1016/j.jcv.2011.03.006
22. Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis. 2016 May;16(5):e64–75. https://doi.org/10.1016/S1473-3099(15)00543-5
23. Karelehto E, Koen G, Benschop K, van der Klis F, Pajkrt D, Wolthers K. Enterovirus D68 serosurvey: evidence for endemic circulation in the Netherlands, 2006 to 2016. Euro Surveill. 2019 Aug;24(35):1800671. https://doi.org/10.2807/1560-7917.ES.2019.24.35.1800671
24. Kamau E, Harvala H, Blomqvist S, Nguyen D, Horby P, Pebody R, et al. Increase in Enterovirus D68 Infections in Young Children, United Kingdom, 2006-2016. Emerg Infect Dis. 2019 Jun;25(6):1200–3. https://doi.org/10.3201/eid2506.181759
25. Hodcroft EB, Dyrdak R, Andrés C, Egli A, Reist J, García Martínez de Artola D, et al. Evolution, geographic spreading, and demographic distribution of Enterovirus D68. PLoS Pathog. 2022 May;18(5):e1010515. https://doi.org/10.1371/journal.ppat.1010515
26. Grädel C, Ireddy NR, Koch MC, Baumann C, Terrazos Miani MA, Barbani MT, et al. Genome Sequences of Rare Human Enterovirus Genotypes Recovered from Clinical Respiratory Samples in Bern, Switzerland. Microbiol Resour Announc. 2022 Sep;11(9):e0027622. https://doi.org/10.1128/mra.00276-22
27. Freeman MC, Wells AI, Ciomperlik-Patton J, Myerburg MM, Yang L, Konopka-Anstadt J, et al. Respiratory and intestinal epithelial cells exhibit differential susceptibility and innate immune responses to contemporary EV-D68 isolates. eLife. 2021 Jul;10:10. https://doi.org/10.7554/eLife.66687
28. Brown DM, Hixon AM, Oldfield LM, Zhang Y, Novotny M, Wang W, et al. Contemporary Circulating Enterovirus D68 Strains Have Acquired the Capacity for Viral Entry and Replication in Human Neuronal Cells. MBio. 2018 Oct;9(5):e01954-18. https://doi.org/10.1128/mBio.01954-18
29. Schubert RD, Hawes IA, Ramachandran PS, Ramesh A, Crawford ED, Pak JE, et al. Pan-viral serology implicates enteroviruses in acute flaccid myelitis. Nat Med. 2019 Nov;25(11):1748–52. https://doi.org/10.1038/s41591-019-0613-1
30. Hixon AM, Yu G, Leser JS, Yagi S, Clarke P, Chiu CY, et al. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog. 2017 Feb;13(2):e1006199. https://doi.org/10.1371/journal.ppat.1006199
31. Vogt MR, Wright PF, Hickey WF, De Buysscher T, Boyd KL, Crowe JE Jr. Enterovirus D68 in the Anterior Horn Cells of a Child with Acute Flaccid Myelitis. N Engl J Med. 2022 May;386(21):2059–60. https://doi.org/10.1056/NEJMc2118155
32. Kane MS, Sonne C, Zhu S, Malhotra A, Van Haren K, Messacar K, et al. Incidence, Risk Factors and Outcomes Among Children With Acute Flaccid Myelitis: A Population-based Cohort Study in a California Health Network Between 2011 and 2016. Pediatr Infect Dis J. 2019 Jul;38(7):667–72. https://doi.org/10.1097/INF.0000000000002276
33. Aliabadi N, Messacar K, Pastula DM, Robinson CC, Leshem E, Sejvar JJ, et al. Enterovirus D68 Infection in Children with Acute Flaccid Myelitis, Colorado, USA, 2014. Emerg Infect Dis. 2016 Aug;22(8):1387–94. https://doi.org/10.3201/eid2208.151949
34. Schindera C, Kraemer AL, Regamey N, Aebi C, Gorgievski-Hrisoho M, Barbani MT. Immunofluorescence versus xTAG multiplex PCR for the detection of respiratory picornavirus infections in children. J Clin Virol. 2010 Jul;48(3):223–5. https://doi.org/10.1016/j.jcv.2010.04.005
35. Rudy MJ, Frost J, Clarke P, Tyler KL. Neutralizing Antibody Given after Paralysis Onset Reduces the Severity of Paralysis Compared to Nonspecific Antibody-Treated Controls in a Mouse Model of EV-D68-Associated Acute Flaccid Myelitis. Antimicrob Agents Chemother. 2022 Aug;66(8):e0022722. https://doi.org/10.1128/aac.00227-22
36. Harvala H, Broberg E, Benschop K, Berginc N, Ladhani S, Susi P, et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J Clin Virol. 2018 Apr;101:11–7. https://doi.org/10.1016/j.jcv.2018.01.008
