Overall survival and role of programmed death ligand 1 expression in patients with metastatic non-small-cell lung cancer and immunotherapy: an observational study from central Switzerland
DOI: https://doi.org/10.57187/smw.2023.40039
Valentina
Allmanna, Daniela
Dyntarb, Dirk
Lehnickc, Marco
Dresslerd, Kristin
Zeidlere, Philipp
Niederbergerf, Jeanne
Godaug, Joachim
Dieboldh, Oliver
Gautschii
a Faculty
of Medicine, University of Basel, Switzerland
b Cancer
Registry of Central Switzerland, Cantonal Hospital Lucerne, Switzerland
c Biostatistics
and Methodology, Department of Health Sciences and Medicine, University of
Lucerne, Switzerland
d Department
of Medical Oncology, Clinic Hirslanden St Anna, Lucerne, Switzerland
e Department
of Medical Oncology, Cantonal Hospital Nidwalden, Stans, Switzerland
f Department
of Medical Oncology, Cantonal Hospital Obwalden, Sarnen, Switzerland
g Department
of Medical Oncology, Cantonal Hospital Uri, Altdorf, Switzerland
h Institute
of Pathology, Cantonal Hospital Lucerne, Switzerland
I University
of Bern and Cantonal Hospital of Lucerne, Switzerland
Summary
BACKGROUND: In
clinical trials, therapy with immune checkpoint inhibitors has improved the
survival of patients with metastatic non-small-cell lung cancer (NSCLC). These
trials were important for drug approval and for defining new treatment
standards but the effect of checkpoint inhibitors in patients treated outside of clinical trials
is not well known. The goal of this study was to assess the effect of
immunotherapy on the overall survival of patients with metastatic NSCLC
in the region of central Switzerland.
MATERIALS
AND METHODS: The study included 274 patients with histologically confirmed
metastatic (stage IV) NSCLC in central Switzerland in the years 2015 to 2018. Patients
with NSCLC and actionable driver mutations were excluded. Patients with checkpoint inhibitor
treatment (immuno-oncology [IO] group, n = 122) were compared with patients
without checkpoint inhibitor treatment (no-IO group, n = 152). Baseline demographics, disease
characteristics and therapies applied were collected retrospectively. The primary
endpoint was median overall survival calculated either from diagnosis or from the
start of checkpoint inhibitor therapy to death or data cut-off (21 July 2021). We used the
Kaplan-Meier method and an adjusted Cox proportional-hazards regression model. The expression of
programmed-death ligand 1 (PD-L1)
on tumour cells was used for exploratory
analysis.
RESULTS: Patients
had a median age of 68.4 years, most were male (61.7%) and more than half were
current or former smokers (65%). A test for PD-L1 expression was available for
55.8% of the tumours. Patients in the IO group were younger than patients in
the no-IO group. Among the 122 patients in the IO group, the median overall
survival was 15 months (95% confidence
interval [CI] 12–20). In the no-IO group, the median overall survival was 4 months
(95% CI 3–7) with chemotherapy and 2 months (95% CI 1–2) with best supportive
care. Patients with high (≥50%) PD-L1 expression and checkpoint inhibitor therapy
had a
slightly longer overall
survival than patients with low PD-L1 and checkpoint inhibitor therapy.
CONCLUSION: These results suggest that treatment with checkpoint inhibitors improves
overall survival in patients with metastatic NSCLC and that PD-L1 expression could
have a predictive value in patients treated outside of clinical trials. Further
studies are needed to study the magnitude of the benefit of checkpoint inhibitors according to
molecular NSCLC subtype.
Introduction
Lung cancer represents a major
global health burden. The main cause of lung cancer is smoking [1, 2]. Although many countries have made notable
efforts to control tobacco use, lung cancer remains the leading cause of
cancer-related deaths in the Western world [3,
4]. In Switzerland, smoking rates remain high and lung cancer is frequent
[5]. According to the statistics of the Swiss
Federal Office of Public Health, approximately 2700 men and 1800 women are
diagnosed with lung cancer each year [6].
Switzerland does not yet have a national lung-cancer screening programme [7]. Approximately 50% of patients with lung
cancer present with metastatic disease at the time of diagnosis [8]. These patients have a poor prognosis [6]. In previous studies, the median overall
survival ranged from 3 to 6 months with best supportive care and from 9 to 12
months with platinum-based chemotherapy [9–12].
Lung cancer includes two
histopathological types: small-cell lung cancer (SCLC) and non-small-cell lung
cancer (NSCLC). NSCLC accounts for 80–90% of all lung cancers and includes the
subtypes adenocarcinoma, squamous-cell carcinoma and large-cell carcinoma [13, 14]. In pulmonary adenocarcinoma, the
discovery of oncogenic driver mutations and the development of targeted therapy
have improved the prognosis of a substantial proportion of patients [15–18]. Today, the routine diagnostic workup
for metastatic NSCLC in Switzerland depends on the histology. For both
adenocarcinoma and squamous-cell carcinoma, the workup includes testing for the
programmed-death ligand 1 (PD-L1), and in cases of newly diagnosed
adenocarcinoma, it also includes sequencing the epidermal growth factor
receptor (EGFR), the anaplastic lymphoma kinase (ALK), the V-RAF murine sarcoma
viral oncogene homologue B1 (BRAF), the Kirsten rat sarcoma homologue (KRAS), the
receptor tyrosine kinase MET proto-oncogene (MET), neurotrophic tropomyosin receptor
kinases (NTRKs), the RET proto-oncogene (RET), and the ROS proto-oncogene 1
(ROS1) [14]. Approved targeted therapies can
be offered to approximately 20–25% of patients with metastatic pulmonary adenocarcinoma
[19–22].
For treating patients with metastatic
NSCLC without actionable driver mutations, monoclonal antibodies targeting the programmed
cell death protein 1 (PD-1) or PD-L1 pathway have been the most important
clinical advance in the last decade
[21]. Checkpoint inhibitors such
as nivolumab, pembrolizumab and atezolizumab were initially tested in patients
with a metastatic NSCLC progressing after first-line chemotherapy. Randomised clinical
trials demonstrated superior activity for checkpoint inhibitors compared with docetaxel in the second
line, with an improvement of overall survival of approximately 9 to 12 months
(measured from the time of randomisation) [10,
11, 23–25]. Based on these results, Swissmedic approved nivolumab in
2015 and pembrolizumab and atezolizumab in subsequent years. Tumour PD-L1
expression has been correlated with the magnitude of clinical benefit from checkpoint inhibitor
therapy in registration trials [25].
Further randomised clinical
trials have studied checkpoint inhibitors in the first line of therapy at different levels of PD-L1
expression. For NSCLC with PD-L1 expression of 50% or more, pembrolizumab was
compared with chemotherapy in the trial KN024 (on squamous and non-squamous
NSCLC), and pembrolizumab (overall survival 95% CI 18.3 months to not reached) exhibited significantly
better overall survival results than chemotherapy (overall survival 14.2 months, 95% CI 9.8–19.0)
[26]. For NSCLC with PD-L1 expression
below 50%, the trials KN189 (on non-squamous NSCLC) and KN407 (on squamous
NSCLC) compared pembrolizumab plus chemotherapy with chemotherapy alone. The combination
therapy was significantly better (overall survival 15.9 and 22.0 months) than
chemotherapy alone (overall survival 10.7 and 11.3 months) [27, 28]. It should be noted that patients with
tumours harbouring EGFR or ALK alterations were excluded from these trials,
because checkpoint inhibitors have limited activity against such tumours [29]. In the IMPOWER-150 study (on non-squamous NSCLC), atezolizumab
plus bevacizumab and chemotherapy (overall survival 21.3 months) produced significantly
better results than chemotherapy (overall survival 16.3 months) [30, 31]. There is a lack of head-to-head
trials testing different checkpoint inhibitors, so therapeutic decisions are based on regional approval
and reimbursement status [21].
The availability of checkpoint inhibitors and
targeted therapies have significantly changed the treatment of NSCLC in the
last decade [32]. Elderly patients over
70 years old, who account for most patients with NSCLC in many countries
including Switzerland, are underrepresented in clinical trials. Moreover,
patients with NSCLC and a poor Eastern Cooperative Oncology Group performance status
(ECOG-PS ≥2), active brain metastases, autoimmune disease, organ dysfunction, or
a life expectancy below 3 months are generally excluded from checkpoint inhibitor registration studies
[33]. Patients with such conditions are very
common in the clinic and are now treated with checkpoint inhibitors [25, 34, 35], but few studies have addressed the impact of these
therapies on patients treated outside of registration trials [36].
In a previous study in central
Switzerland on metastatic NSCLC with actionable driver mutations, we showed
that targeted therapy led to prolonged overall survival [37]. In the present paper, we report the results of a new retrospective
observational study from the same region between 2015 and 2018 in collaboration
with the Cancer Registry of Central Switzerland [38].
Our primary aim was to assess
overall survival from the time of diagnosis of stage IV NSCLC among patients
treated with checkpoint inhibitors in any line of treatment and among patients who did not
receive checkpoint inhibitors in the same period of time. Other aims were to describe the survival
of patients receiving checkpoint inhibitors in the first line and in further lines and to
correlate tumour PD-L1 expression level with survival.
Patients, materials and methods
Study design
This retrospective cross-sectional
study includes clinical data from five non-university hospitals located in four
selected cantons (Lucerne, Uri, Nidwalden and Obwalden) across central
Switzerland. To account for the population density of the canton of Lucerne and
improve data diversity (publicly and privately managed hospitals), we included
the privately managed St Anna Clinic (Lucerne) and the public Cantonal Hospital
of Lucerne as well as the Cantonal Hospital of Uri, the Cantonal Hospital of
Obwalden and the Cantonal Hospital of Nidwalden. The hospitals form a network
and provide health care for approximately 700,000 residents [39, 40].
Patients who received a new
diagnosis of stage IV NSCLC between 1 January 2015 and 31 December 2018 were
identified from the Cancer Registry of Central Switzerland. Data regarding the
diagnosis, vital status and patient characteristics were provided by the cancer
registry. For each patient who met the eligibility criteria, electronic and
paper-based medical records were used to complement and validate the data set.
The observation period was
chosen on the one hand because of the approval and administration of
immunotherapy in Switzerland in 2015 and on the other hand because the
epidemiological data of the cancer registry were validated only until the end
of 2018 at the time of data collection.
Further inclusion criteria were
an age over 18 years, histologically confirmed NSCLC and Union for International
Cancer Control (UICC) stage IV (TNM 7th or 8th edition) at diagnosis. We
excluded patients with SCLC and SCLC-NSCLC mixed histology, neuroendocrine tumours,
pulmonary metastases of other tumour entities, with missing clinical data on histology,
stage or therapy, or with stage I–III NSCLC. We also excluded patients
receiving first-line targeted therapy for NSCLC with actionable driver
mutations, as these tumours are a unique entity regarding biology and treatment
[41].
The oncologists’ choices of
treatment for the patients were in particular based on ECOG-PS, age, sex,
histology, smoking history, the patient’s wish and the availability of checkpoint inhibitors
(according to their approval in Switzerland and reimbursement). Because there
were selection criteria for therapy, these factors (date of incidence, age,
sex, histology, smoking status, and PD-L1 expression) were included as
covariates for the sensitivity analysis.
Our reporting conforms with the
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)
statement [42].
Data collection
To achieve the study goal, the
Cancer Registry of Central Switzerland provided us with a patient list
containing all C34 diagnoses (malignant neoplasms of the bronchus or lung)
according to ICD-10 from 1 January 2015 until 31 December 2018. Data relevant
to the study were extracted from the electronic and paper-based medical records
for each patient who met the eligibility criteria [43].
The eligibility and data collection
criteria were determined by an interdisciplinary team (OG, DD, VA) prior to
data collection. The data collection included the date of diagnosis (date of
pathological examination), the histological type (adenocarcinoma, squamous-cell
carcinoma, not-otherwise specified histology), the TNM stage as defined by the UICC,
the TNM classification (7th edition for the years 2015–2016 and 8th edition for
the incidence years 2017–2018), the date of death, age (at year of diagnosis),
sex, smoking history, the PD-L1 expression status on tumour cells (in %), molecular
tests performed (EGFR, ALK, ROS1, BRAF, KRAS) and the type of therapy received (palliative
surgery and radiotherapy, type of systemic therapy) with specification of the drug
names. Additionally, hospital medical record databases were systematically
searched for the type of therapy, the duration of therapy in days, the number
of therapy lines and the reasons for discontinuation of therapy in cases with immunotherapy.
Following an intention-to-treat
approach, even a single administration of checkpoint inhibitors was considered sufficient to
include the patient in the checkpoint inhibitor group. The patient survival status was provided
by the health registry office of each canton in central Switzerland. Patients who
moved outside the cantons of central Switzerland or discontinued the therapy
were censored at the date of their last contact or 21 July 2021, whichever
occurred first.
Data were collected and curated
by the first author, supported by DD and the team of the Cancer Registry of
Central Switzerland. In cases of inconsistent or missing data, each case was
reassessed and adjusted based on the electronic and paper-based medical records
and the database located at the Cancer Registry of Central Switzerland in Lucerne,
and unclear cases were discussed with a medical expert (OG). In cases where the
data remained unclear or missing, the corresponding patient was excluded from
the study (n = 9). Only
patients without any missing information on histology, stage, treatment and
outcome were included in the outcome analysis. Unfortunately, ECOG-PS and
smoking status were not available for a large proportion of the population,
because these are not routinely
collected in Swiss hospitals and the cancer registry. Each patient for whom the
data were complete was assigned a consecutive number in order to anonymise the
data. The key for the data set was kept independently of the data records.
Histological diagnosis and molecular testing
The Institute of Pathology at the
Cantonal Hospital of Lucerne examined all except one specimen and preformed the
histological and molecular analyses. Light microscopy and immunohistochemistry
(thyroid transcription factor-1, TTF-1) were used to confirm the diagnosis of
NSCLC [37]. Routine testing for PD-L1 was
implemented in 2015. For the PD-L1 analysis of tumour cells, the antibody SP263
from Roche-Ventana was used, and immunohistochemistry was performed on a benchmark
automated stainer (Roche-Ventana, USA). Tumours with at least 1% expression of
PD-L1 were considered PD-L1 positive. The PD-L1 stained slides were all assessed
by the same pathologist (JD).
Statistical analysis
Three groups of patients were
compared: one group of patients received immunotherapy with an checkpoint inhibitor (nivolumab,
pembrolizumab, atezolizumab) in any line of treatment via regular approval, an early-access
programme or a clinical trial. For comparison, two groups of patients were
considered who received only chemotherapy or best supportive care without an checkpoint inhibitor.
Patient and treatment characteristics were analysed descriptively.
Median overall survival was determined by treatment group and, where appropriate, by subgroups
together with corresponding 95% confidence intervals (CIs). Overall survival was described using Kaplan–Meier survival
curves and is presented with risk tables of the number of subjects at risk and
event counts. Treatment outcomes are reported separately for patients treated
in first-line or second-/further-line settings. Overall survival was calculated
from the date of a stage IV NSCLC diagnosis to the date of death, with living
subjects censored on the date of their last follow up (21 July 2021).
To avoid a survivorship bias for
checkpoint inhibitors used in the second and further lines, overall survival was also calculated
from the start of a line of therapy with an checkpoint inhibitor to death or the last follow-up.
A Cox proportional-hazards regression model, adjusted for the year of incidence
and age, was used to estimate hazard ratios (HRs) and 95% CIs. Sensitivity
analyses were performed utilising further covariates including sex, smoking
status and histology. Missing data were categorised and reported as such
(smoking status unknown in 16.4%; PD-L1 status not tested in 44.2%).
Because of the exploratory
nature of this study, there was no formal sample-size determination. To avoid
selection bias, all patients who met the criteria of a predefined selection
strategy were included in the study as described above. The sample size
resulting from this process was considered sufficient to support the
exploratory objectives of this study. All statistical analyses were performed
with STATA version 17.0 (StataCorp LLC, College Station, Texas, USA) [44].
Ethical considerations
This study was performed in
line with the principles of the Declaration of Helsinki and was reviewed and approved
by the Ethikkomission Nordwest- und Zentralschweiz (EKNZ, BASEC identification
number 2019-01865). General consent forms were available from the electronic
medical records (EMRS) of patients treated at the Cantonal Hospital of Lucerne
from August 2016 onward. Patients who withheld informed consent were excluded
from the study.
Results
Patient selection
The Cancer Registry of Central
Switzerland provided electronic records for 978 patients with a diagnosis of a
malignant disease of the chest (ICD-10 code C34) between 2015 and 2018. After the
exclusion of 520 patients with UICC stage I–III NSCLC, 127 patients with
histology other than NSCLC or missing histology, a total of 331 patients with a
diagnosis of stage IV NSCLC (adenocarcinoma, squamous-cell carcinoma, NSCLC-NOS
(NSCLC-not otherwise specified), but excluding those with a mixed histology)
from January 2015 to December 2018 remained for further investigation. Another 12
patients were excluded because of withholding of informed consent, 9 patients
because of missing data and/or inconclusive histology and 36 patients because
of targeted therapies. Ultimately, 274 patients were included in the
statistical analysis. (fig. 1)
According to the systemic
therapy received, patients were divided into three groups. The immune-oncology
(IO) group (n = 122) included patients who received treatment with an checkpoint inhibitor
regardless of the line of therapy, and a comparator group (n = 152, no-IO),
which was divided into two subgroups, treated either with conventional
chemotherapy only (n = 70) or best supportive care (n = 82). The median
follow-up for the patients was 6.5 months.
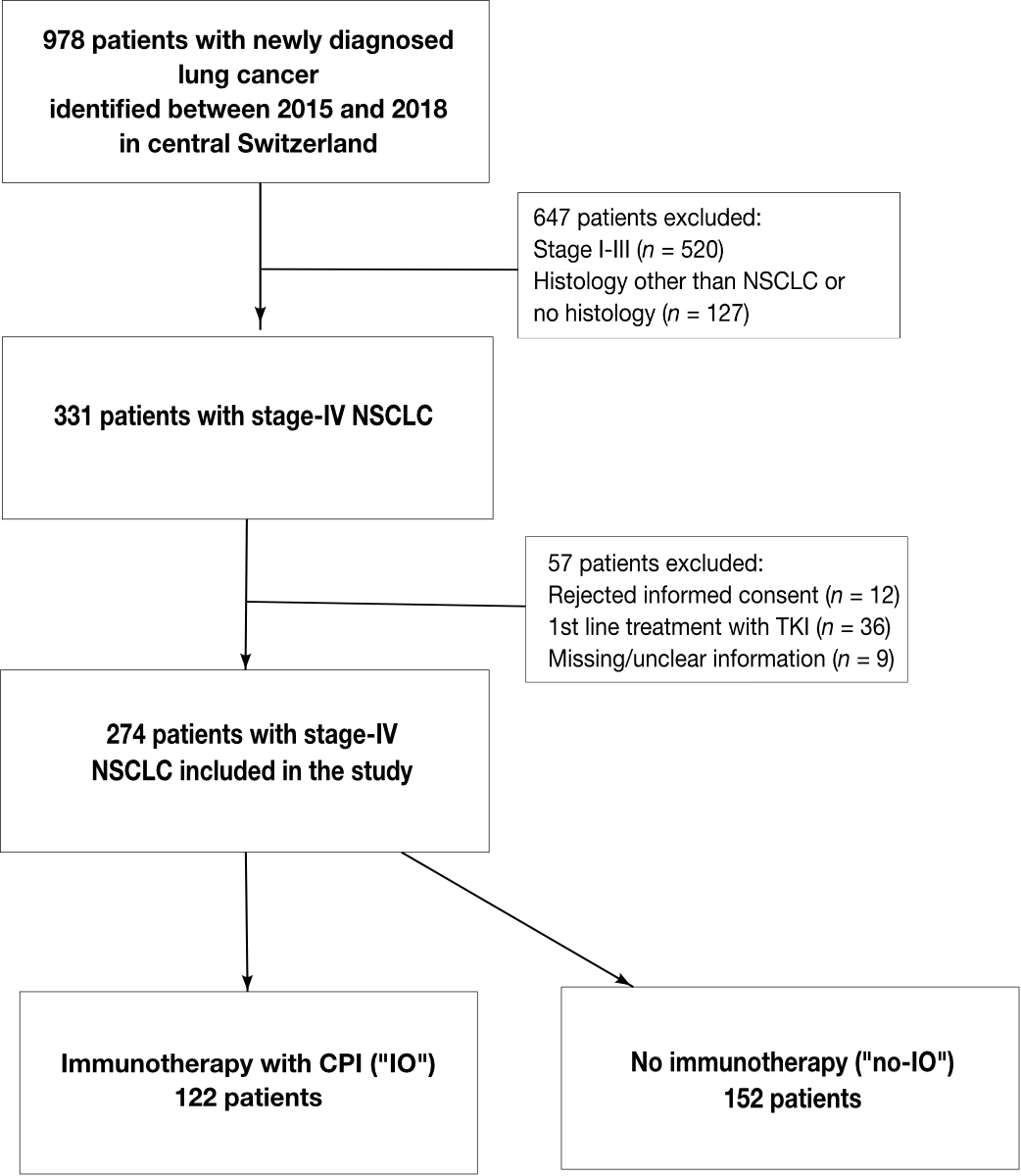
Figure 1 Flowchart of patient inclusion and
exclusion criteria.
Patient selection and division of
patients with metastatic non-small-cell lung cancer (NSCLC) into two groups
according to the type of therapy. CPI: checkpoint inhibitor; IO:
immuno-oncology; no-IO: no immuno-oncology; NSCLC: non-small-cell lung cancer; TKI:
tyrosine kinase inhibitor
Clinical characteristics
Patient characteristics are
shown in table 1. Across the study population (n = 274), the median age at
diagnosis was 68.4 years (range 39–89). Most of the patients were male (61.7%)
and 65.0% were current or former smokers. The most frequent tumour histology
was adenocarcinoma (73.0%), followed by squamous-cell carcinoma (20.4%) and NSCLC-NOS
(6.6%). Bone metastases were present in 45.5% of the patients and brain
metastases in 33.6%. PD-L1 expression was documented for 55.8% of the tumours, and
44.2% of the tumours had not yet been tested for PD-L1 expression.
Less than half of the study
population (44.5%, n = 122) received checkpoint inhibitors, and these patients were assigned to
the IO group. With a median age of 65.5 years (range 43–85), these patients
were younger than the patients who did not receive checkpoint inhibitors (median 70.7 years,
range 39–89).
The majority of patients in the
IO group had adenocarcinoma (72.1%). Central nervous system (CNS) metastases were
present in 40.2% and adrenal metastases in 24.3%. Both were more frequent in
this group than in the no-IO group (28.3% and 19.1%, respectively). Hepatic
metastases were less frequent in the IO group than in the no-IO group (17.8% vs
23.5%).
In the majority of patients (79.5%,
n = 97) in the IO group, tumours were tested for PD-L1, whereas testing for PD-L1
was not performed in 20.5% (n = 25). Among the tested tumours, 42.3% (n = 41)
had high PD-L1 (≥50%), while 57.7% (n = 56) had low or no PD-L1 (<50%).
In the first-line IO subgroup,
all patients (n = 31) had tumours tested for PD-L1 expression, and 87.1% (n = 27)
scored PD-L1 ≥50%. In the second-line IO subgroup (n = 91), the majority (57.1%,
n = 52) of patients had tumours with low or no PD-L1 expression (<50%). High
tumour PD-L1 expression (≥50%) was seen in 14 patients (15.4%), whereas 25
patients (27.5%) had no tumour testing and therefore had an unknown PD-L1
expression status.
Table 1Baseline and
disease characteristics of included patients with metastatic (stage IV)
non-small-cell lung cancer (NSCLC) between 2015 and 2018.
|
Characteristics
|
All (n = 274)
|
IO (n
= 122)
|
No-IO (n = 152)
|
|
CTX, n = 70
|
BSC, n = 82
|
| Median age, years (range) |
68.4 (39–89) |
65.5 (43–85) |
66.1 (39–88) |
74.6 (43–89) |
| Sex, n (%) |
Male |
169 (61.7) |
82 (67.2) |
45 (64.3) |
42 (51.2) |
| Female |
105 (38.3) |
40 (33) |
25 (35.7) |
40 (48.8) |
| Geographic
region, n (%) |
Lucerne |
198 (72.3) |
82 (67.2) |
59 (84.3) |
57 (69.5) |
| Uri |
26 (9.5) |
10 (8.2) |
7 (10.0) |
9 (11.0) |
| Nidwalden |
28 (10.2) |
18 (14.8) |
2 (2.9) |
8 (9.8) |
| Obwalden |
22 (8.0) |
12 (9.8) |
2 (2.9) |
8 (9.8) |
| Smoking
status, n (%) |
Never smoker |
51 (18.6) |
25 (20.5) |
9 (12.9) |
17 (20.7) |
| Former or current smoker |
178 (65.0) |
85 (69.7) |
44 (62.9) |
49 (59.8) |
| Unknown |
45 (16.4) |
12 (9.8) |
17 (24.3) |
16 (19.5) |
| Histology,
n (%) |
Adenocarcinoma |
200 (73.0) |
88 (72.1) |
53 (75.7) |
59 (72.0) |
| Squamous-cell carcinoma |
56 (20.4) |
27 (22.1) |
13 (18.6) |
16 (19.5) |
| Other (e.g., NSCLC-NOS) |
18 (6.6) |
7 (5.8) |
4 (5.7) |
7 (8.5) |
| Metastases
at diagnosis, n (%) |
Central nervous
system |
92 (33.6) |
49 (40.2) |
21 (30.0) |
22 (26.8) |
| Bone |
92 (45.5) |
39 (38.6) |
27 (52.9) |
26 (52.0) |
| Pleura |
42 (18.9) |
22 (20.6) |
11 (19.6) |
9 (15.3) |
| Pulmonary |
56 (25.2) |
28 (26.2) |
15 (26.8) |
13 (22.0) |
| Hepatic |
46 (20.7) |
19 (17.8) |
15 (26.8) |
12 (20.3) |
| Adrenal |
48 (21.6) |
26 (24.3) |
7 (12.5) |
15 (25.4) |
| Other |
59 (26.6) |
25 (23.4) |
15 (26.8) |
19 (32.2) |
| PD-L1 status, n
(%) |
Negative |
51 (18.6) |
25 (20.5) |
17 (24.3) |
9 (11.0) |
| Positive |
102 (37.2) |
72 (59.0) |
17 (24.3) |
13 (15.9) |
| 1–49% |
45 (16.4) |
31 (25.4) |
10 (14.3) |
4 (4.9) |
| ≥50% |
57 (20.8) |
41 (33.6) |
7 (10.0) |
9 (11.0) |
| Not tested |
121 (44.2) |
25 (20.5) |
36 (51.4) |
60 (73.2) |
Therapy
Across all patients, 58.8% (n =
161) received chemotherapy as a first line of therapy. Carboplatin and
pemetrexed (n = 98) was the most frequently administered regimen (approved for
non-squamous NSCLC), followed by carboplatin and paclitaxel (n = 22) and by
carboplatin and gemcitabine (n = 21). Of all patients, 11.3% (n = 31) had checkpoint inhibitors as
a first line of therapy (3 patients out of this group received a combination of
chemotherapy and checkpoint inhibitors), while 29.9% of patients received only best supportive
care.
Among the 122 patients treated
with checkpoint inhibitors, 31 patients (25.4%) received them in the first line and 91 patients
(74.6%) had checkpoint inhibitors in further lines. The most commonly administered checkpoint inhibitor in the first
line was pembrolizumab (93.6%, n = 29). In further lines, 58.2% (n = 53) of
patients received nivolumab, 28.6% (n = 26) pembrolizumab, and 13.2% (n = 12) were
treated with atezolizumab.
Survival
The median overall survival for
all patients receiving checkpoint inhibitor therapy (IO group) was 15 months (95% CI 12–20).
Patients treated with chemotherapy had a median overall survival of 4 months
(95% CI 3–7), and patients with best supportive care had a median overall
survival of 2 months (95% CI 1–2) (fig. 2a).For the analysis from the time
of diagnosis, the adjusted risk (for the date of incidence and age) of death
for patients treated with chemotherapy was approximately 2.7 times higher (adjusted
HR [aHR] 2.67, 95% CI 1.92–3.71; p <0.001), and it was approximately 7 times
higher (aHR 6.96, 95% CI 4.95–9.79; p <0.001) for patients treated with best
supportive care compared with those treated with anti-PD-1/PD-L1 in any line.
The median overall survival
calculated from the time of diagnosis of metastatic NSCLC (fig. 2b) was 15
months (95% CI 4–37) in patients receiving checkpoint inhibitor therapy in the first line and
also 15 months (95% CI 12–20) in patients receiving checkpoint inhibitor therapy in further
lines. The median overall survival from the start of checkpoint inhibitor therapy (fig. 2c) was
15 months (95% CI 4–37) in the first line and 7 months (95% CI 5–10) in further
lines. The calculated and adjusted risk for death in patients with IO in
further lines was 1.6 times higher than in patients receiving checkpoint inhibitor therapy in the
first line (aHR 1.60, 95% CI 0.91–2.80; p = 0.102). The unadjusted HR was also
1.67 (95% CI 1.01–2.74; p = 0.044).
The median overall survival for
PD-L1 ≥50% from the time of diagnosis of metastatic NSCLC was 15 months (95% CI
8–29) and for PD-L1 <50% it was 13 months (95% CI 11–20) (fig. 2d). The
calculated and adjusted risk of death for PD-L1 expression <50% was 1.2
times higher than for PD-L1 ≥50% (aHR 1.21, 95% CI 0.75–1.96 p = 0.442) (see supplementary
table S1in the appendix).

Figure 2 Overall survival of
included patients with NSCLC stage IV from 2015 to 2018.
(a) Overall survival of patients
treated with checkpoint inhibitor (IO, blue), chemotherapy (red), and BSC (green). Kaplan–Meier
curve shows a median OS of 15 months (95% CI 12–20 months) for IO, a median OS
of 4 months (95% CI 3–7 months) for chemotherapy, and a median OS of 2 months
(95% CI 1–2 months) for BSC.
(b) Overall survival (from diagnosis of metastatic NSCLC)
with checkpoint inhibitor in the first line (IO_1L, blue) or in the second line (IO_2L, red).
Kaplan–Meier curves show a median OS of 15 months (95% CI 4–37 months) for IO
in the first line and a median OS of 15 months (95% CI 12–20 months) for IO in
the second line.
(c) Overall survival (from the start of checkpoint inhibitor therapy) with checkpoint inhibitor
in the first line (IO_1L, blue) or in the second line (IO_2L, red).
Kaplan–Meier curves show a median OS of 15 months for IO in the first line (95%
CI 4–37 months) and median OS of 7 months (95% 5–10 months) for IO in the
second line.
(d) Overall survival with checkpoint inhibitor
therapy according to PD-L1 expression (50% threshold). Kaplan–Meier curve shows
a median OS of 15 months (95% CI 8–29 months) for PD-L1 ≥50% and a median OS
of 13 months (95% CI 11–20 months) for PD-L1 <50%.
BSC: best supportive care; CPI: checkpoint inhibitor; NSCLC:
non-small-cell lung cancer; IO: immuno-oncology; IO_1L: immuno-oncology first
line; IO_2L: immuno-oncology first line second line; OS: overall survival;
PD-L1: programmed death ligand 1
Discussion
The primary aim of this
retrospective study was to assess overall survival and treatment patterns in an
unselected population of patients with stage IV NSCLC receiving immunotherapy
or standard care (chemotherapy or best supportive care) in central Switzerland since
the first approval of checkpoint inhibitors in Switzerland in 2015.
Until 2015, patients with
metastatic NSCLC had access only to chemotherapy and targeted therapy [45]. All patients included in this study had
histologically proven stage IV NSCLC. In this group of patients, 58.7% (n = 161)
had received chemotherapy, 11.3% (n = 31) had received immunotherapy, and
approximately one third of all patients (29.9%, n = 82) had been treated with
no systematic therapy as a first-line treatment. Most of the patients treated
with chemotherapy had received, according to clinical guidelines,
platinum-based chemotherapy as the first line, as this had been the standard of
care for several years [14]. Furthermore, most of the newly implemented checkpoint inhibitors, namely nivolumab,
required disease progression after platinum-based therapy.
Between 2015 and 2018, three
checkpoint inhibitors were approved for the treatment of metastatic NSCLC by Swissmedic. First,
nivolumab was available in an early-access programme and atezolizumab in the
OAK trial for patients with previous chemotherapy. Then, nivolumab and
atezolizumab were approved by Swissmedic, and pembrolizumab was approved for
PD-L1-positive NSCLC. Later, pembrolizumab was approved as a first-line
monotherapy for PD-L1-high tumours and in combination with chemotherapy for
PD-L1-low tumours. The results of our study reflect this timeline, in that
nivolumab was the most frequently used checkpoint inhibitor, whereas pembrolizumab and
atezolizumab were used less in the study period [27].
The results of this study, conducted after the availability of checkpoint inhibitor monotherapy
for metastatic NSCLC in Switzerland, indicate that most therapies administered
were in accordance with the national guidelines (according to the ESMO;
European Society for Medical Oncology guidelines) for the observed study period
(January 2015 to December 2018).

Figure 3 Timeline of approval of checkpoint inhibitors for NSCLC in Switzerland by
Swissmedic.
Timeline from 2015 to 2020 (left
to right) with drug names, date and criteria of approval of the different checkpoint inhibitors
in Switzerland according to approval of Swissmedic. CPIs: checkpoint
inhibitors; NSCLC: non-small-cell lung cancer; PD-L1: programmed death ligand 1.
Source: compendium.ch
Our study results suggest that
patients have a longer overall survival when treated with checkpoint inhibitors compared with
patients who are not treated with checkpoint inhibitors and that patients with high PD-L1
expression receiving checkpoint inhibitor treatment have a slightly longer overall survival than
patients with low PD-L1 receiving checkpoint inhibitor treatment, as PD-L1 expression has been
described as a predictive marker in treating NSCLC [46, 47]. Although expected and described previously, this finding
is relevant because cancer registry data in this field are limited.
The baseline characteristics of
the patients enrolled in our study − related to median age, sex, non-squamous
histology and the presence of brain metastases − were comparable to those
included in other cancer-registry populations. Our findings of median overall
survival with first- and second-line treatments with checkpoint inhibitors were generally
consistent with reports of other national and international studies [48–51].
Compared with randomised clinical
trials, the median overall survival with checkpoint inhibitors in the first line and in further
lines observed in this study (15 months and 7 months, respectively) were
shorter than the reported median overall survival in clinical trials, where an overall
survival of 15.9–30 months for the first line [27,
32, 52–57] and of 9.2–13.8 months for the second line [10, 11, 23, 58] have been described.
The difference in overall
survival may be explained by the differences in the characteristics of the
patients included in this study versus clinical trials, as we analysed an
unselected study population. In comparison with the eligibility criteria of
randomised clinical trials, our study population (median age 68.4 years),
especially the group of patients receiving checkpoint inhibitors (median age 65.5 years) was
older than the patients included in clinical trials. Since age-related immune
dysregulation may affect the effectiveness of immunotherapy, this could be a
reason for the different results regarding overall survival [59]. Furthermore, patients with active brain
metastases, clinically relevant comorbidities and previous malignancies are generally
excluded from clinical trials [10]. The
presence of liver, bone or brain metastases is related to poor prognosis in
NSCLC; they correlate with a shorter overall survival compared with metastases
in other organs [18]. These could be possible
explanations for the differences in overall survival between our data and the
results of clinical trials, as many of our patients had tumour metastases at
different sites (33.6% brain, 45.5% bone, 20.7% liver).
In comparison with randomised
clinical trials, it was noticeable that our study population was treated for a
shorter period of time with checkpoint inhibitors. The observed median duration of checkpoint inhibitor therapy
in our study was 127 days (4.2 months) in the first line and 81.5 days (2.7
months) in the second line, whereas patients in registration trials received up
to 7 months of checkpoint inhibitor therapy [11, 25, 52]. Given
that nivolumab (240 mg every 2 weeks) was the most frequent agent used in
second-line therapy and pembrolizumab (200mg every 3 weeks) in first-line
therapy in this study, patients received overall approximately five or six doses
before the discontinuation of therapy. The most frequent reason for the discontinuation
of checkpoint inhibitor treatment in this study was tumour progression and a lack of a response
to treatment in 53.3% (n = 65). The second most frequent reason was because of
severe treatment- and immune-related adverse events (n = 13, 10.7%), and in
9.8% (n = 12) treatment was stopped because of deterioration of the patient’s general
condition that was not clearly attributed to checkpoint inhibitor treatment. Only 10 patients (=
8.2%) among the group receiving checkpoint inhibitor treatment were treated for the entire recommended
therapy duration of 2 years [60]. In the
group of patients with checkpoint inhibitor treatment and tumour progression (n = 65), only 30
(46.2%) patients were treated with subsequent systemic therapy and none of the
patients with immune-related adverse events were re-exposed to checkpoint inhibitors. The high
frequency of immune-related adverse events and the fact that no subsequent
therapy was administered after such events suggest that these patients had poor
ECOG-PS and relevant comorbidities, and that oncologists were cautious
regarding the newly introduced checkpoint inhibitors and their toxicity profiles.
With regard to the limitation
that no data on ECOG-PS was collected, indirect evidence shows that our study
population, especially in the chemotherapy group, was in poor general health.
Despite the availability of PD-L1 testing and the use of second-line checkpoint inhibitors
(nivolumab) from 2015 on, approximately half of the included patients (51.4%, n
= 36) treated with chemotherapy had neither PD-L1 testing nor checkpoint inhibitor therapy after
a failure of chemotherapy, which could be interpreted as a selection bias in
patients unwilling or unfit to undergo therapy, as the choice of treatment is
not only determined by histology but also by ECOG-PS, which is an important and
independent factor for response [61–63].
These findings could explain the fact that that overall survival of patients
with chemotherapy was shorter compared with the results of other national and
international clinical trials. [23, 28, 64, 65]
Despite the introduction of
targeted therapies and immunotherapy and their impact on overall survival, a significant
proportion (29.9%) of the patients in our study received best supportive care
and not tumour-directed systemic therapy. This proportion is in line with other
studies [49, 66] and with the results of our
previous study on targeted therapies from the same region covering the years
2010–2014 [37]. The rate of best supportive care is consistent with the
high proportion of patients with ECOG-PS ≥2 and with the age distribution of
our population, which reflects the high proportion of patients with metastatic
NSCLC who are elderly and frail and are therefore not considered by clinicians
for active treatment of NSCLC [67, 68].
In the present study, patients
with tumours with known actionable driver mutations were excluded from the analysis.
However, during data selection and analysis, we observed an additional KRAS
codon 12 mutation in about 20.4% (n = 56) of the included patients with
adenocarcinoma. According to the literature, approximately 20–25% of all non-squamous
NSCLC and 6% of all squamous NSCLC harbour an additional KRAS mutation [69, 70]. KRAS is the most common oncogenic
mutation detected in patients suffering from NSCLC, but the role of KRAS as a
prognostic or predictive factor remains unclear. Of the KRAS-mutated patients
in this study, 53.5% (n = 30) received immunotherapy, 21.4 % (n = 12) were treated with chemotherapy
and 25% (n = 14) received no systematic therapy. A meta-analysis of 43 selected
studies (including clinical trials and observational studies) suggested that
KRAS is a negative prognostic factor for survival and response to therapy in
metastatic NSCLC [71]. The role of KRAS
in the overall survival of patients with NSCLC who are treated with
immunotherapy remains unclear [72]. A
meta-analysis by Landre et al. suggested that treatment with anti-PD-L1 seemed
to achieve a longer overall survival than chemotherapy for patients with
KRAS-mutant NSCLC [73]. Since the role of
KRAS in the therapeutic landscape as a prognostic and prospective factor needs
to be defined and is debated in the literature [74–77],
we are currently unable to draw any conclusions about this finding for the
moment; further prospective studies are needed. The KRAS G12c mutation is of
particular interest because there is a specific inhibitor (Sotorasib, Lumykras®) with approval in Switzerland since January 2022 and with proven clinical activity
[78, 79], so further explorations
regarding its role in diagnostic and therapeutic strategies and regarding its
potential field of application in pretreated NSCLC are necessary [80].
One of the strengths of this
study is that it represents an unselected population of patients in central
Switzerland with biopsy-confirmed metastatic NSCLC and with a complete data set
on the applied therapies. But there are several limitations of this study. As
this was a retrospective observational study, the effect of selection and
allocation bias needs to be addressed. According to the national guidelines in central
Switzerland, clinicians selected the appropriate systemic therapy based on age,
histology, PD-L1 status, ECOG-PS, relevant comorbidities, and patients’
preferences [60]. This approach may have led
to a selection of patients with better health conditions (ECOG-PS 0−1, fewer
comorbidities) and therefore also to a selection of patients with better
prognoses (namely patients with IO therapy). As a consequence, the measured
effect on overall survival cannot be assigned to treatment with checkpoint inhibitors alone. The
additional sensitivity analyses showed that, although we included covariates as
mentioned above (sex, histology, smoking status), the hazard ratios (HRs) did
not differ much from the unadjusted hazard ratios, so we consider the HRs and
results robust.
Since ECOG-PS and smoking
status are not routinely collected by clinicians, these variables are missing
in the clinical databases as well as in the database of the cancer registry. On
account of this, important adjustments to verify the effects of immunotherapy
could not be made. Furthermore, data on patients’ comorbidities and medications
prescribed outside of the oncology-clinic setting were not recorded, so
additional analyses could not be performed. A further limitation is the small
number of patients with first-line checkpoint inhibitor therapy, as this therapy was only available
from 2017 and only for tumours with a high expression of PD-L1. As a consequence,
follow-up was significantly shorter than in patients with second-line checkpoint inhibitor
therapy. In addition, we admit that this study was conducted at selected
cantons and clinical centres of central Switzerland and that the results may
not represent the entire patient population in Switzerland.
Conclusion
Considering the limitations of
this retrospective and population-based study, the results suggest an
improvement in overall survival in unselected patients with metastatic NSCLC from
the introduction of checkpoint inhibitors, regardless of the line of therapy. The reported
outcomes regarding overall survival were shorter compared with published results
from randomised clinical trials but were in line with other registry data. This
is one of the first cancer-registry-based studies about the use of checkpoint inhibitors in unselected
patients with metastatic NSCLC in central Switzerland. To confirm the effect of
checkpoint inhibitor therapy on overall survival and to identify further prognostic and
predictive factors beyond PD-L1 expression, larger studies at a national level
are needed.
Data availability
Anonymised raw data and the
associated statistical analyses are stored securely at the Institute of
Pathology of the Cantonal Hospital of Lucerne and can be provided upon
reasonable request by interested researchers. Data will be available
immediately after publication.
Acknowledgment
We thank Anja Burgherr and her
team for their technical support in obtaining data from the database of the
Cancer Registry of Central Switzerland; the pathology staff at the Cantonal
Hospital of Lucerne for performing central PD-L1 testing; and Dr. sc. ETH Irène
Frank from the clinical trial unit for her mediation of valuable contacts that
made this project possible.
Author contributions: Conceptualisation: Valentina Allmann (VA), Oliver Gautschi (OG),
Joachim Diebold (JD), Daniela Dyntar (DD). Methodology: VA, OG, JD, DD. Data
curation: VA, DD. Formal analysis and investigation: VA, OG, Dirk Lehnick (DL).
Writing−original draft preparation: VA, OG. Writing−review and editing: VA, OG,
JD. Resources: OG, JD, DD, Marco Dressler (MD), Kristin Zeidler (KZ), Philipp
Niederberger (PN), Jeanne Godau (JG). Supervision: OG, JD, DL. All authors read
and approved the final manuscript.
Valentina Allmann
Faculty of Medicine
University of Basel
Klingelbergstrasse 61
CH-4056 Basel
valentina.allmann[at]icloud.com
References
1. Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA. 2005 Sep;294(12):1505–10. https://doi.org/10.1001/jama.294.12.1505
2. Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. BMJ. 1950 Sep;2(4682):739–48. https://doi.org/10.1136/bmj.2.4682.739
3. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013 Apr;49(6):1374–403. https://doi.org/10.1016/j.ejca.2012.12.027
4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. https://doi.org/10.3322/caac.21492
5. Gmel G. K.H., Notari L, Gmel C. Suchtmonitoring Schweiz - Konsum von Alkohol, Tabak und illegalen Drogen in der Schweiz im Jahr 2016. Lausanne, Schweiz: Sucht Schweiz; 2017.
6. Pasquale., C., et al., Schweizerischer Krebsbericht 2021. Schweizerischer Krebsbericht. 2021: Bundesamt für Statistik (BFS), Nationale Krebsregistrierungsstelle (NKRS). 57-61.
7. Casutt A, Lovis A, Selby K, Noirez L, Peters S, Beigelman-Aubry C, et al. [Lung cancer screening in Switzerland : Who? How? When? ]. Rev Med Suisse. 2020 Nov;16(715):2224–6. https://doi.org/10.53738/REVMED.2020.16.715.2224
8.Chen, V.W., et al., Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer, 2014. 120 Suppl 23(0 0): p. 3781-92 DOI: https://doi.org/10.1002/cncr.29045.
9. NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008 Oct;26(28):4617–25. https://doi.org/10.1200/jco.2008.17.7162
10. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015 Jul;373(2):123–35. https://doi.org/10.1056/NEJMoa1504627
11. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015 Oct;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643
12. Ganz PA, Figlin RA, Haskell CM, La Soto N, Siau J. Supportive care versus supportive care and combination chemotherapy in metastatic non-small cell lung cancer. Does chemotherapy make a difference? Cancer. 1989 Apr;63(7):1271–8. https://doi.org/10.1002/1097-0142
13. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. https://doi.org/10.3322/ca.2007.0010
14. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al.; ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016 Sep;27 suppl 5:v1–27. https://doi.org/10.1093/annonc/mdw326
15. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009 Sep;361(10):947–57. https://doi.org/10.1056/NEJMoa0810699
16. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al.; PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014 Dec;371(23):2167–77. https://doi.org/10.1056/NEJMoa1408440
17. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al.; ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017 Aug;377(9):829–38. https://doi.org/10.1056/NEJMoa1704795
18. Passaro A, Attili I, Morganti S, Del Signore E, Gianoncelli L, Spitaleri G, et al. Clinical features affecting survival in metastatic NSCLC treated with immunotherapy: A critical review of published data. Cancer Treat Rev. 2020 Sep;89:102085. https://doi.org/10.1016/j.ctrv.2020.102085
19. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015 Aug;5(9):2892–911.
20. Hirsch FR, Suda K, Wiens J, Bunn PA Jr. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016 Sep;388(10048):1012–24. https://doi.org/10.1016/S0140-6736(16)31473-8
21. Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021 Oct;18(10):625–44. https://doi.org/10.1038/s41571-021-00520-1
22. Aisner DL, Marshall CB. Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol. 2012 Sep;138(3):332–46. https://doi.org/10.1309/ajcpfr12wjkceezz >
23. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al.; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017 Jan;389(10066):255–65. https://doi.org/10.1016/s0140-6736(16)32517-x
24. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al.; POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016 Apr;387(10030):1837–46. https://doi.org/10.1016/s0140-6736(16)00587-0
25. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016 Apr;387(10027):1540–50. https://doi.org/10.1016/S0140-6736(15)01281-7
26. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol. 2021 Jul;39(21):2339–49. https://doi.org/10.1200/JCO.21.00174
27. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2020 May;38(14):1505–17. https://doi.org/10.1200/jco.19.03136
28. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al.; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018 May;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005
29. Addeo A, Passaro A, Malapelle U, Banna GL, Subbiah V, Friedlaender A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat Rev. 2021 May;96:102179. https://doi.org/10.1016/j.ctrv.2021.102179
30. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al.; IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018 Jun;378(24):2288–301. https://doi.org/10.1056/NEJMoa1716948
31. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol. 2021 Nov;16(11):1909–24. https://doi.org/10.1016/j.jtho.2021.07.009
32. Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2017 Aug;377(9):849–61. https://doi.org/10.1056/NEJMra1703413
33. Al-Baimani K, Jonker H, Zhang T, Goss GD, Laurie SA, Nicholas G, et al. Are clinical trial eligibility criteria an accurate reflection of a real-world population of advanced non-small-cell lung cancer patients? Curr Oncol. 2018 Aug;25(4):e291–7. https://doi.org/10.3747/co.25.3978
34. Pasello G, Pavan A, Attili I, Bortolami A, Bonanno L, Menis J, et al. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020 Jul;87:102031. https://doi.org/10.1016/j.ctrv.2020.102031
35. Spigel DR, McCleod M, Jotte RM, Einhorn L, Horn L, Waterhouse DM, et al. Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non-Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J Thorac Oncol. 2019 Sep;14(9):1628–39. https://doi.org/10.1016/j.jtho.2019.05.010
36. Andreano A, Bergamaschi W, Russo AG. Immune checkpoint inhibitors at any treatment line in advanced NSCLC: real-world overall survival in a large Italian cohort. Lung Cancer. 2021 Sep;159:145–52. https://doi.org/10.1016/j.lungcan.2021.06.019
37. Schwegler C, Kaufmann D, Pfeiffer D, Aebi S, Diebold J, Gautschi O. Population-level effect of molecular testing and targeted therapy in patients with advanced pulmonary adenocarcinoma: a prospective cohort study. Virchows Arch. 2018 Apr;472(4):581–8. https://doi.org/10.1007/s00428-017-2268-y
38. Cancer Registry of Central Switzerland. Available from: https://www.zentralschweizer-krebsregister.ch/
39. Organization chart (LUKS). Available from: https://www.luks.ch/ihr-luks/organisation
40. History of the Cantonal Hospital of Lucerne. Available from: https://www.luks.ch/informationen-fuer-besuchende/geschichte-des-luzerner-kantonsspitals
41. Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013 May;105(9):595–605. https://doi.org/10.1093/jnci/djt072
42. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X
43. WHO Organization The ICD-10 classification of mental and behavioural disorders, Version 2019. Geneva, Switzerland: World Health Organization; 1993.
44. StataCorp LLC, College Station, Texas, USA. Available from: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/
45. Ryser CO, Gautschi O, Diebold J. Bronchialkarzinom - Neue Ansätze in der Therapie. Info Onkologie & Hämatologie 201912–7
46. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer. 2020 Jan;126(2):260–70. https://doi.org/10.1002/cncr.32468
47. Sacher AG, Gandhi L. Biomarkers for the Clinical Use of PD-1/PD-L1 Inhibitors in Non-Small-Cell Lung Cancer: A Review. JAMA Oncol. 2016 Sep;2(9):1217–22. https://doi.org/10.1001/jamaoncol.2016.0639
48. Ivanović M, Knez L, Herzog A, Kovačević M, Cufer T. Immunotherapy for Metastatic Non-Small Cell Lung Cancer: Real-World Data from an Academic Central and Eastern European Center. Oncologist. 2021 Dec;26(12):e2143–50. https://doi.org/10.1002/onco.13909
49. Wallrabenstein T, Del Rio J, Templeton AJ, Buess M. Much has changed in the last decade except overall survival: A Swiss single center analysis of treatment and survival in patients with stage IV non-small cell lung cancer. PLoS One. 2020 May;15(5):e0233768. https://doi.org/10.1371/journal.pone.0233768
50. Alonso-García M, Sánchez-Gastaldo A, Muñoz-Fuentes MA, Molina-Pinelo S, Boyero L, Benedetti JC, et al. Real-World Analysis of Nivolumab and Atezolizumab Efficacy in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer. Pharmaceuticals (Basel). 2022 Apr;15(5):533. https://doi.org/10.3390/ph15050533
51. Figueiredo A, Almeida MA, Almodovar MT, Alves P, Araújo A, Araújo D, et al. Real-world data from the Portuguese Nivolumab Expanded Access Program (EAP) in previously treated Non Small Cell Lung Cancer (NSCLC). Pulmonology. 2020;26(1):10–7. https://doi.org/10.1016/j.pulmoe.2019.06.001
52. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al.; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 Nov;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774
53. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019 Mar;37(7):537–46. https://doi.org/10.1200/jco.18.00149
54. Mok TS, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al.; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019 May;393(10183):1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7
55. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol. 2020 Oct;15(10):1657–69. https://doi.org/10.1016/j.jtho.2020.06.015
56. Jassem J, et al. IMpower110: Clinical safety in a phase III study of atezolizumab (atezo) monotherapy (mono) vs platinum-based chemotherapy (chemo) in first-line non-small cell lung cancer (NSCLC). American Society of Clinical Oncology; 2020.
57. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019 Jul;20(7):924–37. https://doi.org/10.1016/S1470-2045(19)30167-6
58. Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, et al. Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1‒Positive, Advanced Non‒Small-Cell Lung Cancer in the KEYNOTE-010 Study. J Clin Oncol. 2020 May;38(14):1580–90. https://doi.org/10.1200/JCO.19.02446
59. Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005 Jun;174(11):7446–52. https://doi.org/10.4049/jimmunol.174.11.7446
60. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al.; ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct;29 Suppl 4:iv192–237. https://doi.org/10.1093/annonc/mdy275
61. Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010 May;5(5):620–30. https://doi.org/10.1097/JTO.0b013e3181d2dcd9
62. Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, et al. Prognosis in advanced lung cancer—A prospective study examining key clinicopathological factors. Lung Cancer. 2015 Jun;88(3):304–9. https://doi.org/10.1016/j.lungcan.2015.03.020
63. Socinski MA, et al. Treatment of Non-small Cell Lung Cancer, Stage IV: ACCP Evidence-Based Clinical Practice Guidelines (2nd Edition). Chest, 2007. 132(3, Supplement): p. 277S-289S DOI: https://doi.org/https://doi.org/10.1378/chest.07-1381
64. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017 Dec;35(35):3924–33. https://doi.org/10.1200/JCO.2017.74.3062
65. Peters S, Danson S, Hasan B, Dafni U, Reinmuth N, Majem M, et al. A Randomized Open-Label Phase III Trial Evaluating the Addition of Denosumab to Standard First-Line Treatment in Advanced NSCLC: The European Thoracic Oncology Platform (ETOP) and European Organisation for Research and Treatment of Cancer (EORTC) SPLENDOUR Trial. J Thorac Oncol. 2020 Oct;15(10):1647–56. https://doi.org/10.1016/j.jtho.2020.06.011
66. Cramer-van der Welle CM, Verschueren MV, Tonn M, Peters BJ, Schramel FM, Klungel OH, et al.; Santeon NSCLC Study Group. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. 2021 Mar;11(1):6306. https://doi.org/10.1038/s41598-021-85696-3
67. Bittoni MA, Arunachalam A, Li H, Camacho R, He J, Zhong Y, et al. Real-World Treatment Patterns, Overall Survival, and Occurrence and Costs of Adverse Events Associated With First-line Therapies for Medicare Patients 65 Years and Older With Advanced Non-small-cell Lung Cancer: A Retrospective Study. Clin Lung Cancer. 2018 Sep;19(5):e629–45. https://doi.org/10.1016/j.cllc.2018.04.017
68. Seung SJ, Hurry M, Walton RN, Evans WK. Real-world treatment patterns and survival in stage IV non-small-cell lung cancer in Canada. Curr Oncol. 2020 Aug;27(4):e361–7. https://doi.org/10.3747/co.27.6049
69. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013 Sep;24(9):2371–6. https://doi.org/10.1093/annonc/mdt205
70. Ruiz-Patiño A, Rodríguez J, Cardona AF, Ávila J, Archila P, Carranza H, et al.; ONCOLGroup; CLICaP. p.G12C KRAS mutation prevalence in non-small cell lung cancer: contribution from interregional variability and population substructures among Hispanics. Transl Oncol. 2022 Jan;15(1):101276. https://doi.org/10.1016/j.tranon.2021.101276
71. Goulding RE, Chenoweth M, Carter GC, Boye ME, Sheffield KM, John WJ, et al. KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: A systematic literature review and meta-analysis. Cancer Treat Res Commun. 2020;24:100200. https://doi.org/10.1016/j.ctarc.2020.100200
72. Cefalì M, Epistolio S, Ramelli G, Mangan D, Molinari F, Martin V, et al. Correlation of KRAS G12C Mutation and High PD-L1 Expression with Clinical Outcome in NSCLC Patients Treated with Anti-PD1 Immunotherapy. J Clin Med. 2022 Mar;11(6):1627. https://doi.org/10.3390/jcm11061627
73. Landre T, Justeau G, Assié JB, Chouahnia K, Davoine C, Taleb C, et al. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: a meta-analysis of randomized-controlled trials. Cancer Immunol Immunother. 2022 Mar;71(3):719–26. https://doi.org/10.1007/s00262-021-03031-1
74. Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017 Jun;23(12):3012–24. https://doi.org/10.1158/1078-0432.CCR-16-2554
75. Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, et al. Effects of Co-occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res. 2018 Jan;24(2):334–40. https://doi.org/10.1158/1078-0432.CCR-17-1841
76. Biton J, Mansuet-Lupo A, Pécuchet N, Alifano M, Ouakrim H, Arrondeau J, et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti-PD-1 in Lung Adenocarcinoma. Clin Cancer Res. 2018 Nov;24(22):5710–23. https://doi.org/10.1158/1078-0432.CCR-18-0163
77. Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non–small cell lung cancer: A meta-analysis and individual patient–level analysis. JAMA Netw Open. 2019 Jul;2(7):e196879–196879. https://doi.org/10.1001/jamanetworkopen.2019.6879
78. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p. G12C mutation. N Engl J Med. 2021 Jun;384(25):2371–81. https://doi.org/10.1056/NEJMoa2103695
79. Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019 Nov;575(7781):217–23. https://doi.org/10.1038/s41586-019-1694-1
80. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020 Sep;383(13):1207–17. https://doi.org/10.1056/NEJMoa1917239
Appendix
Table S1Adjusted
and not adjusted hazard ratios (HR) from the results of figure 2.
|
Therapy
|
Not adjusted
|
Adjusted
for years of incidence and age at diagnosis
|
Additionally
adjusted for further covariates (sex, smoking, histology)
|
|
Hazard ratio (95% CI)
|
Hazard ratio (95% CI)
|
Hazard ratio (95% CI)
|
| HR for overall survival (from date of incidence to death) − figure 2a ( n =
274). |
Chemotherapy
only (no-IO) vs IO |
2.66 (1.92−3.67), p <0.001 |
2.67 (1.92−3.71), p <0.001 |
2.75 (1.97−3.85), p <0.001 |
| Best
supportive care (BSC) vs IO |
7.18 (5.15−10.01), p <0.001 |
6.96 (4.95−9.79), p <0.001 |
7.54 (5.31−10.72), p <0.001 |
| HR for overall survival (from date of incidence to death) − figure 2b ( n = 122). |
IO
second line vs IO first line |
1.04 (0.63−1.71), p = 0.871 |
1.03 (0.59−1.80), p = 0.912 |
1.04 (0.59−1.84), p = 0.889 |
| HR for overall survival (from date of first immunotherapy to death) −
figure 2c ( = 122) |
IO
second line vs IO first line |
1.67 (1.01−2.74), p =
0.044 |
1.60 (0.91−2.80), p =
0.102 |
1.66 (0.93−2.95), p =
0.086 |
| HR for overall survival (OS according to PD L1–expression) −
figure 2d (n = 97). |
PD-L1
≥ 50% vs PD-L1 <50% |
1.21 (0.74−1.96), p = 0.444 |
1.21 (0.75−1.96), p = 0.442 |
1.27 (0.77−2.11), p = 0.354 |
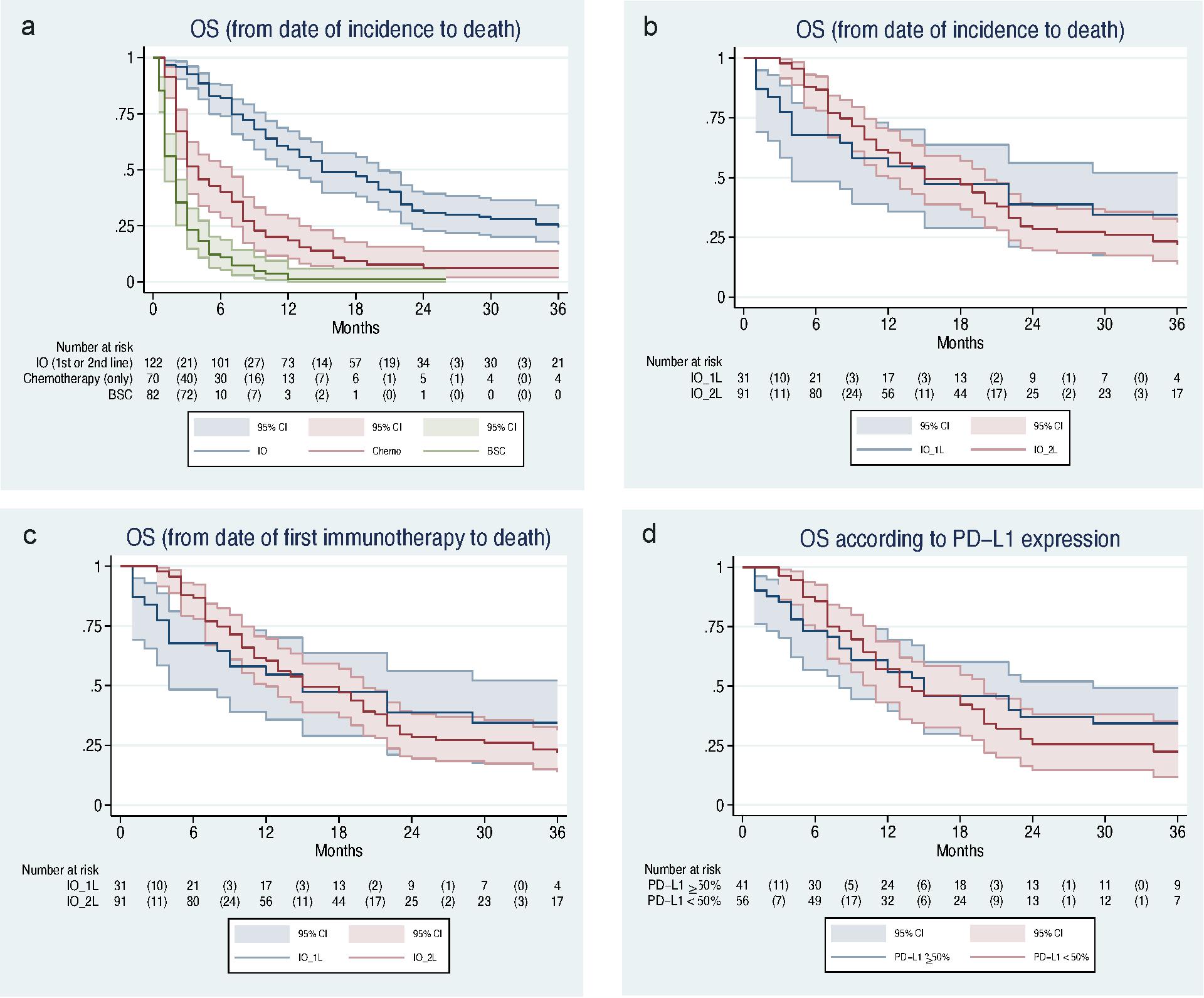
Figure S1 Figure S1 a-d: Overall survival of included patients with NSCLC stage IV from 2015 to 2018 (figure 2 with displayed 95% CI) .
BSC: best supportive care; CPI: checkpoint inhibitor; NSCLC: non-small-cell lung cancer; IO: immuno-oncology; IO_1L: immuno-oncology first line; IO_2L: immuno-oncology first line second line; OS: overall survival; PD-L1: programmed death ligand 1



