Heterogeneous evolution of SARS-CoV-2 seroprevalence in school-age children
DOI: https://doi.org/10.57187/smw.2023.40035
Results from the school-based cohort study Ciao Corona in November–December 2021 in the canton of Zurich
Sarah
R.
Haile, Alessia
Raineri, Sonja
Rueegg, Thomas
Radtke, Agne
Ulyte, Milo
A.
Puhan, Susi
Kriemler
Epidemiology, Biostatistics and Prevention Institute (EBPI), University of Zurich, Zurich, Switzerland
Prof.
Susi Kriemler
Epidemiology,
Biostatistics and Prevention Institute (EBPI), University
of Zurich
Hirschengraben
84
CH-8001 Zürich
susi.kriemlerwiget[at]uzh.ch
Summary
BACKGROUND: Much remains unknown regarding the
evolution of SARS-CoV-2 seroprevalence and variability in seropositive children
in districts, schools and classes as only a few school-based cohort studies
exist. Vaccination of children, initiated at different times for different age
groups, adds additional complexity to the understanding of how seroprevalence
developed in the school aged population.
AIM: We investigated the evolution of SARS-CoV-2
seroprevalence in children and its variability in districts, schools and
classes in Switzerland from June/July 2020 to November/December 2021.
METHODS: In this school-based cohort study, SARS-CoV-2
antibodies were measured in primary and secondary school children from randomly
selected schools in the canton of Zurich in October/November 2020, March/April
2021 and November/December 2021. Seroprevalence was estimated using Bayesian
logistic regression to adjust for test sensitivity and specificity. Variability of seroprevalence between school classes
was expressed as maximum minus minimum seroprevalence in a class and summarised
as median (interquartile range).
RESULTS: 1875 children from 287 classes in 43 schools
were tested, with median age 12 years (range 6–17), 51% 12+ vaccinated.
Seroprevalence increased from 5.6% (95% credible interval [CrI] 3.5–7.6%) to
31.1% (95% CrI 27.0–36.1%)
in unvaccinated children, and 46.4% (95% CrI 42.6–50.9%) in all children
(including vaccinated). Earlier in the pandemic, seropositivity rates in
primary schools were similar to or slightly higher (<5%) than those in
secondary schools, but by late 2021, primary schools had 12.3% (44.3%) lower seroprevalence for unvaccinated (all)
subjects. Variability in seroprevalence among districts and schools increased
more than two-fold over time, and in classes from 11% (95% CrI 7–17%) to 40% (95%
CrI 22–49%).
CONCLUSION: Seroprevalence in children increased
greatly, especially in 2021 following introduction of vaccines. Variability in
seroprevalence was high and increased substantially over time, suggesting
complex transmission chains.
Trial Registration: ClinicalTrials.gov NCT04448717
Introduction
More than two years into the SARS-CoV-2 global
pandemic, it remains unclear to what extent transmission takes place in schools
[1]. The predominant opinion currently is that children of
all ages appear to be equally susceptible to SARS-CoV-2 infection compared to
adults and that transmission in children occurs primarily in the community in
which the children live, including at school, at home and in other settings.
Fortunately, symptomatic or severe disease, hospitalisation and death are much
less common in children [1–3]. This pattern holds true even under the higher
transmissibility and dominance of the delta and omicron variants of concern
(VOCs) over other SARS-CoV-2 strains [4–6]. Yet, studies have shown an elevated risk of SARS-CoV-2
infection for adults living in households with children attending schools
in-person [7, 8], especially for higher school grades [8], although this was not a consistent finding [9]. Moreover, the number of reported outbreaks in schools
after the summer break of 2021 increased compared with earlier times, in part owing
to the greater transmissibility of the delta and omicron VOCs, but possibly
also to higher testing rates including repetitive pool testing in the school
setting [10, 11]. The infection risk may also have varied depending on
control measures in schools, such as wearing masks, social distancing, hygiene,
symptomatic or repetitive testing and the vaccination status of families, peers
and teachers [8, 12]. Overall, several studies documented a low probability
of children getting infected within schools under the delta VOC [13], as reported also during the pre-delta period of the
pandemic [14–18].
The rate of previous natural infection in children can
be estimated from seroprevalence studies and varies between countries and
populations. Several studies show that seroprevalence had risen sharply up to
15–42% in children by summer 2021 [19–22], and is expected to be even higher with the emergence
of delta and omicron and the parallel increase in vaccination rates among
children, adolescents and adults. Most countries, including Switzerland,
started vaccination of adolescents above 12 years from summer 2021 on, whereas
the vaccine was not approved for the 5- to 11-year-old group until late 2021
(in Switzerland mid-December 2021). The variability of vaccination rates among
parents, teachers and children below and above 12 years combined with the
ever-changing transmissibility of new variants, differences in mitigation
measures at school as well as in the community, and testing attitudes make
surveillance of infection rates in school-age children a major challenge.
Specifically, more evidence is needed regarding absolute levels of
seroprevalence.
The Ciao Corona study examines SARS-CoV-2
seroprevalence among children in primary and secondary schools in one of the
largest cantons of Switzerland [23]. In this school-based prospective cohort study, so far
there have been four rounds of antibody testing, in June–July 2020 (T1) [24], October–November 2020 (T2) [17], March–April 2021 (T3) [16] and November–December 2021 (T4), to assess the proportion of seropositive children and adolescents within cantonal districts,
and by school, school level and class. Seroprevalence increased from 1.5 to
16.4% from T1 to T3, and clustering of seropositive children within school
classes was low and mostly reflected community transmission [16]. Up to T3, seroprevalence in primary schools was
similar to or slightly higher than in
secondary schools (T2: primary 5.6% vs secondary 5.7%; T3: 19.5% vs 15.1%),
possibly due to closer contact with parents [16, 17].
The aim of this study was to describe the evolution of
seroprevalence in children and adolescents from randomly selected schools of
the canton of Zurich from October 2020 to December 2021, and to assess changes
and variability of seropositive children within and across districts, schools,
school levels and classes in our cohort. Because of low seroprevalences and
recent school closures that may have led to infections more from households
than from schools in June–July 2020 and generally a lower positive predictive
value in a low seroprevalence setting, we do not report T1 results here.
Materials and methods
The protocol for this school-based cohort study has
previously been reported (ClinicalTrials.gov identifier: NCT04448717) [23], as well as the results of the first three rounds of
Ciao Corona testing [16, 17, 24]. Ciao Corona, as part of the Swiss-wide research
network Corona Immunitas [25], examines a randomly selected cohort of public and
private schools and classes in the canton of Zurich, Switzerland. With 1.5
million inhabitants, the canton of Zurich is largest of 26 cantons in
Switzerland by population and is home to a linguistically and ethnically
diverse population in both urban and rural settings. A similar daily incidence
of diagnosed SARS-CoV-2 cases until December 2021 in the canton of Zurich and
Switzerland (see appendix) show that the canton of Zurich is quite
representative for Switzerland as a whole. The study was approved by the Ethics
Committee of the Canton of Zurich, Switzerland (2020-01336). All participants
provided written informed consent before being enrolled in the study.
After an initial lockdown period during which schools
were closed from March to May 2020 across Switzerland, children have been
physically in school without interruption. Preventive measures such as hygiene
and social distancing rules, and mask requirements were implemented in public
and private schools according to cantonal rules, but with some variation within
and among cantons. Children were required to stay at home if they had a fever
or other than minor cold symptoms. School personnel were required to wear masks
starting in October 2020, whereas secondary school children (7th–9th grades)
wore masks from November 2020, and the older primary school children (4th–6th
grades) in the early months of 2021, and again from December 2021.
School-specific contact tracing was introduced in August 2020, where testing
and quarantine recommendations depended on the situation. If at least two
children in a class simultaneously tested positive, then the whole class would
be quarantined (existing policy from May to February 2022). However, if children were
wearing masks, then quarantine was restricted to close contacts. Some schools
started to participate in weekly pooled polymerase chain reaction (PCR) testing
in spring 2021, with optional participation from children. By the time of T4
testing, approximately 80% of participating schools took part in repetitive
testing, with variable participation rates of individual children, but at least
80% in most schools. In the case of a positive pool of up to 10 students, each
child in that pool had a second individual test, and students remained at
school but wore masks until the results were available. In the case of three or
more positive cases in a class, negative-tested children who participated in
pool testing could continue to attend school, and children who did not
participate in repetitive testing were then required to quarantine for 10 days.
Population
As described elsewhere [23], public and private primary schools in the canton of
Zurich were randomly selected in May 2020, and the geographically closest
secondary school was also invited. The 55 participating schools (among them 2
private schools) were distributed in each of the 12 geographic districts
proportional to the population size. Within participating schools, classes were
randomly selected, stratified by school level: lower level (grades 1–3, age 6–9),
middle level (grades 4–6, age 9–12), and upper level (grades 7–9, age 12–16).
The aim was to invite at least three classes, with at least 40 children in each
school level at a school. The invited sample is representative of the
school-age population in the canton of Zurich.
Eligible children and adolescents (hereafter, children)
in the selected classes could participate in any of the testing rounds and were
reinvited to later testing periods. In the fourth round of testing, from
November to December 2021, some schools declined to continue, reducing the
total number of schools included from 55 to 43. Additional classes within the
43 participating schools were invited with the aim of obtaining a similar
sample size to previous rounds. This resulted in 71 classes with only new
children at T4 and 119 classes with a mix of new children at T4 or from
previous rounds and 97 classes with only previously tested children. The main
exclusion criterion was having a suspected or confirmed SARS-CoV-2 infection at
the time of testing, which precluded attendance at school.
Serological testing
Venous blood samples were collected at schools from 26
October to 19 November 2020 (T2), 15 March to 16 April 2021 (T3) and 15 November
to 14 December 2021 (T4). Blood samples were analysed using the Sensitive Anti-SARS-CoV-2
Spike Trimer Immunoglobulin Serological (SenASTrIS) test [26]. The test uses Luminex technology to detect IgG and
IgA antibodies binding to the entire trimeric S protein of SARS-CoV-2, and has 94.0%
sensitivity and 99.2% specificity for testing for IgG. It should be noted that
previous publications of the Ciao Corona study reported results of a different
Luminex-based test (e.g., ABCORA 2.3 binding assay), and therefore they will vary slightly from what is
reported here.
At T4, two different definitions of seroprevalence
were considered: (a) seroprevalence in tested children without documented
vaccination, or (b) Seroprevalence in all tested children (including those
reporting vaccination). Because subjects who were both infected and vaccinated
are not included in the first definition, seroprevalence will be
underestimated. The true infection rate will be somewhere between the two
definitions.
Statistical analysis
Statistical analysis included descriptive statistics
and Bayesian hierarchical modelling to estimate seroprevalence. The Bayesian
approach accounts for the sensitivity and specificity of the SARS-CoV-2
antibody test and the hierarchical structure of cohort (individual and school
levels). The model (Bayesian logistic regression) was adjusted for
participants’ grade and geographic district of the school and included random
effects for school levels. We applied poststratification weights, which adjusted
for the total population size of the specific school level and the geographic
district. The model and weighting procedure are described in detail elsewhere [24].
Variability in seroprevalence among
districts, communities, schools, school level and classes was examined using
variance partition coefficient, which describes the proportion of total
variation that can be attributed to within unit variability[25]. With the introduction of the COVID-19
vaccine to this age group in Switzerland, it is no longer possible to examine
clustering as we have done in previous analyses of this study. Official
statistics of SARS-CoV-2 infections in children aged 7–17 years of age in the
canton of Zurich were retrieved to calculate the cumulative incidence of
diagnosed SARS-CoV-2 cases by T2 to T4, and to compare with the proportion of
seropositive children by 6 November 2020 (median time point of T2), 29 March
2021 (T3) and 29 November 2021 (T4) [27].
Socioeconomic
status of the school was measured with a composite measure, Social Index (SI), which reflects the
socioeconomic status (e.g., unemployment, immigrant population) of the location
of the school and is provided by the Educational Directorate of Canton Zurich.
Scores range from 100 to 120 with lower scores indicating less disadvantaged
schools. All statistical analysis was performed
using the R programming language [28]. and Bayesian models were fit using the
RSTAN package [29].
Results
Participant characteristics for T2, T3 and T4 are
presented in table 1.
Table 1Characteristics of study participants over time.
|
Oct-Nov 2020,
T2
|
Mar-Apr 2021,
T3
|
Nov-Dec 2021,
T4
|
| N total |
2500 |
2450 |
1875 |
| N (%)
primary school (grades 1–6) |
1594
(64%) |
1606
(66%) |
1050 (56%) |
| N (%) secondary school (grades
7–9) |
906 (36%) |
837 (34%) |
825 (44%) |
| Age
(years), median (range) |
12 (7–17) |
12 (7–17) |
12 (7–17) |
| Sex male,
n (%) |
1211
(48%) |
1166
(48%) |
882 (47%) |
| Socioeconomic
status, n (%) |
High |
1621
(70%) |
1616
(72%) |
1211
(73%) |
| Low–medium |
687 (30%) |
633 (28%) |
443 (27%) |
| Vaccinated,
aged 12+ |
0 |
0 |
472/930 (51%) |
| Seroprevalence
adjusted* |
Infected
only |
5.6% (3.5–7.6) |
18.4%
(15.1–21.9) |
31.1% (27.0–36.1) |
| Vaccinated ± infected |
|
|
46.4%
(42.6–50.9) |
| Ratio of diagnosed to
seropositive |
1:5.8 |
1:3.5 |
1:3.5 |
Among 930 participants aged 12 or older, 51% (n = 472) had been vaccinated at least 2 weeks prior to T4 serological testing. Among seropositive children, 50% in primary and 91% in secondary school were vaccinated. Of the 55 schools participating prior to T4, 43 continued to participate (table 2).
Table 2Participation
rates and school-level characteristics of the Ciao Corona study at T2, T3 and
T4.
|
Oct-Nov 2020, T2 |
Mar-Apr 2021, T3 |
Nov-Dec 2021, T4 |
| Number of
schools |
55 |
55 |
43 |
| Number of
classes |
276 |
275 |
287 |
| Median
number of classes per school (min–max) |
5 (2–10) |
5 (2–10) |
8 (3–18) |
| – IQR |
3–6 |
3–6 |
5–8 |
| Median
number of participants per school (min–max) |
41 (13–101) |
37 (15–102) |
39 (16–94) |
| – IQR |
31–56 |
30–59 |
[27–58] |
| Median
number of participants per class (min–max) |
9 (1–20) |
9 (1–21) |
6 (1–17) |
| – IQR |
5–12 |
5–12 |
2–8 |
| % Participation within a class, median (min–max) |
47% (5–94%) |
50% (4–96%) |
33% (4–89%) |
| – IQR |
30–62% |
32–63% |
22–47% |
| Number of
classes with five or more participants |
222 |
221 |
203 |
The total number of included classes increased from
275 to 287, and the median number of classes per school increased from five to seven
(range 2–10). The number of participants per school remained
fairly constant, but participation at the class level was lower at T4 than
during previous rounds. Socioeconomic status was comparable between schools
that did participate and those that no longer participated in T4 (median 105
among schools not participating vs 107 among participating schools, p = 0.33).
Evolution of seroprevalence
Seroprevalence at T2 was 5.6% (95% credible interval [CrI] 3.5–7.6%) and at T3 18.4% (95% CrI 15.1–21.9%) (table 1). At T4, it was 31.1% (95% CrI 27.0–36.1%) in non-vaccinated children and 46.4% (95% CrI 42.6–50.9%) in all children (including vaccinated). Based on an overall PCR-positivity rate of 133 positive tests per 1000 inhabitants in the canton of Zurich aged 6–17 years, we estimate a ratio of diagnosed to seropositive children in late 2021 of 1: 3.5 which was similar toMarch to April 2021 (T3), though T2 had a higher proportion of children undiagnosed, 1:5.8.
At T2 seroprevalence was similar across school levels
(primary 5.5%, 95% CrI 3.4–7.8% vs secondary 5.6%,
95% CrI 2.8–8.7%), whereas at T3, primary school children had a
higher seroprevalence (19.5%, 95% CrI 16.0–23.7%)
than secondary school children (15.1%, 95% CrI 10.7–19.6).
At T4, when only children with SARS-CoV-2 antibodies due to infection were
counted and vaccinated subjects excluded, primary students had lower
seroprevalence than secondary school
students (28.7% vs
41.0%). However, when both infected and vaccinated participants were included,
secondary school students had an average seroprevalence of 75.8% (95% CrI 69.6–82.4%), compared with 31.5% (95% CrI 27.1–36.1%) among primary school students (grades 1–6) (see
table 1).
Variability of seroprevalence within and across
districts, schools, and school classes
District level seroprevalence ranged from 30.4% to
88.5% at T4 (fig. 1a-c) indicating a substantial increase in variability over
both T2 (2.1–18.0%) and T3 (11.1–27.2%).
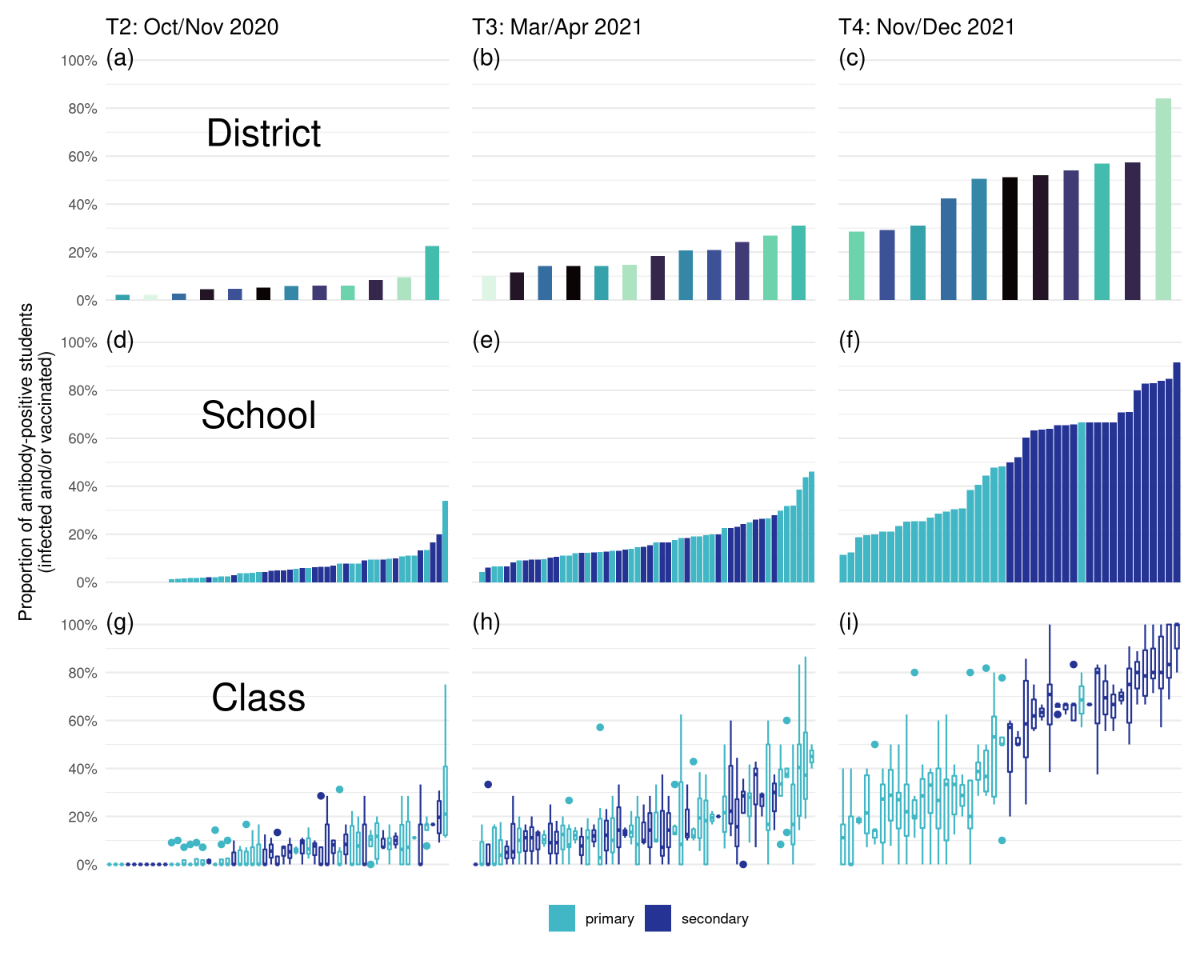
Figure 1 Proportion of ever-seropositive children in the canton of Zurich among districts (upper), schools (middle) and classes (lower) at T2 (October-November 2020), T3 (March-April 2021) and T4 (November-December 2021). Each district has an individual colour in the upper panels, primary school children (grades 2–6) in light blue and secondary school children in dark blue (medium and lower panels). Boxplots in the lower panels denote median and describe variability of seroprevalence on a school level expressed as maximum seroprevalence in a class minus minimum seroprevalence and summarised as median (interquartile range). Whiskers of boxplots denote ± 1.5 standard deviation (SD). Proportion of ever-seropositive children in the canton of Zurich among districts (upper), schools (middle) and classes (lower) at T2 (October-November 2020), T3 (March-April 2021) and T4 (November-December 2021). Each district has an individual colour in the upper panels, primary school children (grades 2–6) in light blue and secondary school children in dark blue (medium and lower panels). Boxplots in the lower panels denote median and describe variability of seroprevalence on a school level expressed as maximum seroprevalence in a class minus minimum seroprevalence and summarised as median (interquartile range). Whiskers of boxplots denote ± 1.5 standard deviation (SD).
At T2 and T3, we observed only small differences
between the school-level seroprevalence rates of primary and secondary schools (T2: primary 5.6% vs
secondary 5.7%; T3: 19.5% vs 15.1%) (fig. 1d-f). However, by
T4 in the full sample (infected and/or vaccinated), all secondary schools had a
higher seroprevalence than all but one of the primary schools (fig. 1d-f).
Within schools there was large variation between seroprevalence of classes (fig.
1g-i). At T2, median between-class variability was 11% (IQR 7–17%), at T3, 24% (IQR 17–37%),
and at T4 median between-class variability had increased to 40% (IQR 22–49%). For example, in the primary school with the
lowest seroprevalence at T4 (fig. 1i, leftmost bar), had classes with a minimum
of 0% and a maximum of 40% seropositive subjects, thus a between class
variability of 40%. Results were similar with a range of different inclusion
criteria of classes in the analysis (two or three children tested per class; two
participants per class and at least two classes per school; at least three
participants and at least 50% participation in class). Even within a single
geographic district, seroprevalence varied widely between and within schools (fig.
2).
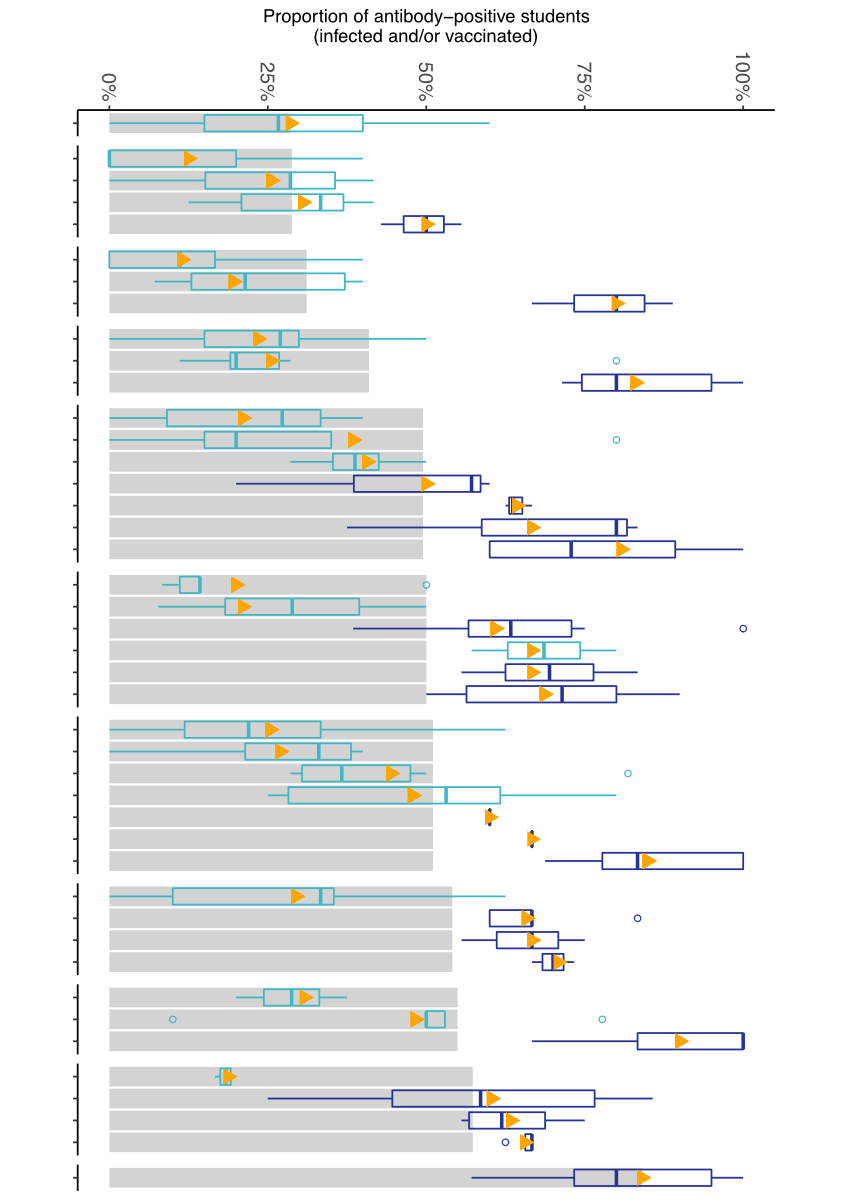
Figure 2 Variability of seroprevalence of children and
adolescents at T4 (November–December 2021) among 11 districts (grey bars,
mean), 43 schools (triangles, mean) and 200 classes (boxplots) within the same
schools and districts. Along the horizontal axis, schools are displayed without
names for reasons of confidentiality. Only classes where more than five
children participated were included. Boxplots describe variability of
seroprevalence on a school level, with middle bar indicating median
seroprevalence, box representing the interquartile range (middle 50% of
classes) and whiskers the more extreme classes.
The overall explained
variance was at most 25% (fig. 3, supplementary table S1 in the appendix).
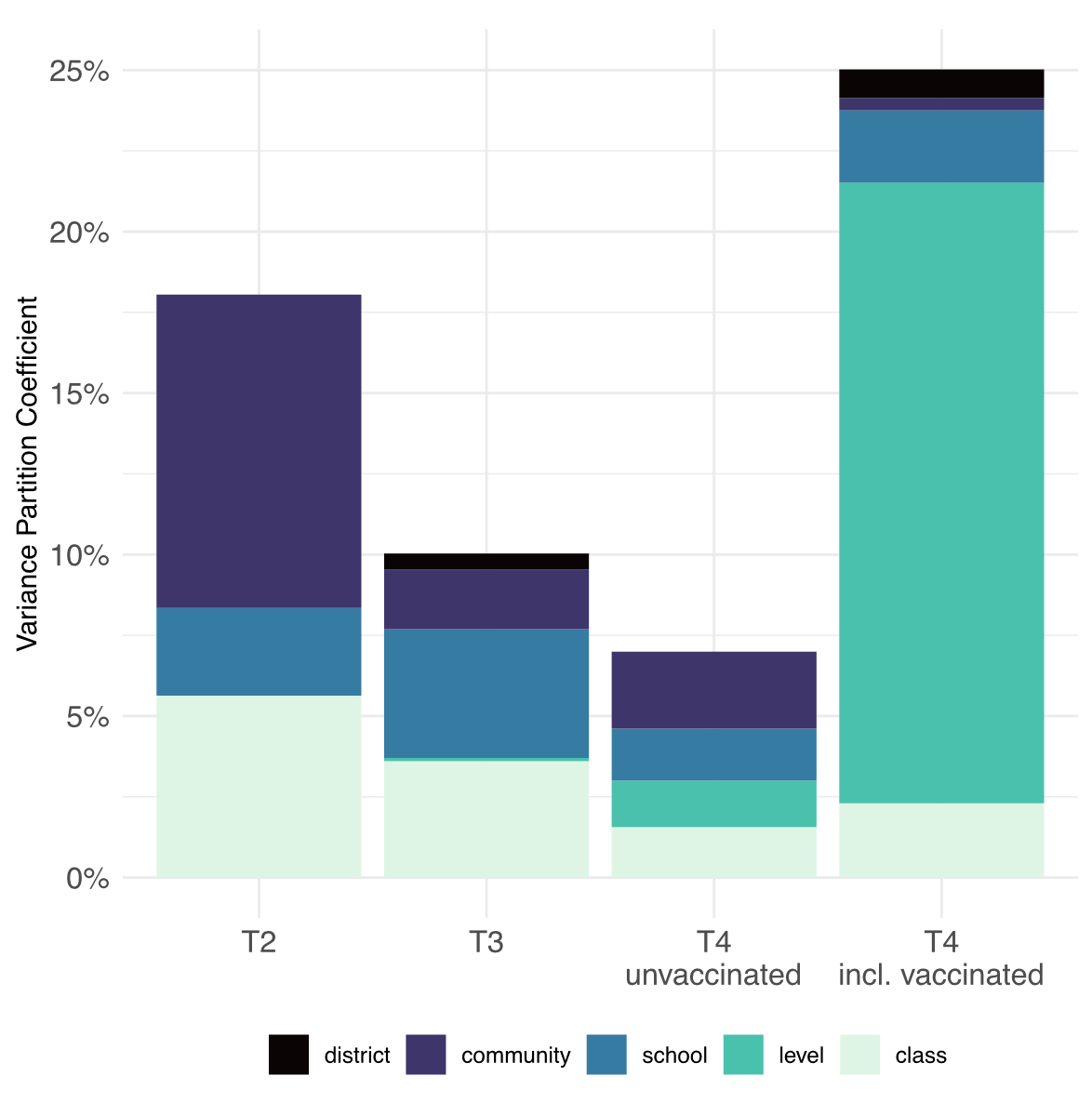
Figure 3 Composite
variability in seroprevalence of children and adolescents at T2
(October–November 2020), T3 (March–April 2021) and T4 (November–December 2021)
using variance partition coefficients. The total length of the bars shows the
total proportion of variance in seroprevalence explained by class, school level
(primary versus secondary), school (ID), community, and district. The remaining
residual variation is attributable to unmeasured factors.
Among unvaccinated
children only, explained total variance and variance explained by community,
school and class were fairly similar but waned over time from T2 to T4. When
the vaccinated children were also included, explained variability increased
markedly from T3 to T4 with most variability explained by school level, i.e., primary
versus secondary school level.
Discussion
In this school-based cohort study of more than 1800
school children from randomly selected schools of the most populous canton in
Switzerland, SARS-CoV-2 seroprevalence in children and adolescents increased
from 6% in October/November 2020 to 46% in November/December 2021. The ratio of
diagnosed to seropositive children in late 2021 (T4) of 1:3.5 was similar to
March to April 2021 (T3).
These key points will be discussed in further detail
below. Secondary school children had much higher seroprevalence rates than
primary school children by the end of 2021, a difference which was not
previously observed and only partially explained by higher vaccination rates.
Variability of seroprevalence between districts, schools and classes within the
same school increased substantially between the end of 2020 and the end of 2021
two- to four-fold. Explained variability over time decreased among unvaccinated
subjects. This evolution mirrors an increasingly complex system of SARS-CoV-2
spread and transmission, which is highly variable both in and outside of the
school system, even in a relatively small part of the same country.
The increase in seroprevalence in children and
adolescents in our study from 6% in October/November 2020 (T3) to 46% in
November/December 2021 (T4) is consistent with other seroprevalence studies of
Europe and the USA [20–22]. Yet between-country variability is substantial and is
driven by multiple processes: vaccination status of children, adolescents and
adults [1], some youth being vaccinated despite infection,
heterogeneity of immune responses to SARS-CoV-2 with occasional failure to
seroconvert after infection [30], and background community transmission [31].
Until March–April 2021, primary school children
consistently showed similar or slightly higher seroprevalence (<5%) than
secondary school students, but this pattern had clearly changed by November-December
2021. The 44% higher seropositivity rates among secondary school compared with
primary school children are certainly explained by the introduction of the
SARS-CoV-2 vaccination. In our analysis, 49% of those eligible to be vaccinated
had completed the two-dose regimen, and close to 100% of those were seropositive.
It is not possible to determine which of these children also had antibodies due
to infection from COVID-19. Considering that some of the vaccinated children
were seropositive prior to vaccination (reflecting the seroprevalence of
unvaccinated-infected children), the true seroprevalence in infected children
would be expected to be higher than what was measured (extrapolating from the
infected only children by about 10% to 15%). Not even N-antibodies (reflecting
natural infection rather than vaccination) specifically found in infected
individuals would have helped to estimate seroprevalence in infected children,
as they wane after a few months, much faster than our 8-month time window
between T3 and T4 [32, 33]. Even after those vaccinated were excluded, the higher
seroprevalence in secondary school children was also documented in other
countries [1, 12, 33] and was among the highest among age groups under the
delta variant. Adolescents of this age group show social behaviour different
from their younger counterparts, with more social contacts outside the home and
school setting, and they may also have been weary of the mitigation measures
that were constantly in place since the beginning of the pandemic. In previous
rounds, seroprevalence was higher in primary than secondary schools, but this
was partially explained by differences in mask-wearing policies [16]. Masks were initially required only for the older age
group, which was associated with a 5% lower seroprevalence in adolescents than
in younger children in early 2021 [16]. The reversed trend in seroprevalence between primary
and secondary school in late 2021 with the mask mandate changed to a general
masking rule at school during most of the period between testing in 2021
reinforces this previous result [16] and could also be supported by a different social
behaviour of adolescents and a higher susceptibility compared with the younger
age group in and outside school [34].
Interestingly, explained variability was rather low
and decreased over time when vaccinated children were excluded, but increased
when all children, including the vaccinated children, were considered. In the
unvaccinated group, the dominant part of explained variance at T2 was the
community where the child lived and his or her class [17, 24]. School became more important at T3, [16] whereas at T4 neither community, nor school, school
level or class explained much variability. With the vaccination of 48% of
participating secondary school children, school level became the dominant
factor, as the age of children in secondary school corresponded well to the age
group where vaccination was available, 12 years and older. Even though the
spread in school increased with the more infectious variants, variation was not
explained by school or class identity. Other factors such as the household or
close contacts outside class and school could still be more important in the
infection spread [34].
From a
public health perspective, Ciao Corona is unique as it repeatedly measures
seroprevalence, an important variable to document the spread of infection in
children and adolescents in the school setting, as well to assess the impact of
children’s seroprevalence and vaccination on SARS-CoV-2 spread in schools. Ciao Corona is one of the few large studies reporting
variation in seroprevalence over time in children within districts, schools and
school classes from randomly selected schools in a country where the general
lock-down at a population level was mild and short (6 weeks in 2020), and school closure lasted only for 2 months at the start of
the pandemic. We were able to perform four assessments covering the major
SARS-CoV-2 variants (wild type at T1/T2, alpha in T3, delta in T4). The overall
retention rate remained comparatively high through March/April 2021 (89% and
87%) although it decreased by T4 (34%), which mainly reflected decisions not to participate in T4 on a school rather than
individual level. Use of serological testing implies that children with
asymptomatic infections were also detected.
This
study also has limitations. Due to the nature of serological testing, exact
timing of infections cannot be determined. Therefore, examination of associated
infections, in the sense of outbreaks or temporal clusters of infections, is
not possible. We used a highly accurate serological test and adjusted for
inaccuracy using Bayesian models on a population level, but it is not possible
to avoid some false positive or false negative results on an individual level. Additionally, there were likely vaccinated children and
adolescents who were also infected, and therefore some underestimation of
seroprevalence of the infected-only cohort is likely. Including these children
also as infected would increase the estimates of seroprevalence due to
infection rather than vaccination. Moreover, the addition of anti-N IgG
antibodies that document natural infection with SARS-CoV-2 also in vaccinated
children might have helped to tease out the percentage of vaccinated children
who also had a natural infection, although children seem to develop lower levels of anti-nucleocapsid
IgG antibodies with a faster decline than adults and the temporal sequence of
natural infection and vaccination would still be missing [30, 35]. Participation bias in
studies like Ciao Corona can occur at the individual level of the child, or on
the class or school level; it can be balanced over time or differential
non-participation at some time periods might have occurred. Due to the nature of
repetitive testing with fear of venous blood sampling in mind, possible
participation bias is unavoidable. Yet we managed to have much higher
participation rates than other similar studies (33 vs 9%) [36]. Overall, we had
comparable study populations of higher socioeconomic status than the general
Swiss population at each round, potentially leading to some underestimation of
seroprevalence in each testing round as more disadvantaged populations show
higher SARS-CoV-2 seroprevalence [36, 37]. Selective
non-participation of children with known previous infections compared with
previously seronegative children did not occur (e.g., 66 vs 62% of seropositive
and seronegative children at T3 participated again at T4), but some
misclassification could have taken place as antibody levels may wane over time [38]. Yet, based on the
relatively higher participation of secondary compared to primary school
children and lower participation rates on the class level at T4 compared to
previous rounds (see table 2), some overestimation of seroprevalence is not
excluded. On the school level, we examined the social index of the schools, a
composite measure which reflects socioeconomic status (e.g., unemployment, immigrant population, parental support
by the social system) of the location of the school provided by the Educational
Directorate of Canton Zurich. The social index was representative for the
canton of Zurich, did not change over time and was comparable among those
schools which participated at T4 and those that did not. Because of a lower
participation on the class level, with about half of classes newly entering the
study at T4 (with unknown previous serological status of these children) and a
high number of vaccinated children in whom concurrent natural infections could
not be defined with our design, clustering as indication of intra-class or
intra-school transmission could no longer be determined.
Conclusion
We observed a large increase in seroprevalence from October 2020 through November 2021, especially between March 2021 and November 2021 following introduction of the vaccine for children 12 years and older. Up to March 2021, primary school children had higher seroprevalence, but by November 2021, secondary school children were more likely to be seropositive. This shift was in part due to introduction of the COVID-19
vaccine, but possibly also due to different behaviour,
with more social contacts of older children outside schools and households. Variability in seroprevalence among districts, schools
and classes was high and increased over time, even between different schools of
the same district and among classes in the same school. Since this variability
was not explained by school or class, other factors not captured (e.g., family
members and other close contacts outside of the school setting) could still be
more important in the spread of infection.
Data sharing statement
Data
are still being collected for the longitudinal cohort study Ciao Corona. Upon study completion in 2023, de-identified and
potentially aggregated participant data, together with required data
dictionaries, will be available on reasonable request by email to the
corresponding author. The purpose and methods of data analysis will be
evaluated by the study team first to ensure that it complies with the ethics
approval.
Author contributions
SK
and MAP initiated the project and preliminary design. SK, MAP, AU, TR, SRH
developed the design and methodology. SK, TR, AU, AR, SR recruited study
participants, collected, and managed the data. SRH performed statistical
analysis and wrote the first draft of the manuscript. All authors contributed
to the design of the study and interpretation of its results and revised and
approved the manuscript for intellectual content. SK, SR, AR and SRH had access
to and verified all underlying data. The corresponding author SK attests that
all listed authors meet authorship criteria and that no others meeting the
criteria have been omitted.
References
1. ECDC. Interim public health considerations for COVID-19 vaccination of children aged 5-11 years, 1 December 2021. [cited 2022 August 1]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/TGU-20211119-1925_final-for-publication.pdf
2. Viner RM, Mytton OT, Bonell C, Melendez-Torres GJ, Ward J, Hudson L, et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2021 Feb;175(2):143–56. [cited 2021 Feb 1] https://doi.org/10.1001/jamapediatrics.2020.4573
3. Dawood FS, Porucznik CA, Veguilla V, Stanford JB, Duque J, Rolfes MA, et al. Incidence Rates, Household Infection Risk, and Clinical Characteristics of SARS-CoV-2 Infection Among Children and Adults in Utah and New York City, New York. JAMA Pediatr. 2022 Jan;176(1):59–67. [cited 2022 Jan 1] https://doi.org/10.1001/jamapediatrics.2021.4217
4. DGPI. Infektions- und Übertragungsrisiken von SARS-CoV-2 und die Marbidität und Mortalität bei Kindern und Jugendlichen. Einfluss von saisonalem Verlauf, Virusvarianten und Impfeffekten. Stellungnahme der Deutschen Gesellschaft für Krankenhaushygiene (DGKH) und der Deutschen Gesellschaft für Pädiatrische Infektiologie (DGPI). Berlin 2021 [cited 2021 September 30]. Available from: https://dgpi.de/wp-content/uploads/2021/09/2021-09-13-Stellungnahme-DGPI-DGKH_kurz.pdf
5. Ben-Shimol S, Livni G, Megged O, Greenberg D, Danino D, Youngster I, et al. COVID-19 in a Subset of Hospitalized Children in Israel. J Pediatric Infect Dis Soc. 2021 Aug;10(7):757–65. [cited 2021 Aug 17] https://doi.org/10.1093/jpids/piab035
6. Lorthe E, Bellon M, Berthelot J, Michielin G, L’Huillier AG, Posfay-Barbe KM, et al.; SEROCoV-Schools Study Group. A SARS-CoV-2 omicron (B.1.1.529) variant outbreak in a primary school in Geneva, Switzerland. Lancet Infect Dis. 2022 Jun;22(6):767–8. [cited 2022 Jun] https://doi.org/10.1016/S1473-3099(22)00267-5
7. Vlachos J, Hertegård E, B Svaleryd H. The effects of school closures on SARS-CoV-2 among parents and teachers. Proc Natl Acad Sci USA. 2021 Mar;118(9):e2020834118. [cited 2021 Mar 2] https://doi.org/10.1073/pnas.2020834118
8. Lessler J, Grabowski MK, Grantz KH, Badillo-Goicoechea E, Metcalf CJ, Lupton-Smith C, et al. Household COVID-19 risk and in-person schooling. Science. 2021 Jun;372(6546):1092–7. [cited 2021 Jun 4] A https://doi.org/10.1126/science.abh2939
9. Calvani M, Cantiello G, Cavani M, Lacorte E, Mariani B, Panetta V, et al. Reasons for SARS-CoV-2 infection in children and their role in the transmission of infection according to age: a case-control study. Ital J Pediatr. 2021 Sep;47(1):193. [cited 2021 Sep 27] https://doi.org/10.1186/s13052-021-01141-1
10. RKI. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19). Berlin 2021 [cited 2021 October 28]. Available from: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2021-10-28.pdf?__blob=publicationFile
11. ECDC. COVID-19 in children and the role of school settings in tranmission - second update. 2021 [cited 2021 October 28]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-in-children-and-the-role-of-school-settings-in-transmission-second-update.pdf
12. Chadeau-Hyam M, Eales O, Bodinier B, et al. REACT-1 round 15 final report: Increased breakthrough SARS-CoV-2 infections among adults who had received two doses of vaccine, but booster doses and first doses in children are providing important protection.; London 2021 [cited 2021 October 28]. Available from: https://spiral.imperial.ac.uk/handle/10044/1/92501
13. Ladhani SN, Ireland G, Baawuah F, Beckmann J, Okike IO, Ahmad S, et al. Emergence of the delta variant and risk of SARS-CoV-2 infection in secondary school students and staff: prospective surveillance in 18 schools, England. EClinicalMedicine. 2022 Feb;45:101319. [cited 2022 Mar] https://doi.org/10.1016/j.eclinm.2022.101319
14. Ladhani SN, Ireland G, Baawuah F, et al. Emergence of SARS-CoV-2 Alpha (B.1.1.7) variant, infection rates, antibody seroconversion and seroprevalence rates in secondary school students and staff: Active prospective surveillance, December 2020 to March 2021, England. J Infect 2021 [cited 2021 August 30].
15. Ismail SA, Saliba V, Lopez Bernal J, et al. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis 2020 [cited 2020 Dec 8].
16. Ulyte A, Radtke T, Abela IA, Haile SR, Ammann P, Berger C, et al. Evolution of SARS-CoV-2 seroprevalence and clusters in school children from June 2020 to April 2021: prospective cohort study Ciao Corona. Swiss Med Wkly. 2021 Nov;151(4546):w30092. [cited 2021 Oct 25] https://doi.org/10.4414/SMW.2021.w30092
17. Ulyte A, Radtke T, Abela IA, Haile SR, Berger C, Huber M, et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ. 2021 Mar;372(616):n616. [cited 2021 Mar 17] https://doi.org/10.1136/bmj.n616
18. Vardavas C, Nikitara K, Mathioudakis AG, Hilton Boon M, Phalkey R, Leonardi-Bee J, et al. Transmission of SARS-CoV-2 in educational settings in 2020: a review. BMJ Open. 2022 Apr;12(4):e058308. [cited 2022 Apr 5] https://doi.org/10.1136/bmjopen-2021-058308
19. Stringhini S, Zaballa ME, Pullen N, Perez-Saez J, de Mestral C, Loizeau AJ, et al.; Specchio-COVID19 study group. Seroprevalence of anti-SARS-CoV-2 antibodies 6 months into the vaccination campaign in Geneva, Switzerland, 1 June to 7 July 2021. Euro Surveill. 2021 Oct;26(43):2100830. [cited 2021 Oct] https://doi.org/10.2807/1560-7917.ES.2021.26.43.2100830
20. FDA. Epidemiology of COVID19 in Children Aged 5 – 11 years. Event Materials for Vaccines and Related Biological Products Advisory Committee Meeting October 26. 2021 [cited 2021 2022 August 1]. Available from: https://www.fda.gov/media/153508/download
21. Merckx J, Roelants M, Callies M, et al. Prevalence and incidence of antibodies against SARS-CoV-2 in children and school staff measured between December 2020 and June 2021: Findings of the third testing period – brief summary. Brussels 2021 [cited 2021 August 1]. Available from: https://www.sciensano.be/sites/default/files/report_seroprev_sars-cov-2_schools_t2_jun2021_1.pdf
22. Scotland. Population-based seroprevalence surveillance 13 October 2021. Scotland 2021 [cited 2021 October 30]. Available from: https://publichealthscotland.scot/publications/enhanced-surveillance-of-covid-19-in-scotland/enhanced-surveillance-of-covid-19-in-scotland-population-based-seroprevalence-surveillance-13-october-2021/.
23. Ulyte A, Radtke T, Abela IA, Haile SR, Braun J, Jung R, et al. Seroprevalence and immunity of SARS-CoV-2 infection in children and adolescents in schools in Switzerland: design for a longitudinal, school-based prospective cohort study. Int J Public Health. 2020 Dec;65(9):1549–57. [cited 2020 Dec] https://doi.org/10.1007/s00038-020-01495-z
24. Ulyte A, Radtke T, Abela IA, Haile SR, Blankenberger J, Jung R, et al. Variation in SARS-CoV-2 seroprevalence across districts, schools and classes: baseline measurements from a cohort of primary and secondary school children in Switzerland. BMJ Open. 2021 Jul;11(7):e047483. [cited 2021 Jul 26] https://doi.org/10.1136/bmjopen-2020-047483
25. West EA, Anker D, Amati R, Richard A, Wisniak A, Butty A, et al.; Corona Immunitas Research Group. Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health. 2020 Dec;65(9):1529–48. [cited 2020 Dec] https://doi.org/10.1007/s00038-020-01494-0
26. Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C, et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol. 2021 Jan;95(3):e01828-20. [cited 2021 Jan 13] https://doi.org/10.1128/JVI.01828-20
27. Zurich Co. Numbers and facts on COVID-19 [Kanton Zürich. Zahlen & Fakten zu COVID-19]. 2021: https://www.zh.ch/de/gesundheit/coronavirus/zahlen-fakten-covid-19.zhweb-noredirect.zhweb-cache.html?keywords=covid19#/. [cited 2021.
28. R Core Team. R: A language and environment for statistical computing. In: R Foundation for Statistical Computing, ed. Vienna, Austria 2020. [cited 2020 Date]. Available from: https://www.r-project.org/
29. Stan Development Team. R Stan: the R interface to Stan. 2020 [cited 2020. Available from: https://www.r-project.org/
30. Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021 Jan;22(1):25–31. [cited 2021 Jan] https://doi.org/10.1038/s41590-020-00826-9
31. Tagarro A, Sanz-Santaeufemia FJ, Grasa C, Cobos E, Yebra J, Alonso-Cadenas JA, et al.; EPICO-AEP Working Group. Dynamics of Reverse Transcription-Polymerase Chain Reaction and Serologic Test Results in Children with SARS-CoV-2 Infection. J Pediatr. 2022 Feb;241:126–132.e3. [cited 2022 Feb] https://doi.org/10.1016/j.jpeds.2021.09.029
32. Lumley SF, Wei J, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, et al.; Oxford University Hospitals Staff Testing Group. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin Infect Dis. 2021 Aug;73(3):e699–709. [cited 2021 Aug 2] https://doi.org/10.1093/cid/ciab004
33. Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM; CMMID COVID-19 working group. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205–11. [cited 2020 Aug] https://doi.org/10.1038/s41591-020-0962-9
34. Irfan O, Li J, Tang K, et al. Risk of infection and transmission of SARS-CoV-2 among children and adolescents in households, communities and educational settings: A systematic review and meta-analysis. J Glob Health 2021;11:05013. [cited 2021.
35. Gentles LE, Kehoe L, Crawford KH, et al. Dynamics of infection-elicited SARS-CoV-2 antibodies in children over time. medRxiv 2022 [cited 2022 Jan 25]. https://doi.org/10.1101/2022.01.14.22269235
36. Zinszer K, McKinnon B, Bourque N, Pierce L, Saucier A, Otis A, et al. Seroprevalence of SARS-CoV-2 Antibodies Among Children in School and Day Care in Montreal, Canada. JAMA Netw Open. 2021 Nov;4(11):e2135975. [cited 2021 Nov 1] https://doi.org/10.1001/jamanetworkopen.2021.35975
37. Ladhani SN, Baawuah F, Beckmann J, Okike IO, Ahmad S, Garstang J, et al. SARS-CoV-2 infection and transmission in primary schools in England in June-December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021 Jun;5(6):417–27. [cited 2021 Jun] https://doi.org/10.1016/S2352-4642(21)00061-4
38. Bloise S, Marcellino A, Testa A, Dilillo A, Mallardo S, Isoldi S, et al. Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults. Eur J Pediatr. 2021 Nov;180(11):3335–42. [cited 2021 Nov] https://doi.org/10.1007/s00431-021-04124-w
Appendix: Supplementary data
Table S1Proportion
of variance in seroprevalence explained by geographic district, community,
school, school level and class, expressed using variance partition coefficient
(percent of total variation explained by within cluster variation).
|
Oct–Nov 2020 (T2) |
Mar–Apr 2021 (T3) |
Nov–Dec 2021, unvaccinated* (T4) |
Nov–Dec 2021, vaccinated or unvaccinated* (T4) |
| Regional |
district |
0% |
0.5% |
0% |
0.9% |
| community |
9.7% |
1.9% |
2.4% |
0.4% |
| School |
School ID |
2.7% |
4.0% |
1.6% |
2.3% |
| School level |
0% |
1.0% |
1.4% |
19.2% |
| class |
5.6% |
3.6% |
1.6% |
2.3% |
| Total |
18.0% |
10.0% |
7.0% |
25.1% |
Figure S1 Average daily cases per 100,000 inhabitants in the canton of Zurich
(blue) and across Switzerland (red).



