Procedures of brain death diagnosis and organ explantation in a tertiary medical centre – a retrospective eight-year cohort study
DOI: https://doi.org/10.57187/smw.2023.40029
Pascale
Grzonkaa, Sira
M
Baumanna, Kai
Tisljara, Sabina
Hunzikerbc, Stephan
Marschac, Raoul
Sutteracd
a Clinic for Intensive Care Medicine, University Hospital Basel, Switzerland
b Medical Communication and Psychosomatic Medicine, University Hospital Basel, Switzerland
c Medical faculty, University of Basel, Switzerland
dDepartment of Neurology, University Hospital Basel, Switzerland
Summary
AIMS OF
THE STUDY: To assess the frequency and variables associated with the need for ancillary
tests to confirm suspected brain death in adult patients, and to assess the
time from brain death to organ explantation in donors. We further sought to
identify modifiable factors influencing the time between brain death and start
of surgery.
METHODS:
Medical records and the Swiss organ allocation system registry were screened
for all consecutive adult patients diagnosed with brain death at an intensive
care unit of a Swiss tertiary medical centre from 2013 to 2020. The frequency
and variables associated with the performance of ancillary tests (i.e.,
transcranial doppler, digital subtraction angiography, and computed tomography
angiography) to confirm brain death were primary outcomes; the time from death
to organ explantation as well as modifying factors were defined as secondary outcomes.
RESULTS: Among
91 patients with a diagnosis of brain death, 15 were not explanted and did not undergo
further ancillary tests. Of the remaining 76 patients, who became organ donors
after brain death, ancillary tests were performed in 24%, most frequently in
patients with hypoxic-ischaemic encephalopathy. The leading presumed causes of
death (not mutually exclusive) were haemorrhagic strokes (49%), hypoxic-ischaemic
encephalopathies (33%) and severe traumatic brain injuries (22%). Surgery for
organ explantation was started within a median of 16 hours (interquartile range
[IQR] 13–18) after death with delay increasing over time (nonparametric test
for trend p = 0.05), mainly due to organ allocation procedures. Patients with
brain death confirmed during night shifts were explanted earlier (during night
shifts 14.3 hours, IQR 11.8–16.8 vs 16.3 hours, IQR 13.5–18.5 during day
shifts; p = 0.05).
CONCLUSIONS: Ancillary tests to confirm brain death
are frequently performed, mainly in resuscitated patients. The delay to surgery
for organ explantation after confirmed brain death was longer during day
shifts, increased over time and was mainly determined by organ allocation
procedures.
The trial was
registered on clinical trials.gov (identifier:
NCT03984981)
Introduction
Brain death has been accepted as a definition of death for over five decades. However, procedures needed to establish such a serious diagnosis, as well as the management that subsequently enables the safest and quickest possible organ explantation, still vary significantly among countries and institutions [1–4]. The fact that, despite international attempts [5], guidelines for the confirmation of brain death and the procedures regarding organ donation are standardised neither nationally nor internationally can lead to misunderstandings, mystifications, and mistrust among the public [6]. This, in turn, could lead to lower organ donation rates, which already lag significantly behind demand. Thorough review of the diagnostic pre- and post-mortem procedures including organ allocation not only serves as quality control and evaluation of optimal resource utilisation but will also improve understanding and acceptance of this concept. However, despite the growing need for organ transplantation and the increasing number of potential donors, scientific data are very limited. This scarcity of data might, at least partly, be explained by the concern of researchers about further fuelling controversies by publishing studies in this context. Notwithstanding these aspects, we decided to elucidate several key aspects in the context of brain death confirmation, ante- and post-mortem diagnostic workup and care, and surgery, as we believe that such studies are key to quality assurance and optimisation of procedures in this context.
We therefore aimed to assess the frequency and variables associated with the use of ancillary diagnostic tests needed to confirm clinically suspected brain death in adult patients treated in the intensive care unit (ICU). We further sought to assess the time from brain death diagnosis to surgery for organ explantation, and to identify potentially modifiable factors of this time span.
Methods
Setting, study design and ethics
This observational single-centre cohort study
was performed at the University Hospital of Basel, a Swiss tertiary academic
medical care centre. We followed the STROBE guidelines to enhance the quality
and standardisation for the reporting of observational studies [7]. The study was registered prior to initiation (identifier:
NCT03984981). The local ethics
committee (Ethikkommission Nordwest- und Zentralschweiz) approved the study in
compliance with the ethical standards laid down in the 1964 Declaration of
Helsinki and its later amendments (EKNZ No.2019-00244). Based on the ethical
review, patients’ consent was waived.
Data collection
Digital medical records and protocols used for procedures
to diagnose brain death of adult (≥18 years of age) patients treated in the
ICUs from 1 January 2013 to 31 December 2020 were retrospectively assessed.
Clinical, laboratory and radiological data of all consecutive patients with confirmed
brain death were systematically collected and entered into a predefined digital
case report form. The following data were collected from the prospectively
recording digital medical records and the ICU information system MetaVision (Version
5.46.44, iMDsoft, Wakefield, MA) and the digital storage system for patients’
clinical data outside the ICU ISMED (Version 21.02a, ProtectData AGⒸ, Boswil, Switzerland): sex, age, presumed aetiology
of brain death, the Charlson comorbidity index quantifying patients' morbidity [8], level of consciousness at admission, the
availability of written advance directives and organ donor cards, treatment
characteristics including intubation,
use of vasopressors and their maximum dose administered after confirmation of
brain death, and duration of ICU stay. In addition, neurological diagnostic
workup was assessed including neuroimaging, electroencephalography (EEG),
somatosensory evoked potentials (SSEPs) and ancillary neuroradiological tests.
The latter included transcranial Doppler,
digital subtraction angiography and computed tomography angiography, which were
performed to confirm cerebral circulatory arrest if clinical assessment was
inconclusive. Core body temperature (in °C) during clinical neurological
examination regarding brain death was documented, as well as the time without
administration of anaesthetic, analgetic and muscle relaxing drugs before
neurologic examination.
Finally,
data on successfully transplanted organs as well as the duration of specific post-mortem procedures (such as
activating local organ transplant coordinators, registration processes,
assessment of organ quality and organ allocation processes) were extracted from
the national database of organ donations.
Standardised diagnostic workup to confirm brain
death
The standardised
clinical neurological examination at the bedside was as recommended by the
Swiss Academy of Medical Sciences in
their guideline from 2011 and the later updated versions including the most recent update from 2017, which is comparable to most international guidelines. All patients with
clinically suspected brain death had to have a known cause of coma as well as neuroimaging
studies revealing signs of intracranial hypertension (i.e., severe cerebral oedema
or mass lesions with consecutive herniation) and a clinical context that was compatible
with such a diagnosis. Potential confounders, such as hypothermia (e.g., core
body temperature <35.1°C), any type of shock, central nervous system (CNS)
infection or polyradiculitis, rhombencephalitis, acute demyelinating
encephalomyelitis (ADEM), persisting metabolic derangements, severe hypothyroidosis,
large brainstem strokes from basilary thrombosis or haemorrhages of the
brainstem, locked-in-syndrome (top-of-the-basilary-syndrome), acute obstructive
hydrocephalus, intoxications, and other conditions that may mimic brain death,
as previously compiled [9], must have been
excluded. In short, the clinical examination had to confirm bilateral absence
of pupillary reaction to light, absence of oculocephalic reflexes (cervico-ocular
and/or vestibulo-ocular reflexes), bilateral absence of corneal reflexes,
absence of any reaction to strong pain tested by applying pressure on the nerve
exit points of the trigeminal nerve at the orbital rim, and absence of
swallowing and cough reflexes following the stimulation of the posterior
pharyngeal and/or tracheal mucosa. In addition, an apnoea test had to be
performed. For this, the patient had to be hyperoxygenated with 100% oxygen for
at least 5 minutes followed by a disconnection from the ventilator once the partial
pressure of CO2 in arterial blood was confirmed to be above 8 kPa
and the pH below 7.3. Two to four litres of oxygen per minute were then
administered via a transtracheal probe in order to avoid desaturation. After
disconnection from the ventilator, the physicians had to confirm absence of any
breathing attempts (defined by activation of respiratory muscles) for at least
60 seconds. Two physicians were required and they had to fulfil the following
criteria in order to be allowed to perform the examination: (1) both had to be
either board certified neurologists or intensivists (or both); (2) at least one
of the two had to have performed five or more previous neurological examinations
to confirm brain death, supervised by experts in this context; (3) at least one
of them was not allowed to be involved in the treatment of the patient. Brain
death could only be clinically confirmed without ancillary tests if both
physicians agreed that the clinical neurological examination, neuroimaging, and
the clinical context were all compatible with brain death. If potential
confounders could not be excluded with certainty, ancillary neuroradiological
tests as described above were performed to confirm brain death.
Outcomes
The frequency and variables associated with the
performance of ancillary tests (such as transcranial doppler, digital subtraction
angiography and computed tomography angiography) to confirm suspected brain
death were primary outcomes, the time from confirmed brain death to organ
explantation as well as modifying factors were defined as secondary outcomes.
Sample size considerations
As
this was an exploratory retrospective observational study, no formal sample
size calculations were performed.
Statistics
Patients
were categorised into those with and without ancillary testing. Chi-square and Fisher
exact tests (where appropriate) were used for univariable comparisons of
proportions. Continuous variables were analysed using the Mann-WhitneyU-test. Discrete variables were
expressed as counts (percentage), and continuous variables as medians and
interquartile ranges (IQRs). The Kruskal-Wallis test (also known as a
non-parametric alternative to the "one-way ANOVA on ranks" test) was
performed to compare the time from diagnosis of brain death to start of surgery
for organ explantation over the study period categorised in years. To analyse
time from organ allocation to start of surgery over the study period (categorised
as years), a nonparametric test for trend developed by Cuzick, which is an
extension of the Wilcoxon rank-sum test, was applied [10]. Two-sided p-values ≤0.05 were considered
significant.
Statistical
analysis was performed with STATA®16.1 (Stata Corp., College
Station, TX, USA).
Results
Between January 2013 and December 2020, brain
death was diagnosed clinically in 91 patients. Fifteen patients were not
explanted, had no further examination with ancillary tests and were excluded. Demographics,
baseline clinical characteristics, the presumed aetiologies of brain death, as
well as the neurological workups of the remaining 76 patients who became organ
donors after brain death (DBD) are presented in table 1.
Table 1Demographics,
baseline characteristics, treatment and diagnostic measures of patients who
were organ donors after brain death (DBD).
|
|
Patients with DBD (n = 76)
|
|
n/median
|
%/IQR
|
| Demographics and baseline characteristics |
Age (years; n, %) |
57 |
48–68 |
| Female (n, %) |
38 |
50 |
| Charlson comorbidity index
(median, IQR) |
1 |
0.5–3 |
| Coma at admission (n, %) |
46 |
61 |
| Presumed aetiology of brain death (not mutually
exclusive; n, %) |
Haemorrhagic stroke |
37 |
49 |
| Hypoxic-ischaemic encephalopathy after cardiac
arrest |
25 |
33 |
| Severe traumatic brain injury |
17 |
22 |
| Malignant ischaemic stroke |
7 |
9 |
| Generalized oedema from CNS infection |
3 |
4 |
| Generalized oedema from intoxication |
2 |
3 |
| Generalized oedema from severe metabolic derangement
|
1 |
1 |
| Hypoxic encephalopathy from strangulation |
1 |
1 |
| Medical treatment |
Time from stop of muscle relaxation to brain death diagnosis (hours;
median, IQR) |
28 |
16–51 |
| Time from stop of anaesthetics to brain death diagnosis (hours; median,
IQR) |
19 |
11–30 |
| Time from stop of analgesics to brain death diagnosis (hours; median,
IQR) |
20 |
13–28 |
| Patients with vasopressors (noradrenaline) post mortem (n, %) |
66 |
87 |
| Maximum doses of noradrenaline post mortem (ug/min; median, IQR) |
10 |
8–20 |
| Neurological diagnostics |
Cerebral lesions on computed tomography (n, %) |
75 |
99 |
| Cerebral lesions on magnetic
resonance tomography (n, %) |
1 |
1 |
| EEG performed prior to
brain death diagnosis |
10 |
13 |
| – Days of EEG recording prior to brain death
diagnosis (days; median, IQR) |
3 |
4 |
| – Alpha coma pattern |
1 |
1 |
| – Generalised delta slowing or suppression |
6 |
8 |
| – Burst-suppression on EEG |
4 |
5 |
| – Isoelectric EEG |
1 |
1 |
| Somatosensory evoked
potentials performed |
3 |
4 |
| – Absence of cortical N20 potentials |
3 |
4 |
| Ancillary tests to confirm absence of cerebral
perfusion (n, %) |
18
|
24
|
| – CT angiography |
16 |
21 |
| – Transcranial Doppler sonography |
2 |
3 |
| – Digital subtraction angiography |
0 |
0 |
| – Confirmation of absent cerebral perfusion
on neuroimaging |
18 |
24 |
| Organ donation |
Time from diagnosis of
brain death to start of organ explantation (hours; median, IQR) |
15.8 |
12.9–18.3 |
The most frequent aetiology of brain death was massive
intracranial haemorrhage. The 76 DBD patients were treated on the ICU for a
median of 34 hours (IQR 14.0–79.5). External ventricular drains were implanted
in 27.6% before a brain death diagnosis was established, craniotomy was
performed in 2.6% and decompressive craniectomy in 11.8% (of which brain death
was confirmed after further swelling in 2%).
In all patients, clinical neurological
examination at the bedside was performed by two board-certified and trained
experts in intensive care and/or neurology. Median core body temperature at brain death
diagnosis was 36.1°C (IQR 35.6–36.6). In addition to bedside clinical
neurological examination, ancillary neuroradiological tests were performed in 24%,
the majority being computed tomography angiography (performed in 21%) to detect
cessation of brain perfusion and thus confirm suspected brain death. Written advanced
directives and organ donor cards clearly stating organ donation as the patient’s
will were available in 5%. In all other patients, information regarding the
presumed patient’s best interest was provided by closest relatives or healthcare
agents. Overall, 124 kidneys, 64 livers, 29 hearts, 25 lungs and 18 pancreases
were transplanted. Surgery was started within a median of 16 hours (IQR 13–18)
after death. Median time from diagnosis of brain death to start of surgery and
its increase over time is presented in figure 1a.
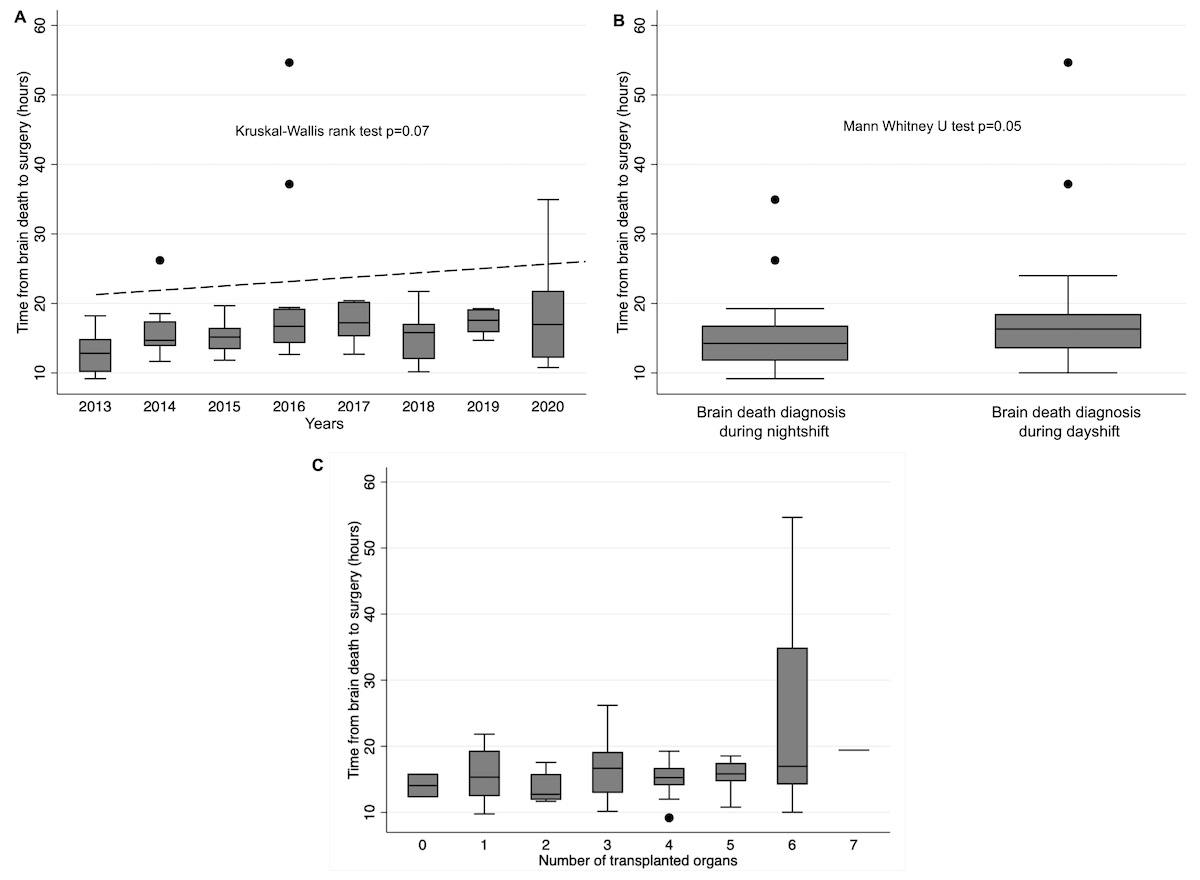
Figure 1 (a)
Median time from diagnosis of
brain death to start of surgery over time (n = 76); (b) median time from
diagnosis of brain death to start of surgery during day shifts versus night
shifts (n = 76); (c) time from death
to surgery in relation to the number of organs explanted (n = 76).
Table 2 presents the univariable comparisons of characteristics of patients with and without the need for ancillary tests to confirm the clinical diagnosis.
Table 2Univariable comparisons of patients with and without the
need of ancillary neuroradiological tests for absence of cerebral perfusion (n
= 76).
|
Patients'
characteristics
|
Ancillary tests
(n = 18)
|
No ancillary tests (n = 58) (i.e.,
brain death confirmed by clinical assessments) |
|
|
n/median
|
%/IQR
|
n/median
|
%/IQR
|
p-value*
|
| Age (years; median, IQR) |
53 |
32–67 |
57 |
50–69 |
0.130 |
| Female (n, %) |
10 |
55.6 |
28 |
48.3 |
0.788 |
| Presumed aetiology of
brain death (n, %) |
Haemorrhagic stroke |
6 |
33.3 |
31 |
53.5 |
0.180 |
| Hypoxic-ischaemic encephalopathy after cardiac
arrest |
11 |
61.1 |
14 |
24.1 |
0.008
|
| Severe traumatic brain injury |
3 |
16.7 |
14 |
24.1 |
0.747 |
| Malignant ischaemic stroke |
0 |
0.0 |
7 |
12.1 |
0.188 |
| Generalised oedema from CNS infection |
2 |
11.1 |
1 |
1.7 |
0.138 |
| Generalised oedema from intoxication |
2 |
11.1 |
0 |
0.0 |
0.054 |
| Generalised oedema from severe metabolic derangement
|
1 |
5.6 |
0 |
0.0 |
|
| Hypoxic encephalopathy from strangulation |
0 |
0.0 |
1 |
1.1 |
|
| Charlson comorbidity index
(median, IQR) |
1 |
1–2 |
2 |
1–3 |
0.131 |
| Coma at admission |
11 |
61.1 |
35 |
60.3 |
1.000 |
| Time from stop of muscle relaxation
to brain death diagnosis (hours; median, IQR) |
36 |
21–85 |
27 |
14–50 |
0.189 |
| Time from stop of anaesthetics
to brain death diagnosis (hours; median, IQR) |
25 |
13–34 |
17 |
10–29 |
0.139 |
| Time from stop of analgesics
to brain death diagnosis (hours; median, IQR) |
26 |
13–34 |
17 |
13–27 |
0.165 |
| Core temperature at brain
death diagnosis (°C; median, IQR) |
36.0 |
35.4–36.5 |
36.2 |
35.6–36.6 |
0.489 |
The only group of patients with a significantly increased
use of ancillary tests were those who initially survived resuscitation after
cardiac arrest and suffered from hypoxic-ischaemic encephalopathy. Of these
patients, twice as many were examined with ancillary tests compared with those
with other presumed aetiologies of brain death (61% versus 24%, p = 0.008). As the
regulations of the SAMW were revised in 2017, we performed additional analyses
regarding the use of ancillary tests before and after this revision, which
revealed no significant differences between these groups (before 2018 20.8%
with ancillary tests versus 30.4% since 2018; p = 0.389).
The univariable comparisons of the time from
diagnosis of brain death to start of surgery in relation to specific patient characteristics
are presented in table 3.
Table 3Univariable comparisons of time from diagnosis of brain
death to start of surgery in relation to specific patient characteristics (n =
76).
|
|
Median
(hours)
|
IQR
(hours)
|
p-value*
|
|
Demographics
|
Age |
|
|
0.367 |
| – Age ≥65 years |
14.7 |
12.7–18.5 |
|
| – Age <65 years |
15.9 |
13.4–18.2 |
|
| Sex |
|
|
0.831 |
| – Female |
15.7 |
12.9–17.8 |
|
| – Male |
15.8 |
12.8–19.0 |
|
| Patient directives |
|
|
0.422 |
| – Patients with advance
directives |
14.6 |
10.9–17.6 |
|
| – Patients without advance
directives |
15.8 |
13.0–18.5 |
|
| Organ donation information |
|
|
0.271 |
| – Organ donation card
available |
17.3 |
15.1–22.2 |
|
| – No organ donation card
available |
15.7 |
12.8–18.1 |
|
|
Clinical characteristics
|
Presumed aetiologies |
|
|
0.470** |
| – Haemorrhagic stroke |
15.8 |
13.0–18.5 |
|
| – Hypoxic-ischaemic encephalopathy
after cardiac arrest |
15.3 |
12.2–17.0 |
|
| – Severe traumatic brain
injury |
16.7 |
15.3–19.0 |
|
| – Malignant ischaemic
stroke |
15.0 |
12.7–18.2 |
|
| – Generalized oedema from
CNS infection |
14.3 |
11.9–17.5 |
|
| – Generalized oedema from intoxication |
19.5 |
12.7–26.2 |
|
| – Generalized oedema from
severe metabolic derangement |
18.1 |
1 case |
|
| – Hypoxic encephalopathy from strangulation |
10.8 |
1 case |
|
| Comorbidities |
|
|
0.195 |
| – Charlson comorbidity
index ≥3 |
13.6 |
11.9–17.3 |
|
| – Charlson comorbidity
index <3 |
16.0 |
13.9–18.1 |
|
| Time of brain death
diagnosis |
|
|
0.05
|
| – Brain death confirmed
during day shifts (i.e., 8:00 to 20:00) |
16.3 |
13.5–18.5 |
|
| – Brain death confirmed
during night shifts (i.e., 20:01 to 7:59) |
14.3 |
11.8–16.8 |
|
Most patient characteristics assessed had no significant influence on the time elapsed until surgery; however, we found that patients with brain death confirmed during night shifts were explanted more rapidly (table 3 and fig. 1b). Of note, there was no significant difference between patients with and without the need for ancillary tests, neither in terms of burden of comorbidities that might have triggered extensive post-mortem examination of organ function nor in terms of core body temperature at the time of clinical examination or time without CNS-active medication (such as analgesics and anaesthetics), as these could have acted as confounding factors in this context. Remarkably, vasopressors were administered to achieve haemodynamic stability in 72 of 76 patients (95%), with noradrenaline administered in all 72 cases (median maximum dosage of 10.5 µg/min) and additional vasopressors administered in 9 (12%). Analyses regarding the duration of specific post-mortem procedures prior to surgery are presented in figure 2.
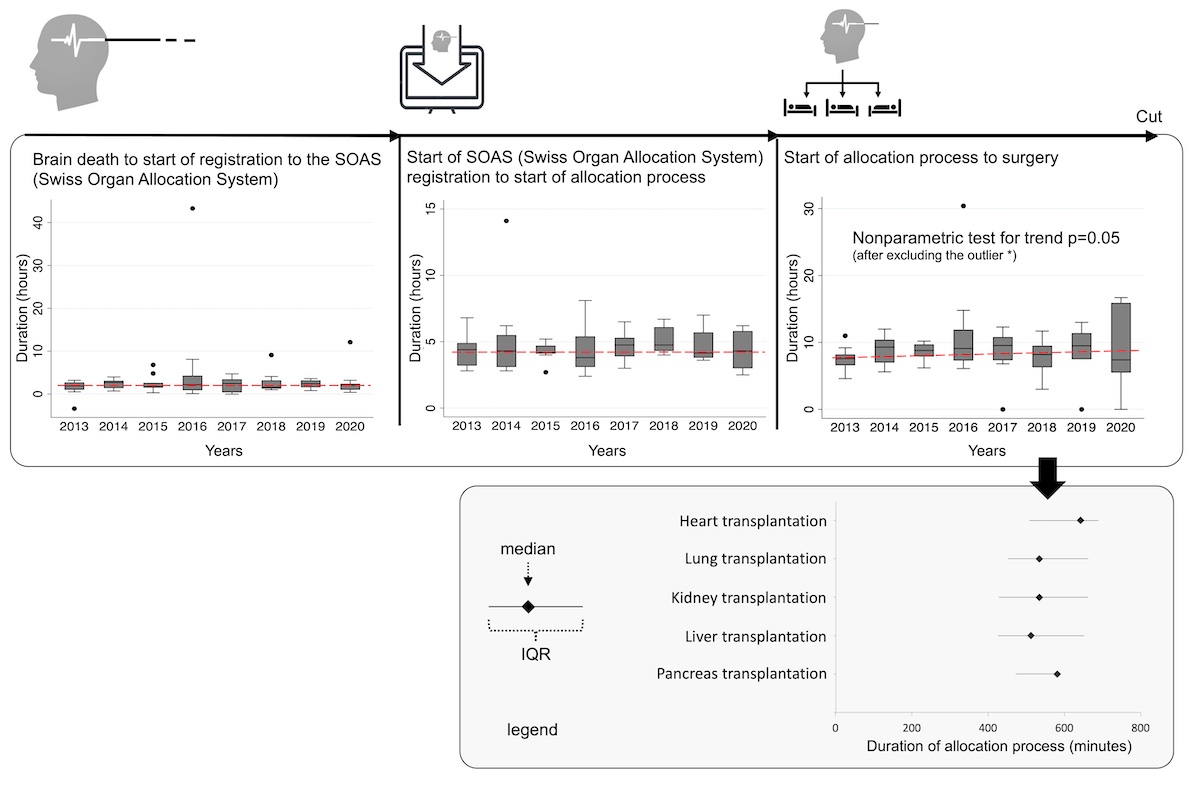
Figure 2 Duration of specific post-mortem procedures to
surgery over the study period. IQR: interquartile range.
Organ allocation was found to be the most time-consuming
step, especially for donated hearts, and its duration increased over the study
period (nonparametric test for trend p = 0.05) with a median of 8.6 hours (IQR 7.0–10.7).
Other procedures such as activating the organ transplant coordinator or
completing the registration process were shorter and of stable duration over
time.
Discussion
Our study on a large cohort at a Swiss tertiary medical care centre provided insights into the certainty or uncertainty of presumed brain death based on the clinical neurological examination at the bedside, expressed by the surrogate of ancillary tests performed. Moreover, it identified clinical factors that are linked to the use of such ancillary tests. According to our data, physicians appear to be uncertain in almost every fourth patient and especially often in the context of hypoxic-ischaemic encephalopathy. Our data further revealed that in the vast majority of cases, the presumed patient’s will was ascertained by relatives or health-care agents, as written directives regarding post-mortem organ donation were available in only 5%.
As stated above, ancillary tests to confirm suspected brain death were used in almost every fourth patient in our institution, despite involving board-certified and trained experts in this context. This underscores the challenging nature of such diagnostic procedures. Interestingly, the use of ancillary tests was not associated with the burden of comorbidities or the time without CNS depressing drugs prior to diagnostic workup, but frequent in the context of generalised oedema from hypoxic-ischemic encephalopathy. Due to the retrospective study design, underlying causes for this association can only be hypothesised. One possible explanation may be the fact that cerebral oedema after severe hypoxic-ischaemic neuronal and glial damage can occur without herniation or other more prominent cerebral lesions. Moreover, oedema is potentially reversible. These facts urge the clinician to demonstrate a lack of cerebral perfusion by ancillary neuroradiological tests. However, prospective studies are needed to confirm these hypotheses.
Computed tomography angiography was the ancillary neuroradiological test most frequently performed, due to considerable disadvantages of transcranial Doppler sonography (transtemporal bone window not always available, examiner-dependent quality) and digital subtraction angiography (more invasive and time consuming) [11]. Despite these advantages, the available evidence cannot support the use of computed tomography angiography as a mandatory test, or as a complete replacement for neurological testing. However, it can be useful as a confirmatory or add-on diagnostic measure following a clinical diagnosis of death, assuming that clinicians are aware of the relatively low overall sensitivity according to a systematic review of eight studies including 337 patients [12]. Overall, studies on the use of ancillary tests in the context of brain death diagnosis are rare, which makes sound comparisons of our results with other studies difficult. In 2008, a survey comparing guidelines for brain death determination in 50 top ranked neurology and neurosurgery institutions, according to the American Academy of Neurology (AAN) revealed that physicians were required to conduct repeat testing in 44% of guidelines [13]. In addition, guidance regarding specific situations in which to pursue ancillary testing was included in only 66% of guidelines [13]. The latter seems problematic in light of the frequent uncertainty of experts as revealed by our results. At the other extreme, a later study analysing hospital policies of 52 organ procurement organisations revealed that ancillary tests were mandatory in up to 7% [3].
Surgery for organ explantation in our donors was mostly started within 13–18 hours after diagnosis of brain death, with a more pronounced delay in patients whose death was confirmed during day shifts as compared with night shifts. This delay also slightly increased over the study period, mainly owing to a longer organ allocation process. However, patient-related characteristics were not found to have a significant impact.
A longer delay till surgery increases the ICU team’s workload and can cause considerable distress for the bereaved families. Moreover, it may lead to the loss of potential organ donors due to hemodynamic instability and cardiac arrest before organ explantation can be achieved. In light of these aspects, further studies are needed to confirm our results and to find ways to reduce this delay.
Strengths and limitations
The strength of our study is the relatively large cohort of patients with a specific and rare clinical situation, the observation period of 8 years at a Swiss tertiary academic medical care centre, and the use of comprehensive prospectively monitored and stored clinical data during the entire study period with the digital ICU information system MetaVision (iMDsoft, Wakefield, MA). The single-centre observational study design, however, limits the generalisability of our results. Another limitation is the inconsistent documentation of the precise reason for performing ancillary tests, which could therefore not be retrospectively assessed. However, as these tests are time consuming and costly, it seems more than plausible that their use was restricted to cases where physicians were uncertain regarding brain death. Furthermore, it remains unclear if some ancillary tests were performed to resolve a lack of consensus among the examining physicians – an important question that must be answered in future prospective studies, as national guidelines differ regarding the recommended number of examining physicians, with some countries requiring two while others require just one [5]. In addition, due to the limited sample size of our study, multivariable comparisons were not performed, so analyses regarding potential predictors are not available.
Conclusion
Ancillary testing to confirm clinically suspected brain death was performed in almost every fourth patient, most commonly in resuscitated patients with hypoxic-ischaemic encephalopathy – the second most frequent aetiology of brain death in our cohort, following haemorrhagic strokes. Although organ explantation in donors is performed frequently in our Swiss tertiary medical care centre, there is a substantial delay from brain death diagnosis to surgical organ explantation. Our data suggest that this time span is not significantly influenced by patient-related characteristics but rather by organisational aspects. Further studies are needed to confirm our results and to find ways to reduce this delay.
Data availability statement
The corresponding author has full access to all the data in the study. He takes full responsibility for the integrity of the data, the accuracy of the data analysis and interpretation, and the conduct of the research. The authors have the right to publish any and all data, separate and apart from guidance of any sponsor. On reasonable request, we are happy to share the data.
Acknowledgements
We
thank our colleagues at SwissTransplant for sharing their data regarding organ
allocation and transplantation, and Sarah Tschudin-Sutter, MD, MSc (University
Hospital Basel), for her statistical assistance.
Pascale Grzonka, MD
Clinic for Intensive Care Medicine
University Hospital Basel
Petersgraben 4
CH-4031
Basel
PascaleSusanne.Grzonka[at]usb.ch
References
1. Powner DJ, Hernandez M, Rives TE. Variability among hospital policies for determining brain death in adults. Crit Care Med. 2004 Jun;32(6):1284–8. https://doi.org/10.1097/01.CCM.0000127265.62431.0D
2. Wahlster S, Wijdicks EF, Patel PV, Greer DM, Hemphill JC 3rd, Carone M, et al. Brain death declaration: practices and perceptions worldwide. Neurology. 2015 May;84(18):1870–9. https://doi.org/10.1212/WNL.0000000000001540
3. Greer DM, Wang HH, Robinson JD, Varelas PN, Henderson GV, Wijdicks EF. Variability of Brain Death Policies in the United States. JAMA Neurol. 2016 Feb;73(2):213–8. https://doi.org/10.1001/jamaneurol.2015.3943
4. Junn A, Hwang DY. Practice Variability in Determination of Death by Neurologic Criteria for Adult Patients. Yale J Biol Med. 2019 Dec;92(4):719–24.
5. Greer DM, Shemie SD, Lewis A, Torrance S, Varelas P, Goldenberg FD, et al. Determination of Brain Death/Death by Neurologic Criteria: The World Brain Death Project. JAMA. 2020 Sep;324(11):1078–97. https://doi.org/10.1001/jama.2020.11586
6. De Georgia MA. History of brain death as death: 1968 to the present. J Crit Care. 2014 Aug;29(4):673–8. https://doi.org/10.1016/j.jcrc.2014.04.015
7. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X
8. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8
9. Grzonka P, Tisljar K, Rüegg S, Marsch S, Sutter R. What to exclude when brain death is suspected. J Crit Care. 2019 Oct;53:212–7. https://doi.org/10.1016/j.jcrc.2019.06.030
10. Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. https://doi.org/10.1002/sim.4780040112
11. Welschehold S, Boor S, Reuland K, Thömke F, Kerz T, Reuland A, et al. Technical aids in the diagnosis of brain death: a comparison of SEP, AEP, EEG, TCD and CT angiography. Dtsch Arztebl Int. 2012 Sep;109(39):624–30.
12. Taylor T, Dineen RA, Gardiner DC, Buss CH, Howatson A, Pace NL. Computed tomography (CT) angiography for confirmation of the clinical diagnosis of brain death. Cochrane Database Syst Rev. 2014 Mar;2014(3):CD009694. https://doi.org/10.1002/14651858.CD009694.pub2
13. Greer DM, Varelas PN, Haque S, Wijdicks EF. Variability of brain death determination guidelines in leading US neurologic institutions. Neurology. 2008 Jan;70(4):284–9. https://doi.org/10.1212/01.wnl.0000296278.59487.c2

