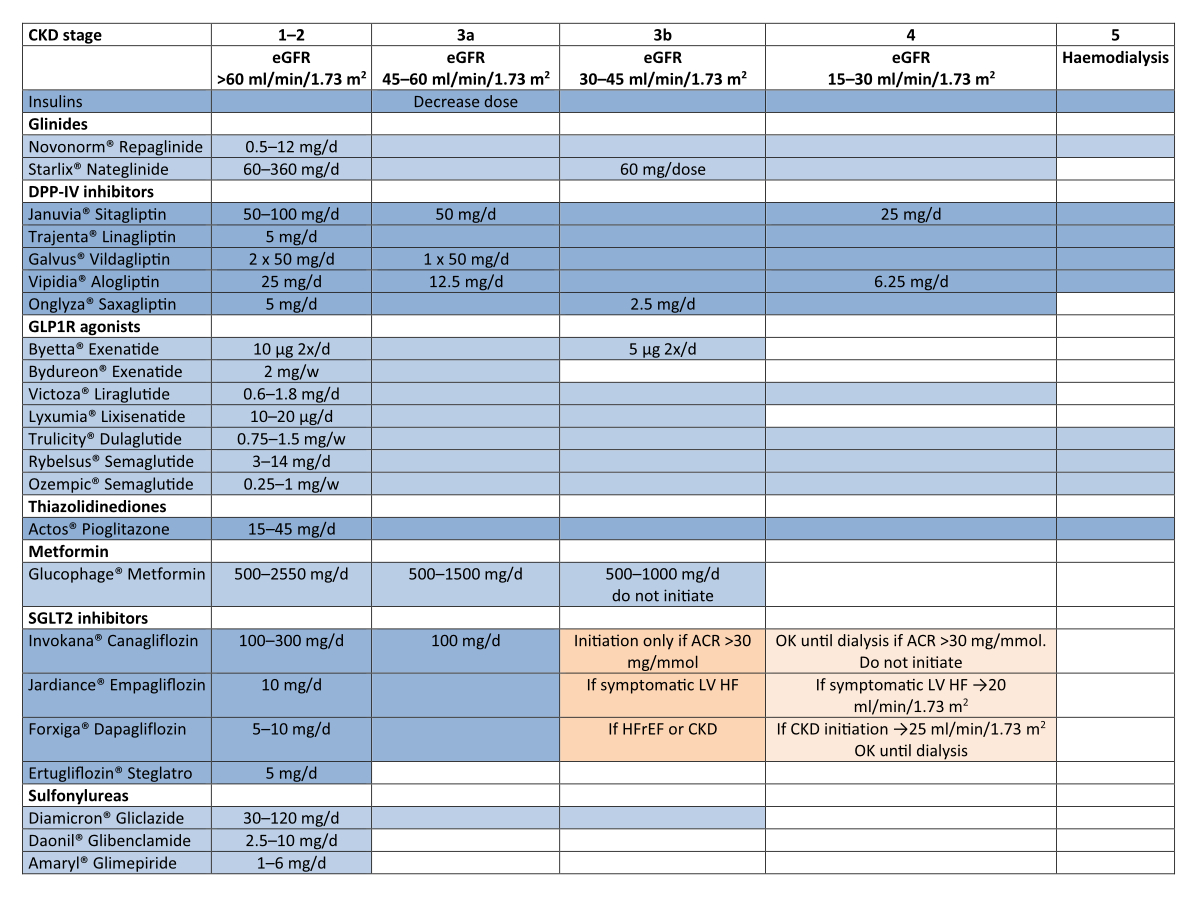
Figure 1 Adjustment of dosages according to eGFR (Swissmedic [Switzerland], for other countries refer to local restrictions).
DOI: https://doi.org/10.57187/smw.2023.40004
For more than 20 years, the standard therapy of patients at risk of or with diabetic kidney disease included efficient glucose and blood pressure control and the use of renin-angiotensin system inhibitors. Although these therapies slow the decline in renal function, the number of patients with end-stage renal disease secondary to diabetes is still on the rise all around the world due to the high prevalence of diabetes, obesity and an aging population. Recently, large studies have demonstrated remarkable renal protective properties of new classes of drugs in type 2 diabetes. For this reason, prior recommendations published in this journal in 2014 need an update.
Patients with diabetic kidney disease are among the most complex patients in diabetes care. Their care is multifactorial and multidisciplinary, involving different groups of healthcare providers. The primary care physician, the diabetologist, the nephrologist, the nutritionist and the specialised nurse, among others, need to rely on a common view while treating these patients.
It has become evident that a consensus document is necessary to help all healthcare providers involved in the care of patients with diabetic kidney disease. With this consensus, we propose a concise document summarizing the important topics around diabetic kidney disease. It includes therapies with proven efficacy and which are available in Switzerland. It largely extends the document in 2014 endorsed by the Swiss Society of Endocrinology and Diabetes (SGED/SSED). This consensus will be updated yearly on its digital platform (diabetic kidney disease SSED/SGED (www.sgedssed.ch) and Swiss society of Nephrology (SSN) (www.swissnephrology.ch) guidelines; www.guidelines.ch).
The working group included diabetologists and nephrologists across Switzerland and extended between 2019 and 2022. Those participating in the workshop are co-authors of the consensus. Before its publication, it was reviewed by the Swiss Society of Endocrinology and Diabetes and by the Swiss Society of Nephrology.
References for this section: [1–11]
Screening for diabetic kidney disease (DKD) is important because it is a silent disease and symptoms develop only at very late stages. Primary care physicians and endocrinologists remain central to the screening process. The yearly recommended screening of creatinine-based estimated glomerular filtration rate (eGFR) and urine albumin/-creatinine ratio has not changed for many decades and will identify patients with significant kidney disease. Yet the urine albumin/creatinine ratio is often lacking in the annual workup, as is the calculation of creatinine-based eGFR. Therefore, there is a constant need to improve physician awareness of diabetic kidney disease by implementing systematic screening and clear classification of patients with diabetes and nephropathy. New biomarkers identifying patients with early renal function decline are actively being investigated (such as tumour necrosis factor [TNF] receptors 1 and 2, kidney injury molecule-1 [KIM-1]). They will hopefully provide a tool for better stratification of patients and intervention in the early stages of diabetic kidney disease.
Diabetic kidney disease
Diabetic nephropathy
Yearly screening in all (table 1).
Classification: KDIGO G1–5, A1–3 (table 1).
Expert opinion: dynamics over time are important to document as:
Table 1KDIGO 2012 classification and recommended frequency of monitoring per annum (modified from: Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020 Oct;98(4S):S1-S115 [1]).
| Guide to frequency of monitoring (number of times per year) by GFR and albuminuria category | Persistent albuminuria categories. Description and range | |||||
| A1 | A2 | A3 | ||||
| Normal to mildly increased | Moderately increased | Severely increased | ||||
| <3 mg/mmol | 3–30 mg/mmol | >30 mg/mmol | ||||
| GFR categories (ml/min/1.73 m2): description and range | G1 | Normal or high | ≥90 | 1 if CKD | 1 | 2 |
| G2 | Mildly decreased | 60–89 | 1 if CKD | 1 | 2 | |
| G3a | Mildly to moderately decreased | 45–59 | 1 | 2 | 3 | |
| G3b | Moderately to severely decreased | 30–44 | 2 | 3 | 3 | |
| G4 | Severely decreased | 15–29 | 3 | 3 | 4+ | |
| G5 | Kidney failure | <15 | 4+ | 4+ | 4+ | |
GFR: glomerular filtration rate
––Twenty-four-hour urine collection is only recommended in situations where creatinine values are less accurate in the estimation of GFR (see above). However it has several caveats (errors in urine collection, tubular creatinine secretion with declining renal function).
References for this section: [1, 2, 12–32]
The 1990s demonstrated that tight glycaemic control prevents the early stages of diabetic kidney disease in type 1 diabetes (DCCT, EDIC), which was confirmed in type 2 diabetes later on. Recently, new classes of antidiabetic drugs have been proven to have powerful renal protective effects in type 2 diabetes, particularly the sodium-glucose cotransporter-2 (SGLT2) inhibitor class. Their effects are beyond glucose control, opening an exciting period in the field of chronic kidney disease. This section highlights the important facts around glycaemic control and antidiabetic drugs in diabetic kidney disease. For general information on antidiabetic therapy in type 2 diabetes, we refer to www.sgedssed.ch. Only frequently prescribed antidiabetic drugs are discussed.

Figure 1 Adjustment of dosages according to eGFR (Swissmedic [Switzerland], for other countries refer to local restrictions).
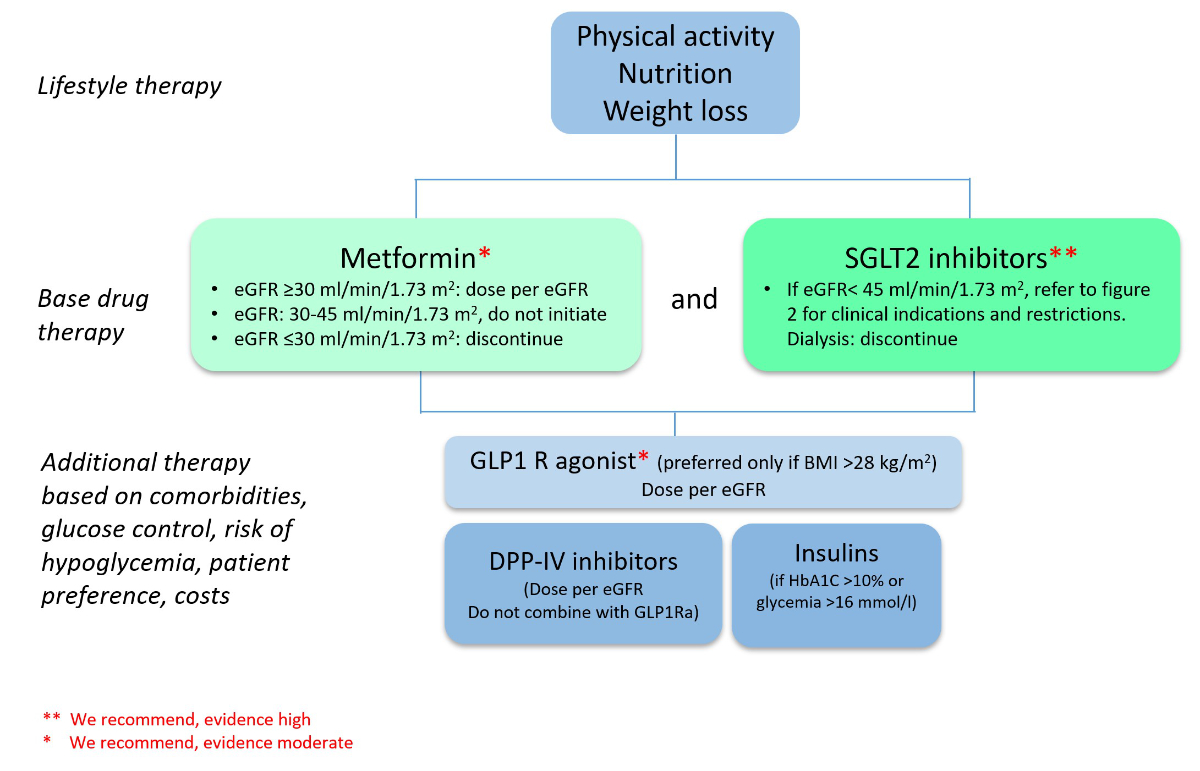
Figure 2 Antidiabetic therapy in chronic kidney disease stage G1–3 A2–3 (modified from: Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020 Oct;98(4S):S1-S115 [1]).
Per eGFR see figure 1.
Comment: dual SGLT2 inhibitor – GLP1 agonist therapy is under investigation. Preliminary results demonstrate additional effects on weight, blood glucose and blood pressure control.
References for this section: [33–40]
Hypertension and diabetes coexist in a vast majority of patients with type 2 diabetes. Antihypertensive therapy is beneficial for both cardiovascular and renal outcomes in patients with type 2 diabetes and is central to the standard of care in this population. The exact goal of blood pressure control remains unclear as studies with specific blood pressure goals in diabetic kidney disease are lacking. The UKPDS, HOT and ADVANCE BP trials, dedicated to patients with type 2 diabetes, failed to achieve a systolic blood pressure goal of <130 mm Hg. The ACCORD BP study showed no clear evidence that a systolic blood pressure goal of <120 mm Hg is beneficial for cardiovascular endpoints, except for stroke reduction and albuminuria progression with, however, more serious adverse events such as hypotension and hyperkalaemia. Thus, proposed goals are based on consensus statements and may differ from each other. A goal of <140/90 mm Hg has a high level of evidence for cardiac and renal protection whereas a goal <130 mm Hg has a high level of evidence for stroke reduction. The ADA 2021 guidelines recommend targets of <140/90 mm Hg in all or <130/80 mm Hg in those at higher cardiovascular risk (established or 10-year atherosclerotic cardiovascular disease [ASCVD] risk >15%). KDIGO 2021 guidelines recommend a systolic blood pressure goal of <120 mm Hg using standardized office blood pressure measurement in patients with chronic kidney disease, with or without diabetes, not undergoing dialysis. However, KDIGO acknowledge that the evidence supporting such a goal is less certain in diabetes.
Both angiotensin converting-enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) reduce the progression of albuminuria in diabetic kidney disease more effectively than other drug classes. The renal protective effects are beyond the blood pressure lowering effects. They are recommended as first line therapy (fig. 3).
Table 2Classification of office blood pressure* and definition of hypertension grade** (modified from: Williams B, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens. 2018;36(10):1953–2041 [36]).
| Category | Systolic (mm Hg) | Diastolic (mm Hg) | |
| Optimal | <120 | and | <80 |
| Normal | 120–129 | and/or | 80–84 |
| High normal | 130–139 | and/or | 85–89 |
| Grade 1 hypertension | 140–159 | and/or | 90–99 |
| Grade 2 hypertension | 160–179 | and/or | 100–109 |
| Grade 3 hypertension | ≥180 | and/or | ≥110 |
| Isolated systolic hypertension | ≥140 | and | <90 |
* Blood pressure category is defined according to seated clinic measurement and by the highest level, whether systolic or diastolic.
** The same classification is used for all ages from 16 years.
Table 3Definition of hypertension according to office, ambulatory and home blood pressure levels (modified from Williams et al. J Hypertens. 2018;36:1953-2041 [36]).
| Category | Systolic (mm Hg) | Diastolic ( mm Hg) | ||
| Office blood pressure* | ≥140 | and/or | ≥90 | |
| Ambulatory blood pressure | Daytime (or awake) mean | ≥135 | and/or | ≥85 |
| Night-time (or asleep) mean | ≥120 | and/or | ≥70 | |
| 24-hour mean | ≥130 | and/or | ≥80 | |
| Home blood pressure mean | ≥135 | and/or | ≥85 | |
* Refers to conventional office blood pressure rather than unattended office blood pressure
Table 4Office blood pressure treatment target ranges (modified from Williams et al. J Hypertens. 2018;36:1953-2041 [36]).
| Diabetes | CKD | ||
| SBP | Age 18–65 | Target to 130 or lower if tolerated, not <120 | Target 130–139 if tolerated |
| Age ≥65 | Target to 130–139 if tolerated | Target 130–139 if tolerated | |
| DBP | 70–79 | 70–79 | |
CKD: chronic kidney disease; DBP: diastolic blood pressure; SBP: systolic blood pressure
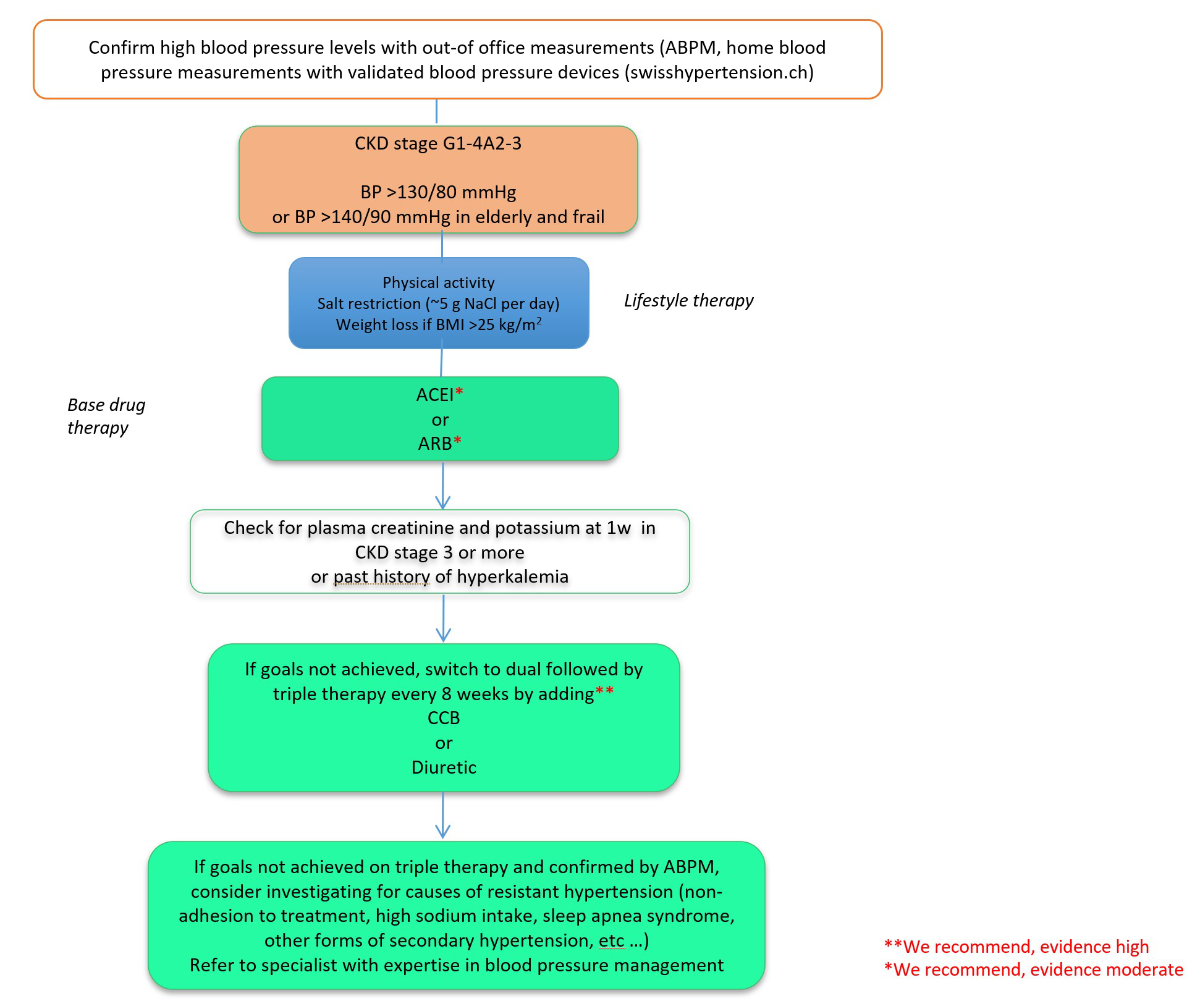
Figure 3 Proposed algorithm of blood pressure target and management in diabetic kidney disease.
References for this section: [41–45]
The mineraolcorticoid receptor is an important contributor to the development of diabetic kidney disease. Mineralocorticoid receptor overactivation is assumed to promote kidney inflammation and fibrosis in diabetic individuals. The steroidal mineralocorticoid receptor antagonists (MRAs) spironolactone and eplerenone reduce albuminuria either as monotherapy or on top of ACE inhibitor or ARB treatment in diabetic kidney disease. They are also indicated for the treatment of HFrEF and refractory arterial hypertension. However, no studies examine the impact of steroidal MRA treatment on hard endpoints in diabetic kidney disease.
Finerenone, a specific and nonsteroidal MRA improves renal (–18%, number needed to treat [NNT] 29, p = 0.001) and cardiovascular outcomes (3PMACE+HHF, –14%, NNT 42, p = 0.03) if given in addition to maximum tolerated RAS blockade, with only a modest effect on blood pressure in patients with type two diabetes, proteinuric diabetic kidney disease and an eGFR of 25–75 ml/min/1.73m2. Treatment doubles the risk of hyperkalaemia. Only 4.6% of the patients were on an SGLT2 inhibitor, therefore the exact treatment effect of combination therapy is unclear. Also, no head-to-head comparisons of the cost-effectivenes of different MRAs exist. Post-hoc analyses suggest that the addition of finerenone to a SGLT2 inhibitor further reduces albuminuria. In addition, cardiorenal protection with finerenone appears to be independent of SGLT2 inhibitor use. Finally, the risk of hyperkalaemia was significantly lower with the SGLT2 inhibitor + finerenone combination.
References for this section: [46–57]
Metabolic factors, among them dyslipidaemia and diabetes, are the most important modifiable cardiovascular risk factors and cardiovascular diseases are the most important causes of death in both patients with diabetes and patients with chronic kidney disease. Therefore, lipid-lowering treatment is among the cornerstones of cardiovascular disease prevention in both diabetes and chronic kidney disease. This has been adopted in recent guidelines, which uniformly advocate the use of lipid-lowering treatment, mostly statins, in patients with diabetes and chronic kidney disease not requiring dialysis.
Table 5Current recommendations on cardiovascular risk stratification in diabetes and chronic kidney disease [51, 58].
| EAS/ESC, 2021 | Very high risk if | Patients with DM with established ASCVD and/or severe target organ damage | eGFR <45 ml/min/1.73 m2 irrespective of albuminuria |
| eGFR 45–59 ml/min/1.73 m2 and microalbuminuria (ACR 3–30 mg/mmol) | |||
| ACR >30 mg/mmol | |||
| Presence of microvascular disease in at least 3 sites (i.e., ACR >3 mg/mmol plus retinopathy plus neuropathy) | |||
| High risk if | Patients with DM of >10 years duration or ≥1 CVD risk factor without ASCVD or target organ damage | ||
| Moderate risk if | Patients with DM and none of the above | ||
ACR: urinary albumin/creatinine ratio; ASCVD: atherosclerotic cardiovascular disease: DM: diabetes mellitus;
Table 6Current recommendations on lipid lowering treatment in diabetes with chronic kidney disease [51].
| CKD stage/risk category | Treatment | LDL cholesterol goal | |
| EAS/ESC, 2021 | Very high >G3b or G3aA2 or A3 | High intensity statin (Class IA) ± ezetimibe (Class IB) / PCSK9 inhibitor (Class IIbC) | Step 1: <1.8 mmol/l and 50% reduction from baseline |
| Step 2: <1.4 mmol/l* (if established ASCVD Class IA, if not IIbC) |
ASCVD: atherosclerotic cardiovascular disease; LDL: low-density lipoprotein
*Based on residual 10-year cardiovascular risk, lifetime cardiovascular risk and treatment benefit, comorbidities, frailty and patient preferences.
Class IA (recommended, high evidence), IB (recommended, moderate evidence), IIbC (may be considered, low evidence).
References for this section: [59–65]
Diabetes mellitus, chronic kidney disease and treatment with blockers of the renin-angiotensin-aldosterone system (RAAS) are major risk factors for hyperkalaemia. For this reason, patients with diabetic kidney disease are at high risk of hyperkalaemia. Up to now, there are no clinical trials studying relevant clinical outcomes in patients with diabetic kidney disease and chronic hyperkalaemia. Novel molecules are or will soon be available for potassium control in chronic kidney disease, and are briefly discussed here. The recommendations are not specific to diabetic kidney disease.
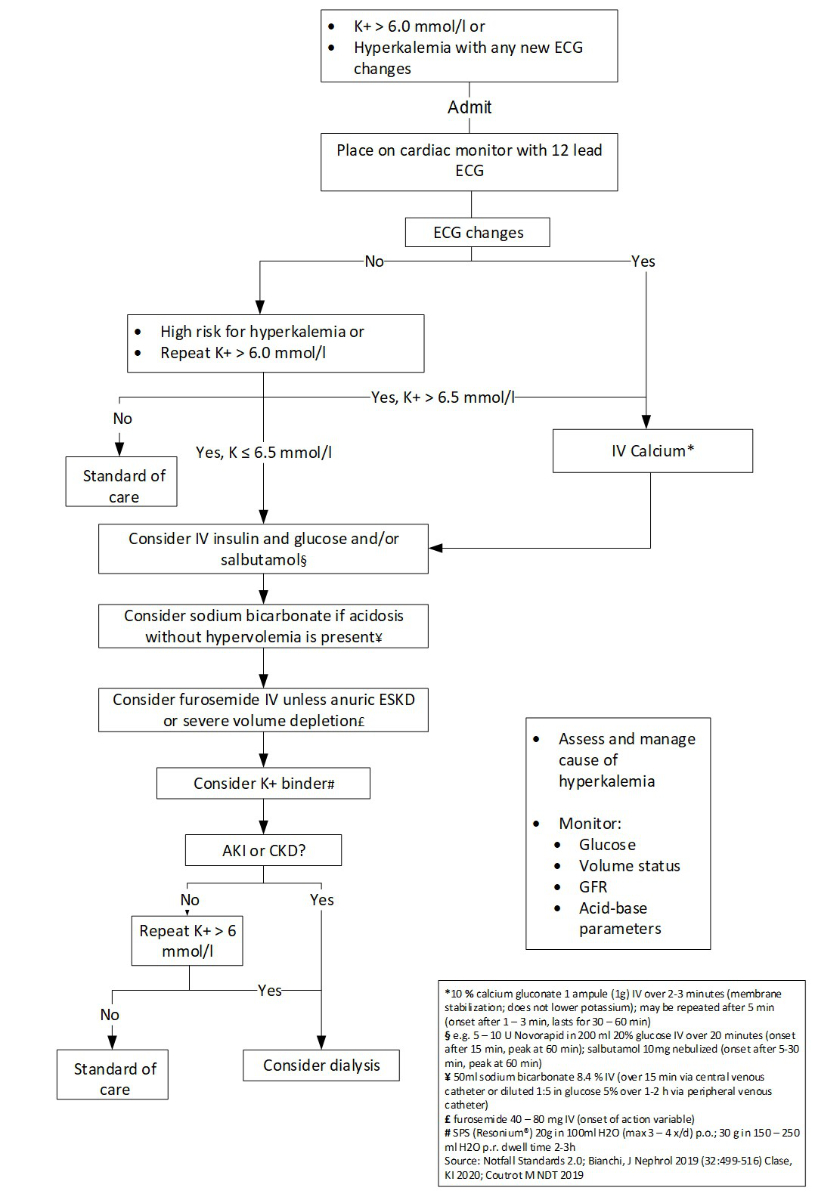
Figure 4 Treatment of acute hyperkalaemia. Suggested management algorithm for acute hyperkalaemia in adult patients (adapted from: Clase CM, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97(1):42–61 [59]). Depending on the patient and the clinical situation, the sequence of procedures may be adapted.
Mild to moderate stable hyperkalaemia (4.5–6 mmol/l)
Severe hyperkalaemia (>6 mmol/l)
Table 7Potassium binders (modified from [61–63]).
| Polystyrene sulphonate (Resonium®) | Patiromer (Veltassa®) | SZC (sodium zirconium cyclosilicate) | |
| Approval in Switzerland | Yes | Yes* | No |
| Formulation | Dissolvable powder | Dissolvable powder | |
| Application | Oral or rectal | Oral | Oral |
| Counterion | Sodium | Calcium | Sodium |
| Cations bound | K+, Mg2+, Ca2+ | K+, Mg2+ | K+ |
| Chemical properties | Polymer; sodium salt of polystyrene sulphonic acid | Polymer; patiromer sorbitex calcium | Non-polymer; non-absorbed zirconium silicate |
| Mechanism of action | Exchanges Na+ for K+, Mg2+, Ca2+ | Exchanges Ca2+ for K+; also binds Mg2+ | Captures K+ in exchange for hydrogen and Na+ |
| Counterion content | Na+: 100 mg/g SPS | Ca2+: 191 mg/g patiromer | Na+: 80 mg/g SZC |
| Site of action | Colon/rectum | Distal colon | Entire GI tract |
| Onset of action | 1–2 h | 4–7 h | 1 h |
| interactions | Lithium, levothyroxin edigitalis, sorbitol | Reduced systemic exposure of coadministered ciprofloxacin, metformin, and levothyroxine. No interaction when patiromer and these drugs were taken 3 h apart | No significant drug-drug interactions |
| Separate from oral medications by at least 3 h before or 3 h after; if gastroparesis, separate other medications by 6 h | Take other oral medications at least 3 h before or 3 h after administration | Take other oral medications with gastric pH-dependent availability at least 2 h before or 2 h after | |
| Side effects | GI: nausea, vomiting, diarrhoea, constipation. Serious GI effects: ileus, intestinal ulcer/necrosis, perforation, haemorrhage, ischaemic colitis. Electrolyte disturbance: hypokalaemia, hypocalcaemia, hypomagnesaemia, oedema and hypertension due to sodium retentionH | Hypomagnesaemia and hypokalaemia; diarrhaea, constipation, nausea, flatulence, abdominal discomfort; potentially calcium overloading | Hypokalaemia, Oedema |
| Setting | Acute hyperkalaemia | Chronic hyperkalaemia | Acute and chronic hyperkalaemia |
| Dosage | 15–60 g (1–4×/d); Rectal: 30–50 g (1–4×/d) | Initial: 8.4 g qd (max.: 25.2 g orally once daily ); dose can be increased by 8.4 g increments at one week intervals | Initial: 10 g orally 3 times daily for 48 h |
| Maintenance dose | 15–60 g once daily | 8.4–25.2g once daily | 10 g |
| Cost | + | +++ | |
* Veltassa® is reimbursed by the health insurance company after consultation with the doctor in charge for adult, non-dialysed patients with CKD (treatment must be started in CKD stage G3 or 4; the glomerular filtration rate must be below 60 ml/min/1.73 m2), who developed chronic recurrent hyperkalemia during therapy with an inhibitor of the renin-angiotensin-aldosterone system, as determined by repeated measurements, and for whom cation exchange resins must be used because the non-drug measures (diet) and the previous drug measures (e.g. potassium-lowering diuretics) were not sufficient to normalize potassium levels (below 5.5 mmol/l). The initial prescription of Veltassa® can only be made by a nephrologist or cardiologists.
References for this section: [66–72]
Anaemia is a common complication of all types of renal disease, occurring usually in advanced stages. Its pathophysiology is multifactorial, involving deficient erythropoietin production, decreased iron availability and inflammation among others. In patients with type 2 diabetes and chronic kidney disease, anaemia is associated with an increased risk of renal and cardiovascular events. Anaemia is also associated with increased mortality and a higher risk for hospitalisation in chronic kidney disease.
There are no specific recommendations for the management of anaemia in diabetic kidney disease as compared to nondiabetic kidney diseases; therefore, anaemia guidelines for chronic kidney disease apply (KDIGO anaemia guidelines).
Definition of anaemia: <12 g/dl in females, <13 g/dl in males.
Frequency of anaemia monitoring:
Investigations:
Consider:
Iron therapy in anaemic patients:
ESA use in anaemic patients
In dialysis and nondialysis patients with chronic kidney disease, several studies have shown that targeting haemoglobin levels ≥13 g/dl increases the risk of adverse outcomes. The TREAT trial was a randomised, double-blind, placebo-controlled study in 4000 nondialysis diabetic patients with chronic kidney disease. Half received darbepoetin alfa to target a haemoglobin level of 13 g/dl, while the other half received placebo and were treated with an ESA only if their hemoglobin fell below 9 g/dl. The group with the higher haemoglobin concentration showed a significant reduction in the need for blood transfusions, but at best a marginal improvement in quality of life. TREAT failed to show a beneficial effect of higher haemoglobin on hard cardiovascular or renal endpoints. In contrast, the risk of venous and arterial thromboembolism increased significantly in the high haemoglobin group, and the risk of stroke was almost double in patients in the higher haemoglobin arm. In the group of patients who had malignant disease at baseline, the number of cancer-related deaths was increased more than tenfold in the higher haemoglobin group.
References for this section: [73–75]
Chronic kidney disease-mineral and bone disorder (CKD-MBD) is a universal complication of progressive loss of kidney function. Biochemical abnormalities, vascular calcification and bone fragility constitute the CKD-MBD syndrome. CKD-MBD is associated with increased risks for morbidity and mortality in observational studies.
For diabetic kidney disease, there are no specific guidelines and recommendations for the management of CKD-MBD. The proposed recommendations reflect guidelines for chronic kidney disease irrespective of aetiology.
The frequency of laboratory tests should consider chronic kidney disease stage and progression, and individual factors to monitor trends and treatment efficacy.
CKD KDIGO stage G3a–G3b:
CKD KDIGO stage G4:
CKD KDIGO stage G5, including G5D:
For chronic kidney disease stage G3a–G5D vascular calcifications should be assessed in an individual approach to detect patients at the highest risk for cardiovascular events.
This topic should be managed by an experienced nephrologist as inadequate therapy may do more harm than good. Patients with diabetes and chronic kidney disease are at increased risk for osteoporosis and renal osteodystrophy. Importantly, osteoporosis and renal osteodystrophy are distinct disorders. Their prevalence depends on the disease stage. Osteoporosis can be present alone or in combination with renal osteodystrophy (fig. 5).
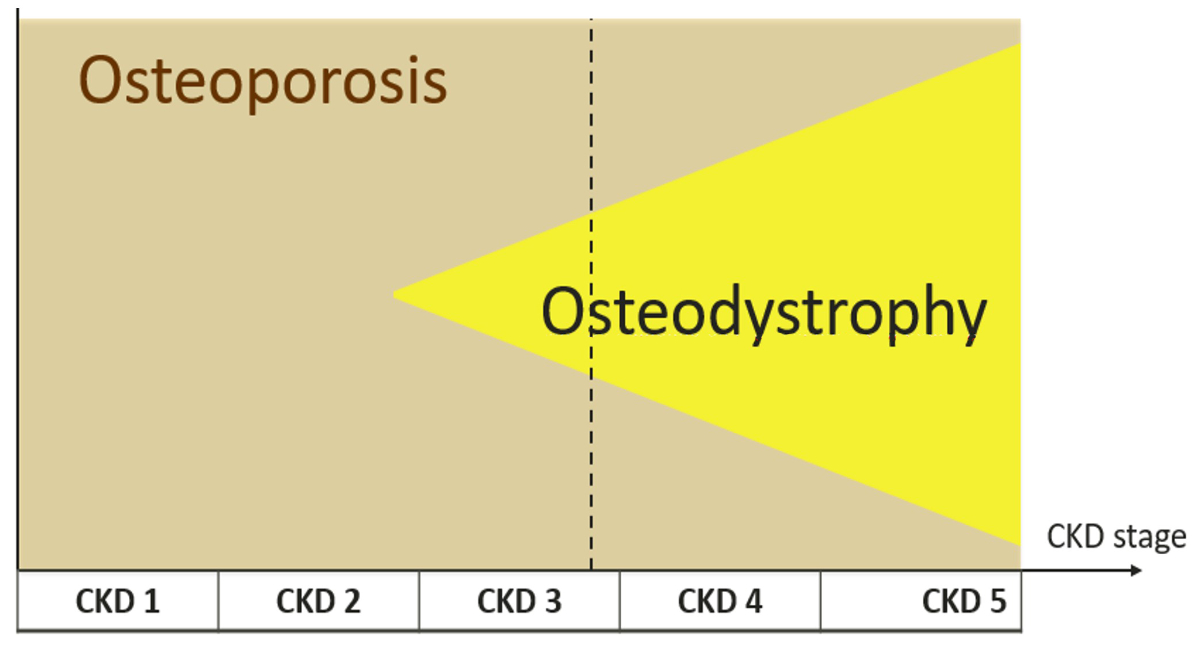
Figure 5 Osteoporosis and osteodystrophy in chronic kidney disease (CKD).
The term “renal osteodystrophy” is used to describe alterations in bone morphology connected to chronic kidney disease detected on bone biopsy. It is classified into five distinct forms, which can overlap: osteitis fibrosa, mild hyperparathyroidism, osteomalacia, adynamic bone disease, and mixed uraemic osteodystrophy.
References for this section: [76–78]
Metabolic acidosis is characterised by a serum bicarbonate level <22 mmol/l in an individual with normal pulmonary function. It is common in chronic kidney disease and represents an independent and modifiable risk factor for progression of the disease. Importantly, even before frank metabolic acidosis occurs, multiple adaptive responses that increase acid excretion are activated. They include activation of pathways, such as the intrakidney RAAS, that mediate the immediate benefit of increased acid excretion, but chronically become maladaptive and promote a decline in kidney function. Importantly, patients with diabetic kidney disease are at increased risk for type IV renal tubular acidosis, with or without hyperkalaemia, caused by hyporeninaemic hypoaldosteronism.
For diabetic kidney disease and chronic metabolic acidosis, there are no specific guidelines. The proposed recommendations reflect current guidelines for chronic kidney disease irrespective of aetiology. This section does not discuss acute metabolic acidosis secondary to SGLT2 inhibitors (euglycaemic ketoacidosis) or metformin (lactic acidosis).
Overt metabolic acidosis commonly develops if GFR declines below 40 ml/min/1.73 m2. Importantly, in individuals with diabetic kidney disease it may manifest earlier due to type IV RTA, which has to be suspected in patients with hyperkalaemia.
Venous blood gas analysis is sufficient to measure bicarbonate concentration.
Suggested monitoring:
CKD KDIGO stage G3a–G3b:
CKD KDIGO stage G4:
CKD KDIGO stage G5, including G5D:
In patients receiving treatments for acidosis or with biochemical abnormalities:
Prevention and therapy of metabolic acidosis: dietary interventions and oral alkali supplements
References for this section: [79–84]
There is an increasing prevalence of diabetes in haemodialysis centres, reaching 30–45% of patients. These patients have variable clinical outcomes and life expectancy. On average, the 5-year mortality of patients with diabetes on haemodialysis is over 50%. Goals of glucose control should be individualised to the patient’s prognosis. Among patients with more stringent goals such as those on the transplantation list, basal bolus therapy is often proposed with 24-hour glucose monitoring.
See figure 2 for therapies indicated in end-stage renal disease.
Comments:
Continuous glucose monitoring has become the standard of care in patients at high risk of hypoglycaemia and on intensive insulin regimens. However, fluid shifts between interstitial and intravascular spaces that occur during dialysis sessions, uraemia and acidosis have the potential to impact the performance of commercially available continuous glucose monitoring devices. Non-therapeutic continuous glucose monitoring for a short period in both haemodialysis and peritoneal dialysis was shown to improve glycaemic control thanks to more frequent treatment adaptations. There are ongoing studies examining the effectiveness of therapeutic continuous glucose monitoring devices with haemodialysis.
Although not all continuous glucose monitoring sensors are validated for haemodialysis, their use outside haemodialysis sessions is highly recommended. With the approval of the patient, the data collected in a cloud can be viewed at all times by healthcare providers. We review the advantages and disadvantages of systems for haemodialysis.
Flash system Freestyle Libre
CGMS Dexcom G6
CGMS Guardian Connect
Implantable sensor Eversense
References for this section: [85–96]
Glycated haemoglobin (HbA1c):
Self-monitoring of blood glucose:
Since icodextrin results in elevated blood levels of maltose, only glucose-specific monitors and test strips that utilise the enzyme glucose dehydrogenase must be used. Pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase test strips are contraindicated because they will give falsely elevated readings leading to insulin misuse and hypoglycaemia events. New test strips have been designed to minimise interference with non-glucose sugars and most glucometers in Switzerland are compatible. Companies providing dialysates have the information on interferences with glucometers.
Continuous glucose monitoring:
Data on the accuracy of therapeutic continuous glucose monitoring in the setting of peritoneal dialysis are still not available.
Indications and contraindications are similar to those discussed in haemodialysis (see above). The main difference relies on the use of antidiabetic drugs to prevent glucose fluctuations induced by peritoneal dialysis solutions. In this respect, insulin regimens offer the most adjustable therapies with a range of duration of action (from 1.5 to 48 hours). Timing of insulin action should take into account the abrupt onset of glucose diffusion at start of dialysis and the abrupt stop when glucose solution is drained.
In most patients on insulin therapy at initiation of peritoneal dialysis, insulin dosages should be increased especially in those receiving hypertonic exchanges. One study showed that diabetic patients receiving a standard 6 l/day dialysis exchange, had a 27% increase in insulin requirements.
References for this section: [97–106]
Solid organ transplantation is an established and routine therapeutic option that has transformed the survival and quality of life of patients with end-organ dysfunction. Post-transplant diabetes mellitus (PTDM), also known as new-onset diabetes after transplantation, is a common and important complication following solid organ transplantation. PTDM in kidney transplant patients is associated with decreased patient and graft survival and other adverse outcomes including increased cardiovascular risk, infection and graft rejection. The reported incidence of PTDM varies from 4% to 25% of kidney transplant recipients. Approximately 50% of kidney transplant recipients need antidiabetic therapy (including pre-existing diabetes and PTDM).
Table 8Risk of post-transplantation diabetes mellitus with different medications.
| Medication | Risk of post-transplantation diabetes mellitus |
| Corticosteroids | Increased |
| Tacrolimus | Increased |
| Ciclosporin | Slightly increased |
| mTOR ihibitor | Increased |
| Mycophenolic acid | Not diabetogenic |
| Azathioprine | Not diabetogenic |
| Belatacept | Not diabetogenic |
| Basiliximab | Probably increased |
| Thymoglobulin | Not diabetogenic |
A diagnosis of PTDM is valid in patients on a stable immunosuppressive regimen, in the absence of infection, and at least 46 days after transplantation. Although the criteria for PTDM are based on criteria for diabetes in the general population, it is unclear whether thresholds for diabetes risk are the same. Some data suggest that criteria for prediabetes and diabetes are all associated with mortality risk in kidney transplant patients.
Encourage self-monitoring of glucose early after transplantation.
References for this section: [107–111]
Life-style management including individualised nutrition therapy, physical activity and interventions for smoking cessation are cornerstones of diabetes management and cardiovascular disease risk reduction and should be reinforced at any time during the course of diabetes and diabetic kidney disease.
In overweight patients with mild to moderate chronic kidney disease, therapies favouring weight loss as treatment with GLP1 receptor agonists or SGLT2 inhibitors and lifestyle changes reinforced by programmes such as Diafit in Switzerland are highly encouraged. However, in more advanced disease (eGFR <30 ml/min/1.73 m2), weight loss may lead to muscle wasting and worse outcomes.
Living with diabetes and chronic kidney disease is a huge challenge for dietary adjustments. The combination of these two conditions makes diet more complicated, as restrictions required by the renal diet may conflict with previous dietary recommendations for diabetes. Successful dietary management requires careful planning, regular assessment of nutritional status and of laboratory values. Poor adherence to the diet puts patients at risk for acute complications such as fluid overload, hyperkalaemia, hyperphosphataemia as well as worsening kidney disease. Diet interventions are recommended to improve adherence to diet and to prevent muscle wasting, sarcopenia and cachexia, which contribute to frailty and morbidity. Early, individualised counselling and nutritional intervention in recently hospitalised patients with chronic kidney disease at nutritional risk are highly recommended with the recent demonstration of decreased mortality and complications at 30 days.
Details of dietary interventions are beyond the scope of these recommendations. All recommendations need to be adapted individually.
The general dietary approach we propose is as follows:
More than 30% of people with diabetes mellitus develop chronic kidney disease. A considerable number of them progress to kidney failure requiring dialysis or transplantation. Hence, there is a great need for efficient evidence-based management of these patients to minimise negative outcomes. Our guidelines address the relevant aspects and provide recommendations for the treatment of diabetic kidney disease and its complications. We also provide advice on screening for and establishing the diagnosis of chronic kidney disease in individuals with type 2 diabetes. Where evidence for diabetic kidney disease is lacking, we have integrated the current recommendations for chronic kidney disease in general.
Developments in recent years have brought effective new therapeutic options such as SGLT2 inhibitors and GLP1 receptor agonists which slow the progression of diabetic kidney disease and/or significantly lower the risk of cardiovascular complications.
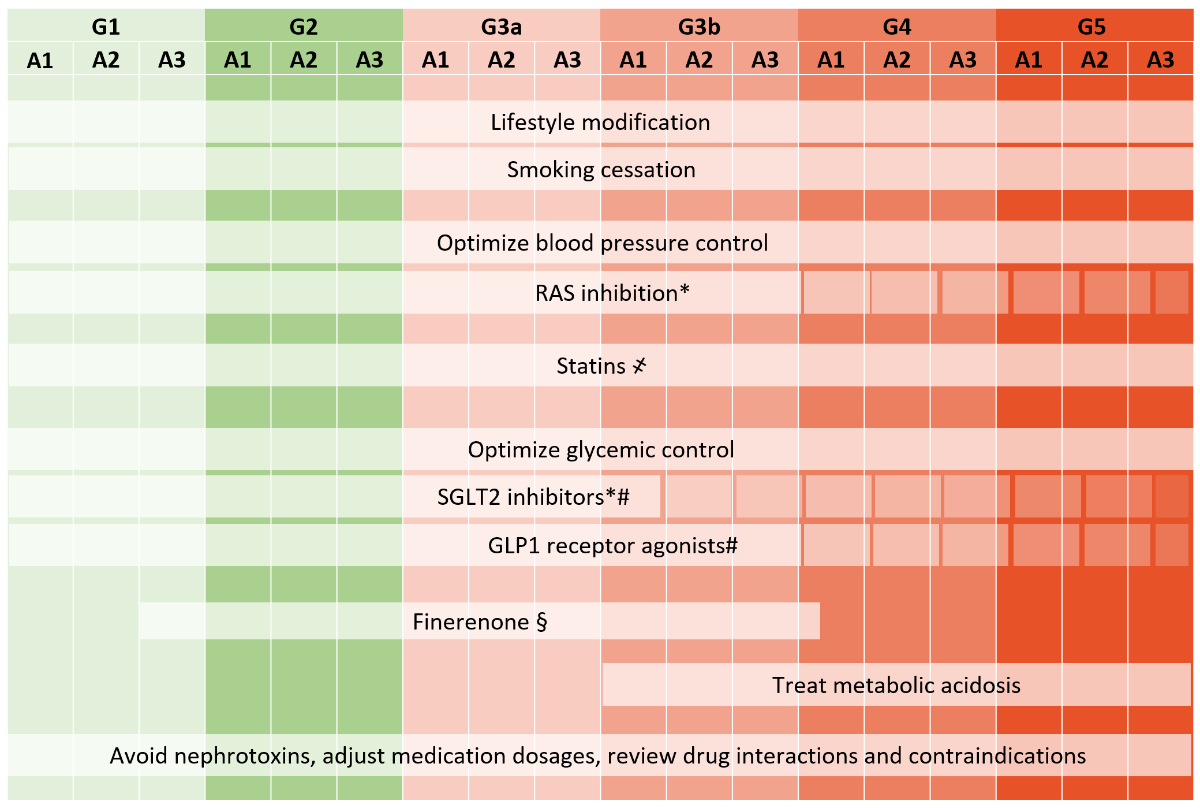
Figure 6 Interventions to slow DKD progression and/or reduce cardiovascular disease (adapted from: Shlipak MG, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99:34–47 [112]).
RAS blockade, SGLT2i and GLP1Ra use in more advanced CKD to be considered individually and based on drug label and figure 2.
҂ No statin initiation in dialysis.
* RAS blockers and SGLT2i slow eGFR decline in albuminuria stage A3.
# SGLT2i and GLP1Ra decrease renal and cardiovascular morbidity in high CV risk patients.
§ Cardiovascular and renal protection with finerenone in albuminuria stage A2 and A3.
Fortunately, the field of diabetic kidney diseases is still rapidly evolving. Several clinical trials of novel agents targeting different pathways in patients with diabetes mellitus are underway. New substances such as nonsteroidal mineralocorticoid receptor antagonists decrease renal and cardiovascular risk in patients with diabetic kidney disease. Endothelin antagonists are in the therapeutic pipeline. These new therapies might be combined with currently available drugs in the future such that an individualised approach can be accomplished. Due to the dynamic development, we plan to publish the guidelines on an electronic platform, so that they can be updated promptly in case of clinically relevant new findings. We are optimistic that our guidelines will significantly contribute to a high-quality multidisciplinary care of patients with diabetic kidney disease in Switzerland in the future.
We thank the SSN and SSED committees for carefully reviewing the document and bringing valuable suggestions.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. SdS participated to advisory boards for Astra Zeneca, Otsuka and Astellas. AZ has participated to advisory boards for NovoNordisk, Bayer, Astra Zeneca, Mundipharma and Boehringher Ingelheim. CC, FL and BV have no conflict of interest. HS participated in advisory boards for Mundipharma, Astra Zeneca, Vifor, Bayer, Astellas and Amgen. AJ has participated to advisory boards for Mundipharma Medical. SB has received advisory board honoraria and speaker fees from Novonordisk, Sanofi, Amgen, Daiichi-Sankyo, Novartis, Boehringer, Bayer, AstraZeneca. No other potential conflict of interest was disclosed.
1. de Boer IH, Caramori ML, Chan JC, Heerspink HJ, Hurst C, Khunti K, et al. Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4):S1–115. https://doi.org/10.1016/j.kint.2020.06.019
2. American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020 Jan;43 Suppl 1:S135–51. https://doi.org/10.2337/dc20-S011
3. Lamine F, Lalubin F, Pitteloud N, Burnier M, Zanchi A. Chronic kidney disease in type 2 diabetic patients followed-up by primary care physicians in Switzerland: prevalence and prescription of antidiabetic drugs. Swiss Med Wkly. 2016 Feb;146:w14282. https://doi.org/10.4414/smw.2016.14282
4. Delanaye P, Jager KJ, Bökenkamp A, Christensson A, Dubourg L, Eriksen BO, et al. CKD: A Call for an Age-Adapted Definition. J Am Soc Nephrol. 2019 Oct;30(10):1785–805. https://doi.org/10.1681/ASN.2019030238
5. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al.; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012 Jul;367(1):20–9. https://doi.org/10.1056/NEJMoa1114248
6. Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, et al.; CKD Biomarkers Consortium and the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Association of Multiple Plasma Biomarker Concentrations with Progression of Prevalent Diabetic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2021 Jan;32(1):115–26. https://doi.org/10.1681/ASN.2020040487
7. Nowak N, Skupien J, Smiles AM, Yamanouchi M, Niewczas MA, Galecki AT, et al. Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int. 2018 May;93(5):1198–206. https://doi.org/10.1016/j.kint.2017.11.024
8. Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, et al.; ADVANCE Collaborative group. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014 Dec;57(12):2465–74. https://doi.org/10.1007/s00125-014-3369-7
9. Gutiérrez OM, Sang Y, Grams ME, Ballew SH, Surapaneni A, Matsushita K, et al.; Chronic Kidney Disease Prognosis Consortium. Association of Estimated GFR Calculated Using Race-Free Equations With Kidney Failure and Mortality by Black vs Non-Black Race. JAMA. 2022 Jun;327(23):2306–16. https://doi.org/10.1001/jama.2022.8801
10. Guessous I, Ponte B, Marques-Vidal P, Paccaud F, Gaspoz JM, Burnier M, et al. Clinical and biological determinants of kidney outcomes in a population-based cohort study. Kidney Blood Press Res. 2014;39(1):74–85. https://doi.org/10.1159/000355779
11. Dubrofsky L, Srivastava A, Cherney DZ. Sodium-Glucose Cotransporter-2 Inhibitors in Nephrology Practice: A Narrative Review. Can J Kidney Health Dis. 2020 Jun;7:2054358120935701. https://doi.org/10.1177/2054358120935701
12. Diabetes C. Complications Trial Research G, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L and Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. https://doi.org/10.1056/NEJM199309303291401
13. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998 Sep;352(9131):837–53. https://doi.org/10.1016/S0140-6736(98)07019-6
14. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun;358(24):2545–59. https://doi.org/10.1056/NEJMoa0802743
15. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al.; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun;358(24):2560–72. https://doi.org/10.1056/NEJMoa0802987
16. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al.; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan;360(2):129–39. https://doi.org/10.1056/NEJMoa0808431
17. de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, et al.; DCCT/EDIC Research Group. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011 Dec;365(25):2366–76. https://doi.org/10.1056/NEJMoa1111732
18. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al.; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720
19. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al.; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016 Jul;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827
20. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al.; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016 Nov;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141
21. Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, et al.; Collaborators on Trials of Lowering Glucose (CONTROL) group. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017 Jun;5(6):431–7. https://doi.org/10.1016/S2213-8587(17)30104-3
22. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020 Oct;383(15):1413–24. https://doi.org/10.1056/NEJMoa2022190
23. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, et al.; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019 Jun;380(24):2295–306. https://doi.org/10.1056/NEJMoa1811744
24. Heerspink HJ, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al.; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020 Oct;383(15):1436–46. https://doi.org/10.1056/NEJMoa2024816
25. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019 Jan;393(10166):31–9. https://doi.org/10.1016/S0140-6736(18)32590-X
26. Heerspink HJ, Karasik A, Thuresson M, Melzer-Cohen C, Chodick G, Khunti K, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020 Jan;8(1):27–35. https://doi.org/10.1016/S2213-8587(19)30384-5
27. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al.; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021 Oct;385(16):1451–61. https://doi.org/10.1056/NEJMoa2107038
28. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019 Nov;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303
29. Wiviott SD, Raz I, Sabatine MS. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. Reply [Reply]. N Engl J Med. 2019 May;380(19):1881–2.
30. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al.; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019 Jul;394(10193):131–8. https://doi.org/10.1016/S0140-6736(19)31150-X
31. Borg R, Persson F, Siersma V, Lind B, de Fine Olivarius N, Andersen CL. Interpretation of HbA1c in primary care and potential influence of anaemia and chronic kidney disease: an analysis from the Copenhagen Primary Care Laboratory (CopLab) Database. Diabet Med. 2018 Dec;35(12):1700–6. https://doi.org/10.1111/dme.13776
32. Hassanein M, Shafi T. Assessment of glycemia in chronic kidney disease. BMC Med. 2022 Apr;20(1):117. https://doi.org/10.1186/s12916-022-02316-1
33. Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al.; HOT Study Group. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998 Jun;351(9118):1755–62. https://doi.org/10.1016/S0140-6736(98)04311-6
34. Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, et al.; ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007 Sep;370(9590):829–40. https://doi.org/10.1016/S0140-6736(07)61303-8
35. Margolis KL, O’Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014 Jun;37(6):1721–8. https://doi.org/10.2337/dc13-2334
36. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al.; Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018 Oct;36(10):1953–2041. https://doi.org/10.1097/HJH.0000000000001940
37. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998 Sep;317(7160):703–13. https://doi.org/10.1136/bmj.317.7160.703
38. American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021 Jan;44 Suppl 1:S125–50. https://doi.org/10.2337/dc21-S010
39. Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021 Mar;99(3):559–69. https://doi.org/10.1016/j.kint.2020.10.026
40. Agarwal R, Sinha AD, Cramer AE, Balmes-Fenwick M, Dickinson JH, Ouyang F, et al. Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease. N Engl J Med. 2021 Dec;385(27):2507–19. https://doi.org/10.1056/NEJMoa2110730
41. Kawanami D, Takashi Y, Muta Y, Oda N, Nagata D, Takahashi H, et al. Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease. Front Pharmacol. 2021 Oct;12:754239. https://doi.org/10.3389/fphar.2021.754239
42. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al.; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020 Dec;383(23):2219–29. https://doi.org/10.1056/NEJMoa2025845
43. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al.; FIGARO-DKD Investigators. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021 Dec;385(24):2252–63. https://doi.org/10.1056/NEJMoa2110956
44. Rossing P, Filippatos G, Agarwal R, Anker SD, Pitt B, Ruilope LM, et al.; FIDELIO-DKD Investigators. Finerenone in Predominantly Advanced CKD and Type 2 Diabetes With or Without Sodium-Glucose Cotransporter-2 Inhibitor Therapy. Kidney Int Rep. 2021 Oct;7(1):36–45. https://doi.org/10.1016/j.ekir.2021.10.008
45. Agarwal R, Joseph A, Anker SD, Filippatos G, Rossing P, Ruilope LM, et al.; FIDELIO-DKD Investigators. Hyperkalemia Risk with Finerenone: results from the FIDELIO-DKD Trial. J Am Soc Nephrol. 2022 Jan;33(1):225–37. https://doi.org/10.1681/ASN.2021070942
46. Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, et al. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol. 2018 Dec;14(12):727–49. https://doi.org/10.1038/s41581-018-0072-9
47. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013 Jul;382(9889):339–52. https://doi.org/10.1016/S0140-6736(13)60595-4
48. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al.; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020 Jan;41(1):111–88. https://doi.org/10.1093/eurheartj/ehz455
49. Cholesterol Treatment Trialists C. Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J and Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–25. https://doi.org/10.1016/S0140-6736(08)60104-X
50. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014 Jun;85(6):1303–9. https://doi.org/10.1038/ki.2014.31
51. Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al.; ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Sep;42(34):3227–337. https://doi.org/10.1093/eurheartj/ehab484
52. Wang N, Fulcher J, Abeysuriya N, Park L, Kumar S, Di Tanna GL, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 2020 Jan;8(1):36–49. https://doi.org/10.1016/S2213-8587(19)30388-2
53. Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu MD, et al.; Cholesterol Treatment Trialists’ (CTT) Collaboration. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016 Oct;4(10):829–39. https://doi.org/10.1016/S2213-8587(16)30156-5
54. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al.; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011 Jun;377(9784):2181–92. https://doi.org/10.1016/S0140-6736(11)60739-3
55. Charytan DM, Sabatine MS, Pedersen TR, Im K, Park JG, Pineda AL, et al.; FOURIER Steering Committee and Investigators. Efficacy and Safety of Evolocumab in Chronic Kidney Disease in the FOURIER Trial. J Am Coll Cardiol. 2019 Jun;73(23):2961–70. https://doi.org/10.1016/j.jacc.2019.03.513
56. Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al.; Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003 Jun;361(9374):2024–31. https://doi.org/10.1016/S0140-6736(03)13638-0
57. Palmer SC, Navaneethan SD, Craig JC, Perkovic V, Johnson DW, Nigwekar SU, et al. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst Rev. 2014 Jan;2014(1):CD005019. https://doi.org/10.1002/14651858.CD005019.pub4
58. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019 Jun;73(24):e285–350. https://doi.org/10.1016/j.jacc.2018.11.003
59. Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, et al.; Conference Participants. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020 Jan;97(1):42–61. https://doi.org/10.1016/j.kint.2019.09.018
60. Natale P, Palmer SC, Ruospo M, Saglimbene VM, Strippoli GF. Potassium binders for chronic hyperkalaemia in people with chronic kidney disease. Cochrane Database Syst Rev. 2020 Jun;6(6):CD013165.
61. Bridgeman MB, Shah M, Foote E. Potassium-lowering agents for the treatment of nonemergent hyperkalemia: pharmacology, dosing and comparative efficacy. Nephrol Dial Transplant. 2019 Dec;34 Suppl 3:iii45–50. https://doi.org/10.1093/ndt/gfz223
62. Georgianos PI, Agarwal R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2018 Feb;93(2):325–34. https://doi.org/10.1016/j.kint.2017.08.038
63. Palmer BF. Potassium Binders for Hyperkalemia in Chronic Kidney Disease-Diet, Renin-Angiotensin-Aldosterone System Inhibitor Therapy, and Hemodialysis. Mayo Clin Proc. 2020 Feb;95(2):339–54. https://doi.org/10.1016/j.mayocp.2019.05.019
64. Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019 Oct;394(10208):1540–50. https://doi.org/10.1016/S0140-6736(19)32135-X
65. Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al.; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015 Jan;372(3):211–21. https://doi.org/10.1056/NEJMoa1410853
66. Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int. 2012 Nov;82(9):952–60. https://doi.org/10.1038/ki.2012.270
67. Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al.; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov;355(20):2071–84. https://doi.org/10.1056/NEJMoa062276
68. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al.; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009 Nov;361(21):2019–32. https://doi.org/10.1056/NEJMoa0907845
69. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al.; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov;355(20):2085–98. https://doi.org/10.1056/NEJMoa065485
70. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis. 1996 Jul;28(1):53–61. https://doi.org/10.1016/S0272-6386(96)90130-4
71. Collins AJ, Ma JZ, Ebben J. Impact of hematocrit on morbidity and mortality. Semin Nephrol. 2000 Jul;20(4):345–9.
72. Xia H, Ebben J, Ma JZ, Collins AJ. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol. 1999 Jun;10(6):1309–16. https://doi.org/10.1681/ASN.V1061309
73. Kidney Disease: Improving Global Outcomes CKDMBDUWG. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2011;2017(7):1–59.
74. Melamed ML, Chonchol M, Gutiérrez OM, Kalantar-Zadeh K, Kendrick J, Norris K, et al. The Role of Vitamin D in CKD Stages 3 to 4: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Am J Kidney Dis. 2018 Dec;72(6):834–45. https://doi.org/10.1053/j.ajkd.2018.06.031
75. Ruospo M, Palmer SC, Natale P, Craig JC, Vecchio M, Elder GJ, et al. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev. 2018 Aug;8(8):CD006023. https://doi.org/10.1002/14651858.CD006023.pub3
76. de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009 Sep;20(9):2075–84. https://doi.org/10.1681/ASN.2008111205
77. Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014 Nov;86(5):1031–8. https://doi.org/10.1038/ki.2014.83
78. Wesson DE, Buysse JM, Bushinsky DA. Mechanisms of Metabolic Acidosis-Induced Kidney Injury in Chronic Kidney Disease. J Am Soc Nephrol. 2020 Mar;31(3):469–82. https://doi.org/10.1681/ASN.2019070677
79. Abe M, Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015 May;11(5):302–13. https://doi.org/10.1038/nrneph.2015.38
80. Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014 Mar;27(2):135–45. https://doi.org/10.1111/sdi.12198
81. Ricks J, Molnar MZ, Kovesdy CP, Shah A, Nissenson AR, Williams M, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012 Mar;61(3):708–15. https://doi.org/10.2337/db11-1015
82. Bally L, Gubler P, Thabit H, Hartnell S, Ruan Y, Wilinska ME, et al. Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Kidney Int. 2019 Sep;96(3):593–6. https://doi.org/10.1016/j.kint.2019.03.006
83. Lu Y, Stamm C, Nobre D, Pruijm M, Teta D, Cherpillod A, et al. Changing trends in end-stage renal disease patients with diabetes. Swiss Med Wkly. 2017 Jul;147:w14458.
84. Navaneethan SD, Schold JD, Jolly SE, Arrigain S, Winkelmayer WC, Nally JV Jr. Diabetes Control and the Risks of ESRD and Mortality in Patients With CKD. Am J Kidney Dis. 2017 Aug;70(2):191–8. https://doi.org/10.1053/j.ajkd.2016.11.018
85. Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017 Feb;13(2):90–103. https://doi.org/10.1038/nrneph.2016.181
86. Uiterwijk H, Franssen CF, Kuipers J, Westerhuis R, Nauta FL. Glucose Exposure in Peritoneal Dialysis Is a Significant Factor Predicting Peritonitis. Am J Nephrol. 2020;51(3):237–43. https://doi.org/10.1159/000506324
87. Meng LF, Yang LM, Zhu XY, Zhang XX, Li XY, Zhao J, et al. Comparison of clinical features and outcomes in peritoneal dialysis-associated peritonitis patients with and without diabetes: A multicenter retrospective cohort study. World J Diabetes. 2020 Oct;11(10):435–46. https://doi.org/10.4239/wjd.v11.i10.435
88. Szeto CC, Chow KM, Leung CB, Kwan BC, Chung KY, Law MC, et al. Increased subcutaneous insulin requirements in diabetic patients recently commenced on peritoneal dialysis. Nephrol Dial Transplant. 2007 Jun;22(6):1697–702. https://doi.org/10.1093/ndt/gfl834
89. Khan SF, Ronco C, Rosner MH. Counteracting the Metabolic Effects of Glucose Load in Peritoneal Dialysis Patients; an Exercise-Based Approach. Blood Purif. 2019;48(1):25–31. https://doi.org/10.1159/000499406
90. Duong U, Mehrotra R, Molnar MZ, Noori N, Kovesdy CP, Nissenson AR, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol. 2011 May;6(5):1041–8. https://doi.org/10.2215/CJN.08921010
91. Li PK, Culleton BF, Ariza A, Do JY, Johnson DW, Sanabria M, et al.; IMPENDIA and EDEN Study Groups. Randomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patients. J Am Soc Nephrol. 2013 Nov;24(11):1889–900. https://doi.org/10.1681/ASN.2012100987
92. Okada E, Oishi D, Sakurada T, Yasuda T, Shibagaki Y. A Comparison Study of Glucose Fluctuation During Automated Peritoneal Dialysis and Continuous Ambulatory Peritoneal Dialysis. Adv Perit Dial. 2015;31:34–7.
93. Yarragudi R, Gessl A, Vychytil A. New-Onset Diabetes Mellitus in Peritoneal Dialysis and Hemodialysis Patients: Frequency, Risk Factors, and Prognosis-A Review. Ther Apher Dial. 2019 Dec;23(6):497–506. https://doi.org/10.1111/1744-9987.12800
94. Qayyum A, Chowdhury TA, Oei EL, Fan SL. Use of Continuous Glucose Monitoring in Patients with Diabetes Mellitus on Peritoneal Dialysis: Correlation with Glycated Hemoglobin and Detection of High Incidence of Unaware Hypoglycemia. Blood Purif. 2016;41(1-3):18–24. https://doi.org/10.1159/000439242
95. Oei E, Samad N, Visser A, Chowdhury TA, Fan SL. Use of continuous glucose monitoring in patients with diabetes on peritoneal dialysis: poor correlation with HbA1c and high incidence of hypoglycaemia. Diabet Med. 2016 Sep;33(9):e17–20. https://doi.org/10.1111/dme.12988
96. Xue C, Gu YY, Cui CJ, Zhou CC, Wang XD, Ruan MN, et al. New-onset glucose disorders in peritoneal dialysis patients: a meta-analysis and systematic review. Nephrol Dial Transplant. 2020 Aug;35(8):1412–9. https://doi.org/10.1093/ndt/gfz116
97. Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992-2000.
98. Jenssen T, Hartmann A. Emerging treatments for post-transplantation diabetes mellitus. Nat Rev Nephrol. 2015 Aug;11(8):465–77. https://doi.org/10.1038/nrneph.2015.59
99. Anderson S, Cotiguala L, Tischer S, Park JM, McMurry K. Review of Newer Antidiabetic Agents for Diabetes Management in Kidney Transplant Recipients. Ann Pharmacother. 2020;•••:1060028020951955.
100. Culliford A, Phagura N, Sharif A. Autosomal Dominant Polycystic Kidney Disease Is a Risk Factor for Posttransplantation Diabetes Mellitus: An Updated Systematic Review and Meta-analysis. Transplant Direct. 2020 Apr;6(5):e553. https://doi.org/10.1097/TXD.0000000000000989
101. Conte C, Maggiore U, Cappelli G, Ietto G, Lai Q, Salis P, et al. Supporting physicians in the management of metabolic alterations in adult kidney transplant recipients: a comment on the joint position statement of the Italian Society of Nephrology (SIN), the Italian Society for Organ Transplantation (SITO) and the Italian Diabetes Society (SID). J Nephrol. 2020 Oct;33(5):887–93. https://doi.org/10.1007/s40620-020-00839-5
102. Valderhaug TG, Jenssen T, Hartmann A, Midtvedt K, Holdaas H, Reisaeter AV, et al. Fasting plasma glucose and glycosylated hemoglobin in the screening for diabetes mellitus after renal transplantation. Transplantation. 2009 Aug;88(3):429–34. https://doi.org/10.1097/TP.0b013e3181af1f53
103. Hecking M, Haidinger M, Döller D, Werzowa J, Tura A, Zhang J, et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol. 2012 Apr;23(4):739–49. https://doi.org/10.1681/ASN.2011080835
104. Eide IA, Halden TA, Hartmann A, Åsberg A, Dahle DO, Reisaeter AV, et al. Mortality risk in post-transplantation diabetes mellitus based on glucose and HbA1c diagnostic criteria. Transpl Int. 2016 May;29(5):568–78. https://doi.org/10.1111/tri.12757
105. Hecking M, Sharif A, Eller K, Jenssen T. Management of post-transplant diabetes: immunosuppression, early prevention, and novel antidiabetics. Transpl Int. 2021 Jan;34(1):27–48. https://doi.org/10.1111/tri.13783
106. Kanbay M, Demiray A, Afsar B, Karakus KE, Ortiz A, Hornum M, Covic A, Sarafidis P and Rossing P. Sodium-glucose cotransporter 2 inhibitors for diabetes mellitus control after kidney transplantation: Review of the current evidence. Nephrology (Carlton). 2021.
107. Ko GJ, Kalantar-Zadeh K, Goldstein-Fuchs J, Rhee CM. Dietary Approaches in the Management of Diabetic Patients with Kidney Disease. Nutrients. 2017 Jul;9(8):9. https://doi.org/10.3390/nu9080824
108. Anderson CA, Nguyen HA. Nutrition education in the care of patients with chronic kidney disease and end-stage renal disease. Semin Dial. 2018 Mar;31(2):115–21. https://doi.org/10.1111/sdi.12681
109. Whitham D. Nutrition for the prevention and treatment of chronic kidney disease in diabetes. Can J Diabetes. 2014 Oct;38(5):344–8. https://doi.org/10.1016/j.jcjd.2014.07.222
110. Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020;49(1-2):202–11. https://doi.org/10.1159/000504240
111. Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019 Jun;393(10188):2312–21. https://doi.org/10.1016/S0140-6736(18)32776-4