Preoperative depression and anxiety as predictors of postoperative C-reactive protein levels in patients undergoing cardiac surgery: a prospective observational study
DOI: https://doi.org/10.57187/smw.2022.40018
Roland
von Känela*, Kim
Rosseletb,
Katharina
Gesslerab,
Achim
Haeusslerb, Jessica
Aschmannab,
Hector
Rodriguezb,
Omer
Dzemalib
aDepartment of Consultation-Liaison Psychiatry and Psychosomatic Medicine, University Hospital Zurich, University of Zurich, Zurich, Switzerland
bDepartment of Cardiac Surgery, City Hospital of Zurich - Triemli, Zurich, Switzerland
*These authors contributed equally to the manuscript
Summary
AIM OF THE STUDY: In patients undergoing cardiac surgery, preoperative depressive and anxiety symptoms and increased postoperative C-reactive protein (CRP) levels have been associated with adverse outcomes. We tested the hypothesis that preoperative depressive and anxiety symptoms predict elevated in-hospital CRP levels after cardiac surgery.
METHODS: The study participants were 96 consecutive patients (mean age [SD], 67.6 [10.3] years, 78.1% men) from a single cardiac surgery centre who underwent either isolated coronary artery bypass grafting (CABG) (n = 34), isolated valve surgery (n = 29), combined procedures (including different combinations of CABG, valve surgery, aortic surgery, and others) (n = 30), or other cardiac surgical procedures (n = 3). Participants self-rated depressive and anxiety symptoms using the Patient Health Questionnaire (PHQ-9) and the General Anxiety Disorder (GAD-7) questionnaire before undergoing elective surgery. CRP levels were measured every 24 h up to 10 days post-surgery. Linear mixed (random effects) regression analysis examined the association between preoperative depressive and anxiety symptoms and CRP levels over time, adjusting for pre-surgery CRP levels, demographics, cardiovascular risk factors, medications, and surgery-related variables.
RESULTS: Before surgery, 32.2% of patients had clinically relevant depressive symptoms (PHQ-9 score ≥5) and 32.2% of patients had clinically relevant anxiety symptoms (GAD-7 score ≥5). More severe depressive symptoms (estimate [95% CI]: 0.081 [0.023, 0.139], p = 0.007) and more severe anxiety symptoms (0.059 [0.005, 0.113], p = 0.032) predicted CRP levels over 10 days, independent of covariates. Furthermore, CRP levels were higher in patients with than in those without clinically relevant depressive symptoms (0.697 [0.204, 1.191], p = 0.006) and were predicted by both more severe somatic (0.132 [0.035, 0.229], p = 0.008) and cognitive (0.128 [0.014, 0.242], p = 0.029) depressive symptoms.
CONCLUSIONS: Preoperative depressive and anxiety symptoms were independent predictors of elevated CRP levels up to 10 days post-surgery. Such a mechanism may help explain the increased morbidity and mortality risk in patients with depression and anxiety who undergo cardiac surgery.
Introduction
In patients undergoing cardiac surgery, including coronary artery bypass grafting (CABG) with and without concomitant cardiac procedures, the prevalence of depression ranges from 14% to 37% depending on the applied measurement tools and thresholds [1–3]. A similar prevalence exists for anxiety before cardiac surgery, which ranges from 7% to 40% [3]. Preoperative depression is associated with a 1.4-fold increased risk of postoperative early and late mortality [3]. These results were similar to depression assessments by self-rated questionnaires and clinical interviews [3]. Furthermore, preoperative depression has been associated with an increased risk of fatal and non-fatal major adverse cardiovascular events [4], hospital readmission, graft disease progression, arrhythmias, and return of angina symptoms [5]. Anxiety is associated with a 1.8-fold increased risk of late postoperative mortality after cardiac surgery [3]. Moreover, preoperative anxiety has been associated with incident atrial fibrillation and acute in-hospital cardiovascular events after CABG [5].
The biological mechanisms underlying the relationships between depression, anxiety, and cardiovascular outcomes are elusive [3]. One plausible explanation is excessive inflammation due to surgical stress. Cardiac surgery induces a systemic inflammatory response with a profound increase in C-reactive protein (CRP) levels [6, 7], reaching a maximum of ~200 mg/L on postoperative days 2 and 3 [8, 9] and declining gradually thereafter until postoperative day 6 [9]. CRP production reflects the trauma caused by the surgical procedure, which probably explains why patients with perioperative events have a similar CRP course as patients with an ordinary perioperative course [9]. Postoperative CRP levels are of prognostic value. For example, in patients who underwent off-pump CABG, the maximum CRP level on postoperative days 1–3 predicted an increased risk of major adverse cardiovascular and cerebral events after a 2-year follow-up [10]. Furthermore, elevation in CRP levels on postoperative days 1–3 is increasingly studied as an early marker of postoperative complications in non-cardiac surgery [11]. For example, in patients who underwent non-cardiac vascular surgery, elevated CRP levels 24 h post-surgery predicted cardiovascular incidents 1 year later [12]. A parallel line of research has revealed the cross-sectional and bi-directional longitudinal associations of depression [13–15] and anxiety [16–18] with low-grade systemic inflammation, including increased levels of CRP. Furthermore, depression [19] and anxiety [20] have been associated with elevated CRP in patients with stable coronary heart disease (CHD).
Considering the existing literature, surprisingly few studies with partially conflicting results have been conducted on the association of depression and anxiety with early postoperative CRP levels in patients who underwent cardiac surgery. One study found that preoperative clinically relevant depressive symptoms significantly predicted increased CRP levels 4–8 days, although not 1–3 days, after CABG surgery [21]. A second study found a significant association between clinically relevant anxiety symptoms before surgery and higher CRP levels 3 days but not 7 days after aortic valve replacement [22]. In contrast, in a third study, more severe anxiety symptoms before surgery were significantly associated with lower CRP levels 24 h after CABG surgery, with no other significant associations on postoperative days 2–5 [23].
Our study aimed to elucidate whether preoperative depressive and anxiety symptoms are predictive of postoperative CRP levels in cardiac surgery patients. We hypothesized that depressive and anxiety symptoms significantly predict elevated CRP up to 10 days post-surgery, adjusting for several other potential predictors of CRP levels. We further explored whether depressive and anxiety symptoms could differently predict the initiation of the acute inflammatory response (postoperative days 1–3) and its subsidence after having reached the maximum response (postoperative days 4–10). Finally, as depression is a heterogeneous disorder with different clinical presentations [24] impacting prognosis in patients with CHD differently [25], we also explored whether somatic and cognitive depressive symptoms differently predict postoperative CRP levels.
Materials and methods
Study design and participants
The Department of Cardiac Surgery, City Hospital of Zurich - Triemli, Zurich, Switzerland, established the single-centre prospective observational PsyCor study, a long-term project with the goal of examining the role of psychosocial factors in consecutive patients undergoing cardiac surgery. The Ethics Committee of the Canton of Zurich, Switzerland, approved the study. This study included data from the first 100 consecutive adult patients who completed the Patient Health Questionnaire (PHQ-9) and General Anxiety Disorder (GAD-7) scale before undergoing any type of elective cardiac surgery between October 2021 and March 2022. Data from four patients could not be included in the analysis: one patient completed only one item of the PHQ-9 questionnaire, one patient did not complete the GAD-7 scale, and data from two patients were omitted due to ethics requirements. The final sample included 96 study participants with complete data for the analysis.
Measures
Patient characteristics and covariates
Several variables were collected before surgery, intraoperatively, or after surgery to characterize the study sample and to serve as covariates in statistical models. The collected/calculated preoperative variables were age, sex, arterial hypertension, diabetes, dyslipidaemia, body mass index (BMI), smoking status (never, former, current), statin use, antidepressant use, left ventricular function (LVEF) assessed by transthoracic echocardiography, and European System of Cardiac Operative Risk Evaluation (EuroSCORE) II. The EuroSCORE was used as a continuous variable in analysis and as a categorical variable to describe the degree of operative risk (low: 0–2, moderate: 2–5, high: >5) [26]. Intraoperative and postoperative collected/calculated variables were type of cardiac surgery, cardiopulmonary bypass (yes/no), type of cardiac access, surgery duration (min), length of stay in the intensive care unit (ICU), and postoperative length of stay in the hospital. The latter two variables were expressed in days and were limited to a maximum of 10 days, corresponding to the maximum length of the observation period of our study. The length of stay in the ICU in days was calculated based on the number of shifts of 8 h each (number of shifts divided by 3). The type of cardiac surgery was categorized into 1) isolated CABG, 2) isolated valve surgery, 3) combined procedures (including different combinations of CABG, valve surgery, aortic surgery, and others), and 4) other procedures. The latter category included only three patients who underwent tumour extirpation on the aortic valve, resection of the left heart appendage, and pericardiectomy. Cardiac access was categorized into median sternotomy and minimally invasive procedures (i.e., right or left lateral thoracotomy, partial upper sternotomy).
Symptoms of depression and anxiety
Patients were sent home the PHQ-9 and GAD-7 questionnaires for the self-assessment of the severity of depressive and anxiety symptoms in the past 2 weeks and were asked to return the completed questionnaire to the hospital (only six patients completed the questionnaire during the preoperative hospital stay). Because test-retest reliability is good, 0.84 for the PHQ-9 [27] and 0.83 for the GAD-7 [28], the number of days between questionnaire completion and surgery was allowed to vary between patients; this was included as a covariate in statistical analysis. The PHQ-9 is an economical and valid instrument to assess the severity of depressive symptoms in cardiac surgery patients [29]. The scale comprises nine symptoms, of which five are cognitive symptoms (depressed mood, loss of interest, feelings of worthlessness, concentration problems, suicidal ideation) and four are somatic symptoms (appetite problems, sleeping difficulties, psychomotor agitation/retardation, fatigue). The GAD-7 comprises seven items and has been used to assess the severity of anxiety symptoms in medical and primary care populations, including in the cardiovascular literature [30, 31]. Typical items are “Not being able to stop or control worrying” and “Feeling afraid as if something awful might happen”. Each item of the PHQ-9 and GAD-7 is rated on a 4-point Likert scale ranging from 0 = “not at all” to 3 = “'nearly every day”. A total PHQ-9 score ranging from 0 to 27 is calculated by summing individual item scores. Total scores of 0–4, 5–9, 10–14, 15–19, and 20–27 define minimal, mild, moderate, moderately severe, and severe symptomatology, respectively [27]. Scores for somatic symptoms (range 0–12) and cognitive symptoms (range 0–15) are also calculated. The total GAD–7 score ranges from 0 to 21, with total scores of 5, 10, and 15 representing cut–offs for mild, moderate, and severe symptomatology [28]. In our study sample, internal consistency was excellent for the GAD–7 total scale (Cronbach's α = 0.91), good for the PHQ–9 total scale (α = 0.84), and acceptable for the somatic (α = 0.72) and the cognitive (α = 0.77) depressive symptom subscales. For the primary analysis, we used continuous scores of the PHQ–9 and GAD–7 scales to examine whether “more severe depressive/anxiety symptoms” were predictive of postoperative CRP levels and to take advantage of the maximum statistical power. To distinguish between patients with and without “clinically relevant depressive/anxiety symptoms”, we additionally formed a binary variable corresponding to the cut-off for “mild depressive/anxiety symptoms” (score ≥5).
C–reactive protein
CRP values were measured in blood samples that were collected before surgery and every 24 h until the patient was discharged from the hospital or on postoperative day 10. In routine clinical practice, CRP levels are usually tested every 24 h for the first 2–3 postoperative days and at the discretion of the attending cardiac surgeon thereafter. CRP was determined in lithium–heparin plasma with a latex immunoturbidimetric assay (Tina-quant® C-Reactive Protein IV, measuring range 0.6–350 mg/L, normal range <5 mg/L) using the Cobas c501 module from Roche Diagnostics (Rotkreuz, Switzerland). The assay was performed according to the manufacturer's instructions at the Institute of Laboratory Medicine, Municipal Hospital Triemli, Zurich, Switzerland, within the clinical routine, applying usual quality standards.
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp; licensing for authorized end users of the University of Zurich, Switzerland) and two-tailed significance at P <0.05. Descriptive statistics are reported as means (M) and standard deviations (SD) for data with normal distribution, median, and interquartile range (IQR) for data with non-normal distribution, or n (%). Due to non-normal distribution, CRP values were box-cox transformed prior to analysis. Box-cox transformation is a parametric power (i.e., non-linear) transformation procedure to find the best exponent λ with which the original values of the dependent variable are exponentiated to reduce the skewness of the data [32]. The procedure does not try to distribute the data normally but instead searches for the data distribution with the smallest standard deviation that has a high probability of being more symmetrical and thus closer to a normal distribution. To calculate λ, which was 0.4 in our sample, we used free software that is accessible via the internet [33]. Group comparisons between patients with and those without clinically relevant depressive symptoms and between patients with and those without clinically relevant anxiety symptoms were performed using the independent-samples t-test, Mann-Whitney U test, Pearson's chi-square test, or Fisher's exact test, where appropriate. Spearman's correlation analysis was used to estimate the correlation between two variables with a non-normal distribution.
The univariable and multivariable longitudinal association of both continuous and categorical measures of depressive and anxiety symptoms and covariates with CRP levels was examined using linear mixed-effects models analysis with random intercepts and restricted maximum likelihood estimation. Mixed model regression is a robust analysis allowing the estimation of an intercept and slope for each patient based on all the available data for that patient (i.e., even when some measurements are missing), augmented by data from the entire sample. In other words, all regression estimates are calculated based on the total number of CRP observations that are contributed by all 96 patients across the 10 measurement time points. The covariates for the multivariable models were selected a priori based on potential associations with depressive/anxiety symptomatology and/or CRP levels. These covariates were age, sex, hypertension, diabetes, dyslipidaemia, BMI, smoking, statin use, antidepressant use, LVEF, EuroSCORE II, pre-surgery CRP level, type of cardiac surgery, cardiopulmonary bypass, type of cardiac access, surgery duration, length of stay in the ICU, postoperative length of stay in the hospital, and time interval between PHQ-9 and GAD-7 completion and surgery. A covariate “time” was entered to account for within-person changes in CRP levels between individual assessments. “Time” was coded from 1 to 10, with “1” for CRP levels 24 h post-surgery, “2” for CRP levels 48 h post-surgery, up to “10” for CRP levels on postoperative day 10. A covariate “time squared” was additionally entered and accounted for non-linear relationships (“up and down”) with CRP levels over 10 days, which improved the model fit according to Akaike's information criterion. There were no missing values for the covariates. All covariates were entered as fixed effects in one block, including “time” and “time squared”, which were both added to the multivariable model. Fixed effects estimates are reported with 95% confidence interval (CI).
The primary analysis used continuous scores of the PHQ-9 and GAD-7 as predictor variables of CRP levels. Additional analyses used depression and anxiety as binary variables. To obtain a more comprehensive understanding of the investigated associations and their dynamics, analyses were additionally performed with somatic depressive symptoms, cognitive depressive symptoms, and for two distinct postoperative periods; the latter also allowed comparison with previous studies [10, 11, 21, 22]. To achieve this, instead of adding a time-by-depression or time-by-anxiety interaction to the multivariable models, we explored whether depressive/anxiety symptoms predicted the initiation of the acute inflammatory response (postoperative days 1–3) and its resolution (postoperative days 4–10), each separately.
According to the ethical approval, the data and analytical code are not available for open access. However, the anonymised dataset used and analysed for this study and the analytical code are available from the corresponding author upon reasonable request.
Results
Patient characteristics
Table 1 shows the characteristics of the study
participants. The overall sample of 96 patients was predominantly male, with a
mean age of 68 years and a high prevalence of cardiovascular risk factors.
Surgical procedures were equally frequent between isolated CABG, isolated valve
surgery, and combined surgical procedures. Two-thirds of the patients underwent
a cardiopulmonary bypass, while invasive and minimally invasive thoracic access
were performed equally frequently. Most patients (85.4%) stayed in the ICU for 1
day or less, and the median postoperative hospital stay was 8 days. According
to the EuroSCORE II, the operative risk was low in 62 (64.6%), moderate in 24
(25.0%), and high in 10 (10.4%) patients.
Table 1Characteristics of study participants.
|
Variable
|
All
participants
|
Clinically
relevant depressive symptoms
|
Clinically
relevant anxiety symptoms
|
|
|
(n = 96)
|
Yes (n = 31)
|
No (n = 65)
|
Yes (n = 33)
|
No (n = 63)
|
| Age, years, M (SD) |
67.6 (10.3) |
67.5 (10.2) |
67.7 (10.4) |
67.1 (9.7) |
67.9 (10.7) |
| Sex, male, n (%) |
75 (78.1) |
25 (80.6) |
50 (76.7) |
21 (63.6) |
54 (85.7) |
| Hypertension, n (%) |
71 (74.0) |
25 (80.6) |
46 (70.8) |
28 (84.8) |
43 (68.3) |
| Diabetes, n (%) |
31 (32.3) |
8 (25.8) |
23 (35.4) |
12 (36.4) |
19 (30.2) |
| Dyslipidaemia, n (%) |
63 (65.6) |
20 (64.5) |
43 (66.2) |
23 (69.7) |
40 (63.5) |
| BMI, kg/m2, M (SD) |
27.7 (3.9) |
28.0 (3.8) |
27.5 (3.9) |
27.4 (4.1) |
27.8 (3.8) |
| Smoking |
Never, n (%) |
47 (49.0) |
13 (41.9) |
34 (52.3) |
17 (51.5) |
30 (47.6) |
| Former, n (%) |
34 (35.4) |
10 (32.3) |
24 (36.9) |
9 (27.3) |
25 (39.7) |
| Current, n (%) |
15 (15.6) |
8 (25.8) |
7 (10.8) |
7 (21.2) |
8 (12.7) |
| Statin use, n (%) |
56 (58.3) |
19 (61.3) |
37 (56.9) |
22 (66.7) |
34 (54.0) |
| Antidepressant use, n (%) |
4 (4.2) |
4 (12.9) |
0 (0) |
3 (9.1) |
1 (1.6) |
| LVEF, median (IQR) |
60.0 (56.5, 65.0) |
63.0 (60.0, 65.0) |
60.0 (55.0, 65.0) |
64.0 (60.0, 65.0) |
60.0 (55.0, 65.0) |
| CRP pre-surgery, mg/L, median (IQR) |
1.40 (0.73, 3.38) |
1.60 (0.8, 4.1) |
1.3 (0.70, 3.25) |
1.10 (0.60, 3.15) |
1.70 (0.80, 3.40) |
| EuroSCORE II, median (IQR) |
1.30 (0.84, 3.05) |
1.75 (0.74, 3.94) |
1.24 (0.86, 2.38) |
1.28 (0.79, 2.66) |
1.39 (0.84, 3.16) |
| Cardiac surgery procedures: |
Isolated CABG, n (%) |
34 (35.4) |
12 (38.7) |
22 (33.8) |
11 (33.3) |
23 (36.5) |
| Isolated valve surgery, n (%) |
29 (30.2) |
8 (25.8) |
21 (32.3) |
12 (36.4) |
17 (27.0) |
| Combined procedures, n (%) |
30 (31.3) |
11 (35.5) |
19 (29.2) |
10 (30.3) |
20 (31.7) |
| Other, n (%) |
3 (3.1) |
0 (0) |
3 (4.6) |
0 (0) |
3 (4.8) |
| Cardiopulmonary bypass, n (%) |
64 (66.7) |
19 (61.3) |
45 (69.2) |
22 (66.7) |
42 (66.7) |
| Cardiac access: |
Sternotomy, n (%) |
48 (50) |
15 (48.4) |
33 (50.8) |
15 (45.5) |
33 (52.4) |
| Minimally invasive, n (%) |
48 (50) |
16 (51.6) |
32 (49.2) |
18 (54.5) |
30 (47.6) |
| Surgery duration, min, M (SD) |
202 (70) |
216 (52) |
196 (76) |
201 (69) |
203 (71) |
| Length of stay in ICU, days, median (IQR) |
1.0 (1.0, 1.0) |
1.0 (1.0, 1.0) |
1.0 (0.67, 1.0) |
1.0 (1.0, 1.0) |
1.0 (0.67, 1.0) |
| Postoperative length of stay, days, median (IQR) |
8.0 (7.0, 10.0) |
8.0 (7.0, 10.0) |
8.0 (7.0, 9.0) |
8.0 (7.0, 10.0) |
8.0 (7.0, 9.0) |
| Questionnaire-to-surgery time, days, median (IQR) |
10.5 (5.3, 20.0) |
10.0 (8.0, 25.0) |
11.0 (5.0, 19.5) |
14.0 (6.0, 30.5) |
10.0 (5.0, 19.0) |
| PHQ-9, total score, median (IQR) |
3.0 (1.0, 5.8) |
7.0 (6.0, 11.0) |
2.0 (0.5, 3.0) |
6.0 (3.5, 10.0) |
2.0 (0, 3.0) |
| PHQ-9, somatic symptom score, median (IQR) |
2.0 (1.0, 3.0) |
4.0 (3.0, 6.0) |
0 (0, 1.0) |
4.0 (2.0, 6.0) |
0 (1.0, 2.0) |
| PHQ-9, cognitive symptom score, median (IQR) |
1.0 (0, 2.0) |
4.0 (2.0, 5.0) |
0 (2.0, 4.0) |
2.0 (1.0, 4.0) |
0 (0, 1.0) |
| GAD-7, total score, median (IQR) |
3.0 (1.0, 6.0) |
7.0 (4.0, 11.0) |
2.0 (0, 4.0) |
7.0 (6.0, 10.5) |
1.0 (0, 3.0) |
Clinically relevant depressive symptoms were prevalent
in 32.3% (mild: 20.8%, moderate: 9.3%, moderately severe: 1.1%, severe: 1.1%)
and clinically relevant anxiety symptoms were prevalent in 34.4% (mild: 25.0%,
moderate: 5.2%, severe: 4.2%) of patients. There were only few significant
differences in participant characteristics between patients with and without
clinically relevant depressive symptoms and between patients with and without
clinically relevant anxiety symptoms. Although antidepressant use was low
overall, it was significantly more frequent in patients with versus those
without clinically relevant depressive symptoms (p = 0.009). There were significantly more females than males in the patient group with clinically relevant anxiety symptoms (p = 0.013), and LVEF was significantly higher in this
group than in the patient group without clinically relevant anxiety symptoms (p
= 0.009). As expected, patients with clinically relevant depressive symptoms showed
more severe anxiety symptoms (p <0.001) and patients with clinically relevant
anxiety symptoms showed more severe depressive symptoms (p <0.001). Furthermore,
depressive and anxiety symptoms were strongly correlated (r = 0.74, p <0.001).
The median time interval between completion of the questionnaires and surgical
intervention was 10.5 days and was not correlated with total PHQ-9 (p = 0.80)
and GAD-7 (p = 0.30) scores.
Postoperative C-reactive protein levels
Table 2 shows the median CRP values at each of the 10 postoperative measurement time points. In total, there were 463 observations for CRP, meaning that each patient contributed an average of 4.8 CRP values. In the overall sample, the maximum CRP level was observed 72 h after surgery, with a steady decline in the subsequent 4 days. A similar pattern of postoperative CRP levels over 10 days was present in the subgroups of patients with and without clinically relevant depressive or anxiety symptoms.
Table 2C-reactive protein
levels
at different time points
post-surgery.
|
Time point
|
All participants
|
Clinically relevant depressive symptoms
|
Clinically relevant anxiety symptoms
|
|
Yes
|
No
|
Yes
|
No
|
| 24 h |
39.4 (28.7, 59.5) |
57.8 (34.6, 65.7) |
38.1 (23.2, 51.3) |
43.7 (32.7, 64.6) |
38.7 (25.8, 53.0) |
| n = 95 |
n = 31 |
n = 64 |
n = 32 |
n = 63 |
| 48 h |
130 (96.2, 175) |
161 (103, 221) |
122 (93.2, 155) |
143 (103, 198) |
122 (92.6, 170) |
| n = 93 |
n = 31 |
n = 62 |
n = 32 |
n = 61 |
| 72 h |
183 (122, 239) |
229 (154, 321) |
143 (116, 198) |
206 (143, 309) |
181 (120, 218) |
| n = 56 |
n = 21 |
n = 35 |
n = 21 |
n = 35 |
| Day 4 |
118 (77.8, 178) |
134 (94.5, 242) |
112 (75.2, 161) |
109 (62.4, 161) |
118 (90.2, 179) |
| n = 63 |
n = 18 |
n = 45 |
n = 18 |
n = 45 |
| Day 5 |
99.0 (65.0, 165) |
125 (81.7, 174) |
85.7 (49.3, 126) |
95.1 (78.3, 164) |
101 (57.6, 172) |
| n = 46 |
n = 19 |
n = 27 |
n = 16 |
n = 30 |
| Day 6 |
68.2 (42.9, 114) |
114 (66.8, 159) |
56.9 (37.2, 90.7) |
99.0 (54.4, 141) |
59.7 (37.4, 106) |
| n = 41 |
n = 14 |
n = 27 |
n = 11 |
n = 30 |
| Day 7 |
59.1 (40.7, 102) |
78.7 (44.1, 124) |
55.6 (39.3, 93.3) |
61.7 (39.6, 85.1) |
56.4 (43.3, 138) |
| n = 32 |
n = 13 |
n = 19 |
n = 13 |
n = 19 |
| Day 8 |
66.6 (28.3, 154) |
169 (44.6, 187) |
56.9 (16.9, 101) |
36.7 (17.3, 67.5) |
101 (32.3, 168) |
| n = 16 |
n = 5 |
n = 11 |
n = 4 |
n = 12 |
| Day 9 |
122 (60.1, 162) |
163 (91.7, 188) |
102 (30.5, 143) |
N/A |
137.0 (66.3, 163) |
| n = 10 |
n = 4 |
n = 6 |
n = 1 |
n = 9 |
| Day 10 |
90.7 (35.6, 143) |
139 (34.1, 159) |
64.9 (33.0, 110) |
41.8 (26.3, N/A) |
94.1 (36.5, 142) |
| n = 11 |
n = 5 |
n = 6 |
n = 3 |
n = 8 |
| Observations |
n = 463 |
n = 161 |
n = 302 |
n = 151 |
n = 312 |
Relationships with measures of depressive symptoms
In univariable analysis, both more severe depressive symptoms (PHQ-9 total score) (estimate = 0.083 [95% CI: 0.026, 0.139], p = 0.005) (table 3) and clinically relevant depressive symptoms (PHQ-9 score ≥5) (0.792 [0.307, 1.277], p = 0.002) predicted significantly higher CRP levels over 10 days.
Table 3Univariable and multivariable relationships with post-surgery C-reactive protein levels over 10 days.
|
Parameter
|
Univariable relationships
|
Multivariable model for depressive symptoms (PHQ-9 total score)
|
Multivariable model for anxiety symptoms (GAD-7 total score)
|
|
Estimate
|
95% CI
|
Estimate
|
95% CI
|
Estimate
|
95% CI
|
| Intercept |
6.157*** |
5.918, 6.397 |
2.177 |
–1.022, 5.376 |
2.084 |
–1.179, 5.347 |
| Time |
0.014 |
–0.048, 0.077 |
1.046*** |
0.851, 1.242 |
1.045*** |
0.850, 1.241 |
| Time squared |
–0.007* |
–0.014, –0.001 |
–0.115*** |
–0.136, –0.094 |
–0.115*** |
–0.136, –0.094 |
| Age |
–0.002 |
–0.026, 0.021 |
–0.001 |
–0.023, 0.021 |
0.002 |
–0.021, 0.025 |
| Male sex |
0.539 |
–0.036, 1.113 |
0.381 |
–0.195, 0.957 |
0.447 |
–0.163, 1.057 |
| Hypertension |
0.194 |
–0.352, 0.739 |
–0.281 |
–0.830, 0.267 |
–0.347 |
–0.913, 0.218 |
| Diabetes |
–0.148 |
–0.665, 0.370 |
–0.348 |
–0.887, 0.181 |
–0.385 |
–0.924, 0.154 |
| Dyslipidaemia |
–0.133 |
–0.641, 0.375 |
0.319 |
–0.319, 0.957 |
0.242 |
–0.408, 0.893 |
| Body mass index |
0.017 |
–0.027, 0.101 |
0.013 |
–0.048, 0.074 |
0.019 |
–0.044, 0.081 |
| Smoking |
0.032 |
–0.298, 0.361 |
–0.151 |
–0.461, 0.158 |
–0.115 |
–0.429, 0.198 |
| Statin use |
–0.250 |
–0.735, 0.235 |
–0.291 |
–0.919, 0.338 |
–0.310 |
–0.951, 0.331 |
| Antidepressant use |
0.471 |
–0.714, 1.656 |
0.187 |
–1.006, 1.380 |
0.572 |
–0.574, 1.717 |
| Left ventricular ejection fraction |
0.007 |
–0.019, 0.033 |
–0.017 |
–0.040, 0.016 |
–0.014 |
–0.043, 0.015 |
| C–reactive protein pre–surgery |
–0.024 |
–0.289, 0.241 |
0.204 |
–0.082, 0.489 |
0.159 |
–0.133, 0.451 |
| EuroSCORE II |
0.008 |
–0.053, 0.070 |
–0.037 |
–0.113, 0.040 |
–0.035 |
–0.113, 0.043 |
| Isolated CABG surgery |
1.164 |
–0.264, 2.593 |
1.436 |
–0.022, 2.894 |
1.382 |
–0.108, 2.873 |
| Isolate valve surgery |
0.399 |
–1.038, 1.837 |
0.772 |
–0.750, 2.294 |
0.708 |
–0.844, 2.260 |
| Combined surgical procedures |
1.634* |
0.201, 3.067 |
2.349** |
0.803, 3.895 |
2.275** |
0.696, 3.854 |
| Other surgical procedures |
0 |
|
0 |
|
0 |
|
| Cardiopulmonary bypass |
–0.191 |
–0.702, 0.320 |
–0.553 |
–1.396, 0.289 |
–0.690 |
–1.551, 0.170 |
| Minimally invasive access |
–0.272 |
–0.752, 0.208 |
0.577 |
–0.065, 1.219 |
0.585 |
–0.072, 1.242 |
| Surgery duration |
0.004* |
<0.001, 0.007 |
0.003 |
–0.001, 0.008 |
0.004 |
–0.001, 0.008 |
| Length of stay in ICU |
–0.101 |
–0.260, 0.058 |
–0.262* |
–0.460, –0.064 |
–0.232* |
–0.435, 0.029 |
| Postoperative length of stay |
–0.028 |
–0.195, 0.139 |
0.042 |
–0.117, 0.202 |
0.050 |
–0.113, 0.213 |
| Questionnaire-to-surgery time |
0.004 |
–0.009, 0.017 |
0.010 |
–0.002, 0.023 |
0.012 |
–0.001, 0.024 |
| PHQ-9 total score |
0.083** |
0.026, 0.139 |
0.081** |
0.023, 0.139 |
|
|
| GAD-7 total score |
0.055* |
0.002, 0.108 |
|
|
0.059* |
0.005, 0.113 |
Figure 1 illustrates the time course of CRP levels in patients with and without clinically relevant depressive symptoms, which was similar to the untransformed and transformed CRP values. Group comparison for individual time points revealed significantly higher CRP levels in patients with than those without clinically relevant depressive symptoms at 24 h (p = 0.012), 48 h (p = 0.042), 72 h (p = 0.001), 5 days (p = 0.028), and 6 days (p = 0.010) post-surgery.
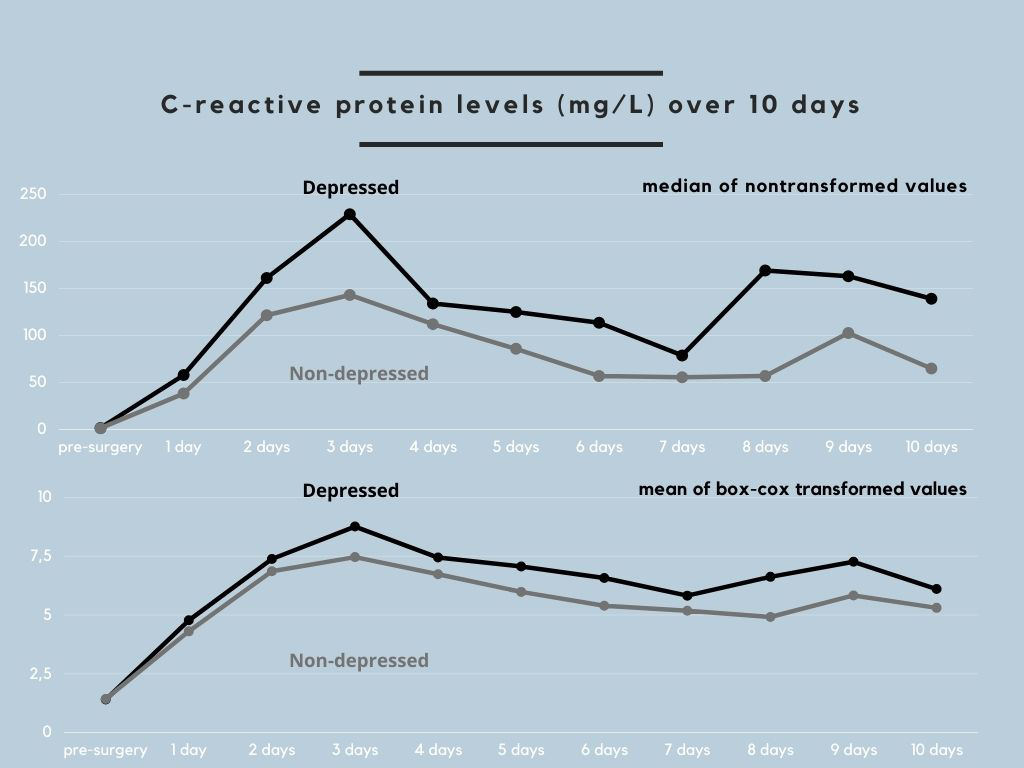
Figure 1 Time course of C-reactive protein
levels
in patients with and without
clinically relevant depressive symptoms.
The upper part of the
figure depicts the median C-reactive protein (CRP) values in original units
before surgery and at each of the 10 measurement time points after surgery in
patients with clinically relevant depressive symptoms (Patient Health
Questionnaire-9 score ≥5; "depressed") and in patients without clinically
relevant depressive symptoms (Patient Health Questionnaire-9 score <5; "non-depressed").
The lower part of the figure shows this group difference in mean CRP values
that were box-cox transformed because of non-normal distribution. Patients with
clinically relevant depressive symptoms had higher absolute CRP levels than those
without clinically relevant depressive symptoms on each of the 10 postoperative
days.
In multivariable analysis, more severe
depressive symptoms (0.081 [0.023, 0.139], p = 0.007) (table 3) and clinically
relevant depressive symptoms (0.697 [0.204, 1.191], p = 0.006) were significantly
predictive of higher CRP levels over 10 days, independent of covariates. More
severe somatic (0.132 [0.035, 0.229], p = 0.008) and cognitive (0.128 [0.014,
0.242], p = 0.029) depressive symptoms were also significantly predictive of
higher CRP levels over 10 days, independent of covariates.
The course of CRP with a maximum level on postoperative
day 3 justified the pre-planned additional analysis in which the 10-day
observation period was divided into the first 72 h post-surgery and postoperative
days 4–10. The analysis revealed that more severe depressive symptoms were significantly
predictive of elevated CRP over the first 72 h post-surgery (0.061 [0.007, 0.115],
p = 0.028) and from postoperative days 4 to 10 (0.109 [0.034, 0.184], p = 0.005).
A separate analysis predicting untransformed CRP values revealed an increase in
CRP of 3.60 mg/L (95% CI: 0.99, 6.22) with each one-point increase in the total
PHQ-9 score over 10 days, when all other covariates in the model remained
constant.
Relationships with measures of anxiety symptoms
Univariable analysis revealed that more severe anxiety
symptoms (total GAD-7 score) (0.055 [0.002, 0.108], p = 0.043) (table 3), but not
clinically relevant anxiety symptoms (GAD-7 score ≥5) (p = 0.60), predicted significantly
higher CRP levels over 10 days. Patients with and without clinically relevant anxiety
symptoms did not show significant differences in CRP levels at any of the 10
measurement time points.
In multivariable analysis, more severe anxiety
symptoms were significantly predictive of CRP levels over the 10 postoperative
days (0.059 [0.005, 0.113], p = 0.032) (table 3), whereas clinically relevant anxiety
symptoms were not (p = 0.44). More severe anxiety symptoms predicted elevated CRP
over the first 72 h with marginal significance (0.048 [–0.001, 0.098], p = 0.055),
although they significantly predicted elevated CRP from days 4 to 10
post-surgery (0.084 [0.015, 0.153], p = 0.018). A separate analysis predicting untransformed
CRP values revealed a 2.83 mg/L (95% CI: 0.44, 5.23) increase in CRP with each
one-point increase in the GAD-7 total score over 10 days.
Relationships with covariates
Combined surgical procedures were significantly associated with higher CRP levels, and longer length of stay in the ICU was significantly associated with lower CRP levels over the 10 days post-surgery, both independent of the other covariates in the model. Longer duration of surgery was also significantly associated with higher CRP levels over the 10 days post-surgery but was not independent of covariates. The significant positive effect of “time’ and the significant negative effect of “time squared’ in multivariable analysis indicated that the effect of time became smaller with an increasing number of days after surgery, reflecting the marked increase in CRP in the first 3 postoperative days.
Discussion
In patients undergoing elective cardiac surgery, we found that both severe preoperative depressive and anxiety symptoms were significant predictors of higher postoperative CRP levels over the 10 days post-surgery. Based on a definition that used a binary questionnaire cut-off, clinically relevant depressive symptoms (PHQ-9 score ≥5) but not clinically relevant anxiety symptoms (GAD-7 score ≥5) before surgery were predictive of higher postoperative CRP levels over 10 days. One parsimonious explanation for this discrepancy could be that clinically relevant anxiety symptoms are a weaker predictor of postoperative CRP levels than clinically relevant depressive symptoms. Such an interpretation is supported by the observation of significant differences in CRP levels between patients with and without clinically relevant depressive symptoms at several of the 10 measurement time points but not between patients with and without clinically relevant anxiety symptoms.
We found that measures of depressive and anxiety symptoms significantly predicted elevated postoperative CRP levels in both the univariable and multivariable adjusted analyses. Because combined surgical procedures and length of stay in the ICU emerged as the only significant covariates, the effects of depressive and anxiety symptoms may be clinically relevant. Although increments in CRP were small overall, clinical relevance is substantiated by the observed increase of 3.60 mg/L and 2.83 mg/L CRP with each point increase on the depressive and anxiety symptom scales, respectively. A 2.2-, 2.5- and 2.8-fold higher risk for major adverse cardiovascular and cerebral events 2 years post-CABG has been reported with maximum CRP on postoperative days 1–3 of 180–220 mg/L, 220–270 mg/L, and ≥270 mg/L, respectively, compared with CRP <180 mg/L. Therefore, a patient with moderate depressive symptoms, based on a total PHQ-9 score between 10 and 13 and a corresponding CRP increase of ~36–47 mg/L, would move up one risk category compared with a patient with no depressive symptoms. A similar argument can be made for the event risk of a patient with severe anxiety symptoms (total GAD-7 score ≥15) compared with a patient with no anxiety symptoms.
Although the prevalence of clinically relevant depressive symptoms (32.3%) and clinically relevant anxiety symptoms (34.4%) in our sample was consistent with the literature [1–3], less severe forms with symptom scores <10 predominated. Therefore, clinical inferences for patients at the upper end of the PHQ-9 and GAD-7 scores should be made with caution. The binary coding of depressive and anxiety symptoms in our study may also lead to misconceptions about homogeneity and heterogeneity across participants. For example, a patient with a PHQ-9 score of 5 may be much more similar to a patient with a score of 4 than to a patient with a score of 27. However, the sample size precluded calculation of a five-category model across the spectrum of minimal, mild, moderate, moderately severe, and severe depressive and anxiety symptoms. A model with all severity levels may be calculated with future data from a larger sample.
Our findings confirmed those of a study in which clinically relevant depressive symptoms, defined by a Beck Depression Inventory score >10 before surgery, significantly predicted elevated CRP on postoperative days 4–8 in 145 patients who underwent elective CABG with or without valve replacement [21]. However, unlike in our study, clinically relevant depressive symptoms were not significantly predictive of CRP levels on postoperative days 1–3 [21]. Differences in the types of surgical procedures performed, measurement tools for depressive symptoms, and covariates could explain this discrepancy. In addition, we found more severe anxiety symptoms as a marginally significant predictor of CRP levels on postoperative days 1–3, perhaps because depressive symptoms may affect the initiation of the acute inflammatory response to a greater extent than anxiety symptoms. Furthermore, our findings concur partially with a study on 73 patients who underwent aortic valve replacement that reported an association between clinically relevant anxiety symptoms before surgery and elevated CRP levels on postoperative day 3 [22]. However, in that study, no significant association was found 7 days post-surgery [22], and another study found an inverse association between more severe anxiety symptoms before surgery and CRP levels 1 day after CABG, with no further significant associations until postoperative day 5 [23]. There may be several explanations for these inconsistent results. Previous studies used different measurement tools (i.e., Hamilton Anxiety Scale and State-Trait Anxiety Inventory), had smaller sample sizes, made no adjustments for covariates, and included patients undergoing one surgical procedure [22, 23].
More severe somatic and cognitive depressive symptoms were both predictive of elevated postoperative CRP levels in our study. This finding contrasts the results of a meta-analysis indicating poorer prognosis in patients with CHD with somatic depressive symptoms [25]. However, this meta-analysis included only one study that examined depressive symptoms before CABG and reported that cognitive, but not somatic depressive symptoms, predicted adverse cardiovascular outcomes [34].
Whether interventions to improve psychological wellbeing before cardiac surgery [35] attenuate postoperative elevation in CRP and the biobehavioural mechanisms linking preoperative depressive and anxiety symptoms with postoperative CRP production is unknown. Alterations in the neuroendocrine and autonomic nervous system might be involved. Both cardiac surgery and acute psychosocial stress activate the innate immune response, with increases in pro-inflammatory cytokines and CRP [36], which is curtailed by cortisol release in a feedback mechanism [37, 38]. In patients with CHD, severe anxiety symptoms were associated with a blunted cortisol response to acute psychosocial stress [39], and clinical depression was associated with insufficient glucocorticoid signalling, resulting in increased CRP levels [40]. Moreover, individuals with clinically relevant depressive symptoms exhibited a stronger relationship between reduced heart rate variability, indicating autonomic dysfunction, and elevated CRP than their non-depressed counterparts [41].
Our study has important limitations. The data were collected as part of routine clinical practice, so CRP was not assessed using a high-sensitivity assay and was not measured every 24 h for every patient, decreasing the number of possible observations and precluding a priori power analysis. We adjusted for a number of important covariates; however, confounding by unmeasured variables remains a possibility. For example, increased sickness behaviour in patients with more severe depressive symptoms due to inflammation may have prompted ordering a chemistry lab at times when CRP was high. Additional clinical factors, which were not accounted for and may have led to increased CRP, include the size of the surgical incision, coagulation activity, extent of inflammation, and wound characteristics. Because of the high correlation of depressive and anxiety symptoms with the resulting multicollinearity in regression analysis, we were unable to investigate whether depressive and anxiety symptoms predicted postoperative CRP levels independently of each other. The sample size did not allow for a moderator analysis to evaluate whether depressive and anxiety were differently predictive of elevated postoperative CRP in patients with isolated CABG compared with isolated valve surgery or combined procedures or in men compared with women.
Therefore, preoperative depressive and anxiety symptoms may independently contribute to elevated CRP up to 10 days after cardiac surgery to an extent that suggests clinical relevance. The findings of our study provide important new information on how depression and anxiety may negatively affect the prognosis of patients after cardiac surgery.
Correspondence
Roland von Känel, M.D.
Department of Consultation-Liaison Psychiatry and Psychosomatic Medicine
University Hospital Zurich
Culmannstrasse 8
CH-8091 Zurich
roland.vonkaenel[at]usz.ch
References
1. Correa-Rodríguez M, Abu Ejheisheh M, Suleiman-Martos N, Membrive-Jiménez MJ, Velando-Soriano A, Schmidt-RioValle J, et al. Prevalence of Depression in Coronary Artery Bypass Surgery: A Systematic Review and Meta-Analysis. J Clin Med. 2020 Mar;9(4):909. https://doi.org/10.3390/jcm9040909
2. Horne D, Kehler S, Kaoukis G, Hiebert B, Garcia E, Duhamel TA, et al. Depression before and after cardiac surgery: do all patients respond the same? J Thorac Cardiovasc Surg. 2013 May;145(5):1400–6. https://doi.org/10.1016/j.jtcvs.2012.11.011
3. Takagi H, Ando T, Umemoto T; ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Perioperative depression or anxiety and postoperative mortality in cardiac surgery: a systematic review and meta-analysis. Heart Vessels. 2017 Dec;32(12):1458–68. https://doi.org/10.1007/s00380-017-1022-3
4. Flaherty LB, Wood T, Cheng A, Khan AR. Pre-existing psychological depression confers increased risk of adverse cardiovascular outcomes following cardiac surgery: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2017 Nov;154(5):1578–1586.e1. https://doi.org/10.1016/j.jtcvs.2017.06.052
5. Tully PJ, Baker RA. Depression, anxiety, and cardiac morbidity outcomes after coronary artery bypass surgery: a contemporary and practical review. J Geriatr Cardiol. 2012 Jun;9(2):197–208. https://doi.org/10.3724/SP.J.1263.2011.12221
6. Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002 Jul;97(1):215–52. https://doi.org/10.1097/00000542-200207000-00030
7. Tomic V, Russwurm S, Möller E, Claus RA, Blaess M, Brunkhorst F, et al. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation. 2005 Nov;112(19):2912–20. https://doi.org/10.1161/CIRCULATIONAHA.104.531152
8. Ayala J, Smith A, Farrar D. C-reactive protein levels following cardiac surgery in adults. Eur J Anaesthesiol. 2006 Jun;23 Supplement 37:196. https://doi.org/10.1097/00003643-200606001-00703
9. Holm J, Cederholm I, Alehagen U, Lindahl TL, Szabó Z. Biomarker dynamics in cardiac surgery: a prospective observational study on MR-proADM, MR-proANP, hs-CRP and sP-selectin plasma levels in the perioperative period. Biomarkers. 2020 May;25(3):296–304. https://doi.org/10.1080/1354750X.2020.1748716
10. Min JJ, Nam K, Kim TK, Kim HJ, Seo JH, Hwang HY, et al. Relationship between early postoperative C-reactive protein elevation and long-term postoperative major adverse cardiovascular and cerebral events in patients undergoing off-pump coronary artery bypass graft surgery: a retrospective study [Erratum in: Br J Anaesth. 2014 Nov;113] [5] [:895. PMID: 24829443]. Br J Anaesth. 2014 Sep;113(3):391–401. https://doi.org/10.1093/bja/aeu099
11. Nakamoto S, Hirose M. Prediction of early C-reactive protein levels after non-cardiac surgery under general anesthesia. PLoS One. 2019 Dec;14(12):e0226032. https://doi.org/10.1371/journal.pone.0226032
12. Kouvelos GN, Milionis HJ, Arnaoutoglou EM, Chasiotis G, Gartzonika C, Papa NK, et al. Postoperative levels of cardiac troponin versus CK-MB and high-sensitivity C-reactive protein for the prediction of 1-year cardiovascular outcome in patients undergoing vascular surgery. Coron Artery Dis. 2011;22(6):428–34. https://doi.org/10.1097/MCA.0b013e3283487d96
13. Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020 Jul;87:901–9. https://doi.org/10.1016/j.bbi.2020.02.010
14. Smith KJ, Au B, Ollis L, Schmitz N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp Gerontol. 2018 Feb;102:109–32. https://doi.org/10.1016/j.exger.2017.12.005
15. Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 2021 Jul;26(7):3302–14. https://doi.org/10.1038/s41380-020-00867-4
16. Costello H, Gould RL, Abrol E, Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open. 2019 Jul;9(7):e027925. https://doi.org/10.1136/bmjopen-2018-027925
17. Liu CH, Hua N, Yang HY. Alterations in Peripheral C-Reactive Protein and Inflammatory Cytokine Levels in Patients with Panic Disorder: A Systematic Review and Meta-Analysis. Neuropsychiatr Dis Treat. 2021 Dec;17:3539–58. https://doi.org/10.2147/NDT.S340388
18. Glaus J, von Känel R, Lasserre AM, Strippoli MF, Vandeleur CL, Castelao E, et al. The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: results from a large longitudinal population-based study. Depress Anxiety. 2018 Apr;35(4):360–71. https://doi.org/10.1002/da.22710
19. Frasure-Smith N, Lespérance F, Irwin MR, Sauvé C, Lespérance J, Théroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007 Aug;62(4):302–8. https://doi.org/10.1016/j.biopsych.2006.09.029
20. Munkhaugen J, Otterstad JE, Dammen T, Gjertsen E, Moum T, Husebye E, et al. The prevalence and predictors of elevated C-reactive protein after a coronary heart disease event. Eur J Prev Cardiol. 2018 Jun;25(9):923–31. https://doi.org/10.1177/2047487318768940
21. Poole L, Kidd T, Leigh E, Ronaldson A, Jahangiri M, Steptoe A. Depression, C-reactive protein and length of post-operative hospital stay in coronary artery bypass graft surgery patients. Brain Behav Immun. 2014 Mar;37(100):115–21. https://doi.org/10.1016/j.bbi.2013.11.008
22. Gao Q, Mok HP, Zhang HY, Qiu HL, Liu J, Chen ZR, et al. Inflammatory indicator levels in patients undergoing aortic valve replacement via median sternotomy with preoperative anxiety and postoperative complications: a prospective cohort study. J Int Med Res. 2021 Feb;49(2):300060520977417. https://doi.org/10.1177/0300060520977417
23. Płotek W, Pielok J, Cybulski M, Samborska R. Emotional processes in patients undergoing coronary artery bypass graft surgeries with extracorporeal circulation in view of selected indicators of the inflammatory condition. Med Sci Monit. 2015 Jan;21:105–17. https://doi.org/10.12659/MSM.892372
24. Goldberg D. The heterogeneity of “major depression”. World Psychiatry. 2011 Oct;10(3):226–8. https://doi.org/10.1002/j.2051-5545.2011.tb00061.x
25. de Miranda Azevedo R, Roest AM, Hoen PW, de Jonge P. Cognitive/affective and somatic/affective symptoms of depression in patients with heart disease and their association with cardiovascular prognosis: a meta-analysis. Psychol Med. 2014 Oct;44(13):2689–703. https://doi.org/10.1017/S0033291714000063
26. Borde D, Gandhe U, Hargave N, Pandey K, Khullar V. The application of European system for cardiac operative risk evaluation II (EuroSCORE II) and Society of Thoracic Surgeons (STS) risk-score for risk stratification in Indian patients undergoing cardiac surgery. Ann Card Anaesth. 2013;16(3):163–6. https://doi.org/10.4103/0971-9784.114234
27. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
28. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May;166(10):1092–7. https://doi.org/10.1001/archinte.166.10.1092
29. Stenman M, Sartipy U. Depression Screening in Cardiac Surgery Patients. Heart Lung Circ. 2019 Jun;28(6):953–8. https://doi.org/10.1016/j.hlc.2018.04.298
30. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–59. https://doi.org/10.1016/j.genhosppsych.2010.03.006
31. Tully PJ, Turnbull DA, Horowitz JD, Beltrame JF, Baune BT, Sauer-Zavala S, et al. Transdiagnostic Cognitive-Behavioral Therapy for Depression and Anxiety Disorders in Cardiovascular Disease Patients: Results From the CHAMPS Pilot-Feasibility Trial. Front Psychiatry. 2022 Apr;13:741039. https://doi.org/10.3389/fpsyt.2022.741039
32. Sakia RM. The Box-Cox transformation technique: a review. Statistician. 1992;41(2):169–78. https://doi.org/10.2307/2348250
33. Hemmerich W. Box-Cox Powertransformation berechnen. 2016. Available from: https://statistikguru.de/rechner/box-cox.html
34. Tully PJ, Winefield HR, Baker RA, Turnbull DA, de Jonge P. Confirmatory factor analysis of the Beck Depression Inventory-II and the association with cardiac morbidity and mortality after coronary revascularization. J Health Psychol. 2011 May;16(4):584–95. https://doi.org/10.1177/1359105310383604
35. Guo P. Preoperative education interventions to reduce anxiety and improve recovery among cardiac surgery patients: a review of randomised controlled trials. J Clin Nurs. 2015 Jan;24(1-2):34–46. https://doi.org/10.1111/jocn.12618
36. Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. 2017 Aug;64:208–19. https://doi.org/10.1016/j.bbi.2017.01.011
37. Galvis D, Zavala E, Walker JJ, Upton T, Lightman SL, Angelini GD, et al. Modelling the dynamic interaction of systemic inflammation and the hypothalamic-pituitary-adrenal (HPA) axis during and after cardiac surgery. J R Soc Interface. 2022 Apr;19(189):20210925. https://doi.org/10.1098/rsif.2021.0925
38. von Känel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006 Jan;20(1):40–8. https://doi.org/10.1016/j.bbi.2005.03.013
39. Julija Gecaite-Stonciene J, Hughes BM, Kazukauskiene N, Bunevicius A, Burkauskas J, Neverauskas J, et al. Cortisol Response to Psychosocial Stress in Coronary Artery Disease Patients: The Role of Mental Distress, Fatigue and Quality of Life. Sci Rep 2022 (preprint under review). doi: https://doi.org/10.21203/rs.3.rs-1519480/v1
40. Nikkheslat N, Zunszain PA, Horowitz MA, Barbosa IG, Parker JA, Myint AM, et al. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun. 2015 Aug;48:8–18. https://doi.org/10.1016/j.bbi.2015.02.002
41. Frasure-Smith N, Lespérance F, Irwin MR, Talajic M, Pollock BG. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun. 2009 Nov;23(8):1140–7. https://doi.org/10.1016/j.bbi.2009.07.005
