A retrospective analysis of blood culture-negative endocarditis at a tertiary care centre in Switzerland
DOI: https://doi.org/10.57187/smw.2022.40016
Roman
Dählera, Silvio D.
Bruggera, Michelle
Frankb, Matthias
Greutmannb, Juri
Sromickic, Ewerton
Marques-Maggiod, Frank
Imkampe, Robert
Bauernschmittc, Thierry
Carrelc, Annelies S.
Zinkernagela, Barbara
Hassea
aDivision of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Switzerland
bDepartment of Cardiology, University Heart Center, University Hospital Zurich, University of Zurich, Switzerland
cClinic for Cardiac Surgery, University Hospital Zurich, University of Zurich, Switzerland
dDepartment of Surgical Pathology, University Hospital Zurich, University of Zurich, Switzerland
eInstitute of Medical Microbiology, University of Zurich, Switzerland
Summary
AIMS OF THE STUDY: Numerous studies from different countries have contributed to an improved understanding of blood culture-negative infective endocarditis. However, little is known about its epidemiology and microbiology in Switzerland. We aimed to assess the epidemiology and microbiology of blood culture-negative endocarditis at the University Hospital Zurich, Switzerland.
METHODS: We screened all patients hospitalised between 1997 and 2020 with possible or definite endocarditis at our institution. Thereof, we identified all cases with blood culture-negative endocarditis and retrospectively retrieved patient characteristics, microbiological, histopathological, radiographic and surgical data from medical records.
RESULTS: Among 861 patients screened, 66 (7.7%) cases of blood culture-negative endocarditis were identified. Thereof, 31 cases could be microbiologically documented or not documented (n = 30), and in five cases a non-infectious aetiology was confirmed. Endocarditis predominantly affected men (77%) and the left heart (79%); predisposing factors were prosthetic valves (42%), congenital heart disease (35%) and prior endocarditis (14%). The most common reasons for negative blood cultures were antibiotic treatment prior to blood culture sampling (35%), fastidious and slow growing microorganisms (30%) and definite non-infective endocarditis (8%). Coxiella burneti
i and Bartonella spp. were the most common fastidious bacteria identified. In addition to serology, identification of causative microorganisms was possible by microbiological and/or histopathological analysis of tissue samples, of which polymerase chain reaction testing (PCR) of the 16S ribosomal RNA proved to be most successful.
CONCLUSIONS: The present study provides a detailed analysis of blood culture-negative endocarditis over a time span of more than 20 years in Zurich, Switzerland. Antibiotic treatment prior to blood collection, and fastidious and slow growing organisms were identified as main reasons for sterile blood cultures. Typical culture-negative bacteria were mainly found by PCR and/or culture of tissue samples.
Introduction
Infective endocarditis is a rare but life-threatening infection of the endocardial surface of the heart which is usually caused by bacteria and rarely by fungi. The global incidence has increased in the last 20 years and ranges nowadays between 1.5 and 11.6 cases per 100,000 person-years [1]. Despite optimal treatment, mortality rates are approximately 25%. Risk factors include previous infective endocarditis, prosthetic valves or an indwelling cardiac device, or a congenital heart disease [2, 3]. Fast detection and identification of the causative pathogen is crucial in order to ensure timely and appropriate therapy. Diagnosis is usually established by combining clinical, imaging, microbiological and histopathological criteria [4]. However, in some cases no causative microorganism grows when applying standard blood culture methods. These cases are called blood culture-negative endocarditis [4]. Compared with infective endocarditis with positive cultures, identification of the aetiological agent is often delayed, which may result in an increased risk of complications such as heart valve destruction, systemic or pulmonary embolisation and even death [6].
The prevalence of blood culture-negative infective endocarditis varies greatly between countries and among different centres within the same country [7]. In Europe, blood cultures in patients investigated for infective endocarditis remain negative in 9% to 25% of cases [8, 9], whereas in developing countries blood culture-negative infective endocarditis prevalence ranges from 31% up to 69% [10, 11]. In a recent Danish nationwide study, blood cultures remained negative in 18.9% of infective endocarditis cases, with a declining temporal trend from 2000–2017 [12]. The difference in frequency might be due to the higher incidence of fastidious zoonotic agents causing human infections, or the application of different analytical techniques influencing the local epidemiology of infective endocarditis [7, 13]. Epidemiological and microbiological data as well as patient characteristics of blood culture-negative infective endocarditis among Swiss patients are scarce. We therefore aimed to characterise patients presenting with culture-negative endocarditis at the University Hospital Zurich, Switzerland.
Materials and methods
Study population and data collection
The University Hospital of Zurich is a tertiary care hospital in the eastern part of Switzerland with approximately 900 beds, 43 clinics and institutes and 43,000 hospitalised patients per year.
We retrospectively screened patients who were admitted to our hospital between January 1997 and December 2020 and had an echocardiography for suspected infective endocarditis. Patients were included if they were hospitalised for blood culture-negative endocarditis (KEK-2014-0461). A majority of patients had been previously enrolled in the “Endovascular and Cardiac Valve Infection cohort study database” (ENVALVE, BASEC 2017-01140) or the “Vascular Graft cohort study database” (VASGRA, KEK-2012-0583; PB 2016-01320 [14]). The VASGRA database served as a source of patients with composite graft endocarditis.
The study protocol was approved by the local ethics committee of Zurich. The participants were included in the study if they provided written informed consent for ENVALVE or VASGRA or if they provided a general consent between the years 2016 and 2020. For subjects with endocarditis from 1997–2016, written informed consent was waived by the ethics committee Zurich since it was decided that it would be disproportionately difficult to obtain informed consent (some patients were already dead at the time of the study).
Clinical data and definitions
We performed a chart review and assessed demographics, echocardiographic parameters, blood culture results, type of antimicrobial treatment, surgery, outcomes and clinical complications. Data was retrospectively extracted from the Clinical Information System (KISIM) in an Excel file according to a structured questionnaire (table S1). Each patient file was again checked for completeness. In the case of missing microbiological and essential data, the patient was not included in the analysis. The two researchers extracting the data were not blinded to the aims of the study. Selected patients were stratified as blood culture-negative infective endocarditis (BCN-IE) if a causative pathogen could be found, as blood culture-negative endocarditis (BCN-E) if no aetiology could be found or as blood culture-negative non-infective endocarditis (BCN-NIE) if a non-infectious underlying systemic disease could be identified [15].
The following disease entities were subsumed in the term “congenital heart disease”: tetralogy of Fallot, transposition of great arteries/vessels, tricuspid atresia, hypoplastic left heart syndrome, Ebstein anomaly, persistent foramen ovale, ventricular septum defect, atrial septum defect, persistent ductus arteriosus, aortic isthmus stenosis, coronary artery anomalies, pulmonic/aortic valve stenosis. Acquired heart disease encompassed all heart diseases not listed as congenital heart disease.
Complications were defined as follows: central nervous system (CNS) embolism: emboli to brain or eye; non-CNS emboli: emboli to kidney, spleen, lung, abdominal/mesenteric and extremity; cardiac complications: valve dehiscence, heart failure, increasing vegetation, heart rhythm and conduction disturbances, myocarditis and myocardial infarction; infectious complication: wound infection or necrosis of operation site, deep wound infection at operation site, sepsis, catheter associated infection, line infection (central venous catheter, peripheral venous catheter, arterial catheter), pneumonia and urinary catheter-associated infection; renal complication: glomerulonephritis and renal failure.
Microbiology and histopathology
The current gold standard for diagnosis of infective endocarditis are the modified Duke criteria [4, 5]. These are based mainly on the evidence of the infective agent (blood cultures) and typical morphological alterations of heart valves and/or intravascular or intracardiac prosthetic material. In most patients with suspected blood culture-negative endocarditis one major criterion for diagnosis of infective endocarditis – positive blood cultures – is lacking [4, 5]. Thus, other diagnostic approaches for identification of an infective agent were applied. This included molecular analyses of resected heart valves by broad range or species-specific polymerase chain reaction (PCR), histopathology, Legionella pneumophila urinary antigen assay and serology (for Coxiella burnetii, Bartonella spp., Brucella spp., Chlamydia spp., Legionella spp, and Mycoplasma pneumoniae). Chronic Q fever was diagnosed in the case of an IgG titre of >1:800 to phase I C. burnetii and infective endocarditis due to Bartonella spp. was diagnosed in the case of IgG titres of >1:800 to B. quintana and/or B. henselae. Since 2014 heparin or sodium citrate blood for mycobacterial cultures and cultures for acid-fast bacilli from extrapulmonary sites were assessed in patients with prosthetic valves [16]. In addition, we assessed results of tests for antinuclear antibodies and rheumatoid factors.
Tissue samples were retrieved at time of cardiac surgery and subjected to histopathological analysis (including staining for microorganisms), culture and PCR if negative. For histopathological analyses, tissue was fixed in 4% buffered formalin solution for at least 12 hours. Fixed samples were processed automatically, which comprised dehydration, clearing, wax infiltration, and paraffin embedding. Histological sections of 2 µm thickness were obtained by microtomy. Subsequently they were deparaffinised and stained according to standard protocols with minor modifications.
Statistical analysis
Qualitative data are presented as absolute numbers and percentages. Continuous variables were analysed as median with interquartile ranges (IQRs) from the 25th to the 75th percentile. We assessed p-values from Fisher’s exact test (categorical variables) and Wilcoxon rank-sum test (age). Data were analysed in Stata/SE 17.0 (StataCorp, College Station, TX, USA).
Results
Patient selection and characterisation
We identified 861 patients with possible or definitive infective endocarditis between 1997 and 2020 (for an overview of year of diagnosis see supplemental figure S1 in the appendix). Of these, a total of 65 patients with 66 episodes of blood culture-negative endocarditis were identified, which accounted for 8% of all admissions (fig. 1).
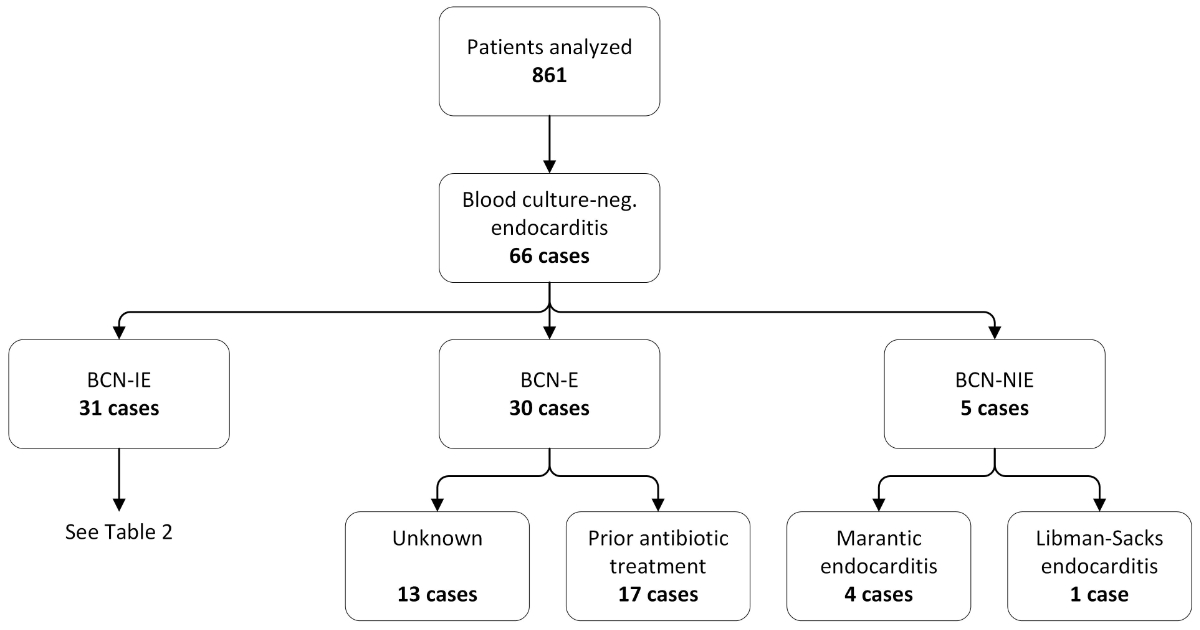
Figure 1 Selection and classification of patients presenting with blood culture-negative endocarditis.
Patients identified with blood culture-negative endocarditis were categorised into three groups, namely blood culture-negative infective endocarditis (BCN-IE), blood culture-negative endocarditis (BCN-E) and blood culture-negative non-infective endocarditis (BCN-NIE). Reasons for negative blood cultures in the BCN-E group are indicated as well as specific diagnoses of BCN-NIE. A detailed analysis of organisms found in BCN-IE can be found in table 2.
According to the modified Duke criteria, 46 were definite infective endocarditis, 15 were possible infective endocarditis and 5 were BCN-NIE. The latter included one case of Libman-Sacks endocarditis and four cases of marantic endocarditis associated with neoplastic diseases (one cervical cancer, one pancreatic cancer, and two lung cancers).
Demographic and clinical characteristic of patients with blood culture-negative endocarditis are presented in table 1.
Table 1Characteristics of the 66 cases with blood culture-negative endocarditis.
|
Characteristics
|
Total n = 66
|
BCN-IE n = 31
|
BCN-E n = 30
|
BCN-NIE n = 5
|
p-value
|
| Median age in years (IQR) |
55 (43–67) |
55 (43–67) |
54 (42–65) |
53 (48–62) |
0.88 |
| Male gender, n (%) |
51 (77.3) |
23 (74.2) |
25 (83.3) |
3 (60.0) |
0.32 |
| Risk factors, n (%) |
Congenital heart disease |
23 (34.8) |
12 (38.7) |
11 (36.7) |
0 (0) |
0.15 |
| Acquired heart disease |
25 (37.9) |
14 (45.2) |
11 (36.7) |
0 (0) |
0.15 |
| Prior cardiac surgery |
28 (42.4) |
13 (41.9) |
15 (50.0) |
0 (0) |
0.07 |
| – Biological valve |
10 (35.7) |
6 (19.4) |
4 (13.3) |
0 (0) |
1.00 |
| – Mechanical valve |
18 (64.3) |
7 (22.6) |
11 (36.7) |
0 (0) |
0.31 |
| Prior endocarditis |
9 (13.6) |
2 (6.5) |
7 (23.3) |
0 (0) |
1.00 |
| IVDU/opiate substitution |
4 (6.1) |
1 (3.2) |
3 (10.0) |
0 (0) |
1.00 |
| Valve affected, n (%) |
Aortic valve |
36 (54.5) |
20 (64.5) |
15 (50.0) |
1 (20.0) |
0.17 |
| Mitral valve |
26 (39.4) |
12 (38.7) |
10 (33.3) |
4 (80.1) |
0.07 |
| Tricuspid valve |
6 (9.1) |
0 (0) |
6 (20.0) |
0 (0) |
1.00 |
| Pulmonary valve |
3 (4.5) |
2 (6.5) |
1 (3.3) |
0 (0) |
1.00 |
| Two valves involved |
5 (7.6) |
3 (9.7) |
2 (6.7) |
0 (0) |
1.00 |
| Left sided endocarditis |
52 (78.8) |
22 (71.0) |
25 (83.3) |
5 (100) |
0.58 |
| Artificial valve |
27 (40.9) |
13 (41.9) |
14 (46.7) |
0 (0) |
0.07 |
| – Mechanical |
18 (66.7) |
7 (22.6) |
11 (36.7) |
0 (0) |
0.31 |
| – Biological |
9 (33.3) |
6 (19.4) |
3 (10.0) |
0 (0) |
1.00 |
| Modified Duke criteria, n (%) |
Definite |
46 (69.7) |
28 (90.3) |
18 (60.0) |
5 (100) |
0.002 |
| Possible |
15 (22.7) |
3 (9.7) |
12 (40.0) |
0 (0) |
0.58 |
| Rejected |
5 (7.6) |
0 (0) |
0 (0) |
0 (0) |
<0.001 |
| Reasons for negative blood culture, n (%) |
Prior antibiotic treatment |
23 (34.8) |
6 (19.4)2
|
17 (56.7) |
0 (0) |
0.15 |
| Fastidious slow-growing organisms1
|
20 (30.3) |
20 (64.5)2
|
0 (0) |
0 (0) |
0.31 |
| Non-bacterial pathogens |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
n.a. |
| Non-infective aetiology |
5 (7.6) |
0 (0) |
0 (0) |
5 (100) |
<0.001 |
| No identified aetiology |
19 (28.8) |
6 (19.4) |
13 (43.3) |
0 (0) |
0.31 |
| Surgery required, n (%) |
39 (59.1) |
24 (77.4) |
15 (50.0) |
0 (0) |
0.01 |
| Complications, n (%) |
Embolism |
33 (50.0) |
14 (45.2) |
16 (53.3) |
3 (60.0) |
1.00 |
| Cardial complication |
28 (42.4) |
15 (48.4) |
11 (36.7) |
2 (40.0) |
1.00 |
| Infectious complication |
19 (28.8) |
11 (35.5) |
8 (26.7) |
0 (0) |
0.31 |
| Renal complication |
17 (25.8) |
5 (16.1) |
11 (36.7) |
1 (20.0) |
1.00 |
| Abscess formation |
8 (12.1) |
5 (16.1) |
3 (10.0) |
0 (0) |
1.00 |
| Death |
10 (15.2) |
1 (3.2) |
6 (20.0) |
3 (60.0) |
0.02 |
| No complications |
8 (12.1) |
3 (9.7) |
3 (10.0) |
2 (40.0) |
|
Median age was 55 years (IQR 43–67) and most participants were male (77%). Predisposing factors were prosthetic heart valves (42%), acquired heart diseases (38%), congenital heart disease (35%), and prior endocarditis (14%). Most cases involved the left heart (79%) with the aortic valve most commonly affected (55%). Antibiotic treatment prior to blood collection was the most common presumed reason for negative blood cultures (35%), followed by later identification of fastidious and slow-growing organisms (30%), and non-infective endocarditis (8%). In 29%, no aetiology for sterile blood cultures could be identified. During hospital admission, 39 cases (59%) underwent cardiac surgery due to heart failure/haemodynamic compromise and/or uncontrolled infection / continuous embolisation. The most frequent complications were episodes with septic embolisation (50%) most commonly to the central nervous system (n = 60%), followed by cardiac complications (42%), and other infectious complications (29%). In-hospital mortality was 15%.
Microbiology
Of 61 cases with definite or possible blood culture-negative infective endocarditis, 31 cases were classified as BCN-IE and 30 cases as BCN-E, with a higher percentage of definite infective endocarditis cases in the BCN-IE group (90%) compared with the BCN-E group (60%) (table 1).
Overall, 17 (55%) BCN-IE cases were caused by fastidious and slow growing bacteria (table 2).
Table 2Bacteria isolated, diagnostic investigation, and reasons for negative blood cultures of patients with BCN-IE.
|
Bacterium isolated
|
Detection methods and sample types
|
Reasons for negative blood cultures
|
|
Fastidious bacterium
|
Prior antibiotic treatment
|
Unknown
|
| Coxiella burnetii (n = 6) |
Serology (n = 6) |
x |
|
|
| PCR aortic valve (n = 1) |
| Bartonella quintana (n = 3) |
Serology (n = 2) |
x |
|
|
| PCR aortic valve (n = 3) |
| Bartonella henselae (n = 1) |
Serology, culture, and PCR aortic valve (n = 1) |
x |
|
|
| Tropheryma whipplei (n = 4) |
PCR aortic valve (n = 3) |
x |
|
|
| PCR aortic- and mitral valve (n = 1) |
| Mycobacterium chimaera (n = 3) |
Culture of bone puncture (n = 2) |
x |
|
|
| PCR aortic valve (n = 1) |
| HACEK (n = 3) |
PCR mitral valve (n = 1) |
(x) |
x |
|
| PCR thrombus (n = 1) |
(x) |
|
x |
| PCR aortic wall (n = 1) |
(x) |
|
x |
| Streptococcus (n = 5) |
Streptococcus agalactiae
|
PCR mitral valve (n = 1) |
|
x |
|
|
Streptococcus pneumoniae
|
Culture and PCR mitral valve (n = 1) |
|
|
x |
|
Streptococcus mitis
|
PCR of knee puncture (n = 1) |
|
|
x |
|
Viridans Streptococci
|
Culture and PCR aortic valve (n = 1) |
|
x |
|
|
Streptococcus sp.
|
Culture and PCR aortic valve (n = 1) |
|
x |
|
| Staphylococcus (n = 3) |
Staphylococcus aureus
|
Culture urine (n = 1) |
|
|
x |
|
Staphylococcus lugdunensis
|
Culture of hip puncture (n = 1) |
|
x |
|
| CoNS (not further specified) |
Culture mitral valve (n = 1) |
|
|
x |
| Cutibacterium acnes (n = 2) |
Culture and PCR mitral valve (n = 1) |
|
|
x |
| PCR thrombus (n = 1) |
|
|
x |
| Enterococcus cecorum (n = 1) |
PCR blood (n = 1) |
|
x |
|
Systematic serological testing yielded six cases of infective endocarditis due to C. burnetii and four cases due to Bartonella spp. (three cases of B. quintana and one case of B. henselae), with PCRs from valve tissue contributing to pathogen identification in 10 of 17 (59%) cases. All of the six patients with infective endocarditis due to C. burnetii had either a congenital or an acquired heart disease and in five patients, infective endocarditis affected an artificial valve (four biological valves and one mechanical valve). The four patients with Bartonella spp. infective endocarditis all suffered from a congenital heart disease, endocarditis affected exclusively the left heart and native valves, and all underwent surgery. Recent travel histories, regular contact with domestic and/or wild animals or asylum status contributed to the final diagnosis of infective endocarditis in five patients with C. burnetii and two patients with B. quintana. Isolation of Mycobacterium chimaera was possible by cultures of bone (spondylodiscitis, osteomyelitis of the distal radius) or Mycobacterium genus-specific PCR from valve tissue. Besides the aforementioned fastidious and slow growing bacteria, 14 (45%) BCN-IE cases were due to the typical IE microorganisms Streptococcus spp., Staphylococcus spp., Enterococcus spp. and HACEK group, as well as Cutibacterium acnes (table 2).
Tissue analysis
We analysed tissue samples from 45 cases, which were derived from cardiac valves (38 cases), peripheral emboli (4 cases), joint punctures (2 cases) and bone biopsy (1 case) (fig. 2).
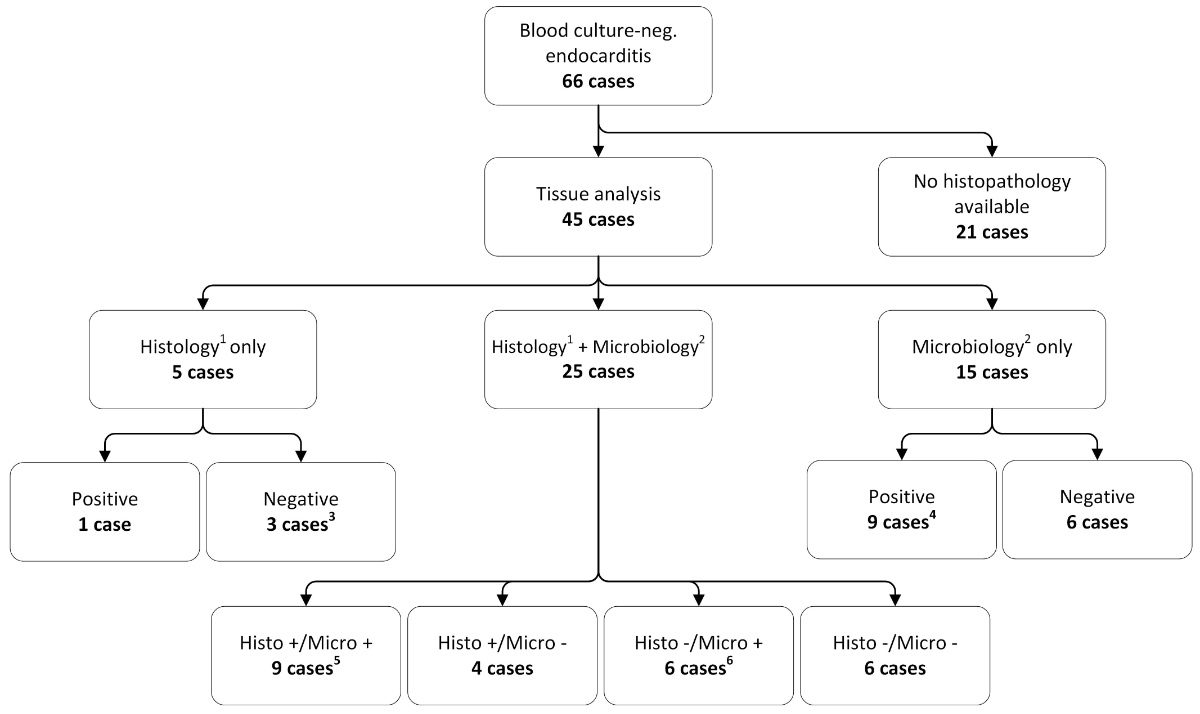
Figure 2 Overview and comparison of the histological and microbiological analysis of tissue samples.
Tissue was available from 45 patients. Histological or microbiological analysis was performed in 5 and 15 patients, respectively. Twenty-five cases were analysed by both microbiology and histology.
1 A positive histology result is defined as a positive signal of the microbiological staining of tissue samples,
2 a positive microbiology result is defined as a positive culture and/or PCR result of tissue samples,
3 one sample was discarded,
4 the nine cases are subdivided as follows: PCR only (n = 5), culture only (n = 1), PCR + culture (n = 3),
5 all congruent histological and microbiological results (PCR only (n = 7), culture only (n = 1), PCR + culture (n = 1)),
6 the six cases are subdivided as follows: PCR only (n = 4), culture only (n = 2).
Tissue samples from five (11%) out of 45 cases were analysed by Brown-Brenn staining which revealed Gram-positive cocci in chains in one patient. Tissue cultures and/or PCR without any histopathological analysis were performed in 15 cases (33%) and the remaining 25 cases (56%) were subjected to both histopathology and microbiological analyses. We found congruent results in 60% of cases.
Among the 30 histologically analysed tissue samples, Brown-Brenn staining detected bacteria in 14 cases (47%) and acute or chronic inflammation was visible in 27 cases (90%) (fig. 3).
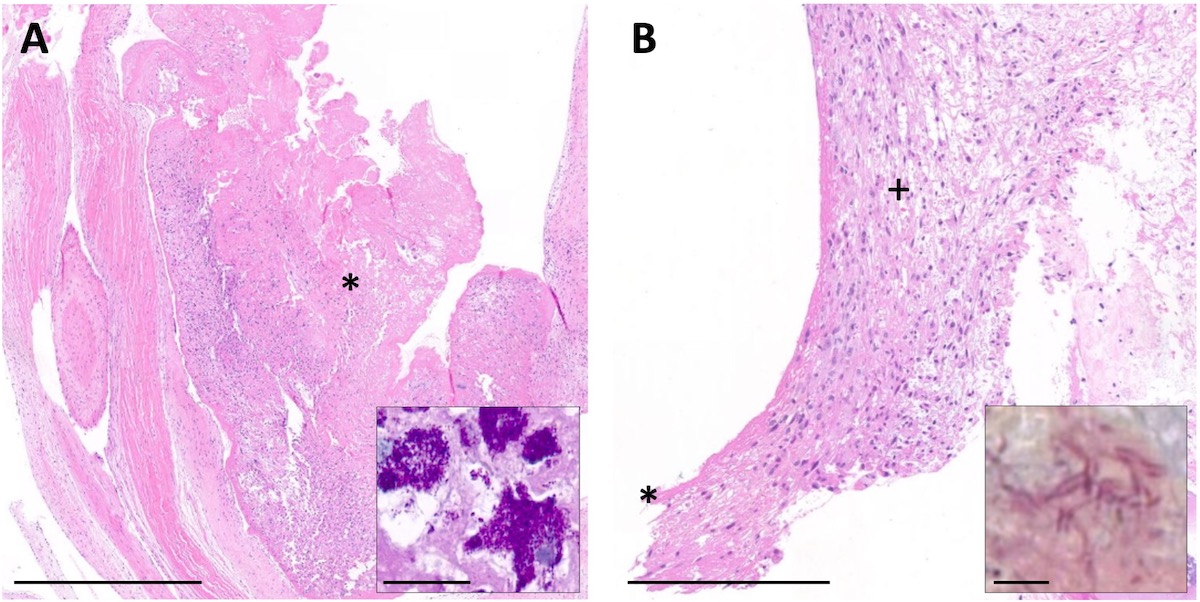
Figure 3 Patient histology of two patients with blood culture-negative aortic valve infective endocarditis.
A: Overview of haematoxylin and eosin-stained aortic valve section showing the vegetation adherent to the surface of the valve (*). Inset depicts typical foamy macrophages loaded with numerous periodic acid Schiff, diastase-resistant positive clumps of intracellular bacteria (Tropheryma whipplei). Scale bar 1 mm and 25 µm inset. B: Overview of H and E-stained aortic valve section showing thickening of the leaflet with granulation tissue (+) and fibrin layer (*). Inset depicts multiple long irregular rods (Cardiobacterium hominis) in Brown-Brenn staining. Scale bar: 250 µm and 5 µm inset.
In the remaining three cases without signs of inflammation, PCR and/or culture were positive.
Discussion
We characterised in this single-centre study patients with blood culture-negative endocarditis over a time span of 23 years. These included 66 cases of blood culture-negative endocarditis, which accounted for 8% of all 861 admitted patients for possible or definite infective endocarditis during the study period. An underlying pathogen was identified in 47% (BCN-IE), and the cause of negative blood cultures remained unknown (BCN-E) in 46%. A non-infective cause of endocarditis was identified (BCN-NIE) in 8% of cases.
Similar analyses were reported in a French study with 819 cases of blood culture-negative endocarditis. Compared with our cohort, the number of BCN-IE cases was higher in the French study by Fournier, with 62.7% of cases, with correspondingly lower rates of BCN-E (36.5%) and BCN-NIE (2.5%) [17]. However, the yield of our diagnostic approach led to a diagnosis in 831/861 infective endocarditis cases (96.5%). Thus, the overall percentage of blood culture-negative endocarditis in our study was lower than previously published (14–31%) [4, 18]. Improvements in diagnostic techniques for detection of microbiological blood stream infections and a decrease of non-targeted antimicrobial therapy might account for this phenomenon.
Administration of empiric antibiotic treatment prior to blood culture sampling was still the most important reason why blood cultures remained without growth (35%), albeit the rate was lower than in previously reported studies (48–80%) [18, 19]. By means of advanced diagnostics, we were able to identify a causative pathogen in approximately one fourth of cases despite antimicrobial therapy prior to sampling for blood cultures. Isolated pathogens were mainly Streptococcus spp., Staphylococcus spp., Enterococcus spp., and C. acnes highlighting the fact that classical infective endocarditis bacteria are also responsible pathogens for BCN-IE.
The majority of cases of Gram-negative infective endocarditis originated from the HACEK group. Although HACEK organisms should grow after a median of 3.4 days when using the automated blood culturing system [20], blood cultures were negative in all three patients of our series. Whereas one patient received amoxicillin / clavulanic acid for two days prior to blood sampling, the reason for negative blood cultures remained unknown in the other two cases.
Numerous studies have shown that fastidious and slow-growing organisms account for a large part of BCN-IE cases with C. burnetii, and Bartonella spp. as leading pathogens [17, 21]. This observation is in alignment with the current study, where the proportion of fastidious organisms was 55% in BCN-IE. Of 10 patients with infective endocarditis due to C. burnetii or Bartonella spp., serological analysis reliably identified nine cases, highlighting the importance of serology in the diagnosis of infective endocarditis due to fastidious pathogens. The number of reported endocarditis cases due to T. whipplei has risen in the last two decades, with an increased incidence in Switzerland, Germany and eastern-central France. Typically, patients with T. whipplei endocarditis complain of arthralgia for several years and may therefore receive immunosuppressive drugs for a potential rheumatic disease [22]. Indeed, three of the four patients with T. whipplei endocarditis in this series reported arthralgia and two patients were on therapy with steroids and methotrexate, respectively.
Among patients with a history of cardiac surgery under cardiopulmonary bypass with fever of unknown origin M. chimaera is a new important differential diagnosis of blood culture-negative endocarditis. Since 2013, over a hundred cases of M. chimaera invasive infections in Switzerland, Germany, the Netherlands, UK, USA and Australia have been reported, which could all be linked to intraoperative transmission of M. chimaera from contaminated heater-cooler units during cardiothoracic surgery [23, 24]. At the University Hospital in Zurich, Switzerland, a total of six patients were diagnosed with prosthetic valve endocarditis or vascular graft infection due to M. chimaera [23, 25]. Three out of these six patients are included in the current study, where the replaced or reconstructed valves affected by BCN-IE were accompanied by spondylodiscitis, osteomyelitis of the distal radius, chorioretinitis, and hepatitis, respectively [26].
Previous studies have identified fungi as important causes of blood culture-negative endocarditis and have suggested systematically searching for these microorganisms [17]. These observations were not in accordance with the present findings as all 11 cases of fungal endocarditis identified in the initial screening had positive blood cultures.
Five cases of non-infective endocarditis were found in the present study. Echocardiography revealed typical rather small and broad-based vegetations on three native mitral valves and one native aortic valve. Thromboembolic complications are a well-known serious complication of marantic endocarditis [27, 28]. Shortly after diagnosis of marantic endocarditis, all of our patients died. If causes of death were multiple embolic events, the advanced malignant disease, or a combination of both is difficult to determine.
As the diagnosis of infective endocarditis according to the modified Duke criteria importantly relies on identification of an infectious agent, the importance of advanced microbiological analysis of potentially infected tissue cannot be overemphasised [29]. Valve histopathology is still considered the gold standard for the diagnosis of infective endocarditis and has an impact on decisions about the duration of antibiotic therapy [30]. In the current study, analysis of tissue by histopathology and culture, which serve as pathological criteria in the modified Duke criteria, changed infective endocarditis diagnosis from probable to definite in 21/66 cases (32%), underlining the importance of histopathology.
Surgical treatment for reasons such as haemodynamic compromise or continuous embolisation is required in approximately half of the patients with infective endocarditis [4], which corresponds with our findings (59%). In 45 cases, tissue samples were available, which predominantly (84%) were derived from cardiac valves. Cultures from valves had a low sensitivity (16%), which is in line with the literature [31]. Since not all samples were concomitantly analysed by histology, PCR and culture, a closer look at the individual diagnostic technique is important. Among the 30 histologically analysed cases, 90% showed acute or chronic inflammation and in almost half of them microbiological staining detected the causative bacterium. Comparable numbers were found in two other studies where microbiological staining of tissue samples revealed a microorganism in 30% [32] and 60% [33], respectively. Cardiac valve tissue is the optimal sample type. By means of molecular analysis and culture of tissue samples, a causative organism could be successfully identified in 77% of BCN-IE cases of the present series. Although several studies have shown the importance of PCR in the aetiological analysis of blood culture-negative endocarditis [32, 34], international infective endocarditis guidelines have yet not incorporated molecular testing of tissue samples into the modified Duke criteria [35].
Lastly, one case of BCN-IE was identified by PCR of a blood sample (Enterococcus cecorum) and one case by urine culture (Staphylococcus aureus). Molecular analysis of serum samples exhibits low sensitivity and is generally not recommended [15]. S. aureus is an uncommon urine isolate and in up to 34% bacteriuria is associated with S. aureus bacteraemia [36]. A recent French study investigated 27 cases of community-acquired S. aureus bacteriuria. All had subsequent S. aureus bacteraemia and were diagnosed with infective endocarditis [37]. In light of these findings, it is very likely that S. aureus was the causative pathogen, despite the fact that PCR and culture of the mitral valve were negative.
To our knowledge, this study represents the first comprehensive report of a Swiss centre over a long observation period. The rate of blood culture-negative endocarditis was low compared with the literature, which could indicate a good diagnostic and clinical approach supported by repeated case discussions in the weekly endocarditis board meetings. Several limitations should also be noted. Since the study was conducted at a tertiary care centre with a large catchment area the current results may be generalised to some extent to the Swiss population, but not necessarily to the European population owing to different diagnostic techniques and prevalence of fastidious organisms, as well as easier access to antibiotics without prescription in some countries. Since data collection occurred at a single tertiary care centre, the sample size was rather small. Despite using a structured questionnaire for chart review, the retrospective design of the study may introduce bias linked to heterogeneous or missing data. Since information technology (IT) and patient documentation has changed greatly over time and only cases with complete data records were selected, we assume that the number of blood culture-negative cases is higher. This finding is reflected in supplemental figure S1 (appendix), where more cases of blood culture-negative endocarditis were found in the second half of the study period than in the first half. Assuming that the rate of endocarditis cases with negative blood cultures remained constant over time, more cases should have been identified between the years 1997 and 2009. Moreover, microbiological techniques may have changed and improved over the years and thus the true prevalence of BCN-IE might be higher than reported in our study. Also, M. chimaera is overrepresented since all cases were identified during an outbreak at the study site.
In conclusion, blood culture-negative endocarditis is a rare entity where a refined diagnostic approach is of upmost importance. This includes prolonged incubation times of blood cultures, serology, species specific PCR from blood as well as analysis of cardiac valve and tissue sample by means of culture, histology and broad-range PCR for bacteria and fungi. A proposed diagnostic algorithm for cases of blood culture-negative endocarditis can be found in supplemental figure S2. Empirical antibiotic treatment prior to blood collection, fastidious and slow growing organisms such as C. burnetii and Bartonella spp. and non-infective endocarditis were identified as main reasons for sterile blood cultures.
Acknowledgements
We would like to thank the participants of the ENVALVE and VASGRA cohort study, and Prof. R. Zbinden for critical reading of the manuscript. We also thank the study nurses C. Mueller and S. Buergin for their excellent work, and Ch. Laich for administrative assistance. We also thank the technicians of the Institute of Medical Microbiology for their dedicated work.
Prof. Barbara Hasse, MD
Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich
Raemistrasse 100
CH-8091 Zurich
barbara.hasse[at]usz.ch
References
1. Talha KM, Baddour LM, Thornhill MH, Arshad V, Tariq W, Tleyjeh IM, et al. Escalating incidence of infective endocarditis in Europe in the 21st century. Open Heart. 2021 Oct;8(2):e001846. https://doi.org/https://doi.org/10.1136/openhrt-2021-001846
2. Hubers SA, DeSimone DC, Gersh BJ, Anavekar NS. Infective Endocarditis: A Contemporary Review. Mayo Clin Proc. 2020 May;95(5):982–97. https://doi.org/https://doi.org/10.1016/j.mayocp.2019.12.008
3. Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG Jr. Infective endocarditis. Nat Rev Dis Primers. 2016 Sep;2(1):16059. https://doi.org/https://doi.org/10.1038/nrdp.2016.59.Infective https://doi.org/https://doi.org/10.1038/nrdp.2016.59
4. Habib G, et al. 2015 ESC Guidelines for the management of infective endocarditis, European Heart Journal, vol. 36, no. 44. Oxford University Press, pp. 3075–3123, 21-Nov-2015, doi: https://doi.org/https://doi.org/10.1093/eurheartj/ehv319
5. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000 Apr;30(4):633–8. https://doi.org/https://doi.org/10.1086/313753
6. Houpikian P, Raoult D. Diagnostic methods. Current best practices and guidelines for identification of difficult-to-culture pathogens in infective endocarditis. Cardiol Clin. 2003 May;21(2):207–17. https://doi.org/https://doi.org/10.1016/S0733-8651(03)00028-6
7. Didier Raoult P. MD and M. Daniel J Sexton, Culture-negative endocarditis: Epidemiology, microbiology, and diagnosis - UpToDate, 2019. Online]. Available from: https://www.uptodate.com/contents/culture-negative-endocarditis-epidemiology-microbiology-and-diagnosis. Accessed: 27-Oct-2020].
8. C. Selton-Suty et al., Preeminence of staphylococcus aureus in infective endocarditis: A 1-year population-based survey, Clinical Infectious Diseases, vol. 54, no. 9. Clin Infect Dis, pp. 1230–1239, 01-May-2012, doi: https://doi.org/https://doi.org/10.1093/cid/cis199.
9. Cecchi E, Forno D, Imazio M, Migliardi A, Gnavi R, Dal Conte I, et al.; Piemonte Infective Endocarditis Study Group. New trends in the epidemiological and clinical features of infective endocarditis: results of a multicenter prospective study. Ital Heart J. 2004 Apr;5(4):249–56.
10. Garg N, Kandpal B, Garg N, Tewari S, Kapoor A, Goel P, et al. Characteristics of infective endocarditis in a developing country-clinical profile and outcome in 192 Indian patients, 1992-2001. Int J Cardiol. 2005 Feb;98(2):253–60. https://doi.org/https://doi.org/10.1016/j.ijcard.2003.10.043
11. Watt G, Lacroix A, Pachirat O, Baggett HC, Raoult D, Fournier PE, et al. Prospective comparison of infective endocarditis in Khon Kaen, Thailand and Rennes, France. Am J Trop Med Hyg. 2015 Apr;92(4):871–4. https://doi.org/https://doi.org/10.4269/ajtmh.14-0689
12. Østergaard L, Voldstedlund M, Bruun NE, Bundgaard H, Iversen K, Køber N, et al. Temporal Changes, Patient Characteristics, and Mortality, According to Microbiological Cause of Infective Endocarditis: A Nationwide Study. J Am Heart Assoc. 2022 Aug;11(16):e025801. https://doi.org/https://doi.org/10.1161/JAHA.122.025801
13. Benslimani A, Fenollar F, Lepidi H, Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis. 2005 Feb;11(2):216–24. https://doi.org/https://doi.org/10.3201/eid1102.040668
14. D. O. Mayer and B. Hasse, Gefäß(endo)protheseninfektionen: Erfahrungen und Lehren aus 8 Jahren prospektiver Begleitung der VASGRA-Kohorte am Universitätsspital Zürich, Gefässchirurgie 2020 258, vol. 25, no. 8, pp. 621–631, Nov. 2020, doi: https://doi.org/https://doi.org/10.1007/S00772-020-00715-3. https://doi.org/https://doi.org/10.1007/s00772-020-00715-3
15. Katsouli A, Massad MG. Current issues in the diagnosis and management of blood culture-negative infective and non-infective endocarditis. Ann Thorac Surg. 2013 Apr;95(4):1467–74. https://doi.org/https://doi.org/10.1016/j.athoracsur.2012.10.044
16. B. Hasse et al., International Society of Cardiovascular Infectious Diseases Guidelines for the Diagnosis, Treatment and Prevention of Disseminated Mycobacterium chimaera Infection Following Cardiac Surgery with Cardiopulmonary Bypass, Journal of Hospital Infection, vol. 104, no. 2. W.B. Saunders Ltd, pp. 214–235, 01-Feb-2020, doi: https://doi.org/https://doi.org/10.1016/j.jhin.2019.10.009.
17. Fournier PE, Thuny F, Richet H, Lepidi H, Casalta JP, Arzouni JP, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis. 2010 Jul;51(2):131–40. https://doi.org/https://doi.org/10.1086/653675
18. Hoen B, Selton-Suty C, Lacassin F, Etienne J, Briançon S, Leport C, et al. Infective endocarditis in patients with negative blood cultures: analysis of 88 cases from a one-year nationwide survey in France. Clin Infect Dis. 1995 Mar;20(3):501–6. https://doi.org/https://doi.org/10.1093/clinids/20.3.501
19. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005 May;84(3):162–73. https://doi.org/https://doi.org/10.1097/01.md.0000165658.82869.17
20. S. L. Sharara, R. Tayyar, Z. A. Kanafani, and S. S. Kanj, HACEK endocarditis: a review, Expert Review of Anti-Infective Therapy, vol. 14, no. 6. Taylor and Francis Ltd, pp. 539–545, 02-Jun-2016, doi: https://doi.org/https://doi.org/10.1080/14787210.2016.1184085.
21. Tattevin P, Watt G, Revest M, Arvieux C, Fournier PE. Update on blood culture-negative endocarditis. Med Mal Infect. 2015;45(1-2):1–8. https://doi.org/https://doi.org/10.1016/j.medmal.2014.11.003
22. Fenollar F, Célard M, Lagier JC, Lepidi H, Fournier PE, Raoult D. Tropheryma whipplei endocarditis. Emerg Infect Dis. 2013 Nov;19(11):1721–30. https://doi.org/https://doi.org/10.3201/eid1911.121356
23. Sax H, Bloemberg G, Hasse B, Sommerstein R, Kohler P, Achermann Y, et al. Prolonged outbreak of mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis. 2015 Jul;61(1):67–75. https://doi.org/https://doi.org/10.1093/cid/civ198
24. van Ingen J, Kohl TA, Kranzer K, Hasse B, Keller PM, Katarzyna Szafrańska A, et al. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis. 2017 Oct;17(10):1033–41. https://doi.org/https://doi.org/10.1016/S1473-3099(17)30324-9
25. Sommerstein R, Rüegg C, Kohler P, Bloemberg G, Kuster SP, Sax H. Transmission of Mycobacterium chimaera from heater-cooler units during cardiac surgery despite an ultraclean air ventilation system. Emerg Infect Dis. 2016 Jun;22(6):1008–13. https://doi.org/https://doi.org/10.3201/eid2206.160045
26. Kohler P, Kuster SP, Bloemberg G, Schulthess B, Frank M, Tanner FC, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J. 2015 Oct;36(40):2745–53. https://doi.org/https://doi.org/10.1093/eurheartj/ehv342
27. I. Steiner, Nonbacterial thrombotic endocarditis--a study of 171 case reports (Article in Czech), Ces. Patol., vol. 29, no. 2, 1993.
28. Asopa S, Patel A, Khan OA, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007 Nov;32(5):696–701. https://doi.org/https://doi.org/10.1016/j.ejcts.2007.07.029
29. Lamas CC, Eykyn SJ. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart. 2003 Mar;89(3):258–62. https://doi.org/https://doi.org/10.1136/heart.89.3.258
30. Brandão TJ, Januario-da-Silva CA, Correia MG, Zappa M, Abrantes JA, Dantas AM, et al. Histopathology of valves in infective endocarditis, diagnostic criteria and treatment considerations. Infection. 2017 Apr;45(2):199–207. https://doi.org/https://doi.org/10.1007/s15010-016-0953-4
31. R. M. Liesman, B. S. Pritt, J. J. Maleszewski, and R. Patela, Laboratory diagnosis of infective endocarditis, Journal of Clinical Microbiology, vol. 55, no. 9. American Society for Microbiology, pp. 2599–2608, 01-Sep-2017, doi: https://doi.org/https://doi.org/10.1128/JCM.00635-17.
32. Lamas CC, Fournier PE, Zappa M, Brandão TJ, Januário-da-Silva CA, Correia MG, et al. Diagnosis of blood culture-negative endocarditis and clinical comparison between blood culture-negative and blood culture-positive cases. Infection. 2016 Aug;44(4):459–66. https://doi.org/https://doi.org/10.1007/s15010-015-0863-x
33. Morris AJ, Drinkovic D, Pottumarthy S, Strickett MG, MacCulloch D, Lambie N, et al. Gram stain, culture, and histopathological examination findings for heart valves removed because of infective endocarditis. Clin Infect Dis. 2003 Mar;36(6):697–704. https://doi.org/https://doi.org/10.1086/367842
34. Harris KA, Yam T, Jalili S, Williams OM, Alshafi K, Gouliouris T, et al. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. Eur J Clin Microbiol Infect Dis. 2014 Nov;33(11):2061–6. https://doi.org/https://doi.org/10.1007/s10096-014-2145-4
35. Godfrey R, Curtis S, Schilling WH, James PR. Blood culture negative endocarditis in the modern era of 16S rRNA sequencing. Clin Med (Lond). 2020 Jul;20(4):412–6. https://doi.org/https://doi.org/10.7861/CLINMED.2019-0342 https://doi.org/https://doi.org/10.7861/clinmed.2019-0342
36. Al Mohajer M, Darouiche RO. Staphylococcus aureus bacteriuria: Source, clinical relevance, and management. Curr Infect Dis Rep. 2012 Dec;14(6):601–6. https://doi.org/https://doi.org/10.1007/s11908-012-0290-4
37. Lafon T, Hernandez Padilla AC, Baisse A, Lavaud L, Goudelin M, Barraud O, et al. Community-acquired Staphylococcus aureus bacteriuria: a warning microbiological marker for infective endocarditis? BMC Infect Dis. 2019 Jun;19(1):504. https://doi.org/https://doi.org/10.1186/s12879-019-4106-0
Appendix: Supplementary data
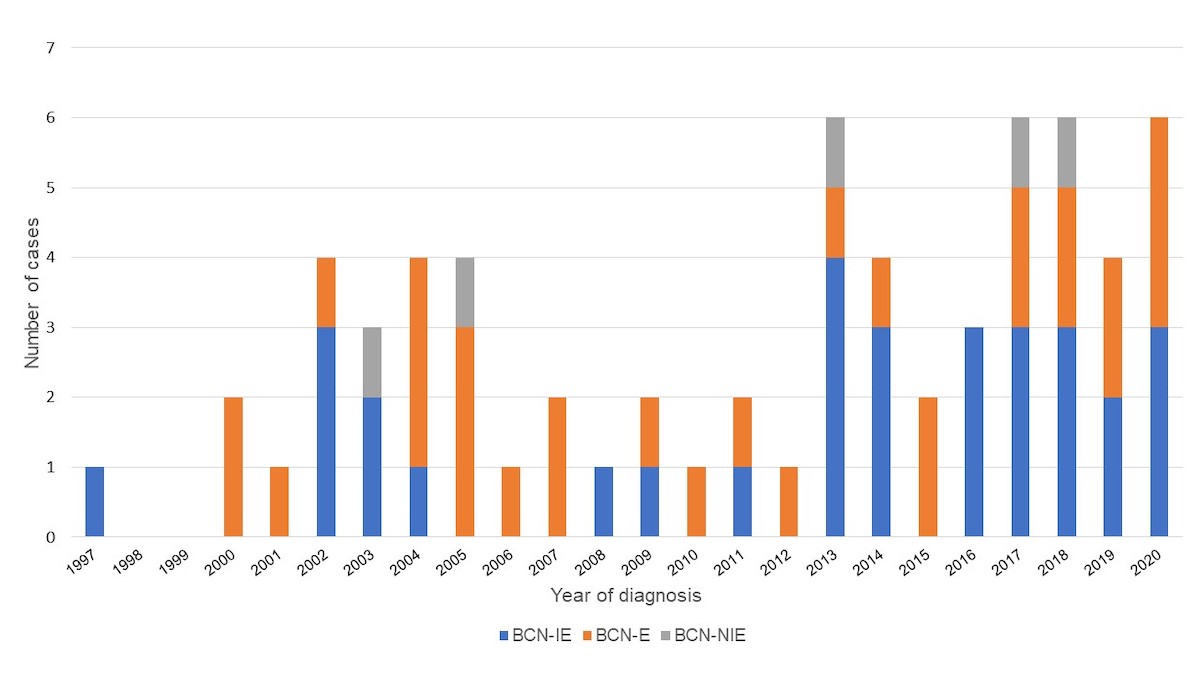
Figure S1 Cases of endocarditis grouped by year of diagnosis.
Depiction of number of cases of blood culture-negative infective endocarditis (BCN-IE), blood culture-negative endocarditis (BCN-E) and blood culture-negative non-infective endocarditis (BCN-NIE) over the study period from 1997–2020.
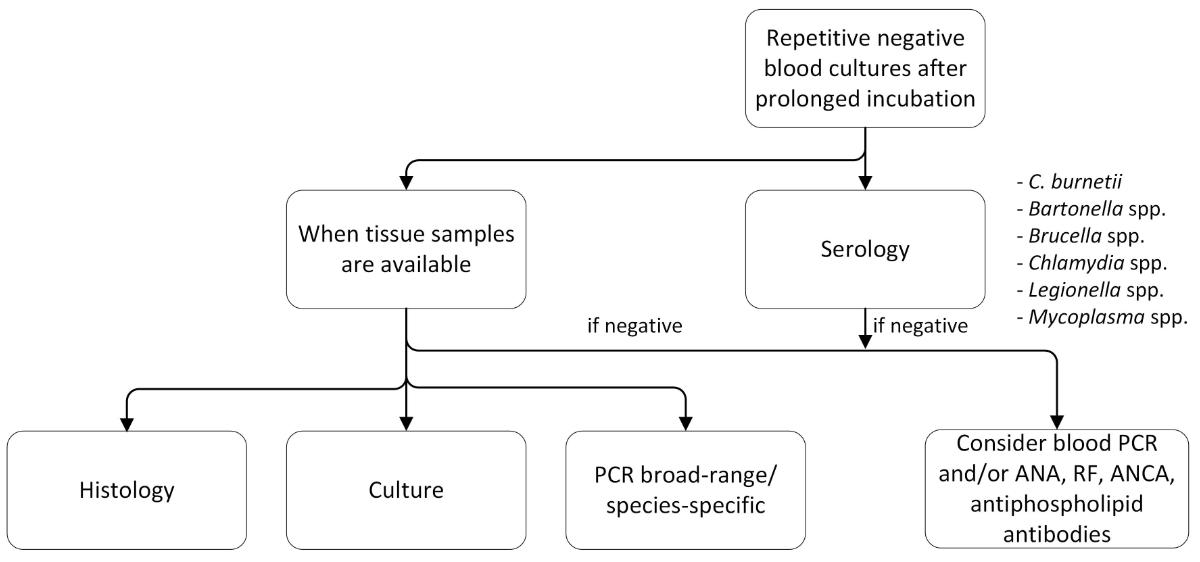
Figure S2 Proposed diagnostic algorithm in case of negative blood cultures.
PCR: polymerase chain reaction; ANA: anti-nuclear antibody; RF: rheumatoid factor; ANCA: anti-neutrophil cytoplasmatic antibody
Table S1Structured questionnaire.
|
Patient data
|
Age at baseline |
| Sex |
| Year endocarditis diagnosed |
|
Risk factors
|
Congenital heart disease |
| Acquired heart disease |
| Prior cardiac surgery |
| Prior endocarditis |
| Intravenous drug use (IVDU) or opiate substitution programme |
| Patient history (travel, contact with animals, special food (consumption of raw dairy products, insufficiently cooked meat), place of origin, occupational and recreational activities (farms, lab, abattoir), living (rural, urban, homeless) |
| Valve/device involvement
|
Which valve |
| Native valve |
| Not native valve (type of material) |
| Device infection |
| Right or left side |
|
Lab
|
Anti-nuclear antibody |
| Anticardiolipin IgG, anti-b2-glycoprotein 1 IgG + IgM |
| Rheumatoid factor |
| Anti ds DNA |
| Modified Duke criteria |
Coxiella burnetii phase-I IgG titre >1:800 |
| Positive echo for vegetation, abscess, pseudoaneurysm, intracardial fistula, valve perforation, new partial dehiscence of a prosthetic valve |
| Positive FDG-PET/CT at new prosthetic valve >3 months postoperative |
| Paravalvular lesion in computed tomography |
| Predisposition heart condition or IVDU |
| Fever >38 °C |
| Vascular phenomena |
| Immunological phenomena |
| Microbiological evidence not meeting major criteria |
| Microorganisms demonstrated by culture or on histological examination of a vegetation, a vegetation that has embolised or an intracardiac abscess specimen |
| Pathological lesions; vegetation or intracardiac abscess confirmed by histological examination showing active endocarditis |
| Reasons for negative blood cultures |
Prior antibiotic treatment |
| Fastidious slow-growing organisms |
| Nonbacterial pathogens |
| Non-infective aetiologies |
| No identified etiology |
| Complications
|
Embolisms |
| Abscess formation |
| Cardial complications |
| Infectious complications |
| Renal complications |
| Other complications |
| Surgery needed |
| Death |




