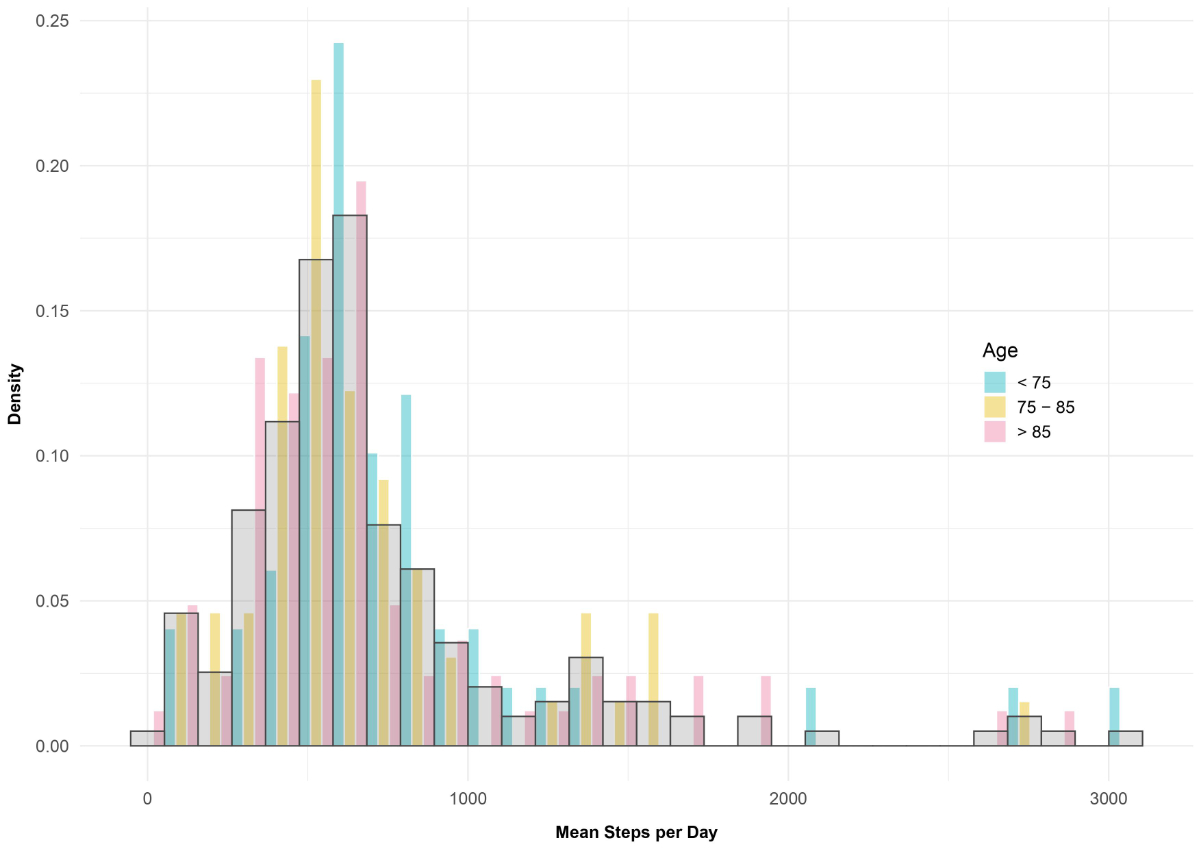
Figure 1 Frequency of mean step per day, stratified by age group.
DOI: https://doi.org/10.57187/smw.2022.40012
Walking is regarded as one of the most effective measures to sustain physical fitness and reduce adverse health events [1, 2]. Still, most elderly patients spend over 80% of their hospitalisation time in bed [3]. Walking aids, comorbidities, medical procedures and lack of motivation are the most relevant factors for a patient’s low activity [4–6]. Low mobility during hospitalisation is associated with an increased probability of death [7], a decline in activities of daily living [8, 9], longer hospital stays [9] and placement in a post-acute facility or other institutionalisation [10, 11]. After hospitalisation from an acute medical illness, approximately 40% of older adults are less able to conduct activities of daily living and only 30% of them return to their pre-admission physical condition within a year of discharge [12].
Physical activity can be quantified by the number of daily steps a patient takes using pedometers or, more recently, accelerometers [13]. A mean of 740 steps [14] and a median of 600–656 steps [15, 16] has been reported in older hospitalised patients, which is two to six times less than a comparable community-dwelling population [17]. Elderly patients walking fewer than 900 steps during hospitalisation have a significant increase in negative outcomes, such as in-hospital acquired functional decline [18].
Thus, to set up future interventional mobility trials aiming at increasing the step-count of elderly patients hospitalised in acute medical wards and decreasing such deleterious outcomes, better knowledge of step parameters is necessary. So far, few studies on step parameters in the acute medical setting have been published and they generally included geriatric or rehabilitation wards, and focused on step counts or raw physical activity measure (mG) rather than on cadence and bout measurements [11, 19–22]. Moreover, since we can hypothesise that inpatient mobilisation’s pattern and quantity is related to the healthcare system and hospital resources, it is of interest to assess, for the first time, step parameters of elderly patients hospitalised in Switzerland. Switzerland has a higher quantity of healthcare personnel per capita than most countries [23], with a high availability of hospital beds and hospital staff. In our previous analysis [11], we assessed levels of physical activity using raw physical activity measure (mG), which is not transposable to clinical practice, since more details about step parameters are necessary to set mobility goals for hospitalised patients.
We therefore aimed to assess in the present analysis step parameters (including step count, cadence and bout duration) of elderly patients hospitalised in a medical ward for acute care of the Lausanne University Hospital, in Switzerland, and to identify factors associated with step parameters.
This study was carried out from February to November 2018 in a 21-bed internal medicine ward at the Lausanne University Hospital (CHUV) in canton Vaud, a French-speaking region of Switzerland. The CHUV has more than 1500 beds and admits over 50,000 patients per year (www.chuv.ch/fr/chuv-home/en-bref/chiffres/). The method used for this study is similar to two previous studies [6, 11] in the same population.
Patients were recruited on a daily basis, Monday to Friday. Briefly, all patients aged ≥65 years admitted directly to the study ward or via the emergency unit were considered as eligible. Participants were excluded if they: (a) had a probable life expectancy of less than 30 days, based on clinical judgement; (b) had insufficient comprehension of the French language, (c) were unable to stand on their feet one week before hospitalisation, as assessed by interview, or (d) were forced to bed rest due to factors unrelated to the cause of hospital admission (e.g., fracture). The selection procedure was applied within the first three days of hospitalisation. If exclusion criteria were not met, patients were invited to participate and the study procedure was explained. If the patient accepted, a written informed consent was signed before the start of the study. Before the beginning of the study, all investigators were trained regarding screening and recruiting methods. The study protocol for the parent study [11] is available upon request.
The ethics committee of canton Vaud (www.cer-vd.ch) approved the study (ref. 2017-01907, decision of 21 December 2017). The full decision of the CER-VD can be obtained from the authors upon request. The study was performed in agreement with the Helsinki declaration and its later amendments, and in accordance with the applicable Swiss legislation. All participants or their legal representatives (in the case of confusion or dementia) provided a signed informed consent before entering the study. If a participant decided to withdraw from the study, data collected until the moment of withdrawal was used as agreed upon in the consent.
Physical activity and step count were assessed using a wrist accelerometer (GENEActiv Original, ActivInsights Ltd, UK), parametrised at 50 Hz sampling rate. These accelerometers provide a reliable and valid measurement of physical activity in adults [24] and were proven to be equivalent to similar devices [25, 26]. The devices were provided to the patients immediately after inclusion and patients could choose on which wrist they preferred to wear the device. Previous studies have shown that wrist choice does not influence results [27]. Patients were asked to wear the device continuously (day and night, including showering). The observation period was limited to the index hospitalisation in internal medicine. At discharge or transfer to another department (e.g., intensive care, surgery unit), the accelerometer was removed by a nurse or one of the investigators. Data regarding the physical activity of the patients has previously been published by our research group [6, 11].
Data was extracted and analysed using MATLAB (MathWorks, USA). A valid day was defined as at least 10 hours of daytime wear, and at least 24 hours of valid data were required for analysis [28]. Non-wearing was identified using a non-wearing detection algorithm on the raw acceleration data [29]. The algorithms used for detection of walking bouts and extraction of related parameters (number of steps, cadence) have been technically validated in previous studies [30–32]. Daily number of steps (mean, standard deviation [SD], min, max) for each patient was estimated as the sum of steps during daily walking bouts. Walking bouts were defined as periods of movement of the patient >10 seconds (s), and then categorised in three groups (<30 s, 30–120 s, and >120 s). The cadence was estimated using a frequency-based approach [31].
Walking bout detection has been previously described [32]. In brief, the wrist-recorded acceleration signal was first enhanced using appropriate signal processing tools, and several biomechanically meaningful features (defined based on intensity, periodicity, posture and noisiness) were extracted. The features were then fed into a classification procedure consisting of a Bayes classifier followed by two smart post-classification blocks. A median sensitivity, specificity, accuracy,and precision, 90.2, 97.2, 96.6 and 80.0 were found (values in %, the highest possible value 100 corresponds to full agreement between the validated algorithm and the ground truth).
The instantaneous cadence (i.e., cadence per second) was estimated using a frequency-based approach, based on a spectral analysis of the acceleration signal during the detected walking bouts. Then, the cadence of each walking bout was obtained as the average of instantaneous cadence [30, 31]. According to the biomechanical definition, the walking bout cadence was defined as:
cad [steps/min] = number of steps / duration walking bout [min]
From this definition, the number of steps during each walking bout was estimated as
cad [steps/min] × duration walking bout [min]
Daily number of steps was estimated as the sum of steps during daily walking bouts. The various statistical metrics characterising walking bout duration and cadence were derived from distribution of all walking bouts detected during the monitoring period.
These analyses were performed using MATLAB (Version R2020a, MathWorks, USA).
Covariates were extracted from the hospital electronic health records. These included demographics and comorbidities in the form of the Charlson index score [33]. At admission investigators recorded self-reported autonomy (physical function) for the 2 weeks before admission, risk of sores using the Braden score, use of walking aids, medical equipment at inclusion (i.e., urinary catheter or oxygen therapy), and isolation precautions. Walking aids were defined as the use of a cane, a walker, or both. Living situation at admission was defined as the patient lived at home with his/her spouse/partner/family (cohabitation), alone, or in a nursing home.
Autonomy was prospectively assessed twice (at admission and at discharge) using the modified Barthel index in a face-to-face interview. The Barthel index score has been reported as being the best scale to assess activities of daily living [34]. It has widespread use, and the modified version [34], which consists of five key questions, has been proven to increase [35] the internal consistency and provide better discrimination of functional ability. For patients with dementia or confusion, the level of autonomy before the hospitalisation was assessed by interviewing their relatives or caregivers, in face-to-face interviews or by telephone call. The patient’s autonomy in performing different activities of daily living was rated as follows: fully independent, with minimal or moderate help, attempts task but unsafe or unable to perform. Maximum score of the Barthel index was 100. A score of 0–20 suggests complete, 21–60 severe, 61–90 moderate and 91–99 slight dependence. A score of 100 indicates that the patient is fully independent of assistance from others. Hospital-acquired functional decline was defined as a ≥5-point decrease/worsening in the Barthel index at discharge [18, 36]. This decrease is equivalent to full dependency in at least one of 10 activities of daily living.
Skin status and risk of bedsores were assessed using the Braden score at inclusion and at discharge [37]. The Braden scale rates patients on six sub-scales: sensory perception, moisture, activity, mobility, nutrition, and friction and shear. The maximum score is 23; a score ≤18 is indicative of high risk of sore development.
The main outcomes of this study were the Barthel index at discharge, the presence of functional decline, the Braden score at discharge, and the destination of the patient at the end of its stay (home, nursing home, rehabilitation clinic, other hospital ward).
Statistical analysis was conducted using the R environment version 4.0.5 [38] and Stata v15.1 (Stata Corp, College Station, TX, USA). Results are expressed as number of patients and (percentage) for categorical variables, and as average (standard deviation, SD) or as median (interquartile range) for continuous variables. Between-group comparisons were performed using chi-square or Fisher's exact test for categorical variables and analysis of variance or Wilcoxon or Kruskal-Wallis nonparametric tests for continuous variables. Statistical significance was assessed for a two-sided test with a p-value <0.05.
Due to the lack of consent to access the medical files of non-participants, it was not possible to compare the characteristics between the non-participants and the participants.
As this was a pilot study stemming from a previously published one [11], no specific sample size was calculated. In the previous study, a sample size of 200 patients allowed a precision of ±1% for most percentage estimates. The current total sample size should allow detection of absolute differences in functional decline of 19%, which is lower than the values reported in the literature (37% and 23%, respectively) [4, 5].
Of the 211 consenting patients aged ≥65 years, 187 met the inclusion criteria for the study and had step parameters available for analysis (supplementary fig. S1 in the appendix). Patients were aged on average 81.6 years (SD 8.5), had on average a Charlson comorbidity index of 4.0 (SD 2.7), and a median Barthel index of 96 (IQR 85–100) at admission. Half were using walking aids at admission and a minority (7%) was living in a nursing home.
Overall, patients wore the wrist accelerometer for a mean of 3.6 days (SD 3.2). Their median number of steps was 603 (IQR 456–809). We categorised the patients into two groups around the median (≥600 and <600 steps). Older patients were less likely to walk ≥600 steps per day (p = 0.050, table 1). Other baseline characteristics did not differ between patients walking ≥600 and <600 steps. The living situation was unknown for two participants.
Table 1Baseline characteristics of the study sample, stratified by the median number of steps.
| Overall n = 187 | <600 steps n = 93 | ≥600 steps n = 94 | p-value | |
| Daily steps, median (IQR) | 603 (456–809) | – | – | – |
| Daily steps, mean (SD) | 722 (511) | – | – | – |
| Cadence (steps/minute), median (IQR) | 100.00 (99.00–101.00) | 100.00 (98.00–101.00) | 101.00 (99.00–102.00) | 0.004 |
| Walking bout duration (seconds), median (IQR) | 33.00 (27.00–37.00) | 33.00 (27.00–38.00) | 33.00 (27.25–36.00) | 0.598 |
| Age (years), mean (SD) | 81.56 (8.51) | 82.78 (7.92) | 80.35 (8.93) | 0.050 |
| Female gender, n (%) | 76 (40.6) | 43 (46.2) | 33 (35.1) | 0.138 |
| BMI (kg/m2), mean (SD) | 24.66 (4.71) | 24.88 (4.54) | 24.46 (4.88) | 0.555 |
| Use of walking aid at admission, n (%) | 95 (51.1) | 49 (53.3) | 46 (48.9) | 0.562 |
| Living Situation at admission, n (%) | 0.935 | |||
| – Cohabitation | 92 (49.2) | 44 (47.3) | 48 (51.1) | |
| – Home alone | 80 (42.8) | 41 (44.1) | 39 (41.5) | |
| – Nursing home | 13 (7.0) | 7 (7.5) | 6 (6.4) | |
| Chronic obstructive pulmonary disease, n (%) | 55 (29.4) | 27 (29.0) | 28 (29.8) | 1 |
| Coronary heart disease, n (%) | 48 (25.7) | 24 (25.8) | 24 (25.5) | 1 |
| Peripheral artery disease, n (%) | 22 (11.8) | 8 (8.6) | 14 (14.9) | 0.256 |
| Cancer, n (%) | 35 (18.7) | 16 (17.2) | 19 (20.2) | 0.708 |
| Depression, n (%) | 29 (15.5) | 11 (11.8) | 18 (19.1) | 0.225 |
| Cognitive impairment, n (%) | 51 (27.3) | 27 (29.0) | 24 (25.5) | 0.625 |
| Stroke, n (%) | 26 (13.9) | 14 (15.1) | 12 (12.8) | 0.678 |
| Braden score at admission, mean (SD) | 17.85 (3.08) | 17.86 (3.23) | 17.85 (2.93) | 0.986 |
| Barthel at admission, median (IQR) | 96.00 (85.00–100.00) | 93.00 (83.00–98.00) | 96.00 (86.50–100.00) | 0.216 |
| Barthel at admission, mean (SD)* | 89 (16) | 88 (15) | 89 (17) | 0.604 |
| Charlson, mean (SD) | 4.02 (2.68) | 3.96 (2.60) | 4.09 (2.78) | 0.745 |
BMI: body mass index; IQR: interquartile range; SD: standard deviation
* 121/187 patients had a Barthel index score at admission of ≥90 and 10 outliers / 187 had a Barthel of ≤50, therefore we used the median and not the mean in the results.
Missing variables: eight participants had a missing Braden score at inclusion. The living situation was unknown for two participants.
Braden score estimates the risk of bedsores and a Braden score ≤18 represents a high risk of sores
Barthel (modified Barthel index) estimates the functional ability (level of autonomy) from 1 to 100, with 100 representing full functional ability.
Charlson is a weighted index representing the risk of death, based on a series of comorbidities. The higher the score, the higher the risk of death or of medical resources use.
There was a significant difference in the median number of steps depending on the destination at discharge (returning home, home with nursing, nursing home, rehabilitation, or other ward; p = 0.046, table 2).
Table 2Hospital-related factors and discharge characteristics of the study sample, stratified by the median number of steps.
| Overall n = 187 | <600 steps n = 93 | ≥600 steps n = 94 | p-value | |
| Prescription of physiotherapy, n (%) | 116 (62.4) | 55 (59.8) | 61 (64.9) | 0.545 |
| Total time of physiotherapy (minutes), mean (SD) | 43.0 (58.3) | 36.8 (54.4) | 49.2 (61.6) | 0.149 |
| Oxygen therapy during hospital stay, n (%) | 22 (11.8) | 14 (15.1) | 8 (8.5) | 0.181 |
| Urinary catheter during hospital stay, n (%) | 21 (11.2) | 13 (14.0) | 8 (8.5) | 0.256 |
| Braden score at discharge, mean (SD) | 17.83 (2.92) | 17.73 (2.83) | 17.91 (3.02) | 0.693 |
| Braden score <18, n (%) | 58 (37.2) | 31 (41.9) | 27 (32.9) | 0.320 |
| Destination at discharge, n (%) | 0.154 | |||
| – Home | 60 (32.1) | 30 (32.3) | 30 (31.9) | |
| – Home with nursing care | 31 (16.6) | 15 (16.1) | 16 (17.0) | |
| – Nursing home | 22 (11.8) | 13 (14.0) | 9 (9.6) | |
| – Other acute care hospital | 2 (1.1) | 2 (2.2) | 0 (0.0) | |
| – Rehabilitation | 55 (29.4) | 21 (22.6) | 34 (36.2) | |
| – Transferred to another ward | 15 (8.0) | 10 (10.8) | 5 (5.3) | |
| – Deceased | 2 (1.1) | 2 (2.2) | 0 (0.0) | |
| Barthel at discharge, median (IQR] | 92.00 (70.50–99.50) | 92.00 (69.00–98.00) | 93.00 (71.50–100.00) | 0.503 |
| Barthel at discharge, mean (SD)* | 80 (25) | 79 (26) | 81 (24) | 0.660 |
| Functional decline, n (%) | 70 (37.4) | 38 (40.9) | 32 (34.0) | 0.367 |
| Length of stay (days), mean (SD) | 7.0(4.68) | 6.1 (4.11) | 7.8 (5.07) | 0.011 |
IQR: interquartile range; SD: standard deviation
* 100/187 patients had a Barthel index score at admission ≥90 and 26 outliers / 187 had a Barthel index of ≤50, therefore we will use the median and not the mean in the results.
Missing variables: 31 participants had a missing Braden score at inclusion.
Functional decline defined as ≥5-point decrease in the Barthel index at discharge.
Braden score estimates the risk of bedsores and a Braden score: ≤18 represents a high risk of sores
Barthel (modified Barthel index) estimates the functional ability (level of autonomy) from 1 to 100, with 100 representing full functional ability.
Charlson is a weighted index representing the risk of death, based on a series of comorbidities. The higher the score, the higher the risk of death or of medical resources use.
Thirty-one patients (17%) walked more than 1000 steps per day (fig. 1).

Figure 1 Frequency of mean step per day, stratified by age group.
Step count per day was not distributed normally (fig. 2).
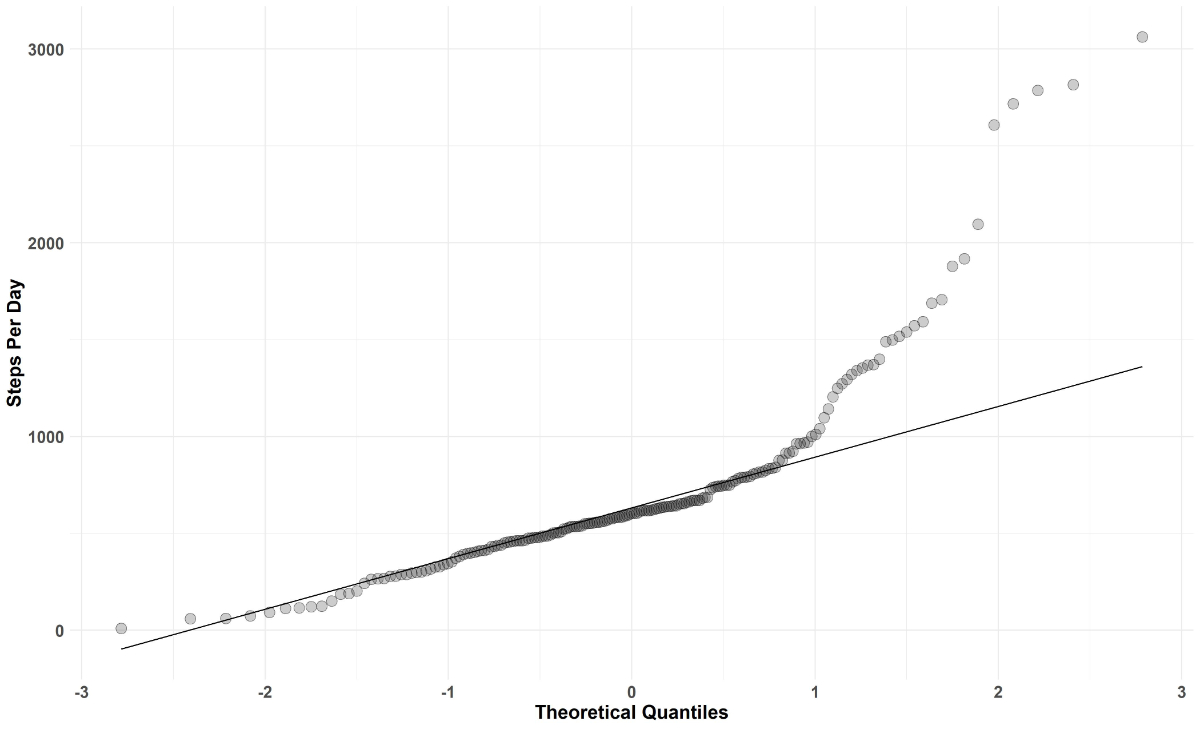
Figure 2 Q-Q plot of steps per day.
Characteristics of patients walking >1000 steps per day are shown in supplementary table S1 (appendix). Their mean age was 81.4 years (SD 8.1) and they had a median Barthel index of 98 (IQR 91–100) at admission. Of these, 17 (55%) had a walking aid and 22 (71%) received a prescription of physiotherapy.
Patients walked at a median cadence of 100 steps/minute (IQR 99–101), and median walking bouts lasting 33 s (IQR 27–37]. Patients had 70% of their walking bouts lasting <30 s, 27% lasting between 30 s and 120 s, and 3% lasting 120 s or more (table 1). Patients walking more than 1000 steps per day had 71% of their walking bouts lasting <30 s, 26% lasting between 30 s and 120 s, and 3% lasting 120 s or more. These patients had a median cadence of 101 steps/minute (IQR 100–102), and median walking bouts of 33 seconds (IQR 27–35).
The cadence differed significantly between patients walking ≥600 vs <600 steps per day (p = 0.004). Duration of walking bouts were similar between patients walking ≥600 and <600 steps.
The majority of patients had a prescription of physiotherapy during their hospital stay, and 37% had a functional decline at discharge. Patients with a prescription of physiotherapy tended to walk more (614 vs 580 steps per day, p = 0.39) than patients without physiotherapy, but without reaching statistical significance. Functional decline was not associated with a significantly lower number of steps per day (table 3).
Thirty percent of the patients were transferred to rehabilitation, 49% were discharged home and 12% were institutionalised, of which 5% were a new institutionalization. Patients transferred to rehabilitation facilities walked the most, with a median of 651 steps, compared with patients discharged home (median 601) or to a nursing home (median 506).
Overall the Braden score did not decrease during the hospital stay (17.83, SD 2.92 at discharge vs 17.85, SD 3.08 at admission). The mean Braden score at discharge did not differ significantly between patients walking <600 or ≥600 steps. However, patients with a Braden score of <18 walked less (median steps per day 564 vs 626, p = 0.042) compared with patients with a lower risk of bed sores. Eight participants had a missing Braden score at inclusion, and 31 at discharge.
The average length of hospital stay was 7.0 (SD 4.7) days. Surprisingly, patients walking ≥600 steps had longer length of stay (6.1 vs 7.8, p = 0.011). Daily step count and length of stay were positively associated (Spearman rank correlation0.190, p = 0.009). A significant difference (p = 0.024) of 96 steps was found when we dichotomised the length of stay over 7 days (table 3).
Table 3Median steps per day for a selection of hospital-related factors and discharge characteristics of the study sample.
| n | Median | IQR | p-value | ||
| Prescription of physiotherapy | Yes | 116 | 614 | 458–846 | 0.393 |
| No | 70 | 580 | 458772 | ||
| Length of stay | <7 days | 105 | 558 | 433744 | 0.024 |
| ≥7 days | 82 | 654 | 486954 | ||
| Braden score at discharge | <18 | 58 | 564 | 394812 | 0.042 |
| ≥18 | 98 | 626 | 526840 | ||
| Functional decline | No | 117 | 617 | 480807 | 0.134 |
| Yes | 70 | 575 | 400806 | ||
| Destination | Home | 60 | 601 | 498732 | 0.046 |
| Home with nursing | 31 | 618 | 468920 | ||
| Nursing Home | 22 | 506 | 409784 | ||
| Rehabilitation | 55 | 651 | 512966 | ||
| Other ward | 15 | 441 | 322738 |
We used Wilcoxon tests for prescription of physiotherapy, length of hospital stay, Braden score at discharge and functional decline, and Kruskall-Wallis for destination.
Our results show that elderly hospitalised patients walked a mere 603 steps daily, at a cadence of 100 steps/minute with walking bouts of 33 s. The majority of patients performed walking bouts lasting less than 30 s. We found that patients walking ≥600 steps per day were 2 years younger and stayed almost 2 days longer than those walking <600 daily steps. Furthermore, patients at low risk of sores at discharge walked 10% more steps than those at higher risk of sores. We did not find other hospitalisation-related factors associated to the number of steps per day.
In our cohort of elderly patients hospitalised in a medical ward for an acute illness, the median step count was 603 steps, which is in accordance with the literature [15, 16, 39]. However, comparison of step parameters of elderly patients hospitalised in internal medicine is limited, since most observational studies examining possible associations between the number of steps and patient characteristics or outcomes have been conducted in the general, non-hospitalised population [40], a few in patients hospitalised for surgery [41] and none in the Swiss healthcare system.
One in six patients walked more than 1000 steps per day during their hospitalisation. These patients were fully autonomous at admission, and nearly three quarters of them had a prescription for physiotherapy. As autonomy is correlated with step count [18] and physiotherapy increases the amount of physical exercise, this may explain the resultant high step count. However, why the most mobile patients have more physiotherapy remains questionable. As previously described, overuse of physiotherapy in patients who would not benefit from it could be an explanation [42].
We report here for the first time the average cadence of elderly patients hospitalised in internal medicine for an acute illness, as the measurement of steps during a minute during normal mobilisation was mostly conducted in the general, community-dwelling population. In our study sample, we found that patients with a higher number of daily steps also had a higher cadence. When observed in the normal environment, mean cadences of older people range between 97 and 105 steps/minute [43–45], which is in accordance with our findings, i.e., 100–101 steps/minute.
We could not assess whether walking bouts of 33 s and the fact that a majority of walking bouts lasted less than 30 s were in accordance with the literature, since we did not find previous publications including measurement of bouts duration in hospitalised patients. However, we expect these values not to be fully reflective of the walking capacity of our study population. Since hospitalised patients are geographically limited and are not accustomed to the environment, their walking duration may be “artificially” shortened.
Among patients walking >1000 steps per day, the cadence, the number of steps in a walking bout, and the time spent walking during each bout were comparable to those found in patients walking less, and we therefore suspect that these “extreme” walkers had more walking events than their peers.
Functional decline was not associated with a significantly lower number of steps per day. Our results do not replicate the findings of two Israeli studies [8, 18] that assessed mobility in elderly patients admitted to a medical unit using a self-reported questionnaire or accelerometry. Possible explanations include a higher level of independency at admission in our study (median Barthel index of 96, IQR 85–100 compared with 78–85) and different methods of measuring patients’ mobility (total number of steps per day using different algorithms vs self-reported mobility levels). The higher Barthel index in our Swiss sample may be associated with a higher than average number of doctor and nurses per capita [23], which benefits the overall accessibility and care of the older Swiss population. There are as yet no studies available to prove this association.
The decline in the Barthel index seems to be inversely related to the Barthel index at admission, as decline is more likely to occur when Barthel index values are high (regression to the mean). We report an overall 37.4% functional decline at a median Barthel index of 92, close to our colleagues in Israel [8] who reported a 46% decline in a sample with a mean Barthel index of 87. In Italy [46], a 17% decline was reported in participants with a mean Barthel index at admission of 59.
Of interest, only 23% of patients walking >1000 steps had a functional decline versus 37% overall. It remains to be studied whether increasing the daily number of steps can reduce functional decline, and what would be the step count needed to achieve this goal [20].
Moreover, patients walking ≥600 steps per day were not significantly more independent in activities of daily living at admission (Barthel index 93 vs 96). The activities of daily living involve the need of walking approximately 400 steps [47] for their correct execution, thus our results are congruent with the literature. Indeed, a high level of independence at admission preclude big variations such as described by Agmon et al., who reported that hospitalised patients walking more than 900 steps daily had a Barthel index 15 points superior to their counterparts [18].
Patients with a lower risk of bedsores walked more than patients at higher risk. Our findings are in accordance with a previous publication using raw physical activity measures (in mG) rather than step counts [6, 11]. This finding is also in accordance with that of a previous retrospective Portuguese study, in which low patient mobilisation was associated with risk of sores independently of the total Braden score [48]. However, the mean Braden score at discharge did not differ significantly between patients walking ≥600 and <600 steps. Indeed, the Braden scale includes a four-point mobility variable (one point for completely immobile, four for no mobility limitations); however patients able to walk and take any step will not be rated as completely immobile, it was therefore not surprising to find no difference between groups.
Patients having a longer hospital stay walked more than those with an earlier discharge. Indeed, patients walking <600 steps stayed for 6 days, patients walking ≥600 steps stayed for 8 and patients walking >1000 steps stayed for 9 days at the hospital. This finding is contradictory to the available literature, as patients walking the most stayed the shortest time in the hospital [49, 50]. Furthermore, and in accordance with our findings, it was reported that the number of steps taken increases with each day of hospitalisation [20, 51], increasing the overall average.
Patients discharged to a rehabilitation facility, most receiving a prescription of physiotherapy during their hospital stay, walked more than patients discharged home. This is in agreement with previous findings, as meta-analyses [52, 53] regarding mobility programmes showed an increase in the physical activity and step count in patients having physiotherapy sessions during their hospital stay. The mobility programmes varied, ranging from simple reminders in the patient’s room and patient education [54], daily 20-minute mobility exercises [55] to grouped high-intensity functional exercises [56]. Overall, most protocols achieved an increase in daily step count with various degrees of success and resources. As the associations between the patient outcomes and the physical activity or step count varied greatly between studies, the impact of enhanced physiotherapy, before transfer to a rehabilitation center, remains uncertain.
Our results provide reliable step counts for the study of a wide variety of clinical characteristics and hospital-related factors. Our approach also included cadence and walking bout duration for a hospitalised sample of elderly patients with acute illness. As patients walking >1000 steps per day had less often a functional decline and a lower risk of bedsores, an interventional trial aiming at reaching 1000 steps would discern if the patients receiving the intervention would have better outcomes. While 1000 steps may be useful as a target to achieve, efforts should be made to motivate patients to walk as much as they can. This may prove useful to find a reliable threshold to see a significant decrease in functional decline and improve the patients’ wellbeing with an even lower risk of bedsores. Since normal activities of daily living in the general population suffering from a chronic disease are believed to account for approximately 400 steps [47], the challenge would be in finding a protocol that would allow a patient to consistently walk at least a supplementary 600 steps each day. Thus, future studies should try to assess the minimum number of steps needed to decrease increased in-hospital morbidity and/or length of stay in elderly hospitalised patients. Important hospital resources may not be required, as a study in Israel [18] had more than 58% of patients walking more than 900 steps per day, while having an inferior nurse-to-bed ratio (1.01) [23] than other developed countries.
Assessment of step parameters using accelerometry requires specific equipment and software and step count extraction algorithms as well as adequate information technology knowledge to extract, process and interpret the results. This approach allows a comprehensive assessment of physical activity behaviour, by extracting a variety of walking-related parameters, not only the amount/volume (step count) but also the manner in which walking is performed in terms of bout duration and intensity (cadence). Accelerometers are also more expensive than pedometers, which might limit their use where resources are scarce.
Conversely, pedometers allow an immediate reading of the data and are easier to use in a general hospital setting. Hence, to increase participants’ compliance and facilitate data collection in non-academic hospitals, pedometers should be preferred [26]. Indeed, a pedometer with a step count visible to the patient and his family or care providers may provide additional incentive to achieve a goal of 1000 steps per day during a hospitalization.
Other strategies to improve patients’ mobility without involvement of doctors or nurses may include inexpensive printed reminders in the patients’ room and on doors to regularly walk [54], clear objectives written on a whiteboard with a daily goal (for example, if the wards’ corridor is 200 steps long, to do it at least five times), or general recommendations for the daily hospital activities (make telephone calls in the patients’ cafeteria 500 steps away instead of in the room, eat the dessert on a bench outside if the weather and physical conditions permit, etc.). The hospital staff can quickly and effortlessly propose and implement these strategies without requiring additional resources.
The strengths of this study are its relatively large sample size, the broad inclusion criteria (e.g., inclusion of patients with cognitive decline or use of walking aids), and the use of a validated accelerometer and algorithms to assess the daily step parameters. When resources allow, use of accelerometers to monitor patient mobility have been shown to be superior to pedometers for comprehensive and repeatable data collection [13]. Furthermore, patients using walking aids [5] are often excluded from mobilisation studies [15, 16, 19], which limits the generalisability of the results. Half of our patients required the use of walking aids before or during the hospital stay, which is in accordance with and representative of an older medical sample of inpatients: estimates from the literature range from 29% in community-dwellers [57, 58] up to 73% in a study of older Danish inpatients [59]. Conversely, this study also presents some limitations. First, the use of walking aids was not prospectively monitored during the hospital stay, thus possibly assigning some patients who did not use their auxiliary tools every day into the walking aid group at admission. As the literature suggests that hospitalised patients with walking aids walk less than their peers [5, 6], we can hypothesise that some patients were wrongly assigned to the walking aids category, artificially lowering the average difference in step counts between groups. Second, a risk of bedsores was used instead of objectively diagnosed bedsores, because bedsores in our division are very rare due to standard preventive measures. Had the number of bedsores been used, the group of interest would have been very small, thus reducing statistical power. Third, other step parameters such as walking speed could not be calculated, as data needed for its calculation (e.g., height) was not collected during the initial survey. Finally, the group of patients walking >1000 step per day was too small (n = 31) to perform valid between-groups comparisons.
Furthermore, reverse causality has not been assessed regarding the patient’s outcomes. For example, although older patients have an increased risk of pressure ulcers if they are not mobilised regularly, we cannot exclude the possibility that those patients with pressure ulcers already formed will walk fewer steps per day due to the pain of the ulcers. Moreover, patients with a rehabilitation project stay longer at the hospital as rehabilitation facilities have a short supply of beds, and a waiting list of two or three days is common. These patients may receive additional resources in the form of enhanced physiotherapy during the waiting period, thus increasing the number of daily steps.
During a hospitalisation for an acute internal medicine illness in a Swiss University hospital, elderly inpatients walk 603 steps daily and most of the time for periods <30 seconds. Cadence is higher in patients walking the most. Patients walking ≥600 steps are younger and have longer hospital stays, while patients with a low risk of bed sores walked 10% more than those at a higher risk. These details about step parameters could be useful for future intervention trials or to set mobility goals for elderly patients hospitalized for acute illnesses in Swiss hospitals.
Individual data cannot be provided as no consent for open data was provided. Aggregated, coded data can be provided upon request by the authors.
All authors have read and approved the manuscript. Concept and design: MM, PMV, PT. Drafting of the manuscript: FT, MM, PMV. Critical revision of the manuscript for important intellectual content: MM, PMV, AI, BK, PT. Data acquisition: PT, PMV, AI. Statistical analysis: FT, PMV, AI
This research did not receive any form of funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Morris JN, Hardman AE. Walking to health. Sports Med. 1997 May;23(5):306–32. https://doi.org/https://doi.org.10.2165/00007256-199723050-00004
2. Lee IM, Buchner DM. The importance of walking to public health. Med Sci Sports Exerc. 2008 Jul;40(7 Suppl):S512–8. https://doi.org/https://doi.org.10.1249/MSS.0b013e31817c65d00195-9131 https://doi.org/https://doi.org.10.1249/MSS.0b013e31817c65d0
3. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009 Sep;57(9):1660–5. https://doi.org/https://doi.org.10.1111/j.1532-5415.2009.02393.x
4. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007 Sep;2(5):305–13. https://doi.org/https://doi.org.10.1002/jhm.209
5. Pedersen MM, Bodilsen AC, Petersen J, Beyer N, Andersen O, Lawson-Smith L, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013 Mar;68(3):331–7. https://doi.org/https://doi.org.10.1093/gerona/gls165
6. Tasheva P, Kraege V, Vollenweider P, Roulet G, Méan M, Marques-Vidal P. Accelerometry assessed physical activity of older adults hospitalized with acute medical illness - an observational study. BMC Geriatr. 2020 Oct;20(1):382. https://doi.org/https://doi.org.10.1186/s12877-020-01763-w
7. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004 Aug;52(8):1263–70. https://doi.org/https://doi.org.10.1111/j.1532-5415.2004.52354.x
8. Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011 Feb;59(2):266–73. https://doi.org/https://doi.org.10.1111/j.1532-5415.2010.03276.x
9. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015 Jan;63(1):55–62. https://doi.org/https://doi.org.10.1111/jgs.13193
10. Hoyer EH, Young DL, Friedman LA, Brotman DJ, Klein LM, Friedman M, et al. Routine Inpatient Mobility Assessment and Hospital Discharge Planning. JAMA Intern Med. 2019 Jan;179(1):118–20. https://doi.org/https://doi.org.10.1001/jamainternmed.2018.5145
11. Tasheva P, Vollenweider P, Kraege V, Roulet G, Lamy O, Marques-Vidal P, et al. Association Between Physical Activity Levels in the Hospital Setting and Hospital-Acquired Functional Decline in Elderly Patients. JAMA Netw Open. 2020 Jan;3(1):e1920185. https://doi.org/https://doi.org.10.1001/jamanetworkopen.2019.20185
12. Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008 Dec;56(12):2171–9. https://doi.org/https://doi.org.10.1111/j.1532-5415.2008.02023.x
13. Godfrey A, Conway R, Meagher D, ÓLaighin G. Direct measurement of human movement by accelerometry. Med Eng Phys. 2008 Dec;30(10):1364–86. https://doi.org/https://doi.org.10.1016/j.medengphy.2008.09.005
14. Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011 Jan;59(1):91–5. https://doi.org/https://doi.org.10.1111/j.1532-5415.2010.03202.x
15. Lim SE, Dodds R, Bacon D, Sayer AA, Roberts HC. Physical activity among hospitalised older people: insights from upper and lower limb accelerometry. Aging Clin Exp Res. 2018 Nov;30(11):1363–9. https://doi.org/https://doi.org.10.1007/s40520-018-0930-0
16. Kolk D, Aarden JJ, MacNeil-Vroomen JL, Reichardt LA, van Seben R, van der Schaaf M, et al.; Hospital-ADL Study Group. Factors Associated with Step Numbers in Acutely Hospitalized Older Adults: The Hospital-Activities of Daily Living Study. J Am Med Dir Assoc. 2021 Feb;22(2):425–32. https://doi.org/https://doi.org.10.1016/j.jamda.2020.06.027
17. Tudor-Locke C, Schuna JM Jr, Barreira TV, Mire EF, Broyles ST, Katzmarzyk PT, et al. Normative steps/day values for older adults: NHANES 2005-2006. J Gerontol A Biol Sci Med Sci. 2013 Nov;68(11):1426–32. https://doi.org/https://doi.org.10.1093/gerona/glt116
18. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association between 900 steps a day and functional decline in older hospitalized patients. JAMA Intern Med. 2017 Feb;177(2):272–4. https://doi.org/https://doi.org.10.1001/jamainternmed.2016.7266
19. Zisberg A, Agmon M, Gur-Yaish N, Rand D, Hayat Y, Gil E ; WALK-FOR team. No one size fits all-the development of a theory-driven intervention to increase in-hospital mobility: the “WALK-FOR” study. BMC Geriatr. 2018 Apr;18(1):91. https://doi.org/https://doi.org.10.1186/s12877-018-0778-3
20. Hamilton AC, Lee N, Stilphen M, Hu B, Schramm S, Frost F, et al. Increasing Mobility via In-hospital Ambulation Protocol Delivered by Mobility Technicians: A Pilot Randomized Controlled Trial. J Hosp Med. 2019 May;14(5):272–7. https://doi.org/https://doi.org.10.12788/jhm.3153
21. Pavon JM, Sloane RJ, Pieper CF, Colón-Emeric CS, Gallagher D, Cohen HJ, et al. Physical Activity in the Hospital: Documentation and Influence on Venous Thromboembolism Prophylaxis. J Aging Phys Act. 2020 Apr;28(2):306–10. https://doi.org/https://doi.org.10.1123/japa.2018-0462
22. Kolk D, Aarden JJ, MacNeil-Vroomen JL, Reichardt LA, van Seben R, van der Schaaf M, et al.; Hospital-ADL Study Group. Factors Associated with Step Numbers in Acutely Hospitalized Older Adults: The Hospital-Activities of Daily Living Study. J Am Med Dir Assoc. 2021 Feb;22(2):425–32. https://doi.org/https://doi.org.10.1016/j.jamda.2020.06.027
23. Organisation for Economic Co-operation and Development. https://data.oecd.org/health.htm
24. Esliger DW, Rowlands A, Hurst TL, Catt M, Murray P, Eston RG; ESLIGER DW. ROWLANDS A v., HURST TL, CATT M, MURRAY P, ESTON RG. Validation of the GENEA Accelerometer. Med Sci Sports Exerc. 2011;43(6):1085–93. https://doi.org/https://doi.org.10.1249/MSS.0b013e31820513be
25. Rowlands A v., Mirkes EM, Yates T, et al. Accelerometer-assessed physical activity in epidemiology: Are monitors equivalent? Medicine and Science in Sports and Exercise. 2018;50(2). https://doi.org/https://doi.org.10.1249/MSS.0000000000001435
26. Lim SE, Ibrahim K, Sayer AA, Roberts HC. Assessment of Physical Activity of Hospitalised Older Adults: A Systematic Review. J Nutr Health Aging. 2018;22(3):377–86. https://doi.org/https://doi.org.10.1007/s12603-017-0931-2
27. Dieu O, Mikulovic J, Fardy PS, Bui-Xuan G, Béghin L, Vanhelst J. Physical activity using wrist-worn accelerometers: comparison of dominant and non-dominant wrist. Clin Physiol Funct Imaging. 2017 Sep;37(5):525–9. https://doi.org/https://doi.org.10.1111/cpf.12337
28. Dillon CB, Fitzgerald AP, Kearney PM, Perry IJ, Rennie KL, Kozarski R, et al. Number of days required to estimate habitual activity using wrist-worn geneActiv accelerometer: A cross-sectional study. PLoS One. 2016 May;11(5):e0109913. https://doi.org/https://doi.org.10.1371/journal.pone.0109913
29. Zhou SM, Hill RA, Morgan K, Stratton G, Gravenor MB, Bijlsma G, et al. Classification of accelerometer wear and non-wear events in seconds for monitoring free-living physical activity. BMJ Open. 2015 May;5(5):e007447. https://doi.org/https://doi.org.10.1136/bmjopen-2014-007447
30. Soltani A, Dejnabadi H, Fasel B, Ionescu A, Gubelmann C, Marques-Vidal PM, et al. Locomotion detection and cadence estimation using 3D wrist accelerometer: an in-field validation. Gait Posture. 2017;57:186–7. https://doi.org/https://doi.org.10.1016/j.gaitpost.2017.06.359
31. Fasel B, Duc C, Dadashi F, Bardyn F, Savary M, Farine PA, et al. A wrist sensor and algorithm to determine instantaneous walking cadence and speed in daily life walking. Med Biol Eng Comput. 2017 Oct;55(10):1773–85. https://doi.org/https://doi.org.10.1007/s11517-017-1621-2
32. Soltani A, Paraschiv-Ionescu A, Dejnabadi H, Marques-Vidal P, Aminian K. Real-World Gait Bout Detection Using a Wrist Sensor: An Unsupervised Real-Life Validation. IEEE Access. 2020;8:102883–96. https://doi.org/https://doi.org.10.1109/ACCESS.2020.2998842
33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/https://doi.org.10.1016/0021-9681(87)90171-8
34. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–9. https://doi.org/https://doi.org.10.1016/0895-4356(89)90065-6
35. Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017 Aug;17(1):131. https://doi.org/https://doi.org.10.1186/s12874-017-0409-2
36. Gill TM, Allore H, Guo Z. The deleterious effects of bed rest among community-living older persons. J Gerontol A Biol Sci Med Sci. 2004 ;59(7):755–61. https://doi.org/https://doi.org.10.1093/gerona/59.7.m755
37. Bergstrom N, Braden BJ, Laguzza A, Holman V. The braden scale for predicting pressure sore risk. Nurs Res. 1987 Jul-Aug;36(4):205–10. https://doi.org/https://doi.org.10.1097/00006199-198707000-00002
38. R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
39. Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010 Nov;170(21):1942–3. https://doi.org/https://doi.org.10.1001/archinternmed.2010.422
40. Hall KS, Hyde ET, Bassett DR, Carlson SA, Carnethon MR, Ekelund U, et al. Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. Int J Behav Nutr Phys Act. 2020 Jun;17(1):78. https://doi.org/https://doi.org.10.1186/s12966-020-00978-9
41. Daskivich TJ, Houman J, Lopez M, Luu M, Fleshner P, Zaghiyan K, et al. Association of Wearable Activity Monitors With Assessment of Daily Ambulation and Length of Stay Among Patients Undergoing Major Surgery. JAMA Netw Open. 2019 Feb;2(2):e187673. https://doi.org/https://doi.org.10.1001/jamanetworkopen.2018.7673
42. Martinez M, Cerasale M, Baig M, Dugan C, Robinson M, Sweis M, et al. Defining Potential Overutilization of Physical Therapy Consults on Hospital Medicine Services. J Hosp Med. 2021 Aug;16(9):553–5. https://doi.org/https://doi.org.10.12788/jhm.3673
43. Brown JC, Harhay MO, Harhay MN. Walking cadence and mortality among community-dwelling older adults. J Gen Intern Med. 2014 Sep;29(9):1263–9. https://doi.org/https://doi.org.10.1007/s11606-014-2926-6
44. Tudor-Locke C, Barreira TV, Brouillette RM, Foil HC, Keller JN. Preliminary comparison of clinical and free-living measures of stepping cadence in older adults. J Phys Act Health. 2013 Nov;10(8):1175–80. https://doi.org/https://doi.org.10.1123/jpah.10.8.1175
45. Jerome GJ, Ko SU, Kauffman D, Studenski SA, Ferrucci L, Simonsick EM. Gait characteristics associated with walking speed decline in older adults: results from the Baltimore Longitudinal Study of Aging. Arch Gerontol Geriatr. 2015 Mar-Apr;60(2):239–43. https://doi.org/https://doi.org.10.1016/j.archger.2015.01.007
46. Palese A, Gonella S, Moreale R, Guarnier A, Barelli P, Zambiasi P, et al.; ESAMED Group. Hospital-acquired functional decline in older patients cared for in acute medical wards and predictors: findings from a multicentre longitudinal study. Geriatr Nurs. 2016 May-Jun;37(3):192–9. https://doi.org/https://doi.org.10.1016/j.gerinurse.2016.01.001
47. Ummels D, Beekman E, Theunissen K, Braun S, Beurskens AJ. Counting steps in activities of daily living in people with a chronic disease using nine commercially available fitness trackers: cross-sectional validity study. JMIR Mhealth Uhealth. 2018 Apr;6(4):e70. https://doi.org/https://doi.org.10.2196/mhealth.8524
48. Sardo PM, Guedes JA, Alvarelhão JJ, Machado PA, Melo EM. Pressure ulcer incidence and Braden subscales: retrospective cohort analysis in general wards of a Portuguese hospital. J Tissue Viability. 2018 May;27(2):95–100. https://doi.org/https://doi.org.10.1016/j.jtv.2018.01.002
49. Liu B, Moore JE, Almaawiy U, Chan WH, Khan S, Ewusie J, et al.; MOVE ON Collaboration. Outcomes of Mobilisation of Vulnerable Elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing. 2018 Jan;47(1):112–9. https://doi.org/https://doi.org.10.1093/ageing/afx128
50. Hoyer EH, Friedman M, Lavezza A, Wagner-Kosmakos K, Lewis-Cherry R, Skolnik JL, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016 May;11(5):341–7. https://doi.org/https://doi.org.10.1002/jhm.2546
51. Ostir GV, Berges IM, Kuo YF, Goodwin JS, Fisher SR, Guralnik JM. Mobility activity and its value as a prognostic indicator of survival in hospitalized older adults. J Am Geriatr Soc. 2013 ;61(4):551–7. https://doi.org/https://doi.org.10.1111/jgs.121700002-8614
52. Smart DA, Dermody G, Coronado ME, Wilson M. Mobility Programs for the Hospitalized Older Adult: A Scoping Review. Gerontol Geriatr Med. 2018 Nov;4:2333721418808146. https://doi.org/https://doi.org.10.1177/2333721418808146
53. Bachmann S, Finger C, Huss A, Egger M, Stuck AE, Clough-Gorr KM. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;340(apr20 2):c1718-c1718. https://doi.org/https://doi.org.10.1136/bmj.c1718
54. Teodoro CR, Breault K, Garvey C, Klick C, O’Brien J, Purdue T, et al. STEP-UP: Study of the Effectiveness of a Patient Ambulation Protocol. Medsurg Nurs. 2016 Mar-Apr;25(2):111–6.
55. Hamilton AC, Lee N, Stilphen M, Hu B, Schramm S, Frost F, et al. Increasing Mobility via In-hospital Ambulation Protocol Delivered by Mobility Technicians: A Pilot Randomized Controlled Trial. J Hosp Med. 2019 May;14(5):272–7. https://doi.org/https://doi.org.10.12788/jhm.3153
56. Raymond MJ, Jeffs KJ, Winter A, Soh SE, Hunter P, Holland AE. The effects of a high-intensity functional exercise group on clinical outcomes in hospitalised older adults: an assessor-blinded, randomised-controlled trial. Age Ageing. 2017 Mar;46(2):208–13. https://doi.org/https://doi.org.10.1093/ageing/afw215
57. Fisher SR, Graham JE, Brown CJ, Galloway RV, Ottenbacher KJ, Allman RM, et al. Factors that differentiate level of ambulation in hospitalised older adults. Age Ageing. 2012 Jan;41(1):107–11. https://doi.org/https://doi.org.10.1093/ageing/afr110
58. Pain H, Gale CR, Watson C, Cox V, Cooper C, Sayer AA. Readiness of elders to use assistive devices to maintain their independence in the home. Age Ageing. 2007 Jul;36(4):465–7. https://doi.org/https://doi.org.10.1093/ageing/afm046
59. Trøstrup J, Andersen H, Kam CA, Magnusson SP, Beyer N. Assessment of Mobility in Older People Hospitalized for Medical Illness Using the de Morton Mobility Index and Cumulated Ambulation Score-Validity and Minimal Clinical Important Difference. J Geriatr Phys Ther. 2019 Jul/Sep;42(3):153–60. https://doi.org/https://doi.org.10.1519/JPT.0000000000000170
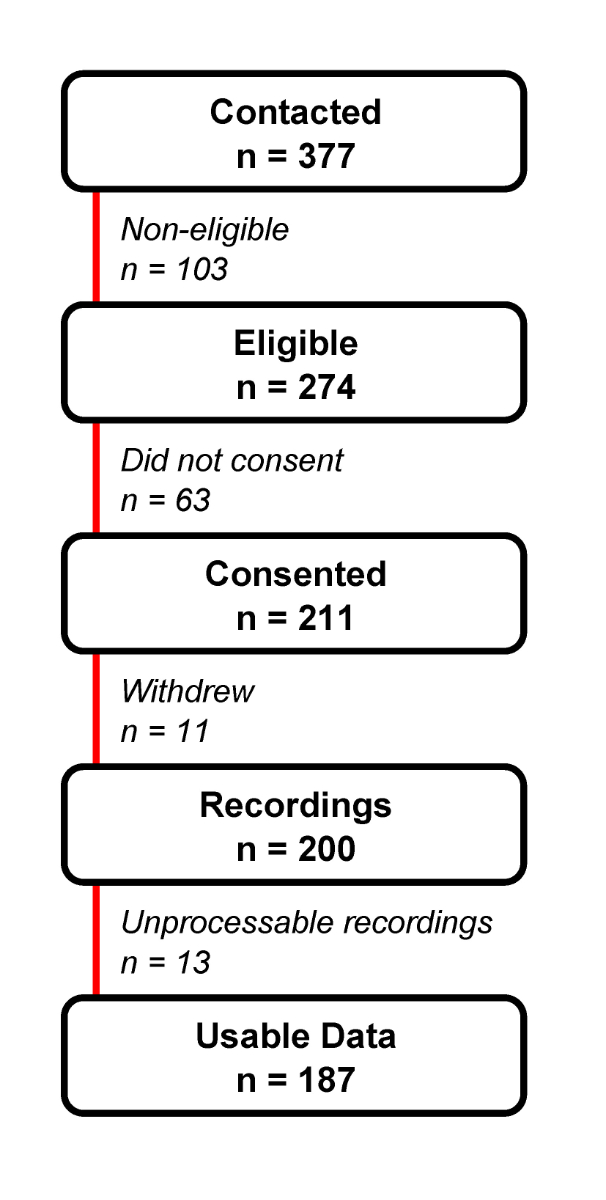
Figure S1 Selection procedures for the study population.
Table S1Characteristics of patients walking more than 1000 steps per day (n = 31).
| Daily steps, median (IQR ) | 1489 (1284–1793) | |
| Daily steps, mean (SD) | 1644 (578) | |
| Cadence median (IQR ) | 101.00 (100.50–102.00) | |
| Walking bout duration, median (IQR ) | 33.00 (27.00–35.00) | |
| Age, mean (SD) | 81.35 (8.07) | |
| Female, n (%) | 7 (22.6) | |
| BMI (kg/m2), mean (SD) | 25.24 (4.59) | |
| Use of walking aids at admission, n (%) | 17 (54.8) | |
| Living situation at admission, n (%) | Cohabitation | 17 (54.8) |
| Home alone | 13 (41.9) | |
| Nursing home | 0 (0.0) | |
| Chronic obstructive pulmonary disease, n (%) | 9 (29.0) | |
| Coronary heart disease, n (%) | 8 (25.8) | |
| Peripheral artery disease, n (%) | 5 (16.1) | |
| Cancer, n (%) | 7 (22.6) | |
| Depression, n (%) | 10 (32.3) | |
| Oxygen therapy, n (%) | 2 (6.5) | |
| Urinary catheter, n (%) | 2 (6.5) | |
| Cognitive impairment, n (%) | 7 (22.6) | |
| Stroke, n (%) | 5 (16.1) | |
| Braden score at admission, mean (SD) | 18.39 (3.40) | |
| Barthel at admission, median (IQR ) | 98.00 (91.00–100.00) | |
| Barthel at admission, mean (SD) | 93 (13) | |
| Charlson, mean (SD) | 4.65 (3.02) | |
| Prescription of physiotherapy, n (%) | 22 (71.0) | |
| Total time of physiotherapy (minutes), mean (SD) | 84.5 (69.9) | |
| Braden score at discharge, mean (SD) | 18.50 (2.03) | |
| Braden score <18 at discharge, n (%) | 7 (25) | |
| Destination at discharge, n (%) | Home | 7 (22.6) |
| Home with nursing care | 7 (22.6) | |
| Nursing home | 1 (3.2) | |
| Rehabilitation | 13 (41.9) | |
| Transfer to other ward | 3 (9.7) | |
| Deceased | 0 (0.0) | |
| Barthel at discharge, median (IQR ) | 95.00 (81.50–100.00) | |
| Barthel at discharge, mean (SD) | 87 (19) | |
| Functional decline, n (%) | 7 (22.6) | |
| Length of stay (days), mean (SD) | 9.35 (7.19) | |
BMI: body mass index; IQR: interquartile range; SD: standard deviation
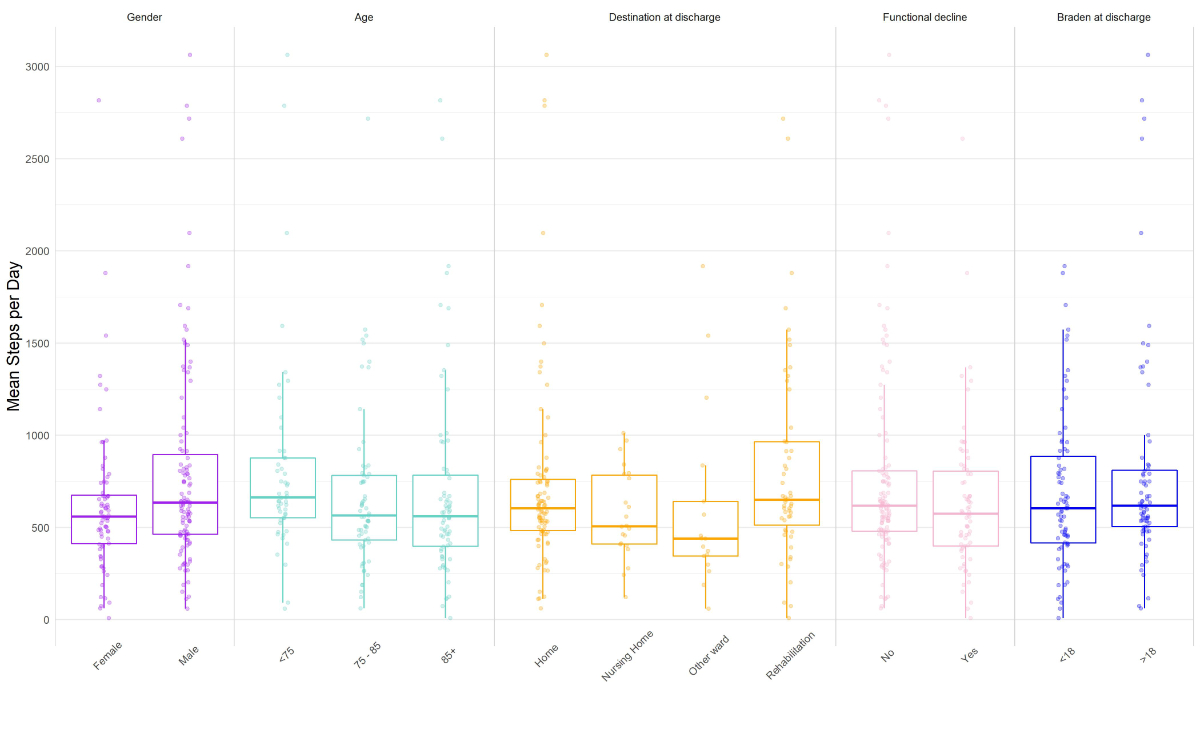
Figure S2 Step characteristics across main patient categories and outcomes.