Atypical pulmonary phaeohyphomycosis due to Aureobasidium spp. – case report and brief literature review
DOI: https://doi.org/10.57187/smw.2022.40011
Davide
Bosettia, David
Spoerlb, Arnaud
Riatc, Claudio
De Vitod, Stavroula
Masouridi-Levrate, Yves
Chalandone, Dionysios
Neofytosa
aDivision of Infectious Diseases, University Hospital of Geneva, Switzerland
bDivision of Immunology and Allergy, Department of Medicine, University Hospital and Faculty of Medicine, Geneva, Switzerland. Division of Laboratory Medicine, Department of Diagnostic, Geneva University Hospitals, Geneva, Switzerland
cDiagnostic Department, Laboratory of Bacteriology, University Hospital of Geneva, Switzerland
dDivision of Clinical Pathology, University Hospital of Geneva, Switzerland
eDivision of Haematology, Bone Marrow Transplant Unit, University Hospital of Geneva and faculty of Medicine, University of Geneva, Switzerland
Summary
We report on a case of probable invasive Auerobasidium spp. pulmonary infection in a patient with myelodysplastic syndrome. The patient was successfully treated with liposomal amphotericin B monotherapy, with transition to orally administered isavuconazole. This case shows an atypical initial radiological presentation with diffuse ground-glass opacities, as previously demonstrated in cases of Aureobasidium spp. hypersensitivity pneumonitis. Moreover this case further highlights the difficulties associated with the diagnosis and complexity in the management of Aureobasidium spp. infections.
Case report
A 63-year-old patient, with a past medical history of large vessel vascultis treated with prednisone, was admitted for the first cycle of induction chemotherapy by idarubicin and cytarabine for myelodysplastic syndrome with excess of blasts type 2 (day 0). The prophylaxis regimen consisted of acyclovir 500 mg three times daily intravenously (IV), co-trimoxazole double-strength (DS) three times weekly orally (PO) and posaconazole 300 mg once daily PO. On day 9 the patient developed persistent neutropenic fever (day 9–15), treated with different broad-spectrum antibiotics, including cefepime, vancomycin, imipenem and levofloxacin. Thoraco-abdominal computed tomography (CT) on day 12 showed diffuse ground-glass opacities with numerous micro-nodules, primarily in the superior and the apical segments of the inferior lobes (fig. 1a). A bronchoscopy was performed on day 13 with a bronchoalveolar lavage (BAL) negative by bacterial and fungal stain, sterile culture, negative viral multiplex polymerase chain reaction (PCR) panel (adenovirus, human metapneumovirus, parainfluenza virus 1–4, rhinovirus, coronaviruses, SARS-CoV-2, influenza A/B, respiratory syncytial virus), Legionella spp. PCR and culture, Pneumocystis jirovecii PCR, Chlamydophila pneumoniae PCR, Mycoplasma pneumoniae PCR, Mycobacterium tuberculosis PCR, and broad-spectrum bacterial and panfungal PCR, and a specific Aspergillus fumigatus and Mucorales spp. PCR. Serum beta-D-glucan (Fungitell) and galactomannan enzyme immunoassay (Platelia Aspergillus Bio-Rad) were negative. Owing to a new episode of neutropenic fever on day 23, another chest CT scan was performed, which showed progression of the diffuse bronchiolitis and a new nodular lesion of 2 cm in the left lower lobe, prompting a second bronchoscopy on day 24 (fig. 1b). The repeat BAL was negative for all previously mentioned diagnostic tests. Because of persistence of neutropenic fever and a new appearing nodular lesion, posaconazole prophylaxis was discontinued and empirical antifungal therapy with liposomal amphotericin B at 5 mg/kg once daily IV was initiated on day 24. Neutropenia resolved by day 29 with rapid clinical improvement and, considering the negative results of both BALs, antifungal treatment was discontinued 10 days later (day 34) without reinitiating antifungal prophylaxis. On the same day, the patient developed a new non-neutropenic fever. A repeat chest CT was performed on day 41, showing the same left lower lobe nodular lesion, unchanged in size. After a seven-day “therapeutic window” with discontinuation of all antifungal treatment, a third bronchoscopy with a transbronchial biopsy was performed on day 42. All BAL stains, cultures and PCR tests (M tuberculosis PCR, Aspergillus spp. PCR, Pneumocystis jirovevii PCR, panfungal PCR, Mucorales spp. PCR) remained sterile and negative, respectively. The transbronchial biopsy was positive for multiple septate branching fungal hyphal forms (fig. 1c). A specific fungal stain (Grocott-Gomori) was positive (fig. 1d).
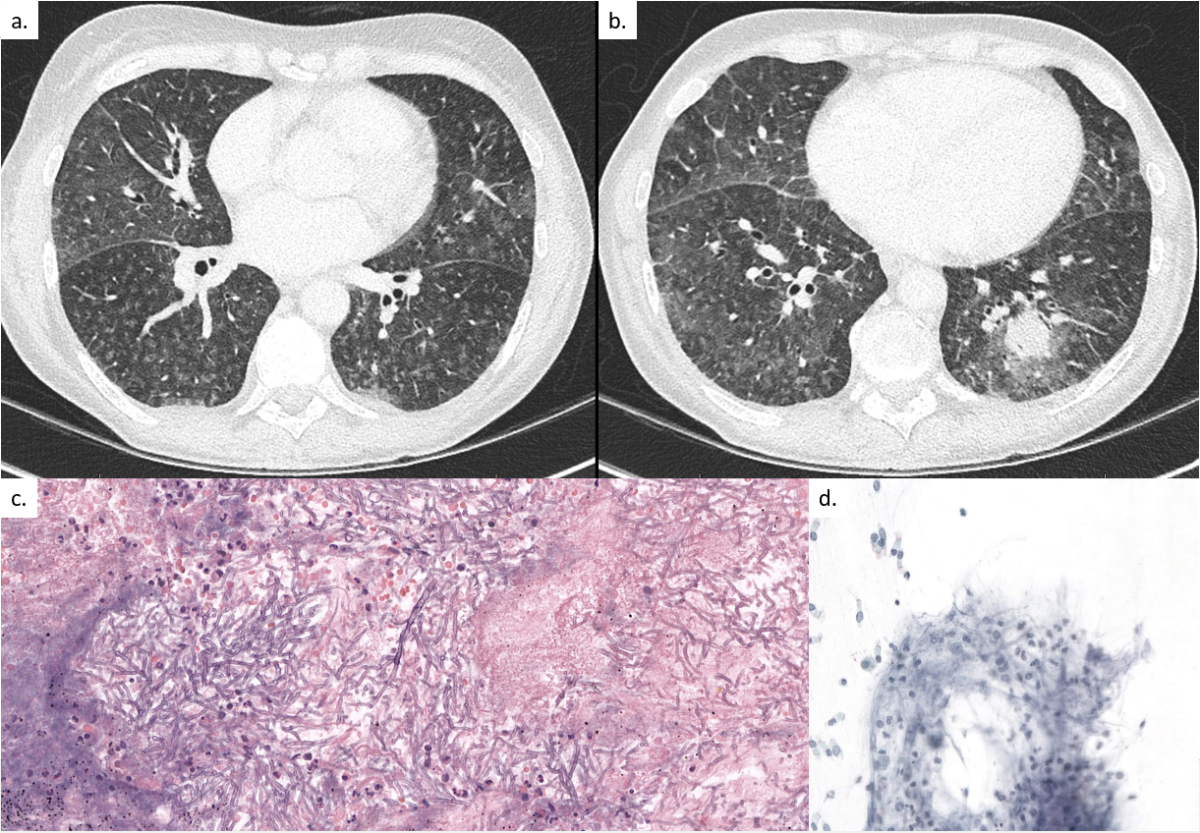
Figure 1 (a) Pulmonary computed tomography (CT) on day 12 showing diffuse ground-glass opacities with numerous micro-nodules, primarily in the superior lobes and the apical segments of the inferior lobes. (b) Pulmonary CT on day 23 showing progression of the diffuse bronchiolitis signs and a new nodular lesion of 2 cm in the left lower lobe with a halo sign. (c) Transbronchial biopsy on day 42 showing multiple, narrow, septated, branching hyphal forms on haematoxylin and eosin stain identified by PCR as Aureobasidium spp.. Abundant presence of lymphocytes, neutrophils, fibrocytes, bronchial cells and alveolar macrophages indicating an inflammatory process. (d) Part of the transbronchial biopsy on Grocott stain showing the fungal hyphal forms identified by PCR as Aureobasidium spp.
A panfungal PCR 18S rDNA on the biopsy performed after extraction from paraffin-embedded tissue was positive for Aureobasidium spp. at >log5 copies/ml, while the culture remained sterile. Serum IgG antibodies specific for Aureobasidium pullulans were negative. Antifungal therapy with liposomal amphotericin B at 5 mg/kg once daily IV was reinitiated on day 46. The patient received the second cycle of induction therapy FLAG-IDA (fludarabine, idarubicine and cytarabine) on day 43 and remained neutropenic until day 78. A repeat chest CT on day 101 (8 weeks after re-initiation of antifungal treatment) showed complete resolution of the nodular lesion. Liposomal amphotericin B was subsequently switched to isavuconazole 200 mg three times daily IV for the first two days, followed by 200 mg once daily PO, to facilitate the discharge from the hospital on day 72. The patient’s clinical course is presented in detail in figure 2. The patient eventually received an allogeneic haematopoietic cell transplant from an unrelated HLA-compatible donor on day 109 (9 weeks after re-initiation of antifungal treatment), developing acute and an overlap graft-versus-host disease, which was successfully treated. Isavuconazole was continued through the haematopoietic cell transplant and during graft-versus-host disease treatment and was stopped after almost two years after the transplant.
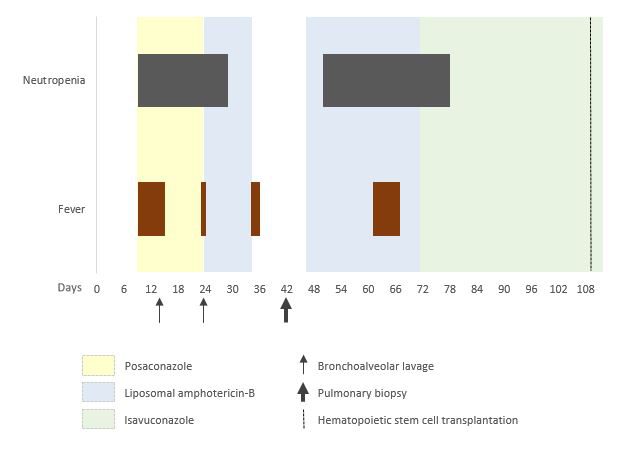
Figure 2 Presentation of patient’s clinical course correlated to the temporal evolution of the administrated antifungal therapies and performed bronchoscopies.
Discussion
To our knowledge, this is the first case of a probable Auerobasidium spp. pulmonary invasive mould infection in a patient with myelodysplastic syndrome prior to a haematopoietic cell transplant in our 10-year single-centre cohort of allogeneic haematopoietic cell transplant recipients [1]. Infections due to this pathogen have been reported but they remain rare, affecting predominately immunocompetent patients in the setting of catheter-related infections and after eye surgery [2, 3]. This case report illustrates the atypical and variable radiological manifestation of lung infections due to Aureobasidium spp., from hypersensitivity pneumonitis to nodular lung lesions.
Aureobasidium genus is a yeast-like dematiaceous (black) mold, ubiquitous in the environment [4, 5]. It is characterised by high concentrations of melanin within its cell walls, which probably plays an important role in pathogenesis, with inactivation of free radicals and hydrolytic enzymes produced by the phagocytic cells [6]. To date, there are four different species described in the literature to cause human infections: A. pullulans, A. proteae, A. mansoni and A. melanigenum, the last appearing to be more pathogenic [7, 8]. A. pullulans is mostly known for the production of pullulan, an extracellular polysaccharide fundamental to resistance to desiccation, used in different biotechnological fields (production of adhesives/oxygen-impermeable films) [9]. A. pullulans has a low pathogenicity and can often colonise the skin, causing onychomycosis or keratitis after traumatic inoculation in healthy individuals, but it can also grow on implanted medical devices such as peritoneal dialysis catheters and central venous catheters [4, 10–14].
However, deep-seated infections may occur in severely immunocompromised patients, with the organism having been isolated in blood, cerebrospinal fluid, BAL fluid and lymph nodes [15–21]. A total of seven prior cases of Aureobasidium spp. infection have been reported in haematology patients (table 1) [3]. There are no reported cases of lower respiratory tract infections due to Aureobasidium spp. in haematology patients, but mostly cases of fungaemia associated with indwelling catheters or meningitis mostly due to intrathecal therapies [3, 22]. Three out of seven (43%) patients died, showing the burden of this rare infection in this fragile population subgroup. In the literature there is a case of Aureobasidium spp. pneumonia reported in a liver transplant recipient [21].
Table 1Case reports of hematological patients with infections due to Aureobasidium spp.
|
Citation |
Pathogen
|
Site of infection
|
Antifungal therapy
|
Predisponsing factors
|
Results
|
| Kaczmarski EB, et al. (1986) |
A. pullulans
|
Fungemia |
d-AMB |
AML, Hickman catheter |
Died |
| Salkin IF, et al. (1986) |
A. pullulans
|
Splenic abcess |
NA |
Lymphoma |
Died |
| Krcméry et al (1994) |
A. mansoni
|
Meningitis |
d-AMB |
ALL |
Survived |
| Huttova et al. (1998) |
A. mansoni
|
Meningitis |
d-AMB for 21 D |
CLL, intrathecal treatment |
Survived |
| Joshi A, et al. (2010) |
A. pullulans
|
Fungemia/Skin |
LF-AMB (3 mg/kg/D ) for 12 D & PO VCZ for 2 months |
Allogeneic HCT, central venous catheter |
Survived |
| Oliveira LR, et al. (2013) |
A. pullulans
|
Skin |
LF-AMB for 3D & ICZ 400mg/D |
CML, severe neutropenia |
Died |
| Wang et al. (2018) |
A. melanigenum
|
Fungemia |
CAS ( 50 mg /D ) for 28 D |
AML, Central venous catheter |
Survived |
The initial radiological presentation of this infection in our patient consisted of diffuse ground-glass opacities, a rather atypical finding in an immunocompromised host with an invasive mould infection. The initial imaging findings in this case were not consistent with an invasive mould infection and might have further delayed the clinical suspicion and diagnosis. However, cases of Aureobasidium spp. hypersensitivity pneumonitis have been reported in the context of contaminated saunas, humidification systems, damp buildings, heating-cooling ventilation units and hydroponics [23–26]. Other studies have also demonstrated the allergic hypersensitivity potential of this mould in the development of rhinitis in children and sinusitis in adults [27]. The absence of specific precipitating antibodies for A. pullulans in the serum of our patient does not corroborate the hypothesis of a severe hypersensitivity reaction during the first phase of this infection, but another Aureobasidium spp. could have been responsible of this infection and the initially high dose corticosteroids given to the patient for his large vessel arteritis might explain such a negative result. We hypothesise that the patient was already colonised with a high burden of Aureobasidium spp. upon presentation, provoking a mild-to-moderate hypersensitivity reaction with diffuse alveolar dissemination of this organism, leading to a deep-seated infection in the setting of prolonged profound neutropenia.
The identification of this pathogen can be challenging. Its morphology is normally characterised by predominately irregular swollen hyphae [6]. A specific stain for melanin, such as the Fontana-Masson stain, can be used to confirm the diagnosis. The fungal culture may take a long time to grow and often the diagnosis and the differentiation between subspecies is made with molecular diagnostics [28]. The conserved rDNA internal transcribed spacer (ITS) is the most analysed region [5, 29]. In our case a real-time PCR against the 18S rDNA was used. This sequence is present in many copies in the fungal genome making it a good-target and the rDNA-based amplification more sensitive, but less specific in comparison to the ITS-based PCRs [30]. It is unclear why the organism was not identified, either by culture or by PCR on multiple BAL samples, before a transbronchial biopsy was performed, but the latter further points to the difficulties associated with the diagnosis of these infections and the need for additional diagnostic procedures. In the literature there are cases of laboratory contamination due to staining solution or reagents (e.g., paraffin) with this fungus, because it is very abundant in the environment [31]. But based on the abundant signs of inflammation on histology (neutrophils), relevant clinical and radiological findings, and the patient’s clinical response to the administered antifungal treatment, we retained a diagnosis of probable invasive fungal infection due to Aureobasidium spp..
There is no standard treatment for this pathogen; treatment is usually guided by susceptibility testing results, if available. However, in the case of negatives cultures, lipid formulation amphotericin B or a mould-acting azole are considered appropriate empirical antifungal treatment, based on their susceptibility profile and existing evidence [2, 12, 13, 32]. Multiple in vitro studies have demonstrated a great variability of susceptibility of this pathogen to the common antifungals (e.g. minimum inhibitory concentration [MIC] 50 for amphotericin B: 0.25 µg/l, MIC for itraconazole: 0.06 µg/l) [33]. A review of the literature concerning the in vitro antifungal activities of voriconazole infive isolates of A. pullulans showed low MICs for voriconazole (0.03 µg/ml), itraconazole (0.03–0.125 µg/ml) and also amphotericin B (0.125–2 µg/ml) [34]. Echinocandins generally have a variable and species dependent fungistatitc activity against Aureobasidium spp. and are not recommended as monotherapy [35]. Dual therapy with voriconazole and lipid formulation amphotericin B or an echinocandin has been considered in a case of meningitis and proposed by international guidelines [2, 7]. One case of amphotericin B treatment failure has been described, in a profoundly immunosuppressed patient [15]. In cases of localised or cutaneous infections, monotherapy with itraconazole or voriconazole combined with surgical excision is recommended [2]. New antifungal agents may be useful in the management of infections due to Aureobasidium spp. in the future, such as F901318 with good activity against some dematiaceous molds [30].
In conclusion, this case report illustrates the first case of an invasive Aureobasidium spp. infection successfully treated with lipid formulation amphotericin B monotherapy, with transition to orally isavuconazole in a high-risk haematology patient who underwent an allogeneic haematopoietic cell transplant. In the era of breakthrough invasive mould infection in allogeneic haematopoietic cell transplant recipients and patients with acute leukemia, vigilance is required for the identification of – until recently – rarely encountered invasive mould infection.
Davide Bosetti
Division of Infectious Diseases
University Hospital of Geneva
Rue Gabrielle-Perret-Gentil 4
CH-1211 Geneva
davide.bosetti[at]hcuge.ch
References
1.
Roth RS,
Masouridi-Levrat S,
Chalandon Y,
Mamez AC,
Giannotti F,
Riat A, et al.
Invasive Mold Infections in Allogeneic Hematopoietic Cell Transplant Recipients in 2020: Have We Made Enough Progress? Open Forum Infect Dis. 2021 Nov;9(1):ofab596. https://doi.org/10.1093/ofid/ofab596
2.
Hoenigl M,
Salmanton-García J,
Walsh TJ,
Nucci M,
Neoh CF,
Jenks JD, et al.
Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis. 2021 Aug;21(8):e246–57. https://doi.org/10.1016/S1473-3099(20)30784-2
3.
Wang SC,
Lo HJ,
Lin LJ,
Chen CH. Port catheter-associated Aureobasidium melanigenum fungemia. J Formos Med Assoc. 2018 Apr;117(4):346–7. https://doi.org/10.1016/j.jfma.2017.06.009
4.
Chowdhary A,
Perfect J,
de Hoog GS. Black Molds and Melanized Yeasts Pathogenic to Humans. Cold Spring Harb Perspect Med. 2014 Nov;5(8):a019570. https://doi.org/10.1101/cshperspect.a019570
5.
Zalar P,
Gostincar C,
de Hoog GS,
Ursic V,
Sudhadham M,
Gunde-Cimerman N. Redefinition of Aureobasidium pullulans and its varieties. Stud Mycol. 2008;61:21–38. https://doi.org/10.3114/sim.2008.61.02
6.
Revankar SG,
Patterson JE,
Sutton DA,
Pullen R,
Rinaldi MG. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002 Feb;34(4):467–76. https://doi.org/10.1086/338636
7.
Kutleša M,
Mlinarić-Missoni E,
Hatvani L,
Voncina D,
Simon S,
Lepur D, et al.
Chronic fungal meningitis caused by Aureobasidium proteae. Diagn Microbiol Infect Dis. 2012 Jul;73(3):271–2. https://doi.org/10.1016/j.diagmicrobio.2012.03.007
8.
Wang M,
Danesi P,
James TY,
Al-Hatmi AM,
Najafzadeh MJ,
Dolatabadi S, et al.
Comparative pathogenicity of opportunistic black yeasts in Aureobasidium. Mycoses. 2019 Sep;62(9):803–11.
9.
Wei X,
Liu GL,
Jia SL,
Chi Z,
Hu Z,
Chi ZM. Pullulan biosynthesis and its regulation in Aureobasidium spp. Carbohydr Polym. 2021 Jan;251:117076. https://doi.org/10.1016/j.carbpol.2020.117076
10.
Maverick KJ,
Conners MS. Aureobasidium pullulans fungal keratitis following LASEK. J Refract Surg. 2007 Sep;23(7):727–9. https://doi.org/10.3928/1081-597X-20070901-15
11.
Huang YT,
Liaw SJ,
Liao CH,
Yang JL,
Lai DM,
Lee YC, et al.
Catheter-related septicemia due to Aureobasidium pullulans. Int J Infect Dis. 2008 Nov;12(6):e137–9. https://doi.org/10.1016/j.ijid.2008.02.004
12.
Clark EC,
Silver SM,
Hollick GE,
Rinaldi MG. Continuous ambulatory peritoneal dialysis complicated by Aureobasidium pullulans peritonitis. Am J Nephrol. 1995;15(4):353–5. https://doi.org/10.1159/000168863
13.
Hawkes M,
Rennie R,
Sand C,
Vaudry W. Aureobasidium pullulans infection: fungemia in an infant and a review of human cases. Diagn Microbiol Infect Dis. 2005 Mar;51(3):209–13. https://doi.org/10.1016/j.diagmicrobio.2004.10.007
14.
Mise N,
Ono Y,
Kurita N,
Sai K,
Nishi T,
Tagawa H, et al.
Aureobasidium pullulans peritonitis: case report and review of the literature. Perit Dial Int. 2008 Nov-Dec;28(6):679–81. https://doi.org/10.1177/089686080802800626
15.
Girardi LS,
Malowitz R,
Tortora GT,
Spitzer ED. Aureobasidium pullulans septicemia. Clin Infect Dis. 1993 Feb;16(2):338–9. https://doi.org/10.1093/clind/16.2.338-a
16.
Arranz Sánchez DM,
de la Calle MC,
Martín-Díaz MA,
Flores CR,
González-Beato MJ,
Pinto PH, et al.
Subcutaneous mycosis produced by Aureobasidium pullulans in a renal transplant recipient. J Eur Acad Dermatol Venereol. 2006 Feb;20(2):229–30. https://doi.org/10.1111/j.1468-3083.2006.01385.x
17.
Joshi A,
Singh R,
Shah MS,
Umesh S,
Khattry N. Subcutaneous mycosis and fungemia by Aureobasidium pullulans: a rare pathogenic fungus in a post allogeneic BM transplant patient. Bone Marrow Transplant. 2010 Jan;45(1):203–4. https://doi.org/10.1038/bmt.2009.111
18.
Kaczmarski EB,
Liu Yin JA,
Tooth JA,
Love EM,
Delamore IW. Systemic infection with Aureobasidium pullulans in a leukaemic patient. J Infect. 1986 Nov;13(3):289–91. https://doi.org/10.1016/S0163-4453(86)91388-5
19.
Krcméry V Jr,
Spánik S,
Danisovicová A,
Jesenská Z,
Blahová M. Aureobasidium mansoni meningitis in a leukemia patient successfully treated with amphotericin B. Chemotherapy. 1994 Jan-Feb;40(1):70–1. https://doi.org/10.1159/000239174
20.
Salkin IF,
Martinez JA,
Kemna ME. Opportunistic infection of the spleen caused by Aureobasidium pullulans. J Clin Microbiol. 1986 May;23(5):828–31. https://doi.org/10.1128/jcm.23.5.828-831.1986
21.
Tan HP,
Wahlstrom HE,
Zamora JU,
Hassanein T. Aureobasidium pneumonia in a post liver transplant recipient: a case report. Hepatogastroenterology. 1997 Jul-Aug;44(16):1215–8.
22.
Kuster S,
Stampf S,
Gerber B,
Baettig V,
Weisser M,
Gerull S, et al.; Swiss Transplant Cohort Study. Incidence and outcome of invasive fungal diseases after allogeneic hematopoietic stem cell transplantation: A Swiss transplant cohort study. Transpl Infect Dis. 2018 Dec;20(6):e12981. https://doi.org/10.1111/tid.12981
23.
Taylor PE,
Esch R,
Flagan RC,
House J,
Tran L,
Glovsky MM. Identification and possible disease mechanisms of an under-recognized fungus, Aureobasidium pullulans. Int Arch Allergy Immunol. 2006;139(1):45–52. https://doi.org/10.1159/000089522
24.
Woodard ED,
Friedlander B,
Lesher RJ,
Font W,
Kinsey R,
Hearne FT. Outbreak of hypersensitivity pneumonitis in an industrial setting. JAMA. 1988 Apr;259(13):1965–9. https://doi.org/10.1001/jama.1988.03720130029025
25.
Engelhart S,
Rietschel E,
Exner M,
Lange L. Childhood hypersensitivity pneumonitis associated with fungal contamination of indoor hydroponics. Int J Hyg Environ Health. 2009 Jan;212(1):18–20. https://doi.org/10.1016/j.ijheh.2008.01.001
26.
Temprano J,
Becker BA,
Hutcheson PS,
Knutsen AP,
Dixit A,
Slavin RG. Hypersensitivity pneumonitis secondary to residential exposure to Aureobasidium pullulans in 2 siblings. Ann Allergy Asthma Immunol. 2007 Dec;99(6):562–6. https://doi.org/10.1016/S1081-1206(10)60387-0
27.
Stark PC,
Celedón JC,
Chew GL,
Ryan LM,
Burge HA,
Muilenberg ML, et al.
Fungal levels in the home and allergic rhinitis by 5 years of age. Environ Health Perspect. 2005 Oct;113(10):1405–9. https://doi.org/10.1289/ehp.7844
28.
Shoham S,
Dominguez EA
; AST Infectious Diseases Community of Practice. Emerging fungal infections in solid organ transplant recipients: Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019 Sep;33(9):e13525. https://doi.org/10.1111/ctr.13525
29.
Prasongsuk S,
Sullivan RF,
Kuhirun M,
Eveleigh DE,
Punnapayak H. Thailand habitats as sources of pullulan-producing strains of Aureobasidium pullulans. World J Microbiol Biotechnol. 2005;21(4):393–8. https://doi.org/10.1007/s11274-004-2237-x
30.
Chan GF,
Puad MS,
Chin CF,
Rashid NA. Emergence of Aureobasidium pullulans as human fungal pathogen and molecular assay for future medical diagnosis. Folia Microbiol (Praha). 2011 Sep;56(5):459–67. https://doi.org/10.1007/s12223-011-0070-9
31.
Hofman V,
Butori C,
Long E,
Le Fichoux Y,
Hofman P. Aureobasidium pullulans contamination in bronchial aspirates mimicking cryptococcosis: a rare diagnostic pitfall. Pathology. 2008 Dec;40(7):729–32. https://doi.org/10.1080/00313020802436782
32.
Revankar SG. Therapy of infections caused by dematiaceous fungi. Expert Rev Anti Infect Ther. 2005 Aug;3(4):601–12. https://doi.org/10.1586/14787210.3.4.601
33.
Cuenca-Estrella M,
Gomez-Lopez A,
Mellado E,
Buitrago MJ,
Monzon A,
Rodriguez-Tudela JL. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob Agents Chemother. 2006 Mar;50(3):917–21. https://doi.org/10.1128/AAC.50.3.917-921.2006
34.
Espinel-Ingroff A,
Boyle K,
Sheehan DJ. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia. 2001;150(3):101–15. https://doi.org/10.1023/a:1010954803886 https://doi.org/10.1023/A:1010954803886
35.
Espinel-Ingroff A. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev Iberoam Micol. 2003 Dec;20(4):121–36.

