HCV elimination in a Swiss opioid agonist therapy programme – a cohort and sequential cross-sectional study
DOI: https://doi.org/10.57187/smw.2022.40009
Andrea
Bregenzera, Cornelia
Krismera, Stefanie
Wendelb, Patrik
Roserbc, Christoph A.
Fuxa
aDepartment of Infectious Diseases and Hospital Hygiene, Cantonal Hospital Aarau, Switzerland
bOutpatient Centre for Opioid Agonist Therapy (HAG), Department of Addictive Disorders, Psychiatric Services Aargau, Brugg, Switzerland
cLVR-Hospital Essen, Department of Psychiatry and Psychotherapy, Medical Faculty, University of Duisburg-Essen, Essen, Germany
Summary
BACKGROUND: In opioid agonist therapy (OAT) programmes, chronic hepatitis C is highly prevalent and directly observed therapy guarantees optimal adherence. Since 2017, all patients with chronic hepatitis C in Switzerland can be treated with pangenotypic direct-acting antivirals irrespective of liver fibrosis stage. Until the end of 2021, however, prescription was restricted to infectious disease specialists, gastroenterologists and certain addiction specialists. Difficult venous access after long-term intravenous drug use and, in the case of referral to a specialist, difficulties keeping appointments are barriers to HCV diagnosis and treatment.
AIMS: To assess whether minimally invasive point-of-care tests and a “test-and-treat / vaccinate on-site” approach can improve human immunodeficiency virus (HIV) / hepatitis C virus (HCV) screening, HCV treatment uptake and immunity against hepatitis A/B.
METHODS: Since September 2018, an infectious disease specialist and a study nurse performed 4-weekly visits in the OAT programme “HAG” (heroin dispensation of the canton Aargau), offering HIV/HCV antibody rapid testing (20 min) and HCV RNA quantification (GeneXpert®, 60 min) from capillary blood, noninvasive liver fibrosis assessment (Fibroscan®, 5–10 min) and HCV treatment prescription on-site. Recommended venous blood draws for HAV/HBV serology and HAV/HBV vaccinations were performed by the staff of the “HAG”. Project performance was assessed by annual cross-sectional chart review.
RESULTS: Of the 128 patients registered in April 2018, 79 (62%) were still present in May 2021. With 72 newly registered, a total of 200 patients could be assessed, of whom 129 (65%) were still present in May 2021. Between April 2018 and May 2021, the proportion ever tested for HIV antibodies increased from 79% (101/128) to 91% (117/129), the proportion ever tested for HCV antibodies from 83% (106/128) to 93% (120/129) and the proportion of those HCV antibody positive ever tested for HCV RNA tested from 89% (47/53) to 98% (56/57). The proportion with adequate HCV management (last HCV antibody test ≤1 year ago, if HCV antibody negative or last HCV RNA test ≤1 year ago, if HCV antibody-positive and RNA-negative) improved from 23% ([15 + 15]/128) to 80% ([55 + 48]/129). Overall, HCV treatment uptake increased from 60% (21/35) to 92% (55/60) and HCV RNA prevalence among the HCV antibody positives decreased from 38% (18/47) to 7% (6/84). Between 2018 and 2021, 19 non-cirrhotic chronic hepatitis C patients were successfully treated on-site (18 sustained virological responses [SVR] 12, 1 SVR4), with excellent adherence (≥93%) and, so far, no reinfection. The proportion with known HAV/HBV serostatus increased from 38%/51% to 64%/76%. Immunity against HAV/HBV improved from 19%/23% to 50%/57%.
CONCLUSION: Capillary blood point-of-care tests and a “test-and-treat / vaccinate on-site” approach remove crucial barriers to diagnosis and treatment, making hepatitis elimination in OAT programmes achievable. A high fluctuation rate requires HIV/HCV/HAV/HBV testing at admission, but also allows more patients to be screened.
Introduction
Hepatitis C is a blood-borne viral infection transmissible by sharing equipment for injection (needle, syringe, water, spoon, filter) [1] or intranasal drug use (snorting straws) [2, 3]. Hepatitis C virus (HCV) infections in Switzerland are mainly related to intravenous drug use [1, 4, 5]. In Switzerland, there are 22,000–27,000 people who use drugs (PWUD) [6]. About 80% are cared for in an opioid agonist therapy (OAT) programme (oral OAT: 18,000, heroin: 1600) [7] and 7700–15,400 are HCV infected [8]. HCV antibody prevalence is 26–48% in oral OAT programmes and 60–80% in heroin substitution programmes [7], which is markedly higher than the 0.7% in the Swiss general population [9]. Twenty-seven percent of the OAT patients have ongoing intravenous drug use [10]. In 60%, OAT is prescribed by a general practitioner (GP) [7]. However, the OAT provider, who sees the patient at least once a week, is in 49% a pharmacy, in 26% the treating physician and in 21% an institution [11]. The respective numbers for the 737 OAT patients of the canton Aargau in 2020 were 82%, 2% and 16% [12]. The Outpatient Centre for Opioid Agonist Therapy (HAG) of the Psychiatric Services Aargau in Brugg with ~130 patients overall is the only institution offering heroin substitution in the canton Aargau.
After HCV infection, 25% of the infected persons spontaneously clear the virus within 6 months [13], and 75% develop chronic disease. Of the latter, about 20% develop liver cirrhosis after 20 years [14], with an annual risk of hepatocellular carcinoma of 1–5% and of hepatic decompensation of 3–6%. In the year following a decompensation episode, 15–20% die [15]. Since approximately 80% of cases of acute HCV infection are anicteric and asymptomatic [16] and chronically infected patients mostly present with nonspecific symptoms slowly appearing over years, such as fatigue, joint pain and neurocognitive disorders [17], hepatitis C may remain undetected for years and become a “silent killer” [18]. In Switzerland, five times more people die from HCV (2.5/100,000) than from HIV (human immunodeficiency virus) (0.5/100,000) or HBV (hepatitis B virus) (0.5/100,000) [9].
In contrast to HAV (hepatitis A virus) and HBV, there are currently no effective vaccines against HCV [19, 20] and HIV [21]. However, harm reduction programmes including OAT and needle and syringe programmes, which have been established in Switzerland since the early 1990s [22, 23], reduce the risk of HCV and HIV infection [24–28]. Besides, since 2012, guidelines recommend antiretroviral treatment (ART) irrespective of CD4 count [29], lowering the community viral load and contributing to reduced HIV transmission (treatment as prevention) [30–33]. For HCV, a mathematical model has shown that OAT and needle and syringe programmes alone are insufficient to substantially reduce HCV RNA prevalence in people who inject drugs, but HCV therapy is required [34].
Since 2017, all patients with chronic hepatitis C in Switzerland can be treated with the well-tolerated pangenotypic direct-acting antivirals, irrespective of liver fibrosis stage [35, 36]. Sofosbuvir/velpatasvir (once daily one tablet, with or without food, for 12 weeks) [37] and glecaprevir/pibrentasvir (once daily three tablets, with food, for 8 weeks) [38, 39] became the new standard. Genotyping became dispensable [40], cure rates increased to 95–100% irrespective of genotype, HIV co-infection, liver cirrhosis and prior non-response to interferon [41], and monitoring could be simplified to HCV RNA determinations at baseline, week 4, end-of-treatment (week 8 or 12) and 12 weeks thereafter (sustained virological response [SVR] 12) [41]. Until the end of 2021, direct-acting antiviral (DAA) prescription was restricted to infectious disease specialists, gastroenterologists and certain addiction specialists experienced in HCV treatment [42, 43]. Referral to a specialist is a barrier to treatment for people who inject drugs, who often have difficulties keeping appointments.
Difficult venous access after long-term intravenous drug use is another barrier to HCV diagnosis and treatment. Accordingly, patients enrolled into the Argovian OAT cohort have been offered HCV/HIV antibody rapid testing of capillary blood since July 2013 [44] and capillary HCV RNA quantification with the GeneXpert® since August 2017 [45–47]. The OraQuick® HCV antibody rapid test was CE-marked for use with oral fluid, fingerstick whole blood, venous whole blood, serum and plasma in December 2009 [48]. Its sensitivity is slightly lower for oral fluid (98.1%) than for fingerstick whole blood (99.7%) [49]. With fingerstick whole blood, its read time can be safely reduced from 20 to 5 minutes without missing HCV RNA positive patients [50]. So far, however, this test is not yet reimbursed in Switzerland. The Xpert® HCV Viral Load Fingerstick test was CE-approved in September 2018 and allows HCV RNA quantification in 100 µl capillary whole-blood within 60 minutes [51, 52].
In Switzerland, the Federal Office of Public Health (FOPH) published official guidelines for HCV management in people who use drugs in March 2019 [53], but their implementation is still a challenge. Key points already mentioned in earlier national [54] and international [55] recommendations are:
- HIV/HCV and HAV/HBV testing of all people who use drugs,
- Yearly HIV/HCV screening for primary HIV/HCV infection (antibody test) or HCV reinfection (RNA test) in persons with ongoing drug use (injection or non-injection) or OAT,
- HCV treatment in the case of chronic hepatitis C,
- HIV treatment irrespective of CD4 count [32], and
- HAV/HBV vaccination in the event of lacking immunity.
To achieve the WHO (World Health Organization) and Swiss Hepatitis Strategy goal of HCV elimination by 2030 [56, 57], 90% of chronic hepatitis C patients must be diagnosed and 80% treated [56]. At the beginning of 2020, there were an estimated 32,100 remaining viraemic HCV infections in Switzerland (0.37% viraemic prevalence). By the end of 2020, 58% (18,600) were diagnosed, 1000 treated and 970 cured, which is still far away from the elimination goal [4].
With the aim to improve HIV/HCV screening, HCV treatment uptake and immunity against hepatitis A/B, we started a hepatitis micro-elimination project in September 2018. An infectious disease specialist and a study nurse visited the OAT programme “HAG” (heroin dispensation of the canton Aargau) in Brugg every four weeks offering HIV/HCV antibody rapid testing and HCV RNA quantification from capillary blood, noninvasive liver fibrosis assessment with mobile Fibroscan®, same-day direct-acting antiviral treatment prescription on-site and recommendations for HAV/HBV serology and vaccination. We hypothesised that capillary point-of-care tests and a “test-and-treat / vaccinate on-site”-approach would remove crucial barriers to diagnosis and treatment, making hepatitis elimination in OAT programmes achievable.
Methods
Ethical considerations
Patients were enrolled into the Argovian OAT cohort study “Management of hepatitis C in drug substitution programmes – canton Aargau”, approved by the cantonal ethics committee (AG/SO 2012/091; PB_2016-02058) [44], and the Swiss Association for the Medical Management in Substance Users (SAMMSU) cohort, approved by the ethics committees of all participating centres (leading ethics committee: St Gallen, EKSG 13/144) [33]. All participants gave written informed consent. Data were analysed in an anonymised way.
Patient recruitment and data collection
All patients older than 18 years already registered in the OAT-program “HAG” in 04/2018 or newly admitted thereafter were eligible (supplementary fig. S1 in the appendix). Once enrolled into the cohort study, patients were followed up even if they later left the institution. After a cross-sectional chart review in April 2018 to assess baseline, the hepatitis elimination project started in September 2018. Every four weeks (on Monday), an infectious disease specialist and a study nurse visited the OAT programme HAG bringing along in a passenger car HIV antibody (Determine®, Alere/Abbott, 15 min) and HCV antibody rapid tests (OraQuick®, Orasure, 5–20 min) (one fingerstick for both tests), mobile GeneXpert® IV (four modules) for capillary HCV RNA quantification (Xpert® HCV Viral Load Fingerstick, Cepheid, 60 min) and mobile Fibroscan® 420 (Echosens) for noninvasive liver fibrosis assessment (transient elastography). All tests/examinations were offered free of charge. Patients were approached by a dedicated nurse of the HAG, who also performed recommended venous blood draws for HAV/HBV serology and the HAV/HBV vaccinations. After Fibroscan® (5–10 min) and a questionnaire regarding drug use, risk behaviour, comorbidities, medication, vaccinations and former laboratory results (10–15 min), HIV/HCV antibody rapid test results from the initial capillary blood draw were available and could be discussed with the patient. In patients known to be HCV antibody positive, we did not perform an HCV antibody rapid test, but directly collected 100 µl capillary whole-blood from the finger with an EDTA Minivette® for HCV RNA quantification with the Xpert® HCV Viral Load Fingerstick test [46]. In patients newly diagnosed with the HCV antibody rapid test, we immediately performed another capillary blood draw for HCV RNA quantification. Patients were informed about the HCV RNA result via telephone by the infectious disease specialist the same day.
To also reach patients not able to attend our four-weekly visits (always on a Monday), we offered two alternatives:
(1) At any time during the opening hours of the OAT programme (7 days a week), the trained staff of the HAG could independently perform HIV and HCV antibody rapid tests and dried blood spot sampling for HCV RNA quantification in the laboratory [47].
(2) Once a year, GeneXpert® and Fibroscan® were left in the OAT programme for a whole week (Monday to Monday) allowing the trained staff of the HAG to use them at any time during the opening hours, supported and supervised by our team.
Since according to guidelines, HIV/HCV screening in OAT patients should be performed at least once a year, patients were re-approached by the HAG staff as soon as their test results became older than one year.
Project performance was assessed by annual cross-sectional chart review (first: April 2018; last: May 2021).
HCV treatment on-site
Direct-acting antivirals were prescribed on-site by the infectious disease specialist visiting the OAT programme every 4 weeks and dispensed with OAT (≥5x/week, 2–3x/week, 1x/week or biweekly) ensuring optimal adherence. HCV RNA monitoring during and after treatment was performed with capillary blood on-site (start, week 4 [adherence assessment by documenting an HCV RNA decrease], end of treatment [week 8 or 12] and SVR12). In those lacking immunity against hepatitis A/B, HAV/HBV vaccination (months: 0, [1] and 6) was linked to direct-acting antiviral treatment (start, [week 4], SVR12). To monitor liver fibrosis regression under treatment, we performed another Fibroscan® at SVR12. At end of treatment and SVR12, patients were educated regarding the risk of reinfection, measures to prevent it, life-long HCV antibody persistence, and the necessity of at least yearly HCV RNA screening to early detect and treat reinfection.
Patients with already established liver cirrhosis (F4) and HIV co-infected patients were not eligible for HCV treatment on-site. They were referred to the Gastroenterology and Infectious Disease Outpatient Clinics of the cantonal tertiary care hospitals Baden and Aarau.
Definitions
Chronic hepatitis C: HCV RNA persistence for >6 months after primary or re-infection or an HCV RNA decline of <2 log U/ml within 4 weeks (“early chronic hepatitis C”) [58, 59, 60]
Spontaneous clearance: confirmed HCV RNA negative without treatment (two HCV RNA measurements at least 4 months apart)
Sustained virological response (SVR): undetectable HCV RNA at least 12 weeks after end of treatment (treatment success); If an SVR12 is not available, SVR4 (undetectable HCV RNA at least 4 weeks after end of treatment) can be used as a surrogate [61].
HCV treatment uptake: proportion of patients with chronic hepatitis C ever treated
Liver fibrosis stage according to Fibroscan®: F0/F1 (no/mild fibrosis): ≤7.0 kPa; F2 (significant fibrosis): >7.0 kPa and ≤9.5 kPa; F3 (severe fibrosis): >9.5 kPa and ≤12.5 kPa; F4 (cirrhosis): >12.5 kPa [62, 63]. Liver cirrhosis was excluded with Fibroscan® ≤12.5 kPa [62], liver biopsy (<F4) or APRI (AST to Platelet Ratio Index)-score <1.0 [64].
Adequate HCV management: HCV antibody test ≤1 year ago if HCV antibody negative or last HCV RNA test negative and ≤1 year ago if HCV-antibody-positive
Inversely, inadequate HCV management was defined as one of the following:
No HCV antibody test
HCV antibody test >1 year ago, if HCV antibody negative
HCV antibody-positive and no HCV RNA
HCV antibody positive and HCV RNA positive (in need of treatment)
HCV antibody positive and HCV RNA negative, but HCV RNA test >1 year ago
HAV serology: anti-HAV IgG
HBV serology: anti-HBs, anti-HBc, HBs-Ag
HAV immunity: completely vaccinated against HAV or past infection (anti-HAV IgG positive)
HBV-immunity: completely vaccinated against HBV and once an anti-HBs titre >100 U/l (or at least >10 U/l if the last vaccination was ≥3 years ago) or past infection (anti-HBc positive, but HBs-Ag negative)
Statistical analysis
For the primary outcome (HCV RNA treatment uptake among those ever chronically HCV infected and consecutive HCV RNA prevalence reduction among the HCV antibody positive patients) (fig. S1, red), we considered all 200 registered OAT patients in an intention-to-treat approach, chronic hepatitis Cpatients lost to follow-up were counted as not treated / still HCV RNA positive.
For the secondary outcomes (fig. S1, blue), the study design was a sequential cross-sectional study comparing the patients registered in the OAT programme at the time-points April 2018 and May 2021.
Ninety-five percent confidence intervals (CIs) for the proportions were calculated with www.openepi.com (Wilson score). Categorical variables were compared with the chi-square and the Fisher’s exact test, respectively. Continuous variables were analysed with the Wilcoxon rank-sum test (unpaired data). A two-sided p-value <0.05 was considered significant.
Statistical analyses were performed with Stata Version 12.0 and OpenEpi (www.openepi.com).
Results
Patient characteristics
Of the 128 OAT patients in April 2018, 79 (62%) were still present in May 2021. Between April 2018 and May 2021, 72 patients were newly registered. Thus, a total of 200 patients could be assessed, of whom 129 (65%) were still present in May 2021 (supplementary fig. S1, table S1 in the appendix).
In spite of an average fluctuation rate of 24 patients (19%) per year, patient characteristics remained relatively stable over the three-year period (table 1).
Table 1Patient characteristics.
|
April 2018 (n = 128) |
May 2021 (n = 129) |
p-value |
| Male |
78% (100) |
77% (99) |
0.791 |
| Age (years), median (IQR) |
42 (33–48), range: 23–65 |
42 (35–49), range: 19–66 |
0.229 |
| Ever intravenous drug use |
72% (81/113) |
71% (84/119) |
0.854 |
| Opioid agonist therapy: |
Heroin |
22% (26/119) |
18% (22/125) |
0.404 |
| Methadone |
21% (25/119) |
26% (33/125) |
0.323 |
| Buprenorphine |
11% (13/119) |
9% (11/125) |
0.578 |
| Slow-release oral morphine |
46% (55/119) |
47% (59/125) |
0.878 |
| HCV positive |
50% (53/106) |
48% (57/120) |
0.708 |
| → Currently HCV RNA positive |
38% (18/47) |
7% (4/56) |
<0.001 |
| HIV positive |
3% (3/101) (all HCV coinfected) |
3% (4/117) (all HCV coinfected) |
0.851 |
| Enrolled into SAMMSU (www.sammsu.ch) |
47% (60/128) |
84% (108/129) |
<0.001 |
Almost 80% were male. The median age was 42 years. Slightly more than 70% had ever used intravenous drugs. Almost half of the patients were substituted with slow-release oral morphine, approximately one-quarter with methadone, around 20% with heroin and 10% with buprenorphine. About half of the patients were HCV antibody positive and 3% HIV-antibody-positive. The HCV RNA prevalence among the HCV antibody positive patients decreased from 38% (95% CI 26–53%) in April 2018 to 7% (95% CI 3–17%) in May 2021 (p <0.001). The proportion of patients enrolled into the SAMMSU cohort increased from 47% to 84% (p <0.001).
HIV and HCV screening
Between April 2018 and May 2021, the proportion ever screened for HIV antibodies increased from 79% (95% CI 71–85%) to 91% (95% CI 84–95%) (fig. 1, table S2), the proportion ever screened for HCV antibodies from 83% (95% CI 75–88%) to 93% (95% CI: 87–96) and the proportion of HCV antibody positive patients ever tested for HCV RNA from 89% (95% CI 77–95%) to 98% (95% CI 91–100%).
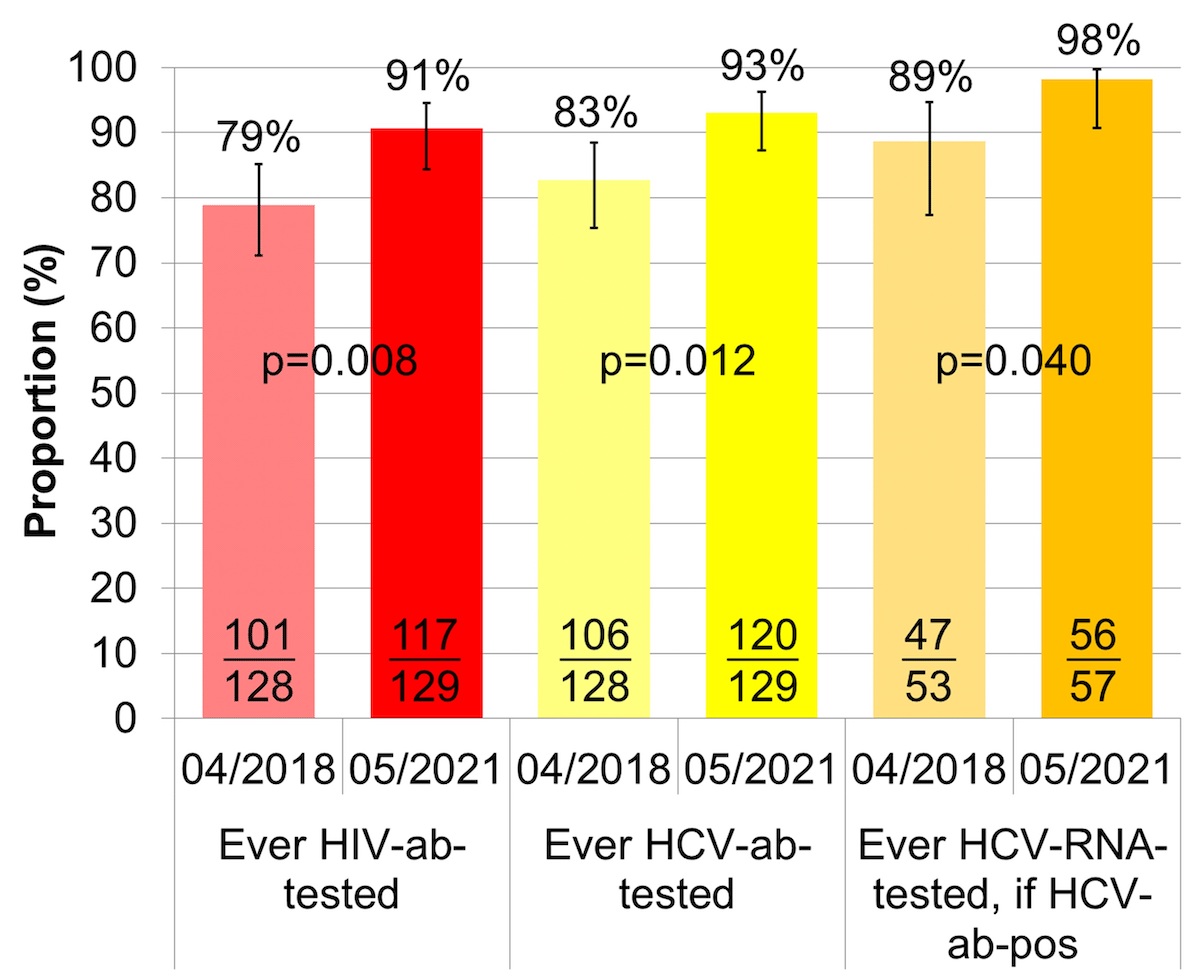
Figure 1 Proportion of patients ever HIV/HCV-antibody-tested and proportion of HCV-antibody-positive patients ever HCV-RNA-tested.
HCV: hepatitis C virus; HIV: human immunodeficiency virus; ab: antibody; RNA = ribonucleic acid
The error bars show the lower and upper limit of the 95% confidence interval (Score [Wilson]).
The proportion of HIV negative patients with a recent HIV screening, i.e., not more than one year ago, improved from 32% (95% CI 23–41%) to 85% (95% CI 77–90%) (fig. 2, table S2). The proportion of HCV negative patients recently screened for HCV antibodies went up from 28% (95% CI 18–42%) to 87% (95% CI 77–93%), and the proportion of HCV antibody positive HCV RNA negative patients with a recent HCV RNA test increased from 50% (95% CI 33–67%) to 92% (95% CI 82–97%).
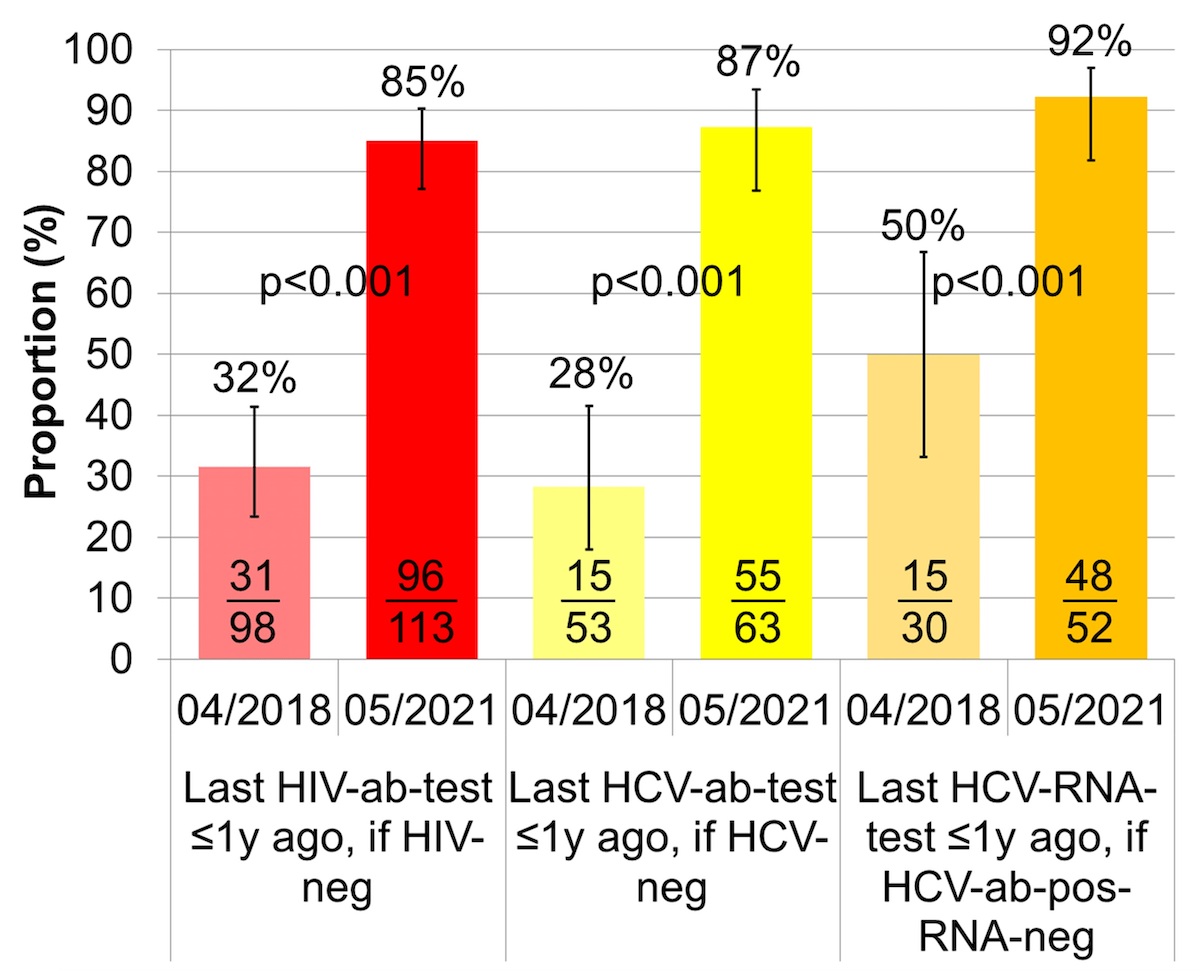
Figure 2 Proportion of HIV/HCV negative patients recently tested for HIV/HCV antibodies and proportion of HCV antibody positive and RNA negative patients recently tested for HCV RNA(i.e., ≤1 year ago).
HCV: = hepatitis C virus; HIV: human immunodeficiency virus; ab = antibody; RNA: ribonucleic acid, y: year
The error bars show the lower and upper limit of the 95% confidence interval (Score [Wilson]).
Adequate HCV management
The proportion of OAT patients with adequate HCV management, defined as either HCV antibody negative with a HCV antibody test not older than one year or HCV antibody positive and RNA negative with a HCV RNA test not older than one year, improved from 23% ([15 + 15]/128; 95% CI 17–31%) to 80% ([55 + 48]/129; 95% CI 72–86%) (figures 2 and 3, table S2).
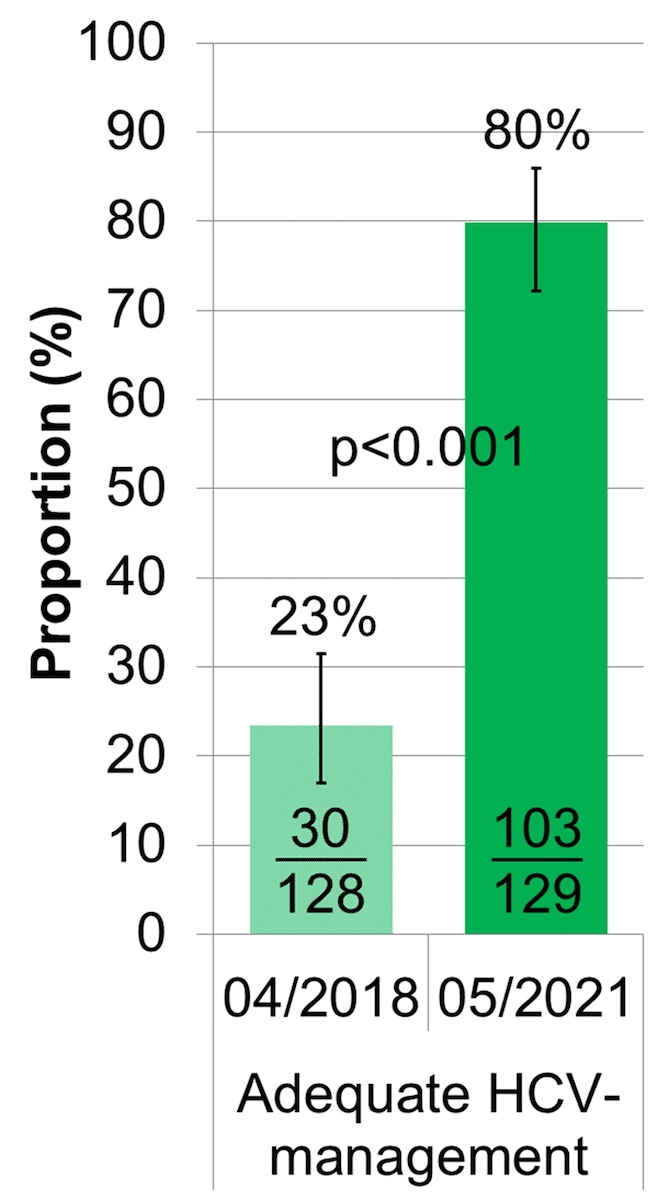
Figure 3 Proportion with adequate HCV management.
HCV: hepatitis C virus; adequate HCV management: = last HCV antibody-test ≤1 year ago, if HCV antibody negative or last HCV RNA test ≤1 year ago, if HCV antibody positive and HCV RNA negative; inadequate HCV management: never tested, HCV antibody/RNA screening >1 year ago or still HCV RNA positive
The error bars show the lower and upper limit of the 95% confidence interval (Score [Wilson]).
HCV treatment uptake and HCV RNA prevalence
Among all 200 patients assessed, HCV treatment uptake increased from 60% (95% CI 44–74%) to 92% (95% CI 82–96%) (fig. 4, table S2). In parallel, the HCV RNA-prevalence among the HCV antibody positive patients decreased from 38% (95% CI 26–53%) to 7% (95% CI 3–15%).
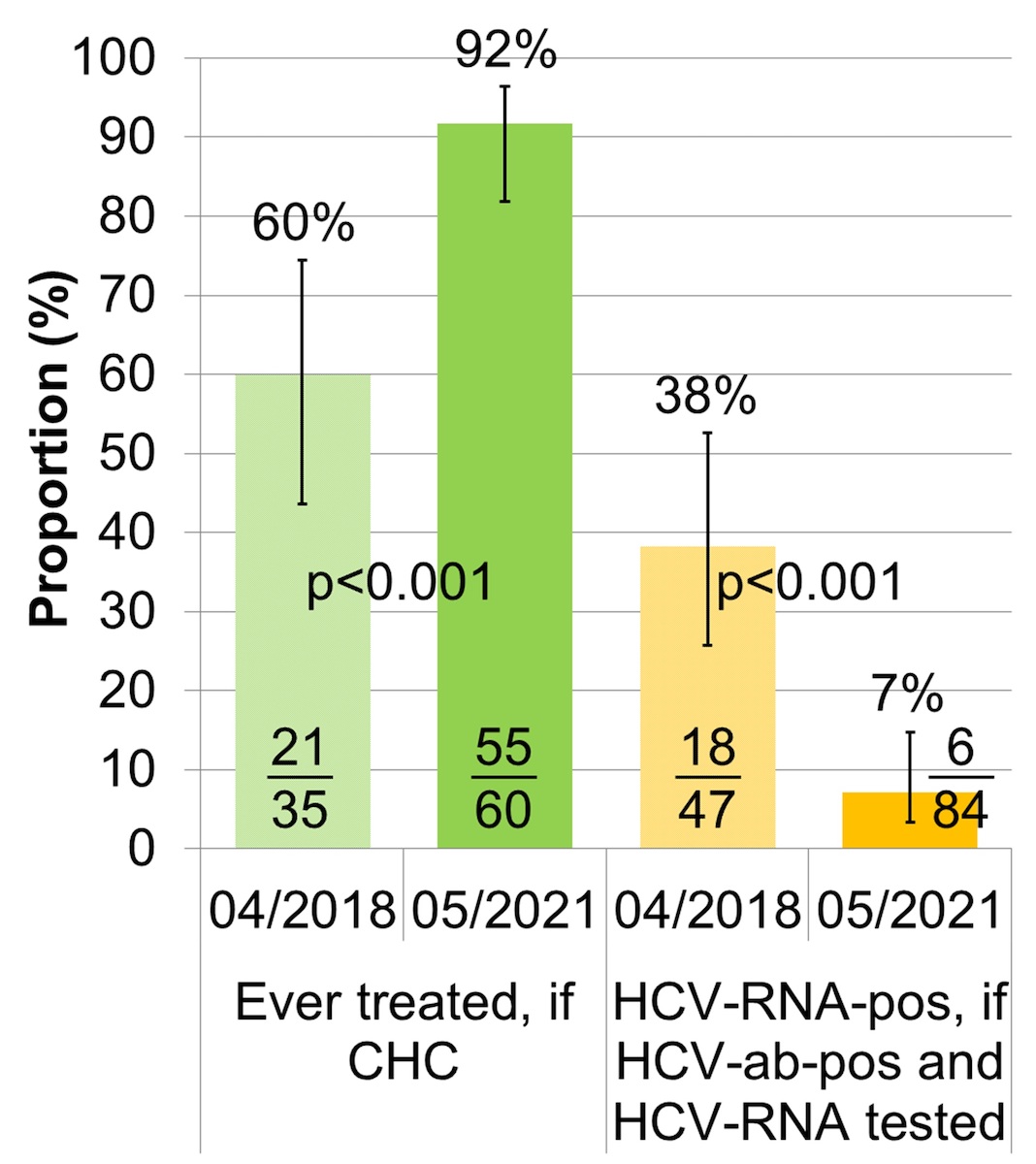
Figure 4 HCV treatment uptake and HCV RNA prevalence (n = 200).
HCV: hepatitis C virus; CHC: chronic hepatitis C; ab: antibody; RNA: ribonucleic acid; HCV treatment uptake: proportion of CHC patients ever treated; HCV RNA prevalence: proportion of HCV antibody positive patients with known HCV RNA that are HCV RNA positive
The error bars show the lower and upper limit of the 95% confidence interval (Score [Wilson]).
Among the six patients still HCV RNA positive in April 2021, two left the OAT programme before they could be treated and are currently “lost to follow-up”, two started direct-acting antiviral treatment in June 2021 and April 2022, respectively, and two are still in the OAT -programme, but currently not interested in treatment. Since one of the two patients leaving the OAT programme prior to treatment had a reinfection after successful interferon/ribavirin treatment, the number of patients never treated in April 2021 was not six, but five.
HCV treatment on-site
Between 2018 and 2021, 19 non-cirrhotic, HIV negative, patients with chronic hepatitis C were treated on-site. Eighty-four percent (16/19) were male. The median age was 36 years (IQR 28–47, range 27-58). All but one patient (95%) had ever used intravenous drugs. Thirty-seven percent (7/19) had continued intravenous drug use in the past 12 months.
Except from one non-responder to interferon/ribavirin-treatment, all patients were HCV treatment naïve. The median time between the first use of intravenous drugs (surrogate of the time of HCV infection) and the start of the current direct-acting antiviral treatment, i.e., the time in which the patients were infectious and could develop cirrhosis, was 13.5 years (IQR 8–20, range 2–33, n = 18). Of the 19 direct-acting antiviral treatments on-site, 12 were started in 2018, 4 in 2019, one in 2020 and 2 in 2021. “Non-compliance with appointments” (6) and “direct-acting antiviral reimbursement restrictions” (4) were the most common reasons why patients were not treated earlier. Four patients were only recently diagnosed, in 2018, 2019 and 2020, and treated within one year after diagnosis.
Of the 17 patients with known HCV genotype, 41% (7) had genotype 1a, 41% (7) had genotype 3, 12% (2) had genotype 4 and one patient had genotype 1b and probably 2. Liver fibrosis stage at start of direct-acting antiviral treatment was known for 18 patients (17 x Fibroscan®, 1 × liver biopsy): 50% (9) had F0-1 (no or mild fibrosis), 28% (5) F2 (significant fibrosis) and 22% (4) F3 (severe fibrosis). In the patient with neither Fibroscan® nor liver biopsy, cirrhosis could be excluded with an APRI score of 0.15.
One patient received sofosbuvir/ledipasvir for 8 weeks. All other patients were treated with sofosbuvir/velpatasvir for 12 weeks. Direct-acting antiviral treatment was dispensed with OAT, i.e., ≥5x/week in 47% (9), 2–3x/week in 26% (5), 1x/week in 21% (4) and biweekly in one patient (5%). Sixty-three percent (12) of the patients had no adherence problems, 32% (6) had delayed or missed intake of one or two doses and one patient, who three times missed two consecutives doses, took only 93% of all doses without a negative effect on treatment success.
All 19 patients had capillary HCV RNA monitoring on-site. After 4 weeks of direct-acting antiviral treatment, HCV RNA was undetectable in 47% (9). For the remaining patients, HCV RNA was at or below the limit of quantification. All 19 patients were HCV RNA negative at EOT and achieved SVR (18x SVR12, 1x SVR4). So far, no reinfection has been observed. For the 19 patients, the total follow-up time between EOT and the last available HCV RNA test was 33.8 years, with a median follow-up time of 1.8 years (IQR 0.6–3.1, range 0.1–3.4).
In 15 patients, a Fibroscan®-measurement had been performed after direct-acting antiviral treatment (at SVR12). Six patients had F0-1 (no or mild fibrosis) both before and after direct-acting antiviral treatment. Four patients improved from F2 (significant fibrosis) to F0-1, three from F3 (severe fibrosis) to F0-1 and one from F3 to F2. The patient with neither Fibroscan® nor liver biopsy before treatment (APRI score 0.15) had F0-1 after treatment.
For 12 patients lacking HAV/HBV immunity, HAV/HBV-vaccination (Twinrix® in five cases, Havrix® in six, Engerix® in one) was linked to the direct-acting antiviral treatment (start, week 4 and SVR12). Only one patient refused the recommended HAV vaccination.
HAV/HBV serostatus and immunity
Between April 2018 and May 2021, the proportion with known HAV/HBV serostatus increased from 38% (95% CI 30–47%) to 64% (95% CI 56–72%) and 51% (95% CI 42–59%) to 76% (95% CI 68–83), respectively (fig. 5, table S2).
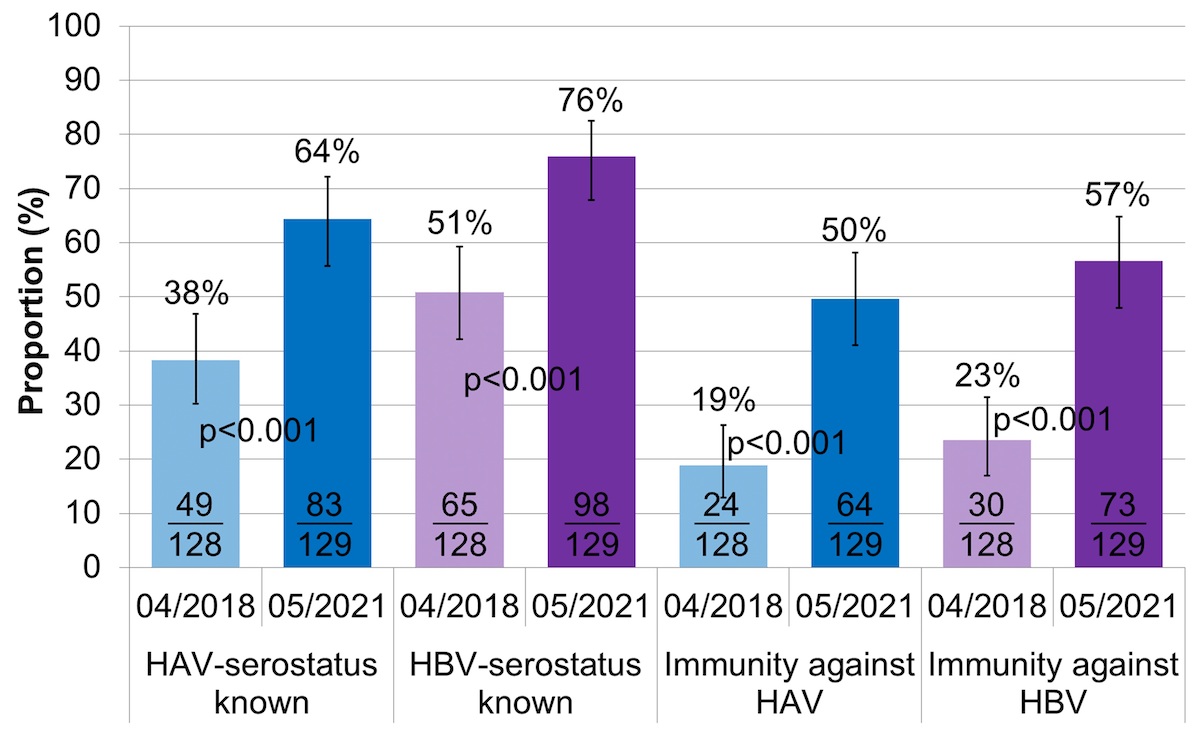
Figure 5 HAV/HBV serostatus and immunity.
HAV: hepatitis A virus; HBV: hepatitis B virus; immunity against HAV: either anti-HAV IgG positive or fully vaccinated against hepatitis A; immunity against HBV: either after vaccination (only anti-HBs positive) or infection (anti-HBc positive)
The error bars show the lower and upper limit of the 95% confidence interval (Score [Wilson]).
Documented HAV/HBV immunity, either after infection or vaccination, increased from 19% (95% CI 13–26%) to 50% (95% CI 41–58%) and 23% (95% CI: 17–31) to 57% (95% CI 48–65%), respectively.
There was only one hepatitis B surface antigen (HBs Ag) positive patient in May 2021 and none in 2018. At both time points, 18 patients (14%) were anti-hepatitis B core antigen (HBc) positive, but HBs Ag negative, with 5 (April 2018) and 4 (May 2021) of them having an anti-HBc only constellation. Between April 2018 and May 2021, the number of patients with documented HBV immunity after vaccination increased from 12 (9%) to 55 (43%) (p <0.001), and the number of patients with HAV immunity after documented vaccination from 9 (7%) to 38 (29%) (p <0.001). Between April 2018 and May 2021, 20 patients were fully vaccinated against HAV and HBV, 19 against HAV only und 6 against HBV only. The proportion of HAV immunity attributable to vaccination increased from 38% (9/24) to 59% (38/64) (p = 0.067), and the proportion of HBV immunity attributable to vaccination from 40% (12/30) to 75% (55/73) (p <0.001).
Discussion
Main findings
OAT programmes are often led by psychiatrists with no somatic physician on-site. Accordingly, HCV screening and treatment is not a priority and patients remain undiagnosed and thus untreated (lack of awareness among healthcare providers and patients). Difficult venous access after long-term intravenous drug use additionally complicates diagnosis. Once diagnosed, patients must be referred to a specialist for treatment, which often results in no-show and thus no treatment. In our hepatitis elimination project, we successfully addressed these barriers using capillary point-of-care tests and a “test-and-treat / vaccinate-on-site”-approach. Offering capillary HIV/HCV antibody and HCV RNA testing increased HIV/HCV screening uptake to >90% and substantially improved the acceptability of a yearly screening. Direct-acting antiviral treatment prescription and capillary HCV RNA monitoring on-site increased HCV treatment uptake to >90%. Thus, the WHO goals for HCV elimination in 2030, “90% of chronic hepatitis C patients diagnosed and 80% treated”, were met in our OAT patients. Since neither adherence problems nor reinfections compromised treatment success, HCV RNA prevalence among the HCV antibody positive patients (about half of the patients) could be reduced to <10%. Systematically offering venous HAV/HBV serology and HAV/HBV vaccination on-site improved documented HAV/HBV immunity from <25% to ≥50%, but capillary HAV/HBV serology remains an unmet need.
HCV screening and treatment uptake, HCV RNA prevalence reduction, adherence, reinfection
At baseline (April 2018), uptake of HIV/HCV antibody and HCV RNA screening was already relatively high at 79%/83% and 89%, respectively, because the OAT programme had already been visited every 4–6 months by our study team since 2014, offering capillary HIV/HCV antibody rapid tests from the beginning [44] and capillary HCV RNA quantification since August 2017 [45, 46]. Among patients enrolled into the Argovian OAT-cohort between July 2013- and July 2015, about one-quarter had never been screened for HIV/HCV antibodies and 19% of those positive for HCV antibodies were never tested for HCV RNA [44]. Intensified presence (every 4 weeks) increased uptake of HIV/HCV antibody- and HCV RNA screening to >90%, meeting the WHO elimination target “90% diagnosed”. Offering treatment on-site for non-cirrhotic, HIV-negative patients combined with capillary HCV RNA monitoring on-site, improved HCV treatment uptake from 60% to 92%, meeting the WHO elimination target “80% treated”. Since treatment success was 100% (no adherence problems, no reinfections), increased treatment uptake (∆32%) directly translated into a reduction in HCV RNA prevalence from 38% to 7% among the HCV antibody positive patients (∆31%, 82% decrease).
Two recently published systematic reviews and meta-analyses confirmed our results [65, 66]. Cunningham et al. found that medical chart reminders, simplified HCV testing (e.g. point-of-care antibody testing, dried blood spot testing, reflex RNA testing, opt-out screening), provider education, patient education, integrated care and patient navigation or care coordination can effectively enhance HCV antibody and HCV RNA testing, linkage to care and HCV treatment initiation [65]. According to Oru et al., linkage to care was better and treatment uptake higher with full decentralisation (testing and treatment at the same site) compared with partial decentralisation (testing at decentralised site and referral elsewhere for treatment) or no decentralisation among people who inject drugs (PWID), especially when HCV testing and treatment was integrated at sites providing harm-reduction services (OAT, needle and syringe programme, or both, and also mental health support), and among people in prisons [66]. In various decentralised settings, including harm-reduction sites, prisons and primary-care or community settings, SVR rates were similar to those in tertiary-level facilities [66]. Pangenotypic direct-acting antivirals allow task-shifting to non-specialists without reducing treatment success [66].
In the SIMPLIFY study (sofosbuvir/velpatasvir in patients with intravenous drug use in the past 6 months), one third of the participants had low adherence (<90%), which was associated with recent or ongoing injection of stimulants (cocaine and/or other amphetamines) [67]. In contrast, adherence was excellent (≥93%) in all our OAT patients treated on-site, although 37% reported continued intravenous drug use in the past 12 months. Remarkably, in the SIMPLIFY study, low adherence did not negatively affect treatment success [68], even when at least seven consecutive doses were missed [69].
In spite of yearly HCV RNA screening, so far, we did not detect any reinfections among our 19 OAT patients treated on-site (total follow-up time 33.8 years, median follow-up time 1.8 years). This is not surprising, because reinfection rates are generally low, especially in OAT patients [70–74]. In the SAMMSU cohort, the reinfection rate after interferon-free treatment (observation period 2013–2021) was 1.6/100 PY [75]. Similarly, the German hepatitis C cohort (GECCO) reported an overall reinfection rate of 1.9/100 PY since 2014 [76], with a lower rate in people who inject drugs than in men who have sex with men (1.1 vs 9.0/100 PY). Anyway, with direct-acting antivirals, reinfections can be treated as successfully as primary infections [77].
Yearly HIV/HCV screening, adequate HCV management
The HCV cascade of care with its four stages – (1) infected, (2) diagnosed, (3) treated and (4) cured – is often used to monitor progress towards HCV elimination [78]. However, it does not reflect adherence to the recommended yearly HCV screening [53, 55, 79], necessary for early diagnosis and treatment of primary infections and reinfections, which prevents further transmissions. Our definition of “adequate HCV management” incorporates it, considering HCV management as adequate only if the last HCV antibody or HCV RNA test is negative and ≤1 year ago.
Offering point-of-care tests on capillary instead of venous blood markedly increased the acceptance and feasibility of a yearly screening in our OAT patients. In HIV/HCV negative patients, the availability of a recent HIV/HCV antibody test (≤1 year ago) increased from 32%/28% in April 2018 to 85%/87% in May 2021, and in HCV antibody positive and RNA negative patients, the proportion with a recent HCV RNA test improved from 50% to 92%. The number of patients with adequate HCV management increased from 30 (23%) to 103 (80%).
Adherence to yearly HIV/HCV screening seems to be generally difficult. In ~50% of the HIV/HCV negatively screened patients of three OAT programmes in St Gallen in 2009, the last test was older than one year [80]. Among patients recruited to the Argovian OAT cohort between July 2013 and July 2015 (~50% in the decentralised setting), only 23% of the HCV antibody negative patients and 40% of the HCV-antibody-positive-RNA-negative patients were tested ≤1 year ago [44]. Similarly, in the Ukraine in 2014/2015, 22% of the PWID had a recent test (in the past 12 months) [81]. Among the non-cohort OAT patients assessed in the canton Aargau in 2018/2019, 38% of those who were HCV antibody negative and 58% of those HCV antibody positive and RNA negative had a screening test ≤1 year ago [45]. The latter might in part reflect recent treatment. In Scotland, 68% of the patients treated in the era of direct-acting antivirals (2015–2018) were tested for HCV RNA within the first year post-SVR, but only 30% in the second year, suggesting that an estimated 200 reinfections (54% of the estimated total) remained undiagnosed [82]. Even in the Swiss-wide SAMMSU cohort study, yearly HCV antibody and HCV RNA screening is not achieved [33].
Hepatitis A/B
OAT patients should be tested for HAV and HBV and vaccinated in the case of lacking immunity [53]. In the three OAT programmes in St Gallen in 2009, among those with available HAV/HBV serology (~90%), 75% were immune against HAV and HBV, but HBV immunity was mainly due to past infection [80]. Former studies in Argovian OAT patients (2013–2015 and 2018/2019) showed that only ~40% had HAV and ~50% interpretable HBV serology [44, 45], with ~50% and two thirds of them, respectively, being immune against HAV and HBV. Again, HBV immunity after infection was more frequent than after vaccination [44, 45]. In the OAT programme HAG (heroin dispensation of the canton Aargau), April 2018 to May 20201, the proportion with HAV serology could be improved from 38% to 64% and the proportion with HBV serology from 51% to 76% by systematically offering venous blood draw for HAV/HBV serology to those with unknown HAV/HBV serostatus. Capillary point-of-care tests for HAV/HBV serology could further improve acceptability. While CE-marked anti-HAV [83] and HBs-Ag [84, 85] point-of-care tests using capillary whole blood are already available, reliable point-of-care tests for anti-HBs and anti-HBc remain an unmet need [86–91]. However, a capillary HBV panel rapid test, simultaneously detecting anti-HBs, HBs Ag and anti-HBc is currently in development [92].
In a study in Irish GPs, two thirds of OAT patients were screened for HBV [93]. In contrast, ≥97% of the chronic hepatitis C patients in a methadone maintenance programme in New York were HAV/HBV tested [94]. However, 49%/63% lacked immunity to HAV/HBV, and vaccination-uptake was low (55%/3%) [94].
HBV vaccines have been available since the early 1980s [95]. However, so far, Switzerland does not meet the 90% coverage goal of the WHO [56]. In 2017–2019, only 74% of 16-year-old adolescents were vaccinated against HBV [96, 97]. Vaccination rates among sexually active adults were even lower [96, 98, 99]. However, systematic HBV vaccination of babies was introduced only in 2019 [100]. Before (1998–2018), 11–15-year-old adolescents were the primary target group [101]. Thus, it was not very surprising that in our OAT patients with a median age of 42 years (IQR 33–48) in April 2018 (born in 1976, IQR 1970–1985), documented HBV immunity was low (<25%) and mainly attributable to past infection. Vaccination uptake was high, with only a few individuals refusing the recommended HAV/HBV vaccinations. Thus, the proportion with documented HAV/HBV immunity could be increased from 19%/23% to 50%/57%, and the proportion of HAV/HBV immunity attributable to vaccination improved from 38%/40% to 59%/75%, which is still unsatisfactory.
Clinical implication and future research
Essentially, we now have the diagnostic and therapeutic tools to achieve HCV elimination by 2030, and guidelines on how to use them [41, 53, 58, 102–107]. In centralised OAT settings such as in our study, HCV micro-elimination is a “low-hanging fruit” [108]. However, overall, only 21% of the OAT patients in Switzerland receive their substitution at an institution (40–60% in Basel, Ticino, Zurich) [11]. In decentralised OAT settings with a low case load [44] (which is associated with low HCV knowledge [109]), implementation is still a challenge. In Switzerland the OAT prescriber is in 60% of cases a GP [7], and the OAT provider is in 49% a pharmacy and in 26% the treating physician [11]; therefore, GPs, psychiatrists and pharmacies should become more involved into the HCV management of OAT patients. Removal of the direct-acting antiviral prescriber restriction in 2022 [42, 43] might be a first step, but there is no guarantee that task shifting automatically occurs [109, 110]. The cantonal physicians (public health officers) could play a key role, because they must approve all OAT treatments and are notified about all new HCV diagnoses.
In a review, Bajis et al. nicely illustrate innovative, simplified and decentralised HCV test and treat models of care bringing hepatitis C care to the patients in OAT and/or needle and syringe programmes, prisons and pharmacies, as well as homelessness and drop-in centres [111].
As our study shows, HAV/HBV immunity of OAT patients can still be improved. We hypothesise that adding reliable capillary point-of-care tests for HAV and HBV serology to the vaccination on-site approach increases the number of OAT patients getting tested and vaccinated.
Strengths and limitations
As a strength, we offered screening and treatment/vaccination in the familiar environment of the OAT patients, where they usually pick up their OAT several times a week. Thus, we avoided stigmatisation, transportation costs and the need to keep appointments in tertiary care hospitals. With capillary blood tests and noninvasive liver fibrosis assessment (Fibroscan®), we removed fear of venous blood draw and liver biopsy as an obstacle regarding HCV diagnosis and treatment. Our presence on-site every four weeks definitely increased HCV awareness among healthcare providers and patients. Instructing the HAG staff how to use HIV/HCV antibody rapid tests, GeneXpert® and Fibroscan® allowed testing of OAT patients beyond our four-weekly visits and ensured sustainability of our project.
As a limitation, the success of such a project depends on dedicated staff in the OAT programme with a trustful relationship to the patients. Besides, the results of our study derive from a relatively small institution with ~130 OAT patients and might not be one-to-one generalisable to bigger institutions with >1000 OAT patients or decentralised settings. Compared with the overall OAT population [11], HAG patients were younger and more often male. Heroin and slow-release oral morphine substitution, ever use of intravenous drugs and HCV antibody positivity were more frequent. Outside academic settings, access to mobile GeneXpert® and Fibroscan® might be another limitation. However, these device-dependent methods can be replaced by dried blood spot sampling for HCV RNA quantification in the laboratory [47] and APRI score [64], respectively.
Implementation of our approach into routine clinical practice is complicated by reimbursement issues, which might jeopardise nationwide HBV/HCV elimination by 2030 [56, 57]. Only since 2022, have all physicians in Switzerland been allowed to prescribe direct-acting antiviral treatment [42], and direct-acting antivirals are reimbursed independently of the flat rate per case for inpatients [112]. In contrast to HIV antibody rapid tests, HCV antibody rapid tests with saliva or capillary whole blood are not yet reimbursed in Switzerland [113]. In some cantons, including the canton Aargau, where in >80%, the pharmacy is the OAT provider [12], pharmacies are not allowed to vaccinate against hepatitis A and B [114]. Besides, since 2020, combined HAV/HBV vaccination with Twinrix® is no longer reimbursed in Switzerland [115], although it is clearly recommended for people who use drugs and men who have sex with men [53, 116].
Conclusion
Capillary point-of-care tests and a “test-and-treat / vaccinate-on-site” approach remove crucial barriers to diagnosis and treatment, making hepatitis elimination in OAT programmes achievable. A high fluctuation rate requires HIV/HCV/HAV/HBV testing at admission, but also allows more patients to be screened.
Acknowledgements
We thank Petra Rippstein and Martin Ooms from the OAT-program “HAG” (heroin dispensation of the canton Aargau) in Brugg for their support.
Andrea Bregenzer
Department of Infectious Diseases and Hospital Hygiene
Cantonal Hospital Aarau
Tellstrasse 25
CH-5001 Aarau
andrea.bregenzer[at]ksa.ch
References
1. Richard JL, Schaetti C, Basler S, Mäusezahl M. The epidemiology of hepatitis C in Switzerland: trends in notifications, 1988-2015. Swiss Med Wkly. 2018 Apr;148:w14619.
2. Aaron S, McMahon JM, Milano D, Torres L, Clatts M, Tortu S, et al. Intranasal transmission of hepatitis C virus: virological and clinical evidence. Clin Infect Dis. 2008 Oct;47(7):931–4. https://doi.org/10.1086/591699
3. Fernandez N, Towers CV, Wolfe L, Hennessy MD, Weitz B, Porter S. Sharing of Snorting Straws and Hepatitis C Virus Infection in Pregnant Women. Obstet Gynecol. 2016 Aug;128(2):234–7. https://doi.org/10.1097/AOG.0000000000001507
4. Bihl F, Bruggmann P, Castro Batänjer E, Dufour JF, Lavanchy D, Müllhaupt B, et al. HCV disease burden and population segments in Switzerland. Liver Int. 2022 Feb;42(2):330–9. https://doi.org/10.1111/liv.15111
5. BAG-Bulletin 45/2019 (4.11.2019). Hepatitis C in der Schweiz, epidemiologische Situation 2015-2018. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/infektionskrankheiten/hepatitis-c/hepatitis-c-epidemiologie-2015-18.pdf (Accessed: 9 June 2022)
6. BAG. 2013. Substitutionsgestützte Behandlung bei Opioidabhängigkeit. Revision 2013. Empfehlungen des Bundesamtes für Gesundheit (BAG), der Schweizerischen Gesellschaft für Suchtmedizin (SSAM), der Vereinigung der Kantonsärztinnen und Kantonsärzte Schweiz (VKS). Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/npp/sucht/hegebe/substitutionsgestuetzte-behandlungen-bei-opioid-abhaengigkeit.pdf.download.pdf/BAG_Brosch_SGB_d(5)_def.pdf (Accessed: 9 June 2022)
7. Bundesamt für Gesundheit (BAG). Substitutionsgestützte Behandlungen bei Opioidabhängigkeit (9.3.2022). Available from: https://www.bag.admin.ch/bag/de/home/gesund-leben/sucht-und-gesundheit/suchtberatung-therapie/substitutionsgestuetzte-behandlung.html (Accessed: 9 June 2022)
8. Cominetti F, Simonson T, Dubois-Arber F, Gervasoni JP. IUMSP, Schaub M., ISGF, Monnat M., SSP. Analyse der Hepatitis-C-Situation bei den drogenkonsumierenden Personen in der Schweiz. Lausanne, Institut universitaire de médecine sociale et préventive, 2014. (Raisons de santé 234b) Available from: https://www.aramis.admin.ch/Default?DocumentID=14464&Load=true (Accessed: 9 June 2022)
9. Zahnd C, Brezzi M, Bertisch B, Giudici F, Keiser O. Analyse de Situation des Hépatites B et C en Suisse (23.3.2017). Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/forschungsberichte/situationsanalyse-hepatitis-bericht.pdf.download.pdf/situationsanalyse-hepatitis-bericht-de.pdf (Accessed: 9 June 2022)
10. Bruggmann P, Blach S, Deltenre P, Fehr J, Kouyos R, Lavanchy D, et al. Hepatitis C virus dynamics among intravenous drug users suggest that an annual treatment uptake above 10% would eliminate the disease by 2030. Swiss Med Wkly. 2017 Nov;147:w14543. https://doi.org/10.4414/smw.2017.14543
11. Nationale Substitutionsstatistik. Jährliche Statistik. Schweiz - 2020. Available from: https://www.substitution.ch/de/jahrliche_statistik.html&year=2020&canton=ch (Accessed: 9 June 2022)
12. Nationale Substitutionsstatistik. Jährliche Statistik. Aargau - 2020. Available from: https://www.substitution.ch/de/jahrliche_statistik.html&year=2020&canton=ag (Accessed: 9 June 2022)
13. Grebely J, Page K, Sacks-Davis R, van der Loeff MS, Rice TM, Bruneau J, et al.; InC3 Study Group. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014 Jan;59(1):109–20. https://doi.org/10.1002/hep.26639
14. Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008 Aug;48(2):418–31. https://doi.org/10.1002/hep.22375
15. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014 Nov;61(1 Suppl):S58–68. https://doi.org/10.1016/j.jhep.2014.07.012
16. WHO. 2021. Fact-sheet Hepatitis C (27 July 2021). Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (Accessed: 11 June 2022)
17. Evon DM, Stewart PW, Amador J, Serper M, Lok AS, Sterling RK, et al. A comprehensive assessment of patient reported symptom burden, medical comorbidities, and functional well being in patients initiating direct acting antiviral therapy for chronic hepatitis C: results from a large US multi-center observational study. PLoS One. 2018 Aug;13(8):e0196908. https://doi.org/10.1371/journal.pone.0196908
18. Edlin BR. Perspective: test and treat this silent killer. Nature. 2011 May;474(7350):S18–9. https://doi.org/10.1038/474S18a
19. Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, et al. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N Engl J Med. 2021 Feb;384(6):541–9. https://doi.org/10.1056/NEJMoa2023345
20. Hartlage AS, Kapoor A, Hepatitis C. Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up. Viruses. 2021 Aug;13(8):1596. https://doi.org/10.3390/v13081596
21. van Heuvel Y, Schatz S, Rosengarten JF, Stitz J. Infectious RNA: Human Immunodeficiency Virus (HIV) Biology, Therapeutic Intervention, and the Quest for a Vaccine. Toxins (Basel). 2022 Feb;14(2):138. https://doi.org/10.3390/toxins14020138
22. Herzig M, Wolf M. Inside Switzerland’s Radical Drug Policy Innovation. Stanford Social Innovation Review 2019. Available from: https://doi.org/https://doi.org/10.48558/MQWP-3277 (Accessed: 9 June 2022)
23. Uchtenhagen A. Heroin-assisted treatment in Switzerland: a case study in policy change. Addiction. 2010 Jan;105(1):29–37. https://doi.org/10.1111/j.1360-0443.2009.02741.x
24. Somaini B, Wang J, Perozo M, Kuhn F, Meili D, Grob P, et al. A continuing concern: HIV and hepatitis testing and prevalence among drug users in substitution programmes in Zurich, Switzerland. AIDS Care. 2000 Aug;12(4):449–60. https://doi.org/10.1080/09540120050123855
25. Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M ; Amsterdam Cohort. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007 Sep;102(9):1454–62. https://doi.org/10.1111/j.1360-0443.2007.01912.x
26. van Santen DK, Boyd A, Matser A, Maher L, Hickman M, Lodi S, et al. The effect of needle and syringe program and opioid agonist therapy on the risk of HIV, hepatitis B and C virus infection for people who inject drugs in Amsterdam, the Netherlands: findings from an emulated target trial. Addiction. 2021 Nov;116(11):3115–26. https://doi.org/10.1111/add.15503
27. Puzhko S, Eisenberg MJ, Filion KB, Windle SB, Hébert-Losier A, Gore G, et al. Effectiveness of Interventions for Prevention of Common Infections Among Opioid Users: A Systematic Review of Systematic Reviews. Front Public Health. 2022 Feb;10:749033. https://doi.org/10.3389/fpubh.2022.749033
28. Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017 Sep;9(9):CD012021. https://doi.org/10.1002/14651858.CD012021.pub2
29. Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012 Jul;308(4):387–402. https://doi.org/10.1001/jama.2012.7961
30. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al.; HPTN 052 Study Team. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016 Sep;375(9):830–9. https://doi.org/10.1056/NEJMoa1600693
31. Baral S, Rao A, Sullivan P, Phaswana-Mafuya N, Diouf D, Millett G, et al. The disconnect between individual-level and population-level HIV prevention benefits of antiretroviral treatment. Lancet HIV. 2019 Sep;6(9):e632–8. https://doi.org/10.1016/S2352-3018(19)30226-7
32. Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2020 Oct;324(16):1651–69. https://doi.org/10.1001/jama.2020.17025
33. Bregenzer A, Bruggmann P, Castro E, Moriggia A, Rothen M, Thurnheer MC, et al. Hepatitis C virus elimination in Swiss opioid agonist therapy programmes - the SAMMSU cohort. Swiss Med Wkly. 2021 Mar;151:w20460. https://doi.org/10.4414/smw.2021.20460
34. Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013;57 Suppl 2(Suppl 2):S39-45
35. Bundesamt für Gesundheit (BAG). BAG erweitert Vergütung von Medikamenten gegen Hepatitis C (27 Apr 17). Available from: https://www.bag.admin.ch/bag/de/home/das-bag/aktuell/medienmitteilungen.msg-id-66508.html (Accessed: 10 June 2022)
36. Bundesamt für Gesundheit (BAG). Hepatitis C: Uneingeschränkte Vergütung der neuen Arzneimittel für alle Betroffenen (25 Sept 2017). Available from: https://www.bag.admin.ch/bag/de/home/das-bag/aktuell/medienmitteilungen.msg-id-68158.html (Accessed: 10 June 2022)
37. Chahine EB, Sucher AJ, Hemstreet BA. Sofosbuvir/Velpatasvir: The First Pangenotypic Direct-Acting Antiviral Combination for Hepatitis C. Ann Pharmacother. 2017 Jan;51(1):44–53. https://doi.org/10.1177/1060028016668897
38. Puoti M, Foster GR, Wang S, Mutimer D, Gane E, Moreno C, et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1-6 patients without cirrhosis. J Hepatol. 2018 Aug;69(2):293–300. https://doi.org/10.1016/j.jhep.2018.03.007
39. Brown RS Jr, Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020 Mar;72(3):441–9. https://doi.org/10.1016/j.jhep.2019.10.020
40. Aghemo A, Colombo M. Glecaprevir/Pibrentasvir: The Final Piece in the Hepatitis C Virus Treatment Puzzle? Gastroenterology. 2018 Mar;154(4):1195–6. https://doi.org/10.1053/j.gastro.2018.01.035
41. Moradpour D, Fehr J, Semela D, Rauch A, Müllhaupt B. Treatment of Chronic Hepatitis C - January 2021 Update. Expert Opinion Statement by SASL, SSG and SSI. Available from: https://www.sginf.ch/files/sasl-ssg-ssi_eos_hepc_jan2021_1.pdf (Accessed: 10 June 2022)
42. Hepatitis Schweiz. News (17.01.2022). Neu können Hepatitis-C-Medikamente auch von Hausärzt:innen verschrieben werden. Available from: https://hepatitis-schweiz.ch/news/neu-koennen-hepatitis-c-medikamente-auch-von-hausaerztinnen-verschrieben-werden (Accessed: 10 June 2022)
43. Bundesamt für Gesundheit (BAG)→Versicherungen→Krankenversicherung→Leistungen und Tarife→Arzneimittel→Referenzdokumente zur Spezialitätenliste→Liste der Ärzte mit Erfahrung in Suchtmedizin und in der Behandlung von chronischer Hepatitis C (01.12.2021). Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/kuv-leistungen/arzneimittel/liste-der-aerzte-mit-erfahrung-in-suchtmedizin-und-in-der-behandlung-von-chronischer-hepatitis-c.pdf (Accessed: 10 June 2022)
44. Bregenzer A, Conen A, Knuchel J, Friedl A, Eigenmann F, Näf M, et al. Management of hepatitis C in decentralised versus centralised drug substitution programmes and minimally invasive point-of-care tests to close gaps in the HCV cascade. Swiss Med Wkly. 2017 Nov;147:w14544.
45. Schürch S, Fux CA, Dehler S, Conen A, Knuchel J, Friedl A, et al. Management of hepatitis C in opioid agonist therapy patients of the Swiss canton Aargau within and outside the cohort study. Swiss Med Wkly. 2020 Aug;150:w20317. https://doi.org/10.4414/smw.2020.20317
46. Bregenzer A, Warmann N, Ottiger C, Fux CA. Rapid point-of-care HCV RNA quantification in capillary whole blood for diagnosing chronic HCV infection, monitoring treatment and detecting reinfection. Swiss Med Wkly. 2019 Oct;149:w20137. https://doi.org/10.4414/smw.2019.20137
47. Bregenzer A, Ottiger C, Krismer C, Sager K, Fux CA. HCV RNA quantification in capillary dried blood spots with the Xpert® HCV Viral Load test for diagnosing chronic HCV infection, monitoring treatment and detecting reinfection. Swiss Med Wkly. 2021 Dec;151:w30089.
48. News NB. (22 February 2011). OraSure Technologies Receives FDA Approval for Its OraQuick(R) HCV Rapid Test Using Fingerstick Whole Blood. Available from: https://www.nbcnews.com/id/wbna41713925 (Accessed: 10 June 2022)
49. Lee SR, Kardos KW, Schiff E, Berne CA, Mounzer K, Banks AT, et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods. 2011 Mar;172(1-2):27–31. https://doi.org/10.1016/j.jviromet.2010.12.009
50. Smookler D, Vanderhoff A, Biondi MJ, Valencia J, Ryan P, Karkada J, et al. Reducing Read Time of Point-of-Care Test Does Not Affect Detection of Hepatitis C Virus and Reduces Need for Reflex RNA. Clin Gastroenterol Hepatol. 2021 Jul;19(7):1451–1458.e4. https://doi.org/10.1016/j.cgh.2020.07.058
51. Lamoury FM, Bajis S, Hajarizadeh B, Marshall AD, Martinello M, Ivanova E, et al.; LiveRLife Study Group. Evaluation of the Xpert HCV Viral Load Finger-Stick Point-of-Care Assay. J Infect Dis. 2018 May;217(12):1889–96. https://doi.org/10.1093/infdis/jiy114
52. Cooper CL. Now Is the Time to Quickly Eliminate Barriers Along the Hepatitis C Cascade of Care. J Infect Dis. 2018 May;217(12):1858–60. https://doi.org/10.1093/infdis/jiy117
53. Bundesamt für Gesundheit (BAG). Hepatitis C bei Drogenkonsumierenden: Richtlinien mit settingspezifischen Factsheets (Mar 2019). Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/infektionskrankheiten/hepatitis-c/richtlinien-hepatitis-c-drogen.pdf (Accessed: 10 June 2022)
54. Bruggmann P, Broers B, Meili D. Hepatitis C-Therapie bei Patienten unter Opioidsusbstitution. Empfehlungen der Schweizerischen Gesellschaft für Suchtmedizin (SSAM). Schweiz Med Forum 2007;7:916-919
55. Grebely J, Robaeys G, Bruggmann P, Aghemo A, Backmund M, Bruneau J, et al.; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015 Oct;26(10):1028–38. https://doi.org/10.1016/j.drugpo.2015.07.005
56. World Health Organization (WHO). Combating hepatitis B and C to reach elimination by 2030 - Advocacy brief (May 2016). Available from: http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf (Accessed: 10 June 2022)
57. Swiss Hepatitis. 2019. Swiss Hepatitis Strategy 2014-2030, Process Paper - A Living Document. January 2019, Version 4. Available from: https://hepatitis-schweiz.ch/data/download/2871/Process_Paper_14_02_2019.pdf (Accessed: 10 June 2022)
58. European AIDS Clinical Society (EACS) Guidelines Version 11.0, October 2021, Available from: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf (Accessed: 11 June 2022)
59. Vogel M, Page E, Matthews G, Guiguet M, Dominguez S, Dore G, et al. Use of week 4 HCV RNA after acute HCV infection to predict chronic hcv infection [abstract 640]. In: 17th Conference on Retroviruses and Opportunistic Infections; 16-19 February 2010; San Francisco, California, USA.
60. Martinello M, Hajarizadeh B, Grebely J, Dore GJ, Matthews GV. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat Rev Gastroenterol Hepatol. 2018 Jul;15(7):412–24. https://doi.org/10.1038/s41575-018-0026-5
61. Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr, Ratziu V, Ding X, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015 Jan;61(1):41–5. https://doi.org/10.1002/hep.27366
62. Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005 Feb;128(2):343–50. https://doi.org/10.1053/j.gastro.2004.11.018
63. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008 May;48(5):835–47. https://doi.org/10.1016/j.jhep.2008.02.008
64. Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007 Sep;46(3):912–21. https://doi.org/10.1002/hep.21835
65. Cunningham EB, Wheeler A, Hajarizadeh B, French CE, Roche R, Marshall AD, et al. Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022 May;7(5):426–45. https://doi.org/10.1016/S2468-1253(21)00471-4
66. Oru E, Trickey A, Shirali R, Kanters S, Easterbrook P. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta-analysis. Lancet Glob Health. 2021 Apr;9(4):e431–45. https://doi.org/10.1016/S2214-109X(20)30505-2
67. Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, et al.; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018 Mar;3(3):153–61. https://doi.org/10.1016/S2468-1253(17)30404-1
68. Cunningham EB, Amin J, Feld JJ, Bruneau J, Dalgard O, Powis J, et al.; SIMPLIFY study group. Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: the SIMPLIFY study. Int J Drug Policy. 2018 Dec;62:14–23. https://doi.org/10.1016/j.drugpo.2018.08.013
69. Cunningham EB, Hajarizadeh B, Amin J, Litwin AH, Gane E, Cooper C, et al.; SIMPLIFY and D3FEAT study groups. Adherence to Once-daily and Twice-daily Direct-acting Antiviral Therapy for Hepatitis C Infection Among People With Recent Injection Drug Use or Current Opioid Agonist Therapy. Clin Infect Dis. 2020 Oct;71(7):e115–24. https://doi.org/10.1093/cid/ciz1089
70. Hajarizadeh B, Cunningham EB, Valerio H, Martinello M, Law M, Janjua NZ, et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J Hepatol. 2020 Apr;72(4):643–57. https://doi.org/10.1016/j.jhep.2019.11.012
71. Akiyama MJ, Lipsey D, Heo M, Agyemang L, Norton BL, Hidalgo J, et al. Low Hepatitis C Reinfection Following Direct-acting Antiviral Therapy Among People Who Inject Drugs on Opioid Agonist Therapy. Clin Infect Dis. 2020 Jun;70(12):2695–702. https://doi.org/10.1093/cid/ciz693
72. Falcato L, Bernardini C, Bruggmann P. Hepatitis C reinfection following successful direct-acting antiviral therapy among patients attending a multidisciplinary treatment centre for people who use drugs in Zurich, Switzerland. Int J Drug Policy. 2021 Oct;96:103434. https://doi.org/10.1016/j.drugpo.2021.103434
73. Cunningham EB, Hajarizadeh B, Amin J, Hellard M, Bruneau J, Feld JJ, et al. Reinfection Following Successful Direct-acting Antiviral Therapy for Hepatitis C Virus Infection Among People Who Inject Drugs. Clin Infect Dis. 2021 Apr;72(8):1392–400. https://doi.org/10.1093/cid/ciaa253
74. Barocas JA. It’s Not Them, It’s Us: Hepatitis C Reinfection Following Successful Treatment Among People Who Inject Drugs. Clin Infect Dis. 2021 Apr;72(8):1401–3. https://doi.org/10.1093/cid/ciaa258
75. Bregenzer A, Scheidegger C. Hepatitis C bei Drogenkonsumierenden im Rahmen der SAMMSU-Kohorte. Abschlussbericht 2022. Im Auftrag des Bundesamtes für Gesundheit (BAG). https://www.aramis.admin.ch/Grunddaten/?ProjectID=45343 (Accessed: 11 June 2022)
76. Ingiliz P, Wehmeyer MH, Boesecke C, Schulze Zur Wiesch J, Schewe K, Lutz T, et al.; European AIDS Treatment Network (NEAT) Study Group; German Hepatitis C Cohort (GECCO) Study Group. Reinfection With the Hepatitis C Virus in Men Who Have Sex With Men After Successful Treatment With Direct-acting Antivirals in Germany: Current Incidence Rates, Compared With Rates During the Interferon Era. Clin Infect Dis. 2020 Aug;71(5):1248–54. https://doi.org/10.1093/cid/ciz949
77. Carson JM, Hajarizadeh B, Hanson J, O’Beirne J, Iser D, Read P, et al.; REACH-C Study Group. Effectiveness of treatment for hepatitis C virus reinfection following direct acting antiviral therapy in the REACH-C cohort. Int J Drug Policy. 2021 Oct;96:103422. https://doi.org/10.1016/j.drugpo.2021.103422
78. Safreed-Harmon K, Blach S, Aleman S, Bollerup S, Cooke G, Dalgard O, et al. The Consensus Hepatitis C Cascade of Care: Standardized Reporting to Monitor Progress Toward Elimination. Clin Infect Dis. 2019 Nov;69(12):2218–27. https://doi.org/10.1093/cid/ciz714
79. Beck T, Bruggmann P, Haemmig R, Caflisch C, Falcato L, Fink A, et al. Medizinische Empfehlungen für Opioidagonistentherapie (OAT) bei Opioidabhängigkeits-Syndrom 2020. Bern: SSAM; 2020. Available from: https://www.ssam-sapp.ch/fachleute/empfehlungen/opioidagonistentherapie-oat (Accessed: 11 June 2022)
80. Witteck A, Schmid P, Hensel-Koch K, Thurnheer MC, Bruggmann P, Vernazza P ; Swiss Hepatitis C and HIV Cohort Studies. Management of hepatitis C virus (HCV) infection in drug substitution programs. Swiss Med Wkly. 2011 May;141:w13193. https://doi.org/10.4414/smw.2011.13193
81. Iakunchykova O, Meteliuk A, Zelenev A, Mazhnaya A, Tracy M, Altice FL. Hepatitis C virus status awareness and test results confirmation among people who inject drugs in Ukraine. Int J Drug Policy. 2018 Jul;57:11–7. https://doi.org/10.1016/j.drugpo.2018.03.022
82. Yeung A, Palmateer NE, Dillon JF, McDonald SA, Smith S, Barclay S, et al. Population-level estimates of hepatitis C reinfection post scale-up of direct-acting antivirals among people who inject drugs. J Hepatol. 2022 Mar;76(3):549–57. https://doi.org/10.1016/j.jhep.2021.09.038
83. Biotech CT. Home / Product Line / Rapid Tests / Hepatitis / HAV IgG/IgM Rapid Test CE. Available from: https://ctkbiotech.com/product/hav-igg-igm-rapid-test-ce/ (Accessed: 11 June 2022)
84. Abbott. Determine™ HBsAg 2. Der diagnostische Schnelltest mit der weltweit höchsten Sensitivität zum Nachweis des Hepatitis-B-Oberflächenantigens aus Serum-, Plasma oder Vollblutproben. Available from: https://www.globalpointofcare.abbott/de/product-details/determine-hbsag-2.html (Accessed: 11 June 2022)
85. Amini A, Varsaneux O, Kelly H, Tang W, Chen W, Boeras DI, et al. Diagnostic accuracy of tests to detect hepatitis B surface antigen: a systematic review of the literature and meta-analysis. BMC Infect Dis. 2017 Nov;17(S1 Suppl 1):698. https://doi.org/10.1186/s12879-017-2772-3
86. Poiteau L, Soulier A, Roudot-Thoraval F, Hézode C, Challine D, Pawlotsky JM, et al. Performance of rapid diagnostic tests for the detection of anti-HBs in various patient populations. J Clin Virol. 2017 Nov;96:64–6. https://doi.org/10.1016/j.jcv.2017.09.012
87. Xiao Y, Thompson AJ, Howell J. Point-of-Care Tests for Hepatitis B: an Overview. Cells. 2020 Oct;9(10):2233. https://doi.org/10.3390/cells9102233
88. El-Ghitany EM, Farghaly AG. Evaluation of commercialized rapid diagnostic testing for some Hepatitis B biomarkers in an area of intermediate endemicity. J Virol Methods. 2013 Dec;194(1-2):190–3. https://doi.org/10.1016/j.jviromet.2013.08.026
89. Wu FY, Liao YW, Wu JF, Chen HL, Hsu HY, Chang MH, et al. A Simple and Rapid Test-card Method to Detect Hepatitis B Surface Antigen and Antibody: Potential Application in Young Children and Infants. Pediatr Neonatol. 2016 Jun;57(3):219–24. https://doi.org/10.1016/j.pedneo.2015.07.003
90. Cruz HM, Scalioni LP, Paula VS, Miguel JC, Ó KM, Milagres FA, et al. Poor sensitivity of rapid tests for the detection of antibodies to the hepatitis B virus: implications for field studies. Mem Inst Oswaldo Cruz. 2017 Mar;112(3):209–13. https://doi.org/10.1590/0074-02760160394
91. Bottero J, Boyd A, Gozlan J, Lemoine M, Carrat F, Collignon A, et al. Performance of rapid tests for detection of HBsAg and anti-HBsAb in a large cohort, France. J Hepatol. 2013 Mar;58(3):473–8. https://doi.org/10.1016/j.jhep.2012.11.016
92. Mag IA. Diagnostics. HBV rapid test for HBsAg, anti-HBc and anti-HBs. Available from: https://www.magia-diagnostics.com/diagnostics/ (Accessed: 11 June 2022)
93. Murtagh R, Swan D, O’Connor E, McCombe G, Lambert JS, Avramovic G, et al. Hepatitis C Prevalence and Management Among Patients Receiving Opioid Substitution Treatment in General Practice in Ireland: Baseline Data from a Feasibility Study. Interact J Med Res. 2018 Dec;7(2):e10313. https://doi.org/10.2196/10313
94. Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010 Oct;29(4):461–5. https://doi.org/10.1080/10550887.2010.509281
95. Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13 Suppl 1:S47–9. https://doi.org/10.1016/0264-410X(95)93547-M
96. BAG-Bulletin 48/2021 (29.11.2021). Sexuell übertragene Infektionen und Hepatitis B/C in der Schweiz im Jahr 2020: eine epidemiologische Übersicht. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/cc/Kampagnen/Bulletin/2021/bu-48-21.pdf.download.pdf/BU_48_21_DE.pdf (Accessed: 11 June 2022)
97. BAG-Bulletin 16/2021 (29.04.2021). Durchimpfung von 2-, 8- und 16-jährigen Kindern und Jugendlichen in der Schweiz, 2017–2019. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/i-und-b/durchimpfung/bu-16-21-durchimpfung-2017-2019.pdf (Accessed: 11 June 2022)
98. Vernazza PL, Rasi M, Ritzler M, Dost F, Stoffel M, Aebi-Popp K, et al. The Swiss STAR trial - an evaluation of target groups for sexually transmitted infection screening in the sub-sample of women. Swiss Med Wkly. 2020 Dec;150:w20393. https://doi.org/10.4414/smw.2020.20393
99. Schmidt AJ, Rasi M, Esson C, Christinet V, Ritzler M, Lung T, et al. The Swiss STAR trial - an evaluation of target groups for sexually transmitted infection screening in the sub-sample of men. Swiss Med Wkly. 2020 Dec;150:w20392. https://doi.org/10.4414/smw.2020.20392
100. Bundesamt für Gesundheit (BAG), Eidgenössische Kommission für Impffragen (EKIF). Schweizerischer Impfplan 2019. Richtlinien und Empfehlungen. Bern: Bundesamt für Gesundheit, 2019. www.bag.admin.ch/impfplan
101. Bundesamt für Gesundheit (BAG), Eidgenössische Kommission für Impffragen (EKIF). Schweizerischer Impfplan 2018. Richtlinien und Empfehlungen. Bern: Bundesamt für Gesundheit, 2018. www.bag.admin.ch/impfplan
102. Loustaud-Ratti V, Debette-Gratien M, Carrier P. European Association for the Study of the Liver and French hepatitis C recent guidelines: the paradigm shift. World J Hepatol. 2018 Oct;10(10):639–44. https://doi.org/10.4254/wjh.v10.i10.639
103. Berzigotti A, Tsochatzis E, Boursier J, Castera L, Cazzagon N, Friedrich-Rust M, et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair; EASL Governing Board representative; Panel members. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021 Sep;75(3):659–89. https://doi.org/10.1016/j.jhep.2021.05.025
104. European Association for Study of Liver Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015 Jul;63(1):237–64. https://doi.org/10.1016/j.jhep.2015.04.006
105. WHO guidelines on hepatitis B and C testing. Geneva: World Health Organization; 2017. Available from https://apps.who.int/iris/rest/bitstreams/1080581/retrieve (Accessed: 11 June 2022)
106. Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members. EASL recommendations on treatment of hepatitis C: final update of the series☆. J Hepatol. 2020 Nov;73(5):1170–218. https://doi.org/10.1016/j.jhep.2020.08.018
107. Ghany MG, Morgan TR ; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020 Feb;71(2):686–721. https://doi.org/10.1002/hep.31060
108. Bruggmann P, Maeschli B. Hepatitis C micro-elimination among people on opioid agonist therapy is a low-hanging fruit. Swiss Med Wkly. 2020 Sep;150:w20348. https://doi.org/10.4414/smw.2020.20348
109. Wade A, Draper B, Doyle J, Allard N, Grinzi P, Thompson A, et al. A survey of hepatitis C management by Victorian GPs after PBS-listing of direct-acting antiviral therapy. Aust Fam Physician. 2017;46(4):235–40.
110. Bruggmann P, Maeschli B, Scheideger C. Hepatitis-C-Behandlungen in der hausärztlichen Praxis. Prim Hosp Care Allg Inn Med. 2020;20(02):67–9.
111. Bajis S, Applegate TL, Grebely J, Matthews GV, Dore GJ. Novel Hepatitic C Virus (HCV) Diagnosis and Treatment Delivery Systems: Facilitating HCV Elimination by Thinking Outside the Clinic. J Infect Dis. 2020 Nov;222 Suppl 9:S758–72. https://doi.org/10.1093/infdis/jiaa366
112. Swiss DR. Fallpauschalenkatalog SwissDRG 11.0 Abrechnungsversion (2022/2022). Anlage 2: Zusatzentgeltkatalog (Zeilen 2349ff (Sofosbuvir und Velpatasvir) und 2678ff (Glecaprevir und Pibrentasvir)). (Genehmigte Version vom 11. Juni 2022, Stand: 06. Dezember 2021) Available from: https://www.swissdrg.org/de/akutsomatik/swissdrg-system-1102022/fallpauschalenkatalog (Accessed: 11 June 2022)
113. Bundesamt für Gesundheit (BAG). Analysenliste (AL). Gesamtliste der AL. Analysenliste per 1. Februar 2022 in Excel Format (23.12.2021). Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/kuv-leistungen/leistungen-und-tarife/Analysenliste/klva-anhang3-gesamtliste-excel-01022022.xlsx (Accessed: 11 June 2022)
114. Pharma Suisse. Schweizerischer Apothekerverband. Liste der Impfungen nach Kanton. (Stand: 01.10.2021) Available from: https://www.ihre-apotheke.ch/data/docs/de/48258/Liste-der-Impfungen-nach-Kanton-Stand-1-10-2021.pdf?v=1.0 (Accessed: 11 June 2022)
115. Bundesamt für Gesundheit (BAG). Spezialitätenliste (SL). SL-Publikationen aktueller Monat. Liste der gestrichenen Packungen seit 01.01.2010. Available from: http://www.xn--spezialittenliste-yqb.ch/File.axd?file=Gestrichene_Packungen_Emballages_radies.xlsx (Accessed: 11 June 2022)
116. Bundesamt für Gesundheit (BAG), Eidgenössische Kommission für Impffragen (EKIF). Schweizerischer Impfplan 2022. Richtlinien und Empfehlungen. Bern: Bundesamt für Gesundheit, 2022. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/i-und-b/richtlinien-empfehlungen/allgemeine-empfehlungen/schweizerischer-impfplan.pdf (Accessed: 11 June 2022)
Appendix: Supplementary material
Table S1Patient characteristics.
|
All (n = 200) |
Present April 18 to May 21 (n = 79) |
Newly admitted after April 18 (n = 72) |
Leaving after April 18 (n = 71) (includes 22 of those newly admitted) |
| Male |
80% (159) |
75% (59) |
81% (58) |
85% (60/71) |
| Age (years), median (IQR) |
42 (34–49) |
46 (37-52) |
37 (32–43) |
39 (33–46) |
| Ever IDU |
67% (121/181) |
77% (60/78) |
59% (36/61) |
60% (37/62) |
| HCV positive |
47% (85/181) |
53% (42/79) |
40% (24/60) |
46% (28/61) |
| → currently HCV RNA positive |
8% (7/84) |
5% (2/42) |
13% (3/23) |
11% (3/28) |
| HIV positive |
2% (4/178) |
4% (3/79) |
2% (1/57) |
0% (0/61) |
| Enrolled into SAMMSU (www.sammsu.ch) |
77% (154) |
94% (74) |
72% (52) |
65% (46) |
| HCV treatments on-site (until May 21)* |
18 |
9 |
4 |
7 |
| Ever CHC |
60 |
31 |
17 |
17 |
|
April 2018 (n = 128) |
May 2021 (n = 129) |
p-value |
| n |
% |
95% CI |
n |
% |
95% CI |
| Ever HIV ab tested |
101/128 |
79 |
71–85 |
117/129 |
91 |
84–95 |
0.008 |
| Ever HCV ab tested |
106/128 |
83 |
75–88 |
120/129 |
93 |
87–96 |
0.012 |
| Ever HCV RNA tested, if HCV-ab pos |
47/53 |
89 |
77–95 |
56/57 |
98 |
91–100 |
0.040 |
| Last HIV-ab test ≤1y ago, if HIV neg |
31/98 |
32 |
23–41 |
96/113 |
85 |
77–90 |
<0.001 |
| Last HCV-ab test ≤1y ago, if HCV neg |
15/53 |
28 |
18–42 |
55/63 |
87 |
77–93 |
<0.001 |
| Last HCV RNA test ≤1y ago, if HCV-ab pos, RNA-neg |
15/30 |
50 |
33–67 |
48/52 |
92 |
82–97 |
<0.001 |
| Adequate HCV management |
30/128 |
23 |
17–31 |
103/129 |
80 |
72–86 |
<0.001 |
| Ever treated, if CHC* |
21/35 |
60 |
44–74 |
55/60 |
92 |
82–96 |
<0.001 |
| HCV RNA pos, if HCV-ab pos and HCV RNA tested* |
18/47 |
38 |
26–53 |
6/84 |
7 |
3–15 |
<0.001 |
| HAV serostatus known |
49/128 |
38 |
30–47 |
83/129 |
64 |
56–72 |
<0.001 |
| HBV serostatus known |
65/128 |
51 |
42–59 |
98/129 |
76 |
68–83 |
<0.001 |
| Immunity against HAV |
24/128 |
19 |
13–26 |
64/129 |
50 |
41–58 |
<0.001 |
| Immunity against HBV |
30/128 |
23 |
17–31 |
73/129 |
57 |
48–65 |
<0.001 |

Figure S1 Patient and study data flow.
HCV: hepatitis C virus; HIV: human immunodeficiency virus; RNA: ribonucleic acid; HAV: hepatitis A virus; HBV: hepatitis B virus
Nine patients were readmitted during the study period (April 2018 to May 2021): five of the 128 present in April 2018 and four of the 72 newly registered between April 2018 and May 2021. All patients receiving their OAT via the institution HAG at the time-point of an annual chart review (April 2018, April 2019, May 2020, May 2021) were registered. Four patients recruited into the SAMMSU cohort in the framework of the 4-weekly visits (August 219, December 2019, February 2020 and July 2020, respectively) left the institution before the next annual chart review.
Between April 2018 (128 patients) and April 2019 (126 patients), 23 patients left the institution and 21 were newly admitted. Between April 2019 (126 patients) and May 2020 (120 patients), 25 patients left the institution and 19 were newly admitted. Between May 2020 (120 patients) and May 2021 (129 patients), 23 patients left the institution and 32 were newly admitted.





