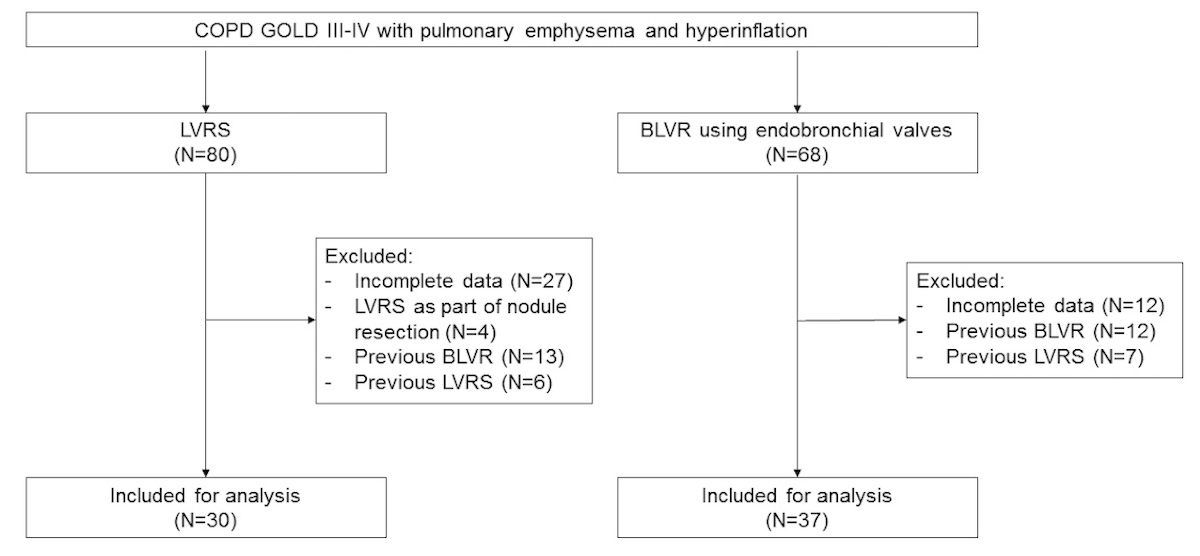
Figure 1 Study flowchart of enrolled patients.
BLVR: bronchoscopic lung volume reduction; LVRS: lung volume reduction surgery
DOI: https://doi.org/10.57187/smw.2022.40008
Chronic obstructive pulmonary disease is a potentially preventable, but progressive and irreversible, lung disease with a global age-standardised prevalence of 3.2% in men and 2.0% in women [1]. It is expected to become the third leading cause of death worldwide by 2030 [1]. However, COPD is not only a relevant and global health problem in terms of prevalence and mortality, it also leads to a massive impairment in quality of life (QoL) [2]. According to comparative studies, QoL in patients with emphysema, the most severe form of COPD, is generally lower than in patients with cancer, coronary heart disease or acquired immune deficiency syndrome (AIDS) [3–5]. It is therefore not surprising that COPD is contributing extensively to global healthcare costs, with disease severity, extent of breathlessness and frequency of exacerbations as the most significant independent drivers of direct healthcare costs [6].
The severity of COPD is divided into four stages according to international consensus of the Global Initiative of Obstructive Lung Disease (GOLD), with GOLD stage 4 representing the highest severity, in most of the cases with development of pulmonary emphysema [7, 8]. The most important prophylactic and therapeutic intervention is a consistent cessation of smoking, since tobacco smoking, in addition to chronic bronchitis, leads to irreversible destruction of the alveoli with loss of the elastic restoring forces of the lung. The result is dynamic and static lung hyperinflation, which can be measured using body plethysmography with an increased residual volume (RV) [9]. Hyperinflation leads to a flattening and loss of function of the diaphragm, adding to an increasing failure of the respiratory muscle pump. In addition to bronchial obstruction, hyperinflation is one of, if not the most significant component of respiratory distress, which in turn limits the physical capacity, the range of activity, and thus also QoL.
By removing or eliminating the most diseased areas of the lung by volume reduction, a "decompressive effect" is achieved, which counteracts the consequences of hyperinflation and improves the patient's shortness of breath. As early as the 1950s, this realisation led to the first efforts to improve hyperinflation by reducing lung volume [10]. Conceptually, lung volume reduction in emphysema can be achieved by surgery or bronchoscopically. Several prospective randomised trials comparing any lung volume reduction technique with best supportive care demonstrated significantly improved QoL, pulmonary function and exercise capacity in the treatment arm [11, 12]. An improved survival time was observed in a subset of patients [11, 13]. bronchoscopic lung volume reduction by means of endobronchial valves was first described in 2002. The clinical effectiveness of the valves is largely comparable to lung volume reduction by surgery, which was also shown in a recent meta-analysis [12]. However, to date there is no direct head-to-head comparison of endobronchial valve treatment with lung volume reduction by surgery within a prospective randomised study – neither for the clinical endpoints nor for an economic analysis.
Since lung volume reduction by surgery and bronchoscopic lung volume reduction using endobronchial valves have identical indications (pulmonary emphysema with hyperinflation) and therapeutic goals (improvement of pulmonary function, dyspnoea, and QoL), it can be assumed that the two methods are alternative and in principle competing procedures. Therefore, the question arises on which basis a decision should be taken regarding the selection of the procedure. Given a comparable indication, safety and effectiveness of two alternative procedures, and disregarding a possible, subjectively biased preference on the part of the patient, economic considerations should be taken into account [14]. However, the consideration of cost-effectiveness analyses for the purpose of resource allocations in the healthcare system may not be assessed uncritically, as they take a strongly utilitarian view, whereby quality-adjusted life years (QALYs) are usually weighed against the respective costs of the intervention [15]. Cost-effectiveness analyses are available for lung volume reduction by surgery [16, 17] as well as for bronchoscopic lung volume reduction with coils [18] and endobronchial valves [19, 20], each in comparison to best supportive care. To the best of our knowledge there is no comparative analysis of lung volume reduction by surgery and bronchoscopic lung volume reduction using endobronchial valves. The aim of this retrospective study performed in one single centre in Switzerland was to provide both a comparative cost-analysis and cost-effectiveness analysis of lung volume reduction by surgery compared with bronchoscopic lung volume reduction using endobronchial valves.
Patients who underwent either surgical lung volume reduction or bronchoscopic lung volume reduction using endobronchial valves for emphysema at the University Hospital Zurich between 01 January 2019 and 31 December 2020 were considered for this economic evaluation. In- and exclusion criteria are presented in the appendix. All patients were discussed at a multidisciplinary conference prior to lung volume reduction. On this occasion, both the indication for lung volume volume reduction was reviewed and the method was chosen. The basis for decision-making was the most up-to-date pulmonary function measurements from spirometry, body plethysmography and CO diffusion measurement (DLCO), 6MWD as well as morphological aspects on single photon emission computed tomography and high-resolution computed tomography of the lung at the time of the conference. The patient's preference for a specific technique was considered in the decision-making process wherever possible. The selection of the target lobe for bronchoscopic lung volume reduction with valves as well as the analysis of the interlobar fissures as a morphological correlate for the absence of collateral ventilation was performed using StratX® analysis (Version 3.2.0.0, PulmonX Inc., Redwood City, CA, USA). The presence of collateral ventilation, which was an absolute contraindication for endobronchial valve treatment, was assumed if the fissure integrity was below 80% or if it could be demonstrated by Chartis® measurement (PulmonX Inc., Redwood City, CA, USA). All baseline values regarding pulmonary function, 6MWD and Chartis® measurement were obtained in an outpatient setting prior to lung volume reduction.
After consensus-based selection and written informed consent, patients usually entered the day before lung volume reduction. Both lung volume reduction by surgery and endobronchial valve treatment were performed under general anaesthesia. If possible, surgical lung volume reduction was performed thoracoscopically and bilaterally on both lungs. In contrast, the bronchoscopic lung volume reduction was carried out unilaterally by deploying three to eight Zephyr® endobronchial valves (PulmonX Inc., Redwood City, CA, USA) into the most diseased lung lobe. Following the respective interventions, patients were transferred to the Intermediate Care Unit. On the first postoperative/postinterventional day, most patients were transferred back to the normal ward to be discharged home with strong advice to participate in an outpatient pulmonary rehabilitation programme or to inpatient pulmonary rehabilitation after 5 to 10 days.
The effectiveness of lung volume reduction was assessed using forced expiratory volume in the first second (FEV1), RV and 6MWD, measured at baseline and at the first outpatient follow-up visit after 4 to 12 weeks. Minimal clinical important differences (MCIDs) of FEV1 and 6MWD are 0.1 L and 26 metres, respectively [21]. As MCID of RV is not known, an MCID of 0.5 L was assumed. In addition, any complications, length of hospital stay (LOS), rehospitalisations and deaths were evaluated. Since no structured measurement of QoL (e.g., St George Respiratory Questionnaire, SGRQ) is usually carried out in everyday clinical practice, no data were collected in this regard.
Despite the obvious disadvantages of retrospective data analysis, an attempt was made to conduct the present economic evaluation according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [22]. In the present study, the cost per effectiveness level is given for FEV1, RV and 6MWD.
All persons residing in Switzerland are obliged to take out basic health insurance (Swiss Health Insurance Act, KVG); the choice of insurer is free, but insurers are obliged to accept patients. In addition to basic insurance, every insured person can take out optional supplementary insurance (private insurance or semi-private insurance). There is no state health insurance. Inpatient hospital costs are covered 55% by the public sector (cantons) and 45% by the health insurance funds. Patients contribute with a self-selected annual deductible of up to 2500 Swiss francs per year as well as a co-payment of 10% up to a maximum amount of 700 Swiss francs plus a fixed amount of 15 Swiss francs per day of hospitalisation [23]. Because of this, the perspective adopted by the payer thus corresponds to a certain extent to the societal perspective, although hospital financing, for example, was not considered in this work.
The present analysis was carried out from the perspective of the payer and the service-providing hospital (University Hospital Zurich), whereas total revenues (cost unit accounting) derived from SwissDRG [24] during the hospital stay in the context of lung volume reduction was used as a surrogate of the costs from the payer’s perspective. It was also considered that in Switzerland an additional charge may be billed for treatment with EBVs depending on the number of valves implanted [25]. The perspective of the service provider is displayed as the net profit.
SwissDRG [24] codes used for LVRS and EBV treatment, respectively, including their cost weights, mean length of stay and their lower and upper limit length of stay are displayed in the Supplement. Outpatient examinations, which are largely identical for both methods, were not included in the cost analysis. Unforeseen costs due to a possible complication that arose after hospital discharge were also not considered. However, complications were considered as secondary clinical outcomes.
Qualitative data are given numerically and as percentages and compared between the two groups using the chi-square test. Quantitative data are presented as median values (interquartile range) or mean values (standard deviation) depending on the distribution (normal versus non-normal distribution). For the comparison between the two groups, the Mann-Whitney test and Student's t-test were used. The comparison of paired clinical outcome data (FEV1, RV, 6MWD) between pre- and posttreatment was made using the Wilcoxon test on continuous variables and the McNemar's test on nominal variables. Since there were very few missing data, imputation of missing data was discouraged. A p-value of 0.05 was assumed as the significance level. SPSS Statistics for Windows, Version 26 (IBM, Armonk, NY, USA) was used as the statistical programme.
This retrospective parallel cohort study was approved by the Ethics Committee of the Canton of Zurich (BASEC-ID 2018-02038), provided that formal consent for anonymous data research (general consent) was obtained from patients.
In total, 67 patients with a mean age of 68.3 ± 7.4 years were included (fig. 1).

Figure 1 Study flowchart of enrolled patients.
BLVR: bronchoscopic lung volume reduction; LVRS: lung volume reduction surgery
Baseline characteristics are summarised in table 1.
Table 1Baseline characteristics regarding demographic data and comorbidities.
| LVRS, n = 30 | BLVR, n = 37 | p-value | ||
| Gender, n (%) | Male | 19 (63.3) | 18 (48.6) | 0.32 |
| Female | 11 (36.7) | 19 (51.4) | ||
| Age, years | 66.2 ±7.1 | 69.9 ±7.3 | 0.042* | |
| BMI, kg/m2 | 23.4 ±4.4 | 22.6 ±4.2 | 0.42 | |
| Pack years | 59.1 ±31.3 | 48.1 ±18.3 | 0.07 | |
| Comorbidities, n (%) | Coronary heart disease | 16 (53.3) | 23 (62.2) | 0.62 |
| Diabetes mellitus | 1 (3.3) | 1 (2.7) | 1.0 | |
| Carcinoma (active) | 1 (3.3) | 3 (8.1) | 0.62 | |
| Pulmonary hypertension | 1 (3.3) | 3 (8.1) | 0.62 | |
| Nutritional deficiency | 0 | 5 (13.5) | 0.06 | |
| Bronchial asthma | 1 (3.3) | 0 | 0.45 | |
| α1-antitrypsin deficiency | 0 | 0 | 1.0 | |
| Emphysema distribution, n (%) | Homogeneous | 2 (6.7) | 4 (10.8) | 0.48 |
| Intermediate | 9 (30.0) | 15 (40.5) | ||
| Heterogeneous | 19 (63.3) | 18 (48.6) | ||
| FEV1, litres | 0.90 ± 0.35 | 0.81 (±0.27) | 0.21 | |
| FEV1, % predicted | 31.7 ± (9.7) | 31.8 ± 10.1 | 0.97 | |
| FVC, litres | 2.54 ± 0.71 | 2.18 ± 0.63 | 0.029* | |
| FVC, % predicted | 69.2 ± 14.6 | 65.8 ± 15.0 | 0.34 | |
| FEV1/FVC | 0.35 ± 1.37 | 0.36 ± 0.06 | 0.39 | |
| RV, litres | 5.20 ± 1.37 | 5.10 ± 1.17 | 0.78 | |
| RV, % predicted | 223.7 ± 53.5 | 225.1 ± 45.3 | 0.90 | |
| RV/TLC | 0.65 ± 0.07 | 0.68 ± 0.08 | 0.21 | |
| DLCO, mmol/min/kPa | 2.54 ± 0.94 | 2.63 ± 1.10 | 0.72 | |
| DLCO, % predicted | 30.6 ±10.5) | 35.0 (±11.5) | 0.13 | |
| 6MWD, metres | 294.9 (± 113.8 | 311.0 ± 115.0 | 0.68 | |
| Borg scale | 5.0 (4.5–7.4) | 4.0 (4.0–6.0) | 0.31 | |
| GOLD stage, n (%) | GOLD 1–2 | 1 (3.3) | 2 (5.4) | 0.63 |
| GOLD 3 | 16 (53.3) | 23 (62.2) | ||
| GOLD 4 | 13 (43.3) | 12 (32.4) | ||
Except for the Borg scale (median value, interquartile range), all continuous data are given as mean values ± standard deviation.
BMI: body mass index; BLVR: bronchoscopic lung volume reduction (with endobronchial valves); FEV1:, first-second capacity; FVC: forced vital capacity; RV: residual volume; TLC: total lung capacity; DLCO: diffusing capacity of the lungs for carbon monoxide; GOLD: Global Initiative of Obstructive Lung Disease; LVRS: lung volume reduction surgery; 6MWD: 6-minute walking distance.
*p <0.05.
Mean length of stay after lung volume reduction surgery was 11.3 ± 4.8 (5–24) days compared with 7.1 ± 4.5 (3–24) days after endobronchial valve treatment (p = 0.001). In-hospital adverse events occurred in 22/30 (73.3%) after lung volume reduction surgery compared with 12/37 (32.4%) after endobronchial valve treatment. In the surgical patients, the most common event was a prolonged air leak, whereas mean drainage time was 8.5 ± 4.7 days (minimum 2 days, maximum 22 days). After bronchoscopic lung volume reduction, the most common complication was pneumothorax, in 18.9%. Of the seven patients with pneumothorax, five were treated with a chest drain for an average of 5.6 ± 4.4 days (minimum 1 day, maximum 11 days). In-hospital complications are summarised in the appendix. All surgical patients were transferred to inpatient rehabilitation compared with one quarter of the bronchoscopically treated patients, who were sent to an outpatient pulmonary rehabilitation programme.
The measurements of clinical effectiveness by pulmonary function and 6MWD was performed as part of a regular follow-up at an average of 80.8 ± 31.8 days after the lung volume reduction procedure; the follow-up after lung volume reduction surgery was significantly later than after endobronchial valve treatment (102.5 ± 22.8 days versus 63.2 ± 26.8 days, p = 0.0001). Comparative pre- and post-treatment effectiveness data are displayed in tables 2 and 3, respectively.
Table 2Change in clinical effectiveness data at 102.5 ± 22.8 days after lung volume reduction surgery.
| Pre-LVRS | Post-LVRS | Difference | p-value | ||
| FEV1, litres | 0.90 ± 0.34 | 1.10 ± 0.46 | 0.20 ± 0.26 | <0.001* | |
| FEV1, % predicted | 31.7 ± 9.7 | 39.6 ± 15.6 | 7.9 ± 9.9 | <0.001* | |
| RV, litres | 5.20 ± 1.37 | 4.80 ± 1.21 | –0.40 ± 0.71 | 0.020* | |
| RV, % predicted | 223.7 (±53.5) | 205.1 (±44.6) | -18.6 (±30.2) | 0.017* | |
| RV/TLC | 0.65 ± 0.07 | 0.62 ± 0.08 | –0.03 ± 0.06 | 0.022* | |
| DLCO, mmol/min/kPa | 2.54 ± 0.94 | 3.20 ± 0.92 | 0.66 ± 0.96 | 0.005* | |
| DLCO, % predicted | 30.6 ± 10.5 | 39.3 ± 10.5 | 8.7 ± 11.7 | 0.004* | |
| 6MWD, metres | 294.9 ± 113.8 | 285.3 ± 144.1 | –9.6 (±135.3) | 0.818 | |
| Borg scale | 5.0 (4.5–7.4) | 6.0 (4.7–8.9) | 1.0 | 0.713 | |
| GOLD stage, n (%) | GOLD 1–2 | 1 (3.3) | 8 (26.6) | 0.021* | |
| GOLD 3 | 16 (53.3) | 15 (50.0) | |||
| GOLD 4 | 13 (43.3) | 7 (23.3) | |||
Except for the Borg scale (median value, interquartile range), all continuous data are given as mean values ± standard deviation.
FEV1: first-second capacity; RV: residual volume; TLC: total lung capacity; DLCO: diffusing capacity of the lungs for carbon monoxide; GOLD: Global Initiative of Obstructive Lung Disease; LVRS: lung volume reduction surgery; 6MWD, 6-minute walking distance.
*p <0.05.
Table 3Change in clinical effectiveness data 63.2 ± 26.8 days after bronchoscopic lung volume reduction using endobronchial valves.
| Pre-BLVR | Post-BLVR | Difference | p-value | |||
| FEV1, litres | 0.81 ± 0.27 | 0.99 ± 0.33 | 0.18 ± 0.19 | <0.001* | ||
| FEV1, % predicted | 31.8 ± 10.1 | 39.5 ± 12.9 | 7.7 ± 8.0 | <0.001* | ||
| RV, litres | 5.10 ± 1.17 | 4.20 ± 1.19 | –0.90 (±0.90) | <0.001* | ||
| RV, % predicted | 225.3 ± 45.3 | 185.8 ± 43.7 | –39.5 (±39.4) | <0.001* | ||
| RV/TLC | 0.68 ± 0.08 | 0.61 ± 0.09 | –0.07 ± 0.08 | <0.001* | ||
| DLCO, mmol/min/kPa | 2.65 ± 1.10 | 2.56 ± 0.80 | –0.09 ± 0.79 | 0.850 | ||
| DLCO, % predicted | 35.0 ± 11.5 | 33.5 ± 8.9 | –1.5 ± 8.6 | 0.460 | ||
| 6MWD, metres | 311.0 ± 115.0 | 336.1 ± 126.3 | 25.1 ± 140.9 | 0.745 | ||
| Borg scale | 4.0 (4.0–6.0) | 4.0 (2.0–5.0) | 0 | 0.328 | ||
| GOLD stage (%) | GOLD 1–2 | 2 (5.4) | 8 (21.6) | 0.046* | ||
| GOLD 3 | 23 (62.2) | 18 (48.6) | ||||
| GOLD 4 | 12 (32.4) | 11 (29.7) | ||||
Except for the Borg scale (median, interquartile range), all continuous data are presented as means ± standard deviation.
FEV1: first-second capacity; RV: residual volume; TLC: total lung capacity; DLCO: diffusing capacity of the lungs for carbon monoxide; GOLD: Global Initiative of Obstructive Lung Disease; BLVR: bronchoscopic lung volume reduction (with endobronchial valves); 6MWD: 6-minute walking distance.
*p <0.05.
Of note, the two methods showed comparable clinical effectiveness, as neither the absolute nor the percentage changes differ significantly from each other when pre- to post-operative or -interventional were compared in the between-group analysis (table 4).
Table 4Vertical (within group) and horizontal (between groups) comparison according to method of lung volume reduction.
| Δ "within" LVRS | Δ "within" BVLR | Δ "between groups" | p -value | |
| FEV1, Δ litres | 0.20 ± 0.26 | 0.18 ± 0.19 | 0.02 | 0.811 |
| FEV1, % baseline | 22.2 | 22.2 | 0 | 0.831 |
| RV, Δ litres | –0.40 ± 0.71 | –0.90 ± 0.90 | 0.50 | 0.348 |
| RV, % baseline | –7.7 | –17.6 | 9.9 | 0.096 |
| 6MWD, Δ metres | –9.6 ± 135.3 | 25.1 ± 140.9 | 34.7 | 0.158 |
| 6MWD, % baseline | –3.3 | 8.1 | 11.4 | 0.179 |
All data are given as mean values ± standard deviation.
FEV1: first-second capacity; RV: residual volume; Δ: difference; 6MWD: 6-minute walking distance
Between hospital discharge and first outpatient follow-up, eight surgical patients (26.7%) had to be rehospitalised because of a pulmonary event, with a median hospital stay of 12 days (minimum 1 day, maximum 24 days). In comparison, 12 patients undergoing bronchoscopic lung volume reduction(32.4%) had to be rehospitalised with a median hospital stay of 5 days (minimum 1 day, maximum 7 days). Neither the difference in the frequency nor in the duration of re-hospitalisations was significant. The reasons for rehospitalisations are summarised in the appendix. There were no deaths in either group within the observation period.
The cost unit accounting (total revenue) and the cost centre accounting, divided according to the two methods of lung volume reduction, are shown in table 5.
Table 5Median cost unit and cost centre accounting by method of lung volume reduction.
| LVRS (n = 30) | BLVR (n = 37) | p-value | ||
| Cost unit accounting | Total revenue, CHF | 28,048 (27,317 to 98,039) | 30,049 (21,536 to 47,184) | 0.910 |
| Cost centre accounting | Patient-related costs, CHF | –21,554 (–40,512 to –10,510) | -22,233 (-65,104- (-6542)) | 0.995 |
| Administrative costs, CHF | –3028 (–10572 to –1701) | –4334 (–17378 to –1432) | 0.984 | |
| Capital costs, CHF | –896 (–2606 to –491) | –1095 (–3611 to –387) | 0.703 | |
| Ancillary costs, CHF | –13 (–33 to –3) | –7.5 (–62 to –3) | 0.481 | |
Patient-related costs include all costs directly derived by the care of the patient (e.g., drugs, devices, operation room or endoscopy suite, etc.). Administrative costs refer to the costs to conduct the business side of healthcare, including billing, marketing, provider and medical management, etc. Capital costs include expenditures for buildings, technology infrastructure, land, and equipment. Ancillary costs include extra-ordinary expenditures, which were not covered by health insurances (e.g., food service for relatives).
LVRS, lung volume reduction surgery; BLVR, bronchoscopic lung volume reduction with endobronchial valves.
All values are given as median values (minimum value-maximum value).
Interestingly, the total revenues of surgical and endobronchial valve treatment were comparable, although the DRG-derived cost weight of 2.58 (5.25–2.83) for lung volume reduction surgery was significantly higher compared with 1.65 (1.59–2.76) for bronchoscopic lung volume reduczion (p <0.001). However, as endobronchial valves generated a considerable additional fee, the revenues of the two methods are similar. The number of patients with private insurance cannot be considered as relevant confounder, as its share was comparable in both groups (p = 0.229). Also, the different items of the cost centre accounting (patient-related costs, administrative costs, capital costs and ancillary costs) were comparable for both procedures. In summary, the cost-cost analysis for lung volume reduction surgery and endobronchial valve treatment revealed that neither the total revenues (cost unit accounting) nor the cost centre accounting differed significantly between the two methods, so that the net profit for the service provider was also comparable for both methods.
Costs per effectiveness level after lung volume reduction according to the method is summarised in table 6.
Table 6Costs per effectiveness level after lung volume reduction.
| LVRS | BLVR | |
| Costs* per ml FEV1, CHF/ml | 140.2 | 166.9 |
| Costs* per % FEV1, CHF/% | 1263.4 | 1353.6 |
| Costs* per ml RV, CHF/ml | 70.1 | 33.4 |
| Costs* per metre (6MWD), CHF/m | 28,048.0** | 1197.2 |
FEV1 : first-second capacity; RV: residual volume; 6MWD: 6-minute walk distance; LVRS, lung volume reduction surgery; BLVR, bronchoscopic lung volume reduction with endobronchial valves.
* Costs (perspective of the payer) result from the median per-capita revenue of the service provider (cost unit accounting), displayed in table 5. Mean effectiveness data were drawn from table 4.
** According to LVRS, there was a slight deterioration in 6MWD. However, an improvement of one metre was assumed.
The incremental cost-effectiveness ratios (ICERs) for bronchoscopic compared with surgical lung volume reduction for FEV1, RV and 6MWD were –101, 4 and 58, respectively, suggesting that bronchoscopic lung volume reduction with endbronchial valves is maybe not cost-effective in terms of FEV1. However, it seems quite a cost-effective alternative to surgery in terms of RV and 6MWD. For the three different measures of effectiveness, a modified cost-effectiveness diagram using a 4-field matrix is displayed in figure 2.
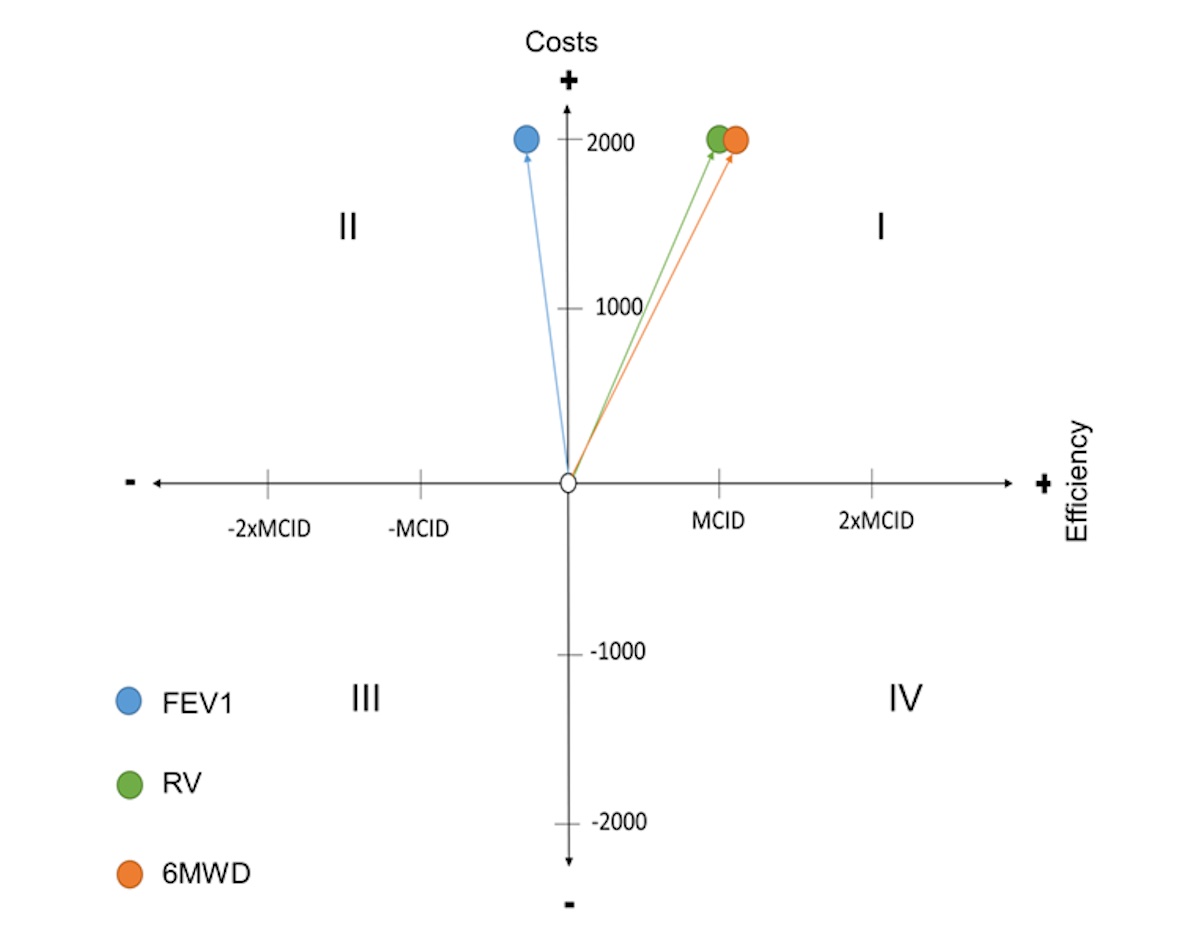
Figure 2 Cost-effectiveness diagram of bronchoscopic lung volume reduction (BLVR) using endobronchial valves (EBVs) compared with lung volume reduction surgery (LVRS).
The 0-point of the diagram corresponds to the LVRS. The coloured points correspond to the different effectiveness qualities of BLVR (FEV1, RV and 6MWD).In general, interventions located in quadrant IV are medically superior and more cost-effective. In this case, BLVR would be superior to LVRS. In terms of FEV1, however, BLVR is in quadrant II, so that the zero-alternative, i.e. LVRS, dominates. If FEV1 were the only measure of effectiveness, BLVR would have to be rejected. In terms of RV and 6MWD, BLVR is in quadrant I, where the better result is offset by higher costs. For interventions located in quadrant I, it is completely unclear whether they should be introduced or not28. According to Schöffski et al. [28], the choice depends on what relationship between costs and outcome is acceptable. The comparison of the angle α does not make sense and was therefore omitted, since it is not possible to choose between several alternatives.Abbreviations: FEV1, forced expiratory volume in first second; RV, residual volume; 6MWD, 6-minute walking distance; MCID, minimal clinical important difference; MCID of FEV1 = 0.2L, MCID of RV = 0.5L (assumption), MCID of 6MWD = 26 metres.
The cost-effectiveness diagram modified by the three qualities illustrates an indifferent result of the cost-effectiveness analysis, as it is not clear which of the three effectiveness qualities is superior.
Both lung volume reduction surgery and bronchoscopic lung volume reduction with endobronchial valves have been demonstrated to significantly improve pulmonary function, 6MWD, breathlessness and QoL in patients with severe emphysema compared with conservative therapy in several trials and cohort studies [12]. The effectiveness of the two methods was also confirmed in two recent Cochrane Network meta-analyses [26, 27]. The present retrospective parallel cohort study was able to confirm the effectiveness of both methods as real-life clinical data by showing a comparable improvement in FEV1 and RV. There was no significant improvement in 6MWD in the present study, which could at best be attributed to the relatively short observation period of around 80 days. A robust statement as to which of the two methods is superior in terms of effectiveness is only possible to a limited extent due to the retrospective nature of the study and the corresponding bias due to various baseline variables (e.g., age). So far, there are no prospective randomised studies comparing lung volume reduction surger with any form of bronchoscopic lung volume reduction. The meta-analysis by van Geffen et al. [12] included a total of 20 prospective randomised studies, each of which compared a lung volume reduction method with conservative therapy. All studies in the meta-analysis showed significant improvements in FEV1, 6MWD and QoL (SGRQ) after lung volume reduction. Thus, in the pooled analysis (independent of the method), there was a 15% improvement in FEV1, an improvement of 43 metres in 6MWD, and a reduction of 0.58 L in RV [12]. In comparison, the present study demonstrated a slightly better effectiveness concerning FEV1 (22%) and RV (0.9 L) after bronchoscopic lung volume reduction, so that these data may be assumed to be plausible. It cannot be overemphasised that a direct comparison of the two methods is hardly possible, neither in the present study nor in the meta-analysis. The results of two prospective randomised studies comparing lungvolume reduction surgery and bronchoscopic lung volume reduction with valves, which are currently still recruiting (CELEB trial; ISRCTN19684749 and SINCERE trial; NCT04537182).
The direct costs of lung volume reduction from the perspective of the service provider were comparable for both methods in the present study and amounted to a median of CHF 28,000 (approx. € 25,760) for surgery and CHF 30,000 (approx. € 27,600) for endobronchial valve treatment. These are only the direct costs of the hospital stay. Indirect costs, follow-up costs (due to possible complications) or even a saving of costs due to a possible decrease in physician consultations after clinical improvement were not considered. However, since the frequency of rehospitalisations after lung volume reductions was comparable for both methods, it could at best be concluded that the follow-up costs were also comparable and can thus be neglected. The immediate follow-up costs (namely inpatient rehabilitation) were also not considered in the present study, although this is problematic, since all patients undergoing lung volume reduction surgery were referred to inpatient rehabilitation following hospitalisation, whereas three quarters of patients having bronchoscopic lung volume reduction were discharged home, participating in an outpatient pulmonary rehabilitation programme.
To date, no cost-effectiveness analysis has been published comparing lung volume reduction surgery with bronchoscopic lung volume reduction with endobronchial valves because, as mentioned above, no results of a prospective randomised study with a direct comparison between the two methods are available. However, the present cost-effectiveness analysis must be regarded with some caution for the reasons mentioned above and because of the retrospective study design. Nevertheless, retrospective observational studies can provide a valuable evidence base for cost-effectiveness analysis [28]. With regard to the two clinical outcomes of RV and 6MWD, it was shown that bronchoscopic lung volume reduction with endobronchial valves is probably a cost-effective alternative to lung volume reduction surgery. However, the results for FEV1 were opposite, as bronchoscopic lung volume reduction was inferior to surgery in this respect. Eventually, the question of whether bronchoscopic lung volume reduction is a cost-effective alternative to surgery cannot be answered conclusively on this basis, since it is not ultimately clear how much additional cost for a given improvement in FEV1, 6MWD or RV can be described as adequate for the Swiss health system. The situation is different with QALYs, as there are suggestions here about how much a QALY may cost. For instance, a guideline value of USD 100,000 per QALY seems to be widely accepted [15].
The customary utilitarian requirement of a cost-effectiveness analysis, which serves to maximise QALYs at the lowest possible cost [15], cannot be directly met in the present study, since there was neither mortality data nor a coherent recording of QoL. However, it is quite arguable that mortality and QoL can be derived indirectly [28] and there is reasonably good evidence that FEV1 and RV correlate with various patient-centred outcomes, for example SGRQ or the Transition Dyspnoea Index [12, 29, 30]. The missing data on QoL, for example, can also be obtained from extrapolated data from the study by van Geffen et al. [12]. Thus, an almost linear correlation between RV and SGRQ was described (r2 = 0.70; p <0.0001), which may indicate that for every 0.1 L decrease in RV, the SGRQ would improve by 2.5 points. Extrapolated to our own data, this would correspond to an improvement in SGRQ of 10 points after lung volume reduction surgery and 22.5 points after endobronchial valve treatment treatment. Eventually, for the calculation of the QALY, a conversion of the SGRQ into the EQ-5D (EuroQol 5 Dimension) is necessary according to the algorithm published by Starkie et al. [31]. Using their formula (EQ-5D = 0.9617–0.0013 * SGRQ – 0.0001 * SGRQ2 + 0.0231) with the above made assumptions, there is an improvement in EQ-5D of 0.80 after lung volume reduction surgery and 0.86 after endobronchial valve treatment treatment. Assuming a discounting of 4% per year, it would therefore indicate a significant improvement in QoL even after five years. The missing mortality data can also be extrapolated as already done in other studies [19, 20]. For example, mortality rates of 6%, 11% and 25% per year have been published for GOLD stages 2, 3 and 4, respectively [32]. An improvement in the GOLD stage should consequently also lead to an improvement in the mortality risk, which was also proven in a large-scale registry study from Scotland with 4885 COPD patients [33]. For example, it was shown that the change from the highest to the second highest GOLD stage was associated with an improvement in the risk of death from 4.31 to 2.96 (p <0.001). In the present study, improvement in GOLD stage was demonstrated in 43.3% of cases after surgery and in 27% of cases after valve treatment. Thus, an improvement in survival can be expected in 2/5 of the surgical patients and 1/3 of the patients having a bronchoscopic lung volume reduction. According to the National Health and Nutrition Examination Survey (NHANES III study), Years of Life Lost (YLL) in COPD patients who quit smoking (which meets the requirements for lung volume reduction) is 1.4 years for GOLD stage 2 compared with 5.6 years for the highest two GOLD stages [34]. Hypothetically, lung volume reduction could thus result in a prolongation of life by 4.2 years in the best case. Similarly, prospective randomised studies have already shown a positive effect on survival time after both lung volume reduction surgery [11] and endobronchial valve treatment [13]. For those patients who had an improvement in GOLD stage after lung volume reduction and thus presumably also achieved a prolongation of survival time, QALYs for surgical as well as for bronchoscopic lung volume reduction can be estimated as follows: 4.2 x 0.80 = 3.36 and 4.2 x 0.86 = 3.61, respectively. These estimated QALYs seem plausible in comparison to previously published QALYs of 2.88 compared to 2.66 for conservatively treated patients after five years, at least for valve treatment [19]. For surgical lung volume reduction, three years after surgery, 1.46 QALY was reported compared to 1.27 with conservative treatment [16]. The ICER in Swiss francs per QALY for the patient collective in this study is shown in table 7.
Table 7Incremental cost-effectiveness results after three months.
| Cost, CHF | Effect, QALYs | ICER, CHF/QALY | |||||
| LVRS | BLVR | Δ | LVRS | BLVR | Δ | ||
| Base case (after 3 months) | 28,048 | 30,049 | 2001 | 3.36 | 3.61 | 0.25 | 8004 |
Several assumptions were made for estimation of QALY and ICER. Quantification of uncertainties was therefore not possible.
QALY: quality-adjusted life year; ICER: incremental cost-effectiveness ratio; LVRS: lung volume reduction surgery; BLVR: bronchoscopic lung volume reduction; Δ: difference.
Based on this table, bronchoscopic lung volume reduction with endobronchial valves was CHF 2000 more expensive after three months lung volume reduction surgery. Given the improvement of 0.25 QALYs, there is an ICER of CHF 8000 per QALY gained.
Two cost-effectiveness analyses have been published to date, comparing lung volume reduction surgery with best supportive care. The most important of these analyses is based on the National Emphysema Treatment Trial (NETT), the largest prospective study to date, in which severely symptomatic emphysema patients were randomised to either lung volume reduction surgery or conservative therapy [11]. The resulting cost-effectiveness analysis has been published twice [16, 17] owing to the controversy regarding the inclusion of lung volume reduction surgery in the US healthcare system at the time. In the first study, Ramsey et al. concluded that lung volume reduction surgery costs USD 190,000 more per QALY gained in the first three years after surgery compared with conservative therapy, regardless of the type of emphysema. If patients who had heterogeneous upper lobe emphysema and were therefore better candidates for this intervention are considered, the cost was USD 98,000 per QALY gained [16]. Based on these results, lung volume reduction surgery was approved by the Centers for Medicare and Medicaid Services (CMS) in the USA in 2004. After the 10-year data of the NETT study became available, an updated version of the cost-effectiveness analysis was finally published [17]. In this analysis, lung volume reduction surgery cost USD 140,000 more per QALY gained at five years and USD 54,000 at 10 years compared with conservative therapy. In patients with upper lobe emphysema, the costs were lower, as expected, at USD 77,000 after five years and USD 48,000 after 10 years [17]. In addition to the long-term results, this study was able to demonstrate a considerable improvement in the cost-effectiveness ratio for patients with upper lobe emphysema. Similar results were obtained in a smaller Canadian study with incremental costs of lung volume reduction surgery compared with conservative therapy of USD 133,000 per QALY after two years [35]. However, with a non-significant increase in QALYs in the surgery group of 0.21 (p = 0.19), it can be assumed that the study was underpowered. Based on the three cost-effectiveness analyses presented, it can be concluded that lung volume reduction surgery is expensive but reasonably cost-effective, especially when compared with other thoracic interventions with ICERs ranging between USD 8300 and USD 216,000 per QALY [36].
The cost-effectiveness ratio of bronchoscopic lung volume reduction compared with conservative therapy has been investigated in two studies to date. The first results are based on the prospective randomised VENT study [37], which was able to prove effectiveness and safety of treatment with endobronchial valves after 12 months. The resulting cost-effectiveness analysis showed incremental costs per QALY gained of € 46,322 after five years, and € 25,142 after 10 years [19]. The second cost-effectiveness analysis was based on the prospective randomised STELVIO trial, which also demonstrated a significant improvement in clinical effectiveness in terms of lung function, 6MWD and SGRQ compared to conservative therapy at six months [38]. The respective cost-effectiveness analysis showed incremental costs of € 39,000 per QALY after five years and € 21,500 after 10 years [20]. In summary, both cost-effectiveness analyses showed that treatment with endobronchiaL VALVES is a cost-effective alternative to conservative therapy, with incremental costs per QALY below the threshold of € 50,000. The relatively high costs in the early THAN IN the later years are mainly due to the costs for endobronchial valves, as well as for the interventions and the necessary hospital stay.
There are several limitations of this study. The results of the present cost-effectiveness analysis are based exclusively on retrospective data, so that the internal validation of the data is not guaranteed and the results are exposed to a significant risk of bias (especially selection bias) [28, 39, 40]. In addition, the observation period in this study is extremely short, with a maximum of 100 days. Since no health costs were recorded postoperatively and beyond the observation period neither mortality nor health data were recorded, no statements can be made about the long-term cost-effectiveness ratio. However, it is already known from randomised studies that both methods of lung volume reduction are cost-effective compared with conservative treatment [16, 17, 19, 20, 36]. The head-to-head comparison constructed from extrapolated data can at best give a vague idea regarding ICER for cost/QALY. Since no costs were considered postoperatively, a discounting of QALY for long-term success cannot be used either. However, QALYs may not be optimal for short-term but effective therapeutic interventions (and lung volume reduction can undoubtedly be counted as such) [14]. Another limitation of the present study is the determination of costs, which is mainly based on the DRG system. As described by Schöffski et al., the average valuation of service bundles, as is the case with DRGs, is no longer sufficient for an analysis from a societal perspective as well as from the point of view of service providers [41]. At least the allocation of overhead costs in the sense of activity-based costing was partially considered.
The present retrospective parallel cohort study was able to confirm the effectiveness for both surgical lung volume reduction and bronschscoppic lung volume reduction using endobronchial valves in the sense of mirroring real life data by showing a comparable improvement in FEV1 and RV. The direct costs of lung volume reduction from the payer's perspective were also comparable for both methods. Concerning RV and 6MWD, it could be shown that endobronchial valve treatment is justified as a cost-effective alternative to surgical lung volume reduction. Based on extrapolated data, it was also shown that endobronchial valve treatment, although slightly more expensive, resulted in an improvement of 0.25 QALYs leading to ICER of CHF 8000 francs (€ 7657) per QALY gained. Nevertheless, a robust statement on the superiority of one of the two lung volume reduction procedures in terms of cost-effectiveness cannot be made from the present study, as the study has some limitations, particularly due to the retrospective design and the short observation period. Therefore, the study is by no means suitable as a basis for resource allocation. Two currently ongoing prospective randomised studies comparing lung volume reduction surgery with endobronchial valve treatment may be able to answer this question in the future.
For insight into the data please contact Christa Bodmer christa.bodmer[at]usz.ch.
Author contributions: Daniel Franzen contributed to the conception and design of the study, statistical analysis, writing original draft and project administration. Christa Bodmer screened the patient. Christa Bodmer, Simon Ehrenbaum prepared the data for statistical analysis. Daniel Franzen, Christa Bodmer, Simon Ehrenbaum, Carolin Steinack, Isabelle Opitz, Katrin Docter, Oliver Schöffski edited and reviewed the article. All the authors have read and approved the final manuscript. Oliver Schöffski supervised the article. Daniel Franzen is the corresponding author.
There is no funding related to this article.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Daniel Franzen received speaker honoraria from Pulmonx. No other potential conflict of interest was disclosed.
1. Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al.; GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017 Sep;5(9):691–706. https://doi.org/10.1016/S2213-2600(17)30293-X
2. Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J. 2006 Apr;27(4):822–32. https://doi.org/10.1183/09031936.06.00145104
3. Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37(2):85–95. https://doi.org/10.1016/0021-9681(84)90050-X
4. Kaplan RM, Anderson JP, Patterson TL, McCutchan JA, Weinrich JD, Heaton RK, et al.; HNRC Group. HIV Neurobehavioral Research Center. Validity of the Quality of Well-Being Scale for persons with human immunodeficiency virus infection. Psychosom Med. 1995 Mar-Apr;57(2):138–47. https://doi.org/10.1097/00006842-199503000-00006
5. Ganiats TG, Palinkas LA, Kaplan RM. Comparison of Quality of Well-Being scale and Functional Status Index in patients with atrial fibrillation. Med Care. 1992 Oct;30(10):958–64. https://doi.org/10.1097/00005650-199210000-00008
6. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int J Chron Obstruct Pulmon Dis. 2020 Feb;15:439–60. https://doi.org/10.2147/COPD.S234942
7. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017 Mar;195(5):557–82. https://doi.org/10.1164/rccm.201701-0218PP
8. Global Initiative for Obstructive Lung Disease. GOLD. Pocket guide to COPD for diagnosis, management, and prevention. A Guide for Health Care Professionals. 2017; http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed 26 June 2021.
9. Kemp SV, Polkey MI, Shah PL. The epidemiology, etiology, clinical features, and natural history of emphysema. Thorac Surg Clin. 2009 May;19(2):149–58. https://doi.org/10.1016/j.thorsurg.2009.03.003
10. Brantigan OC, Mueller E. Surgical treatment of pulmonary emphysema. Am Surg. 1957 Sep;23(9):789–804.
11. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al.; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003 May;348(21):2059–73. https://doi.org/10.1056/NEJMoa030287
12. van Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019 Apr;7(4):313–24. https://doi.org/10.1016/S2213-2600(18)30431-4
13. Garner J, Kemp SV, Toma TP, Hansell DM, Polkey MI, Shah PL, et al. Survival after Endobronchial Valve Placement for Emphysema: A 10-Year Follow-up Study. Am J Respir Crit Care Med. 2016 Aug;194(4):519–21. https://doi.org/10.1164/rccm.201604-0852LE
14. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016 Sep;316(10):1093–103. https://doi.org/10.1001/jama.2016.12195
15. Fleck LM. Controlling Healthcare Costs: Just Cost Effectiveness or “Just” Cost Effectiveness? Camb Q Healthc Ethics. 2018 Apr;27(2):271–83. https://doi.org/10.1017/S0963180117000603
16. Ramsey SD, Berry K, Etzioni R, Kaplan RM, Sullivan SD, Wood DE ; National Emphysema Treatment Trial Research Group. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med. 2003 May;348(21):2092–102. https://doi.org/10.1056/NEJMsa030448
17. Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest. 2007 Mar;131(3):823–32. https://doi.org/10.1378/chest.06-1790
18. Bulsei J, Leroy S, Perotin JM, Mal H, Marquette CH, Dutau H, et al.; REVOLENS study group. Cost-effectiveness of lung volume reduction coil treatment in patients with severe emphysema: results from the 2-year follow-up crossover REVOLENS study (REVOLENS-2 study). Respir Res. 2018 May;19(1):84. https://doi.org/10.1186/s12931-018-0796-x
19. Pietzsch JB, Garner A, Herth FJ. Cost-effectiveness of endobronchial valve therapy for severe emphysema: a model-based projection based on the VENT study. Respiration. 2014;88(5):389–98. https://doi.org/10.1159/000368088
20. Hartman JE, Klooster K, Groen H, Ten Hacken NH, Slebos DJ. Cost-effectiveness of endobronchial valve treatment in patients with severe emphysema compared to standard medical care. Respirology. 2018 Mar;23(9):835–41. https://doi.org/10.1111/resp.13295; Online ahead of print.
21. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014 Feb;189(3):250–5. https://doi.org/10.1164/rccm.201310-1863PP
22. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al.; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013 Mar-Apr;16(2):231–50. https://doi.org/10.1016/j.jval.2013.02.002
23. Bundesamt für Gesundheit [German Federal Institute for Health]. URL: https://www.bag.admin.ch/bag/de/home.html. [Accessed 26 June 2021].
24. Swiss DR. URL: https://www.swissdrg.org/de/akutsomatik/swissdrg-system-1102022/fallpauschalenkatalog [Accessed 26 June 2021].
25. Zusatzentgeltkatalog Swiss DR. URL: https://www.swissdrg.org/application/files/1416/0759/4940/ze_swissdrg_V100_AV_20201209.zip [Accessed 25 June 2021]
26. van Agteren JE, Carson KV, Tiong LU, Smith BJ. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2016 Oct;10(10):CD001001. https://doi.org/10.1002/14651858.CD001001.pub3
27. van Agteren JE, Hnin K, Grosser D, Carson KV, Smith BJ. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 Feb;2:CD012158. https://doi.org/10.1002/14651858.CD012158.pub2
28. Drummond M, Sculpher M, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press, 4th Edition; 2015, Oxford.
29. Martin AL, Marvel J, Fahrbach K, Cadarette SM, Wilcox TK, Donohue JF. The association of lung function and St. George’s respiratory questionnaire with exacerbations in COPD: a systematic literature review and regression analysis. Respir Res. 2016 Apr;17(1):40. https://doi.org/10.1186/s12931-016-0356-1
30. Donohue JF, Jones PW, Bartels C, Marvel J, D’Andrea P, Banerji D, et al. Correlations between FEV1 and patient-reported outcomes: A pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2018 Apr;49:11–9. https://doi.org/10.1016/j.pupt.2017.12.005
31. Starkie HJ, Briggs AH, Chambers MG, Jones P. Predicting EQ-5D values using the SGRQ. Value Health. 2011 Mar-Apr;14(2):354–60. https://doi.org/10.1016/j.jval.2010.09.011
32. Afonso AS, Verhamme KM, Sturkenboom MC, Brusselle GG. COPD in the general population: prevalence, incidence and survival. Respir Med. 2011 Dec;105(12):1872–84. https://doi.org/10.1016/j.rmed.2011.06.012
33. Flynn RW, MacDonald TM, Chalmers JD, Schembri S. The effect of changes to GOLD severity stage on long term morbidity and mortality in COPD. Respir Res. 2018 Dec;19(1):249. https://doi.org/10.1186/s12931-018-0960-3
34. Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III Follow-up Study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–48. https://doi.org/10.2147/COPD.S5237
35. Miller JD, Malthaner RA, Goldsmith CH, Goeree R, Higgins D, Cox PG, et al.; Canadian Lung Volume Reduction Surgery Study. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two-year study from Canada. Ann Thorac Surg. 2006 Jan;81(1):314–20. https://doi.org/10.1016/j.athoracsur.2005.07.055
36. Ramsey SD, Sullivan SD, Kaplan RM. Cost-effectiveness of lung volume reduction surgery. Proc Am Thorac Soc. 2008 May;5(4):406–11. https://doi.org/10.1513/pats.200707-095ET
37. Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al.; International VENT Study Group. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012 Jun;39(6):1334–42. https://doi.org/10.1183/09031936.00161611
38. Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med. 2015 Dec;373(24):2325–35. https://doi.org/10.1056/NEJMoa1507807
39. Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005 Sep-Oct;8(5):521–33. https://doi.org/10.1111/j.1524-4733.2005.00045.x
40. Neumann PJ. Why don’t Americans use cost-effectiveness analysis? Am J Manag Care. 2004 May;10(5):308–12.
41. Schöffski O, Graf von der Schulenberg JM. Gesundheitsökonomische Evaluationen. Springer Verlag, 4th Edition; 2011, Berlin.
Table S1In- and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
| FEV1 less than 55% of target | FEV1 less than 16% of target |
| RV more than 165% of target and/or RV/TLC over 0.56 and/or clinically/radiologically clear hyperinflation | Pulmonary hypertension (high blood pressure) with an echocardiographically estimated RV/RA > 45 mmHg |
| 6MWD less than 550 metres | DLCO less than 18% of target |
| Radiologically documented bilateral emphysema | Lack of pulmonary functional follow-up within 3 months after lung volume reduction |
| LVRS or BLVR with endobronchial valves between 01/01/2019 and 31/12/2020 at the University Hospital Zurich | Missing or incomplete cost statement from the financial department of the University Hospital Zurich (e.g. due to transfer to another hospital). |
| For BLVR only: Lack of collateral ventilation (Chartis® or StratX®). |
RV: residual volume; RV/TLC: ratio of residual volume and total lung capacity; FEV1:first-second capacity; 6MWD: 6-minute walk distance; LVRS: lung volume reduction surgery; BLVR: bronchoscopic lung volume reduction; DLCO: diffusing capacity for carbon monoxide.
Table S2DRG according to SwissDRG [24] for lung volume reduction for emphysema.
| LVR method | DRG | Designation | CW | MLS (days) | Lower limit of length of stay | Upper limit of length of stay | ||
| day | CW/day* | day | CW/day* | |||||
| BLVR (using EBVs) | E02B | Other respiratory system procedures, age >15 years with extensive bronchial surgery | 1.594 | 6.9 | 1 | 0.608 | 15 | 0.186 |
| LVRS | E06A | Other lung resections, biopsies of thoracic organs and procedures on the thoracic wall with extremely severe comorbidities or surgical lung volume reduction | 2.583 | 12.4 | 3 | 0.558 | 24 | 0.193 |
BLVR: bronchoscopic lung volume reduction; DRG: diagnosis-related group; EBVs: endobronchial valves; CW: cost weight; LVRS: lung volume reduction surgery; MLS: mean length of stay.
*Surcharges or deductions
Table S3In-hospital# events after lung volume reduction divided by method.
| LVRS, n = 30 | BLVR, n = 37 | p-value | |
| Pneumothorax | 0 | 7 (18.9) | 0.014* |
| Subcutaneous emphysema | 3 (10) | 0 | 0.045* |
| Pneumonia or exacerbation of COPD | 2 (5.4) | 3 (10) | 0.170 |
| Prolonged fistula > 7 days | 17 (56.7) | 2 (5.4) | 0.018* |
| Death | 0 | 0 | - |
#Average length of stay of 9 ± 5 days; data are presented as n (%).
BLVR: bronchoscopic lung volume reduction (with endobronchial valves); LVRS: lung volume reduction surgery; *p <0.05.
Table S4Reasons for re-hospitalisations after lung volume reduction divided by method.
| LVRS, n = 30 | BLVR, n = 37 | |
| Pneumonia | 6 (20) | 2 (5.4) |
| Exacerbation of COPD | 0 | 1 (2.7) |
| Infection, not further defined | 1 (3.3) | 1 (2.7) |
| Local infection/wound abscess | 1 (3.3) | 0 |
| Pulmonary embolism | 0 | 1 (2.7) |
| Valve revision due to malposition | 0 | 5 (13.5) |
| Valve removal due to lack of effect | 0 | 2 (5.4) |
Data are presented as n (%)
There is no funding related to this article.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Daniel Franzen received speaker honoraria from Pulmonx. No other potential conflict of interest was disclosed.