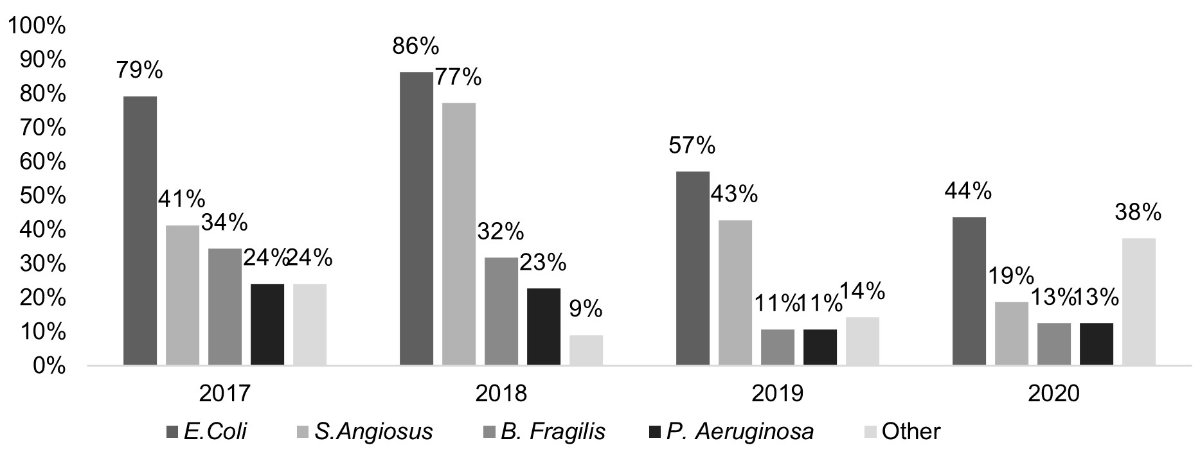
Figure 1 Microorganisms identified in cases of complicated appendicitis (displayed as a percentage per year). This bar chart shows that the most common microorganism found was Escherichia coli peaking at 86% in 2018.
DOI: https://doi.org/10.4414/SMW.2022.w30222
Acute appendicitis is the most frequent paediatric surgical emergency worldwide [1, 2]. Complicated appendicitis, defined as the presence of perforation, peritonitis, gangrene or an intra-abdominal abscess, accounts for 10–40% of cases [3–5]. Complicated appendicitis is inextricably linked to postoperative infectious complications. Frequent postoperative infectious complications includes intra-abdominal abscesses, occurring in up to 25% of complicated appendicitis and are an important source of morbidity [6–9].
The microbiology of acute appendicitis is well documented, implicating aerobic and anaerobic bacteria [2, 10–12]. Microorganisms resistant to or not covered by current empirical antibiotic regimens, such as Pseudomonas aeruginosa , present in 10–30% of cases of complicated appendicitis, are increasingly cited [11, 13–15]. Isolation of amoxicillin-clavulanate-resistant Escherichia coli in peritoneal fluid samples has also been associated with postoperative infectious complications in complicated appendicitis [15, 16].
Intravenous postoperative antibiotics are considered the gold standard of postoperative care in complicated appendicitis; however, treatment protocols vary globally, with little or no official consensus between institutions [17–19]. The optimal selection of an empirical postoperative intravenous antibiotic regimen (EAR) remains contested. Most single antibiotic agents are ineffective alone owing to the polymicrobial nature of complicated appendicitis and postoperative infectious complications [7].
For decades, triple antibiotic administration of ampicillin, gentamicin, and metronidazole/clindamycin was considered the optimal treatment. Several recent publications challenge this combination. A plethora of retrospective studies comparing the efficacy and cost-effectiveness of dual versus triple antibiotic therapy in complicated appendicitis demonstrated no significant difference in the rate of postoperative infectious complications [14, 20, 21]. Some institutions suggest using single broad-spectrum antibiotics in complicated appendicitis [22–24]. Generalised EAR also include the risk of new antimicrobial resistances [25–28].
Until 2013 at our institution, a triple EAR was prescribed (amoxicillin-clavulanate + aminoglycoside + metronidazole). Internal reviews (2012–13) of the bacteriology and local resistances demonstrated low rates of amoxicillin-clavulanate-resistant E. coli (less than 15%). Following SIS-IDSA guidelines, a downgrade from a triple EAR to a monotherapy empirical regimen of amoxicillin-clavulanate was made [28].
Since these changes, we have closely monitored the bacteriology of complicated appendicitis via peritoneal sampling and the rate of postoperative infectious complications. We published a retrospective cohort study of patients treated for complicated appendicitis, which showed an increase in the rate of amoxicillin-clavulanate resistant E. coli and a significant association between these isolates and postoperative infectious complications [16]. Additional findings included an alarming rate of infection with P. aeruginosa. Modification of our EAR from amoxicillin-clavulanate to a bitherapy of ceftriaxone and metronidazole was made as supported by existing literature [20, 21, 28]. There was insufficient evidence from either our study or the literature to introduce a broad spectrum antipseudomonal antibiotic.
This study's primary objective was to describe and analyse microorganisms' microbiology and antibiotic susceptibility in complicated appendicitis since 2017. Secondary objectives included evaluating the change of EAR on the rate of postoperative infectious complications in complicated appendicitis and identifying possible determinants of postoperative infectious complications in our population.
This retrospective, comparative, single-centre cohort study was carried out in a tertiary care hospital (Lausanne University Hospital, Lausanne, Switzerland) in the department of child and adolescent surgery. Inpatient and outpatient medical records for all children and adolescents under the age of 18 who underwent an appendectomy between 01 January 2017 and 31 July 2020 were retrospectively reviewed. Eligibility criteria included the intraoperative diagnosis of complicated appendicitis by the surgeon and peritoneal sampling. Patients not meeting these two criteria were excluded. Standard postoperative follow up occurred, with the only relevant data being hospital readmission. The study was written in accordance with the STROBE (Strengthening The Report of Observational Studies in Epidemiology) guidelines.
Patient electronic records were accessed via the electronic health record system (Soarian – Cerner, North Kansas City, MI, USA) under our hospital's data protection and data mining protocols. The following patient data and variables were extracted manually by retrospective review: demographic information (e.g., gender and age), clinical characteristics (e.g., duration of symptoms), microbiological information (e.g., bacteria isolated, antibacterial resistance), management (e.g., surgical procedure, antibiotic treatment) and complications (infectious versus non-infectious). No data were missing for the variables included in this study.
A paediatric surgeon established the indication for surgery based on their clinical diagnosis of appendicitis or with the help of a radiological examination (i.e., echography, followed by a computed tomography scan in the absence of direct or indirect signs of acute appendicitis). The surgical intervention consisted of a laparoscopic appendectomy or open (McBurney) approach. The surgeon then confirmed the diagnosis of complicated appendicitis based on the presence of perforation, peritonitis, gangrene and/or an intra-abdominal abscess. Visualisation of peritoneal liquid or pus prompted surgeons to take samples for aerobic and anaerobic culture. Intraoperative and postoperative antibiotics were administered following the antibiotic protocol highlighted below. Postoperative infectious complications were defined as new intra-abdominal abscesses (confirmed radiographically) or surgical site infections, with their management being decided individually for each patient by the paediatric surgeon on call. When possible, conservative treatment was preferred. If unsuccessful, percutaneous drainage by the interventional radiologist was favoured, and if not technically possible, a second surgery was performed.
The new EAR was implemented throughout this study but exclusively only from 2018. The anaesthetist gave the first dose of antibiotics at induction or, in cases when surgery was delayed, at diagnosis (NB: administration of this first dose was in all cases within 24 hours before surgery). Antibiotics consisted of a dual intravenous therapy of ceftriaxone (50–75 mg/kg) and metronidazole (15 mg/kg). In the case of acute appendicitis, no further doses were necessary. In complicated appendicitis, the duration of antibiotic therapy depended on the clinical evolution. Modification of the EAR occurred upon receiving the preliminary bacteriological results (within 48 hours after surgery) and again upon publication of the final results. When possible, antibiotics with the narrowest spectrum were chosen. In the event of worsening clinical symptoms in the first 48 hours (e.g., fever, lower right quadrant pain, nausea, and vomiting), piperacillin-tazobactam 100 mg/kg/dose) was administered.
Demographic information, clinical characteristics, microbiological information, and management were compared between groups using either the Student's t-test or the Kruskal–Wallis test for continuous variables, and the chi-square test or Fisher's exact test for categorical variables. All tests were two-tailed, and a resulting p-value <0.05 was statistically significant. Variables with a p-value <0.05 were included in a multivariable logistic regression model for which adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Statistical analyses were computed using STATA software (Stata/SE 16.1 for Mac StataCorp, Lakeway, TX).
Owing to its retrospective and anonymous design, informed consent was not requested with no anticipated harm to the patients. The study was approved by the Human Research Ethics Committee of the Canton of Vaud, Switzerland, and conducted per the principles of the World Medical Association's Declaration of Helsinki, as well as the standards of Good Clinical Practice and in line with Swiss regulatory requirements.
During the study period, 553 patients under the age of 18 underwent an appendectomy. Of these, 458 (83%) presented with acute appendicitis and 94 (17%) met the required inclusion criteria for complicated appendicitis. There was a male predominance with 56 (60%) and a male to female ratio of 1.4:1. The median age was 9years (Interquartile range [IQR] 5–13). The median duration of symptoms before hospital presentation was 2 days (IQR 1–3), with patients staying hospitalised for a median of 6 days (IQR 4–7). The majority of patients with complicated appendicitis (n = 79, 84%) presented with localised perforated appendicitis, with the rest having a collected abscess (n = 8, 9%) or diffuse appendicular peritonitis (n = 7, 8%). Laparoscopy was performed in 88 (94%) patients, with three cases (3%) converted to an open appendectomy. The remaining 3% (n = 3) of patients underwent primary exploratory laparotomies owing to clinical severity or surgeon's preference. A summary of patient characteristics and potential risk factors for postoperative infectious complications is shown in table 1.
Table 1Patient characteristics and potential determinants for post-operative infectious complications.
| Entire population (n = 94) | No postoperative infectious complications (n = 83) | Postoperative infectious complication (n = 11, intra-abdominal abscess formation) | p-value* | ||
| Gender, n male (%) | 56 (60) | 49 (59) | 7 (64) | 0.770 | |
| Age, median years (IQR) | 9 (5–13) | 9 (4–12) | 13 (8–14) | 0.045 | |
| Symptom duration, median days (IQR) | 2 (1–3) | 2 (1–3) | 3 (1–7) | 0.285 | |
| Abscess at presentation, n (%) | 8 (9) | 6 (7) | 2 (18) | 0.448 | |
| Laparoscopy, n (%) | 88 (94) | 78 (94) | 10 (91) | 0.622 | |
| P. aeruginosa positive, n (%) | 19 (20) | 17 (20) | 2 (18) | 0.858 | |
| Co-amoxicillin-resistant E. coli, n/total (%) | 10 (11) | 9 (11) | 1 (9) | 0.859 | |
| ESBL, n (%) | 6 (6) | 6 (7) | – | 0. 357 | |
| Empirical antibiotherapy chosen | Co-amoxicillin, n (%) | 6 (7) | 6 (7) | – | |
| Piperacillin-tazobactam, n (%) | 31 (33) | 25 (30) | 6 (55) | ||
| Ceftriaxone-metronidazole, n (%) | 54 (57) | 49 (59) | 5 (45) | ||
| Other ** | 3 (3) | 3 (3) | - | ||
ESBL: broad-spectrum beta-lactamase; IQR: interquartile range.
*Univariable analysis comparing patients with and without postoperative infectious complications: Student’s t-Test or the Kruskal-Wallis test for continuous variables and chi-squared or Fischer’s exact test for categorical variables.
** Due to known allergy an alternative regimen was administered.
The full classified list of microorganisms identified in peritoneal swab cultures can be seen in table 2. Single isolates were identified in 23 (24%) patients and multiple isolates in 66 (70%) patients. Cultures remained sterile in 11% of cases (n = 10). The most common isolated pathogen was E. coli (n = 65, 68%, fig. 1), followed by Streptococcus anginosus in 47 (48%) of isolates. Bacteroides fragilis was present in 22% (n = 22) of cultures. P. aeruginosa was isolated in 20% (n = 19) of cultures. Multiple isolates demonstrated intermediate or full resistance to one or more antibiotics. Ten of the E. coli isolates (15%) were resistant to amoxicillin-clavulanate, all of which were susceptible to our EAR and did not prove to be significantly associated with postoperative infectious complications (p = 0.859). Six strains of E. coli isolates (9%) demonstrated resistance to cephalosporins and thus to the EAR due to broad-spectrum beta-lactamase (ESBL) production. ESBL-producing strains did not prove to be significantly associated with postoperative infectious complications (p = 0.357). No meropenem or metronidazole resistance existed.
Table 2Microorganisms identified in peritoneal swab cultures sent for aerobic and anaerobic analysis.
| Microorganisms | n (%) | |
| Gram negative | Escherichia coli | 65 (68) |
| Pseudomonas aeruginosa | 19 (20) | |
| Bacteroides fragilis | 22 (23) | |
| Comamonas kerstersii | 3 (3) | |
| Citrobacter koseri | 1 (1) | |
| Proteus gr. Vulgaris | 1 (1) | |
| Klebsiella gr. Oxytoca | 1 (1) | |
| Haemophilus parainfluenzae | 1 (1) | |
| Haemophilus influenzae | 1 (1) | |
| Eikenella corrodens | 1 (1) | |
| Citrobacter freundii | 1(1) | |
| Gram positive | Streptococcus anginosus | 44 (46) |
| Streptococcus pneumoniae serotype 11 | 1 (1) | |
| Streptococcus mitis | 3 (3) | |
| Enterococcus avium | 3 (3) | |

Figure 1 Microorganisms identified in cases of complicated appendicitis (displayed as a percentage per year). This bar chart shows that the most common microorganism found was Escherichia coli peaking at 86% in 2018.
A summary of the antibiotics administered at all phases of this study can be found in table 3. Upon induction, the most frequently administered antibiotic was ceftriaxone/metronidazole (n = 65, 68%). Concerning the EAR, ceftriaxone/metronidazole was predominant (n = 54, 57%), followed by the broad-spectrum antibiotic piperacillin-tazobactam (n = 31, 33%). Based on these figures, the adherence rate to the new EAR for all years combined was 57%. The adherence rate per year was at a low of 21% in 2017, rising to 59%, 79% and 88% for the following years. The median duration of antibiotic treatment in all patients was 10 days (IQR 10–14) and 15 days (IQR 12–22) for the subgroup with postoperative infectious complications (p = 0.0034). EAR lasted for a median duration of 5 days (IQR 4–7), and antibiotics upon discharge lasted for 7 days (IQR 4–10) for all patients. Most patients (n = 83, 87%) were discharged with oral antibiotics, primarily amoxicillin-clavulanate (n = 35, 37%), followed by a dual therapy comprised of a quinolone (most frequently ciprofloxacin) and metronidazole (n = 24, 25%). The EAR correctly covered 74% (n = 69) of cases and their respective pathogens; it was inappropriate for the remaining 26% (n = 25), and therefore a change in antibiotic was necessary, often upgrading to a broad-spectrum antibiotic such as piperacillin-tazobactam. Of the patients with P. aeruginosa, only three (16%) did not need upgrading to a broad spectrum antibiotic due to an unremarkable clinical course. The remaining 84% (n = 16) required an upgrade from ceftriaxone/metronidazole to piperacillin-tazobactam, with the change taking place on postoperative day two.
Table 3Summary of antibiotics administered throughout this study at all possible stages (induction, postoperatively, discharge) for all years combined (2017–2020, n = 94).
| Antibiotic of choice | Induction | Post-op | Discharge |
| Amoxicillin-clavulanate, n (%) | 20 (21) | 6 (6) | 35 (37) |
| Ceftriaxone and metronidazole, n (%) | 64 (68) | 54 (56) | – |
| Piperacillin/tazobactam, n (%) | 5 (5) | 31 (33) | 2 (2) |
| Other, n (%) | 5 (5) | 3 (3) | 57 (60) |
A total of 11 patients (12%) developed postoperative infectious complications. Over the study period, the majority (n = 5, 45%) of cases of postoperative infectious complications occurred in 2017. Patients who developed postoperative infectious complications were significantly older (p = 0.045), with a median age of 13 (IQR 9–14). These patients were also more likely to have an increased length of stay (p = 0.0001), with a median of 10 days (IQR 8–16). Age upon multivariate analysis adjusting for infection with amoxicillin-clavulanate resistant E. coli was on the limit of statistical significance (p = 0.05, table 4). The three most frequently found microorganisms in patients who developed postoperative infectious complications were E. coli (n = 7, 64%), S. anginosus (n = 7, 64%), and B. fragilis (n = 7, 64%). Other bacteria isolated in lower numbers included P. aeruginosa (8%), Citrobacter freundi (9%) and Citrobacter koseri (9%). Two pathogens presented resistance to amoxicillin-clavulanate, an E. coli isolate and a C. freundi isolate. In both cases, isolates were susceptible to our EAR. Both instances of postoperative infectious complications caused by P. aeruginosa received broad spectrum antibiotics without a postoperative delay.
Table 4Multivariable analysis.
| Predictor | Contrast | Odds ratio (95% confidence interval) for postoperative infectious complications | p-value |
| Amoxicillin-clavulanate-resistant E. Coli | Yes vs no | 0.61 (0.07–5.38) | 0.654 |
| Age | One year increase | 1.18 (0.99–1.39) | 0.053 |
| Intercept | – | 0.03 (0.003–0.19) | <0.001 |
The majority (n = 7, 64%) of patients in the postoperative infectious complications subpopulation demonstrated unfavourable clinical evolution during their initial hospitalisation and received treatment immediately. In our study, a total of eight patients (9%) were readmitted, four for non-infectious reasons (postoperative haematomas or ileus;[n = 4/83, 5%) and the remaining four with postoperative infectious complications, leading to a readmission rate of 36% for the subpopulation presenting with postoperative infectious complications (p = 0.006). A total of 5 (45%) were treated conservatively with antibiotics, four (36%) via surgical intervention (exploratory laparotomy), one patient (9%) via interventional radiology, and one patient (9%) underwent an initial surgical intervention later followed by CT-guided drainage.
Postoperative infectious complications such as intra-abdominal abscesses in complicated appendicitis remain a significant source of morbidity in the paediatric population [22, 29]. It follows that the optimisation and streamlining of its treatment and clinical care pathways should be a priority for all paediatric surgical departments. The choice of EAR has been intensely debated in the literature, and despite existing evidence, wide variability remains between surgeons and institutions [24]. Nevertheless, two common trends are becoming apparent. Firstly, antibiotics should address local patterns of microorganisms, choosing to cover the most frequently found pathogens while remaining vigilant to antimicrobial resistance and the increasing risk it poses to global health [15, 26]. Secondly, intravenous antibiotic choice should be simplified from triple to dual therapy or even single broad-spectrum therapy and continuously reviewed, and updated by institutions [30]. Our institution is adamant about following these two trends. Internal reviews and a published study [16] demonstrated that our EAR (amoxicillin-clavulanate) was no longer adequate owing to the significant role that amoxicillin-clavulanate-resistant E. coli played in causing postoperative infectious complications, thus prompting a change to a dual therapy with ceftriaxone/metronidazole. This analysis and conclusion led to the present study.
We retrospectively reviewed cases of complicated appendicitis after introduction of our new EAR. Complicated appendicitis was present in 17% of patients with a postoperative infectious complication rate of 12%, a decrease of 3% and 4%, respectively, versus the previous cohort. These rates are on the lower limit of those published, ranging from 15–40% for complicated appendicitis and 4-20% for postoperative infectious complications [14, 20, 23, 31, 32]. These favourable rates could be due to our definition of complicated appendicitis, which may differ from other institutions. The initial presentation of complicated appendicitis (e.g., abscess, perforation, appendicular peritonitis), the surgical technique used and gender all had no significant impact on the rate of postoperative infectious complications. In line with the current surgical trends in surgery, there was a 38% increase in the rate of laparoscopy as the primary approach, which occurred in 94% of cases in our current study.
E. coli and S. anginosus were the most commonly cultured microorganisms in complicated appendicitis, mirroring our previous study and in line with other published studies [12, 14, 30, 33, 34]. Rate of infection with amoxicillin-clavulanate-resistant E. coli remained similar to the last cohort (15% vs 14%). There was a drop from 28% to 9% in postoperative infectious complications due to amoxicillin-clavulanate-resistant E. coli. These results confirm that the strategic modification of our EAR now provides better coverage of local resistances, demonstrating the importance of making changes at an institutional level based on local resistance patterns. ESBL-producing E. coli increased from 3% in the previous cohort to 9%, with no significant impact on postoperative infectious complications. A study reporting a 57% infection rate of ESBL-producing E. coli failed to demonstrate a statistically significant effect on the rate of postoperative infectious complications [32]. With a small sample size, it is difficult to conclude the role of ESBL-producing E. coli on postoperative infectious complications. Further investigation is warranted as resistant microorganisms are becoming more common in paediatric patients, and their impact must be elucidated [35].
Age was positively correlated with developing postoperative infectious complications (p = 0.045) and concordant with similar studies [36]. Age was on the limit of statistical significance after multivariate analysis adjusting for the presence of amoxicillin-clavulanate-resistant E. coli. Other predictive variables for postoperative infectious complications in complicated appendicitis are described in the literature, such as: C-reactive protein levels at admission superior to 100 mg/dl, higher white blood cell count, and bowel obstruction at presentation [36–38]. These variables were not considered in the present study as they fell beyond the scope of the objectives but prove pertinent for consideration in future prospective studies.
Due to the prolongation and the complexity of treatment, patients with postoperative infectious complications had a significantly longer length of stay and total duration of antibiotic therapy, and a tendency to be readmitted. These factors have an essential role in increasing morbidity. Our patients received intravenous antibiotics for a median of 5 days. The optimal length of EAR in complicated appendicitis remains undecided. Recent studies suggest that 2 days of intravenous antibiotics is not associated with a higher risk of postoperative infectious complications, and in parallel, longer duration of antibiotics do not prevent postoperative infectious complications from occurring [5, 17, 37, 39]. Indeed, the World Society of Emergency Surgery (WSES), in their most recent publication on the diagnosis and treatment of acute appendicitis, recommend an early switch (after 48 hours) to oral antibiotics in children with complicated appendicitis [40]. Moreover, an early transition could decrease the overall morbidity by decreasing patient burden, use of hospital resources and length of stay. Further prospective studies are needed.
Due to a transition period between 2017–2018, after the new EAR was established and introduced, a certain number of patients initially received antibiotics with enhanced anti-pseudomonas activity such as piperacillin-tazobactam. We attribute this heterogeneity to the implementation of and potential initial hesitation with the new EAR. Some surgeons most likely opted for a broader spectrum antibiotic when faced with a severe clinical presentation. We have not excluded these patients. It is noteworthy that the most significant number of postoperative infectious complications was during this transition period. We observed a decrease in postoperative infectious complications when adherence rose. Studies have shown that streamlining clinical care pathways can significantly decrease the rate of postoperative infectious complications and length of stay [41].
P. aeruginosa plays a significant role in postoperative infectious complications [15]. Our previous study demonstrated infection rates between 44% and 9%, depending on the year in question. The presence alone of this microorganism was not correlated with postoperative infectious complications and we, therefore, chose not to introduce an antipseudomonal antibiotic into our EAR [16]. Our current study demonstrated a mean overall infection rate of 20% (3% decrease) with no significant contribution to the rate of postoperative infectious complications. P. aeruginosa may be one of multiple pathogens found in colonic flora of paediatric patients and differs from single P. aeruginosa infections, thus questioning whether its antibiotic coverage is warranted [14].
Multiple retrospective studies have been conducted in children with complicated appendicitis comparing dual EAR versus broader antipseudomonal antibiotics such as piperacillin-tazobactam. The results are often conflicting. Some authors report positive outcomes such as a decrease in the percentage of postoperative infectious complications rates in those receiving broader antipseudomonal antibiotics [42], whereas others report no added advantage [19, 31, 43]. These studies have many drawbacks, notably not detailing the local microbiology and thus the infection rate of P. aeruginosa. Guillet-Caruba et al. [44] proposed providing supplemental pseudomonal coverage with an aminoglycoside for a period of 2 days until microbiological results become available. Institutions would then down-grade or maintain their therapy. Reverting to a triple EAR could increase morbidity, which in our view is not recommended. The risk of multidrug resistance and supplemental side effects such as infection with Clostridium difficile are important to consider when deciding to implement piperacillin-tazobactam as a first-line regimen [13, 25, 31].
Given the shortfall of evidence in the literature proving a clear added benefit of administering antipseudomonal antibiotics in cases of complicated appendicitis, and with our rate of infection of P. aeruginosa stagnating at 20%, we chose not to modify our empirical antibiotic regimen further. Institutions should continue to track and analyse their rates of P. aeruginosa infection and other potentially resistant microorganisms and their influence on postoperative infectious complications. Randomised prospective studies are needed to determine whether P. aeruginosa coverage is warranted.
The role of peritoneal sampling and cultures has been widely disputed, particularly in acute appendicitis. Indeed, certain authors concluded that it is fruitless as they do not impact clinical outcomes [34]. In complicated appendicitis, the role of peritoneal sampling is equally disputed. Since 2000, many authors have promoted systematic sampling due to its integral part in studying local flora and streamlining clinical pathways [12, 13, 32, 37, 44]. The Study for Monitoring Antimicrobial Resistance Trends (SMART) conducted a surveillance study of the epidemiology and antimicrobial susceptibility of pathogens causing intra-abdominal infections in paediatric patients, concluding that EAR should reflect regional and local resistance patterns, as there can be a large variability between institutions [45], thus confirming the importance of peritoneal sampling. We stand by these guidelines and will continue this practice, which permitted us to modify our EAR and better target resistant microorganisms in our local population.
This study has some limitations. Firstly, its retrospective design and small sample size may rationalise the limited statistically significant data and restrict the validity of our research. In our defense, multiple homogeneous factors are present. Our patients were all treated in the same institution, by the same pool of on-call surgeons, and followed the same EAR. We recognise the inherent possibility of potential biases. Secondly, due to its single-centre design, the results may not be generalisable, as inter-institutional pathogen distribution and local resistance rates can vary. We hope that our study can guide others in their future study designs and, eventually, their choice of EAR in the treatment of complicated appendicitis.
The findings of this study emphasis the need to implement evidence-based treatment protocols based on local microbiology profiles and current resistance rates to optimise postoperative EAR in complicated appendicitis. The change in our EAR permitted us to successfully cover resistant isolates that have been problematic in the past. We do not retain an indication to introduce antipseudomonal coverage. Our study adds to the vast body of literature evaluating management strategies for patients with complicated appendicitis who develop postoperative infectious complications. Postoperative infectious complications are likely multifactorial, and numerous prospective studies are needed to understand better their aetiology and the role of EAR in decreasing their existence. Antimicrobial resistance is on the rise, and institutions should play their role in tackling this global phenomenon.
The views expressed in the submitted article are our own and not an official position of the institution.
No funding sources.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Lee SL , Islam S , Cassidy LD , Abdullah F , Arca MJ ; 2010 American Pediatric Surgical Association Outcomes and Clinical Trials Committee . Antibiotics and appendicitis in the pediatric population: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2010 Nov;45(11):2181–5. https://doi.org/10.1016/j.jpedsurg.2010.06.038
2. Salö M , Marungruang N , Roth B , Sundberg T , Stenström P , Arnbjörnsson E , et al. Evaluation of the microbiome in children’s appendicitis. Int J Colorectal Dis. 2017 Jan;32(1):19–28. https://doi.org/10.1007/s00384-016-2639-x
3. Obrist NM , Tschuor C , Breitenstein S , Vuille-Dit-Bille RN , Soll C . Appendectomy in Switzerland: how is it done? Updates Surg. 2019 Jun;71(2):375–80. https://doi.org/10.1007/s13304-019-00654-z
4. Becker P , Fichtner-Feigl S , Schilling D . Clinical Management of Appendicitis. Visc Med. 2018 Dec;34(6):453–8. https://doi.org/10.1159/000494883
5. McGillen PK , Drake FT , Vallejo A , Brahmbhatt TS , Sanchez SE . Retrospective Analysis of Post-Operative Antibiotics in Complicated Appendicitis. Surg Infect (Larchmt). 2019 Jul;20(5):359–66. https://doi.org/10.1089/sur.2018.223
6. Svetanoff WJ , Talukdar N , Dekonenko C , Dorman RM , Osuchukwu O , Fraser JD , et al. Intra-abdominal Abscess After Appendectomy-Are Drains Necessary in All Patients? J Surg Res. 2020 Oct;254:384–9. https://doi.org/10.1016/j.jss.2020.05.016
7. Chen C , Botelho C , Cooper A , Hibberd P , Parsons SK . Current practice patterns in the treatment of perforated appendicitis in children. J Am Coll Surg. 2003 Feb;196(2):212–21. https://doi.org/10.1016/S1072-7515(02)01666-6
8. Pham XD , Sullins VF , Kim DY , Range B , Kaji AH , de Virgilio CM , et al. Factors predictive of complicated appendicitis in children. J Surg Res. 2016 Nov;206(1):62–6. https://doi.org/10.1016/j.jss.2016.07.023
9. Frazee R , Abernathy S , Davis M , Isbell T , Regner J , Smith R . Fast track pathway for perforated appendicitis. Am J Surg. 2017 Apr;213(4):739–41. https://doi.org/10.1016/j.amjsurg.2016.08.006
10. Bhangu A , Søreide K , Di Saverio S , Assarsson JH , Drake FT . Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet. 2015 Sep;386(10000):1278–87. https://doi.org/10.1016/S0140-6736(15)00275-5
11. Chen CY , Chen YC , Pu HN , Tsai CH , Chen WT , Lin CH . Bacteriology of acute appendicitis and its implication for the use of prophylactic antibiotics. Surg Infect (Larchmt). 2012 Dec;13(6):383–90. https://doi.org/10.1089/sur.2011.135
12. García-Marín A , Pérez-López M , Martínez-Guerrero E , Rodríguez-Cazalla L , Compañ-Rosique A . Microbiologic Analysis of Complicated and Uncomplicated Acute Appendicitis. Surg Infect (Larchmt). 2018 Jan;19(1):83–6. https://doi.org/10.1089/sur.2017.210
13. Fallon SC , Hassan SF , Larimer EL , Rodriguez JR , Brandt ML , Wesson DE , et al. Modification of an evidence-based protocol for advanced appendicitis in children. J Surg Res. 2013 Nov;185(1):273–7. https://doi.org/10.1016/j.jss.2013.05.088
14. Ong CP , Chan TK , Chui CH , Jacobsen AS . Antibiotics and postoperative abscesses in complicated appendicitis: is there any association? Singapore Med J. 2008 Aug;49(8):615–8.
15. Obinwa O , Casidy M , Flynn J . The microbiology of bacterial peritonitis due to appendicitis in children. Ir J Med Sci 1971 -. 2014;183(4):585‑91. DOI: https://doi.org/10.1007/s11845-013-1055-2
16. Andrey V , Crisinel PA , Prod’hom G , Croxatto A , Joseph JM . Impact of co-amoxicillin-resistant Escherichia coli and Pseudomonas aeruginosa on the rate of infectious complications in paediatric complicated appendicitis. Swiss Med Wkly. 2019 Apr;149:w20055. https://doi.org/10.4414/smw.2019.20055
17. Loux TJ , Falk GA , Burnweit CA , Ramos C , Knight C , Malvezzi L . Early transition to oral antibiotics for treatment of perforated appendicitis in pediatric patients: confirmation of the safety and efficacy of a growing national trend. J Pediatr Surg. 2016 Jun;51(6):903–7. https://doi.org/10.1016/j.jpedsurg.2016.02.057
18. Bonadio W , Rebillot K , Ukwuoma O , Saracino C , Iskhakov A . Management of Pediatric Perforated Appendicitis: Comparing Outcomes Using Early Appendectomy Versus Solely Medical Management. Pediatr Infect Dis J. 2017 Oct;36(10):937–41. https://doi.org/10.1097/INF.0000000000001025
19. Shang Q , Geng Q , Zhang X , Guo C . The efficacy of combined therapy with metronidazole and broad-spectrum antibiotics on postoperative outcomes for pediatric patients with perforated appendicitis. Medicine (Baltimore). 2017 Nov;96(47):e8849. https://doi.org/10.1097/MD.0000000000008849
20. Dreznik Y , Feigin E , Samuk I , Kravarusic D , Baazov A , Levy I , et al. Dual versus Triple Antibiotics Regimen in Children with Perforated Acute Appendicitis. Eur J Pediatr Surg. 2018 Dec;28(6):491–4. https://doi.org/10.1055/s-0037-1606847
21. St Peter SD , Tsao K , Spilde TL , Holcomb GW 3rd , Sharp SW , Murphy JP , et al. Single daily dosing ceftriaxone and metronidazole vs standard triple antibiotic regimen for perforated appendicitis in children: a prospective randomized trial. J Pediatr Surg. 2008 Jun;43(6):981–5. https://doi.org/10.1016/j.jpedsurg.2008.02.018
22. Pogorelić Z , Silov N , Jukić M , Elezović Baloević S , Poklepović Peričić T , Jerončić A . Ertapenem Monotherapy versus Gentamicin Plus Metronidazole for Perforated Appendicitis in Pediatric Patients. Surg Infect (Larchmt). 2019 Dec;20(8):625–30. https://doi.org/10.1089/sur.2019.025
23. Hurst AL , Olson D , Somme S , Child J , Pyle L , Ranade D , et al. Once-Daily Ceftriaxone Plus Metronidazole Versus Ertapenem and/or Cefoxitin for Pediatric Appendicitis. J Pediatr Infect Dis Soc. 2015;piv082. DOI: https://doi.org/10.1093/jpids/piv082
24. Goldin AB , Sawin RS , Garrison MM , Zerr DM , Christakis DA . Aminoglycoside-based triple-antibiotic therapy versus monotherapy for children with ruptured appendicitis. Pediatrics. 2007 May;119(5):905–11. https://doi.org/10.1542/peds.2006-2040
25. Song DW , Park BK , Suh SW , Lee SE , Kim JW , Park JM , et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018 Apr;33(4):441–7. https://doi.org/10.1007/s00384-018-2992-z
26. Chan KW , Lee KH , Mou JW , Cheung ST , Sihoe JD , Tam YH . Evidence-based adjustment of antibiotic in pediatric complicated appendicitis in the era of antibiotic resistance. Pediatr Surg Int. 2010 Feb;26(2):157–60. https://doi.org/10.1007/s00383-009-2540-6
27. Coccolini F , D’Amico G , Sartelli M , Catena F , Montori G , Ceresoli M , et al. Antibiotic resistance evaluation and clinical analysis of acute appendicitis; report of 1431 consecutive worldwide patients: A cohort study. Int J Surg. 2016 Feb;26:6–11. https://doi.org/10.1016/j.ijsu.2015.12.063
28. Solomkin JS , Mazuski JE , Bradley JS , Rodvold KA , Goldstein EJ , Baron EJ , et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010 Jan;50(2):133–64. https://doi.org/10.1086/649554
29. Reinisch A , Malkomes P , Habbe N , Bechstein WO , Liese J . Bad bacteria in acute appendicitis: rare but relevant. Int J Colorectal Dis. 2017 Sep;32(9):1303–11. https://doi.org/10.1007/s00384-017-2862-0
30. Dahlberg M , Almström M , Wester T , Svensson JF . Intraoperative cultures during appendectomy in children are poor predictors of pathogens and resistance patterns in cultures from postoperative abscesses. Pediatr Surg Int. 2019 Mar;35(3):341–6. https://doi.org/10.1007/s00383-018-04428-3
31. Taleb M , Nardi N , Arnaud A , Costet N , Donnio PY , Engrand C , et al. Simplification of first-line antibacterial regimen for complicated appendicitis in children is associated with better adherence to guidelines and reduced use of antibiotics. Int J Antimicrob Agents. 2018 Aug;52(2):293–6. https://doi.org/10.1016/j.ijantimicag.2018.04.010
32. Turel O , Mirapoglu SL , Yuksel M , Ceylan A , Gultepe BS . Perforated appendicitis in children: antimicrobial susceptibility and antimicrobial stewardship. J Glob Antimicrob Resist. 2019 Mar;16:159–61. https://doi.org/10.1016/j.jgar.2018.09.015
33. Montuori M , Santurro L , Gianotti L , Fattori L . Uselessness of microbiological samples in acute appendicitis with frank pus: to collect or not to collect? Eur J Trauma Emerg Surg. 2020 Aug;46(4):835–9. https://doi.org/10.1007/s00068-018-1031-7
34. Subramanian T , Jerome E , Jones I , Jester I . Streptococcus anginosus is associated with postoperative intraabdominal collections in appendicitis. J Pediatr Surg. 2018 Feb;53(2):237–40. https://doi.org/10.1016/j.jpedsurg.2017.11.009
35. Bassetti M , Peghin M , Mesini A , Castagnola E . Optimal Management of Complicated Infections in the Pediatric Patient: The Role and Utility of Ceftazidime/Avibactam. Infect Drug Resist. 2020 Jun;13:1763–73. https://doi.org/10.2147/IDR.S209264
36. Fraser JD , Aguayo P , Sharp SW , Snyder CL , Holcomb GW 3rd , Ostlie DJ , et al. Physiologic predictors of postoperative abscess in children with perforated appendicitis: subset analysis from a prospective randomized trial. Surgery. 2010 May;147(5):729–32. https://doi.org/10.1016/j.surg.2009.10.057
37. Frongia G , Mehrabi A , Ziebell L , Schenk JP , Günther P . Predicting Postoperative Complications After Pediatric Perforated Appendicitis. J Invest Surg. 2016 Aug;29(4):185–94. https://doi.org/10.3109/08941939.2015.1114690
38. Emil S , Elkady S , Shbat L , Youssef F , Baird R , Laberge JM , et al. Determinants of postoperative abscess occurrence and percutaneous drainage in children with perforated appendicitis. Pediatr Surg Int. 2014 Dec;30(12):1265–71. https://doi.org/10.1007/s00383-014-3617-4
39. Tartaglia D , Fatucchi LM , Mazzoni A , Miccoli M , Piccini L , Pucciarelli M , et al. Risk factors for intra-abdominal abscess following laparoscopic appendectomy for acute appendicitis: a retrospective cohort study on 2076 patients. Updat Surg. 2020; DOI: https://doi.org/10.1007/s13304-020-00749-y
40. Di Saverio S , Podda M , De Simone B , Ceresoli M , Augustin G , Gori A , et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020 Apr;15(1):27. https://doi.org/10.1186/s13017-020-00306-3
41. Yousef Y , Youssef F , Homsy M , Dinh T , Pandya K , Stagg H , et al. Standardization of care for pediatric perforated appendicitis improves outcomes. J Pediatr Surg. 2017 Dec;52(12):1916–20. https://doi.org/10.1016/j.jpedsurg.2017.08.054
42. Lee JY , Ally S , Kelly B , Kays D , Thames L . Once Daily Dosing of Ceftriaxone and Metronidazole in Children With Perforated Appendicitis. J Pediatr Pharmacol Ther. 2016 Mar-Apr;21(2):140–5. https://doi.org/10.5863/1551-6776-21.2.140
43. Hamdy RF , Handy LK , Spyridakis E , Dona D , Bryan M , Collins JL , et al. Comparative Effectiveness of Ceftriaxone plus Metronidazole versus Anti-Pseudomonal Antibiotics for Perforated Appendicitis in Children. Surg Infect (Larchmt). 2019 Jul;20(5):399–405. https://doi.org/10.1089/sur.2018.234
44. Guillet-Caruba C , Cheikhelard A , Guillet M , Bille E , Descamps P , Yin L , et al. Bacteriologic epidemiology and empirical treatment of pediatric complicated appendicitis. Diagn Microbiol Infect Dis. 2011 Apr;69(4):376–81. https://doi.org/10.1016/j.diagmicrobio.2010.11.003
45. Lob SH , Badal RE , Hackel MA , Sahm DF . Epidemiology and Antimicrobial Susceptibility of Gram-Negative Pathogens Causing Intra-abdominal Infections in Pediatric Patients in Europe—SMART 2011–2014. J Pediatric Infect Dis Soc. 2014;2016:piv109. https://doi.org/10.1093/jpids/piv109