Response to the first awake prone positioning relates with intubation rate in SARS-CoV-2 patients suffering from acute respiratory failure with moderate to severe hypoxaemia: a retrospective study
DOI: https://doi.org/10.4414/SMW.2022.w30212
Ermes
Lupieriab, Andrea
Boffia, Zied
Ltaiefa, Antoine
Schneiderab, Samia
Abed-Maillarda, Jean-Daniel
Chicheab, Mauro
Oddoab, Lise
Piquilloudab
aAdult Intensive Care Unit, University Hospital of Lausanne, Switzerland
bAdult Intensive Care Unit, University Hospital of Lausanne and Lausanne University, Lausanne, Switzerland
Summary
AIMS OF THE STUDY: Awake prone positioning (aPP) in non-intubated patients with severe SARS-CoV-2-related pneumonia improves oxygenation and reduces the intubation rate, but no early predictors for success or failure of the strategy have been described. The main objective of this study was to assess whether response to the first aPP in terms of PaO2/FiO2, alveolar-arterial gradient (Aa-O2), respiratory rate and PaCO2 could predict the need for intubation. As secondary objective, we assessed the effects of aPP on the same parameters for all the sessions considered together.
METHODS: Retrospective analysis of consecutive SARS-CoV-2 pneumonia patients suffering from acute respiratory failure with moderate to severe hypoxaemia for whom aPP was performed for at least 45 minutes based on the prescription of the clinician in charge according to predefined criteria. Respiratory rate, blood gases and oxygenation parameters (PaO2/FiO2 and Aa-O2), before and after the first aPP were compared between patients who were subsequently intubated or not. Effects of all the aPP sessions together were also analysed.
RESULTS: One hundred and sixty-six patients were admitted for SARS-CoV-2 pneumonia during the study period. Among them, 50 received aPP lasting at least 45 minutes. Because 17 denied consent for data analysis and 2 were excluded because of a “do not intubate order”, 31 patients (for a total of 116 aPP sessions without any severe adverse events reported) were included. Among them, 10 (32.3%) were intubated. Mean age ± standard deviation (SD) was 60 ± 12 years. At ICU admission, respiratory rate was 26 ± 7/minute, median PaO2/FiO2 94 (interquartile range [IQR] 74–116) mm Hg and median Aa-O2 412 (IQR 286–427) mm Hg (markedly increased). Baseline characteristics did not statistically differ between patients who subsequently needed intubation or not. During the first aPP, PaO2/FiO2 increased and Aa-O2 decreased. When comparing patients who later where intubated or not, we observed, in the non intubated group only, a clinically significant decrease in median Aa-O2, from 294 (280–414) to 204 (107–281) mm Hg, corresponding to a 40% (26–56%) reduction, and a PaO2/FiO2 increase, from 103 (84–116) to 162 (138–195), corresponding to an increase of 48% (11–93%). The p value is <0.005 for both. When all the aPP sessions (n = 80) were considered together, aPP was associated with a significant increase in PaO2/FiO2 from 112 (80–132) to 156 (86–183) mm Hg (p <0.001) and Aa-O2 decrease from 304 (244–418) to 224 (148–361) mm Hg (p = 0.001).
CONCLUSIONS: Awake pronation in spontaneously breathing patients is feasible, and improves PaO2/FiO2 and Aa-O2. Response to the first session seems to be associated with lower intubation rate.
Introduction
Severe hypoxaemia is frequent in SARS-CoV-2-related pneumonia and seems mainly related to ventilation/perfusion ratio mismatch with a variable potential for lung recruitment [1, 2]. Prone positioning in intubated patients with moderate to severe acute respiratory distress syndrome improves oxygenation and outcome [3]. Awake prone positioning (aPP) has been proposed for SARS-CoV-2 pneumonia to improve oxygenation and maybe avoid intubation [4]. The longer the duration of proning, the greater the benefit. However, the ideal duration remains unknown. There is growing evidence on feasibility and effects [5–7], but no early predictors for success or failure of the aPP strategy have been described.
The main aim of the study was to assess whether response to the first aPP in terms of oxygenation (ratio of the arterial partial pressure of oxygen to inspired fraction of oxygen [PaO2/FiO2 ratio] and alveolo-arterial oxygen gradient [Aa-O2]), respiratory rate and arterial partial pressure of carbon dioxide (PaCO2) could predict the need for intubation in patients suffering from SARS-CoV-2-related pneumonia with PaO2/FIO2 <200 mm Hg. Only patients who could be proned for at least 45 minutes were considered. As secondary objectives, we assessed for the same parameters the effects of aPP for all the sessions considered together. We also recorded the adverse events related to aPP to evaluate the feasibility of the treatment strategy.
Materials and methods
Study design, setting and eligibility criteria
We performed a retrospective analysis of all the consecutive patients admitted with SARS-CoV-2 pneumonia in the adult intensive care unit (ICU) of the University Hospital of Lausanne, Switzerland, from 13 March to 29 October 2020 (first wave and period between the first and second waves of the pandemic), who had moderate to severe hypoxaemia defined as PaO2/FiO2 ratio lower than 200 mm Hg, did not require early intubation for severe respiratory distress or rapid clinical deterioration, agreed for data utilisation, received aPP lasting at least 45 minutes according to predefined criteria and had no “do not intubate" order. At that time, in the Lausanne ICU, awake proning was considered for as long as possible in all non-intubated patients with PaO2/FiO2 <200 mm Hg without criteria for early intubation. From a practical point of view, eligible patients were invited to change position from supine to prone by the nursing staff, in autonomy or with healthcare assistance under close monitoring for safety reasons (risk of equipment displacement in particular). The tolerability of the position was a major concern and particular attention was payed to making the patient comfortable, adapting the bed position, adding pillows, and providing recreational means, primarily music. Painkillers were administered prior to the procedure if indicated. Intubation was performed based on clinician judgment, following our ICU standard procedure for COVID-19 patients: worsening or persistent tachypnoea (respiratory rate >35/min), inability to protect airways, important agitation and haemodynamic reasons. Isolated hypoxaemia was not a criterion.
Data collection, variables, calculations and outcomes
Respiratory rate and results of blood gas analyses were collected from the clinical database before and at the end of each pronation. The time of the last blood gas analysis before each session was recorded. PaO2/FiO2 and Aa-O2 were computed for each time point. FiO2 was estimated as in daily clinical practice in our ICU using a bedside conversion table. Practically, the table is used by the nurses to estimate delivered FiO2 according to the interface and set oxygen flow. FiO2 estimated this way is entered manually into the clinical information system for each change of oxygen interface or flow. In the table, the conversion formula applied for low flow systems without reservoir bag is: FiO2 (%) = 4 x oxygen flow (l/min) + 20, up to a flow of 5 l/min [8]. For greater flow and use of a non-rebreather mask with reservoir, the delivered FiO2 is estimated according the American Association for Respiratory Care (AARC) guidelines for oxygen therapy [9].
Favourable response to aPP in terms of oxygenation was defined as an increase of at least 20% in PaO2/FiO2 at the end of the proning session compared with the most recent PaO2/FiO2 available in the supine position before aPP [10]. A clinically relevant decrease in Aa-O2 and increase in PaO2/FiO2 was arbitrarily considered as a minimum 20% change compared with baseline.
Statistics
Data before and after proning were compared using paired t-tests or Wilcoxon/Mann Whitney U-tests as appropriated according to data distribution. Intubated and non-intubated patients were compared for their baseline characteristics and regarding physiological response to the first aPP using t-tests or Mann Whitney tests as appropriate according to the data distribution. A p-value <0.05 was considered as significant. Statistical analysis was performed with IBM SPSS 27.
Results
From 13 March to 29 October 2020, 166 patients were admitted with SARS-CoV-2-related pneumonia into the adult ICU of the Lausanne University Hospital, Switzerland. Among them, 59 patients (35.5%) were intubated early because of disease severity at admission or because of rapid clinical deterioration, following the relatively early intubation strategy that was the standard of care in our ICU at the time of the study. These 59 patients were thus not considered eligible for an aPP trial. Seven other patients were transferred from another hospital already intubated. Among the remaining 100 patients, 50 patients could be proned for more than 45 minutes. Nineteen out of them were excluded from the final analysis, 17 because they denied consent for data utilisation and 2 because they had a “do not intubate" order. The total number of aPP sessions of more than 45 minutes performed was 116, with an average of 3 (interquartile range [IQR] 1–6) sessions per patient (range 1–12 sessions). No adverse events were reported related to the 116 aPP sessions.
Data at admission are presented in table 1. The mean age ± standard deviation (SD) of the included patients was 60 ± 12 years and 23 (74.2%) patients were men. The most prevalent comorbidities were hypertension (54.8%), cardiovascular disease (25.8%), diabetes (25.8%) and obesity (25.8%). Upon admission to the ICU, mean respiratory rate was 26 ± 7/minute, median PaO2/FiO2 was 94 (IQR 74–116) and median Aa-O2 was markedly increased at 412 (286–427) mm Hg; the normal predicted value corrected for age, altitude and FiO
2 is 47 (35–52) mm Hg [11].
Table 1Characteristics at admission for all patients and for the two groups intubated and non-intubated.
|
All patients (n = 31)
|
Ultimately intubated (n = 10, 32%)
|
Not intubated (n = 21, 68%)
|
p-value |
| Age |
60 ± 12 |
62 ± 12 |
60 ± 13 |
0.679 |
| Female sex |
8 (25.8%) |
3 (30%) |
7 (23.8%) |
0.517 |
| BMI (kg/m2) |
28.3 ± 5.7 |
29.5 ± 6.3 |
27.7 ± 5.5 |
0.466 |
| Cardiovascular disease |
8 (25.8%) |
4 (40%) |
4 (19%) |
0.208 |
| Hypertension |
17 (54.8%) |
6 (60%) |
11 (52.4%) |
0.497 |
| Diabetes |
8 (25.8%) |
3 (30%) |
5 (23.8%) |
0.517 |
| Respiratory comorbidities |
6 (19.4%) |
2 (2%) |
4 (19%) |
0.650 |
| Immunosuppression |
3 (9.7%) |
2 (20%) |
1 (4.8%) |
0.237 |
| Obesity |
8 (25.8%) |
3 (30%) |
5 (23.8%) |
0.517 |
| FiO2
|
0.80 (0.60–0.80) |
0.80 (0.80–0.80) |
0.70 (0.52–0.80) |
0.268 |
| SpO2 (%) |
93 ± 3 |
93 ± 5 |
93 ± 3 |
0.723 |
| PaO2 (mm Hg) |
65 (59–71) |
65 (55–75) |
65 (60–70) |
0.758 |
| Aa-O2 (mm Hg) |
412 (286–427) |
415 (382–427) |
295 (235–437) |
0.313 |
| PaO2/FiO2
|
94 (74–116) |
90 (69–97) |
103 (74–118) |
0.261 |
| pH |
7.46 ± 0.03 |
7.45 ± 0.03 |
7.47 ± 0.03 |
0.220 |
| PaCO2 (mm Hg) |
32.9 ± 5.1 |
34.4 ± 5.2 |
32.1 ± 5.0 |
0.263 |
| RR (breath/min) |
26 ± 7 |
25 ± 6 |
27 ± 7 |
0.396 |
| Sessions of aPP |
3 (1–6) |
1 (1–3) |
3 (2–7) |
0.028* |
| Deceased |
2 (6.5%) |
2 (20%) |
0 (0%) |
0.97 |
Out of the 31 included patients, 10 (32.3%) were ultimately intubated (7 after the first aPP, 1 after the third, 1 after the fifth and 1 after the tenth session). Two of these intubated patients (6.4%) died in the ICU. Data at admission for the ultimately intubated and non-intubated patients are also given in table 1. The two groups were not statistically different in terms of sex, age, comorbidities and respiratory parameters at admission. PaO2/FiO2 was, however, slightly lower and Aa-O2 was slightly higher in the patients who subsequently were intubated.
Complete data before and at the end of aPP were available for 27 patients for the first aPP and for 80 of the 116 aPP sessions performed (68.9%). Median time between the last available blood gas analysis before aPP and the start of proning was 37 (23–64) min for the first session. Minimum and maximum times were 2 and 157 minutes, respectively.
Data describing the effects of the first aPP are shown in table 2 for all the patients and for the patients who subsequently were intubated or not. Following the first aPP, in the overall population, we observed a clinically significant Aa-O2 decrease of 86 (3–180) mm Hg, corresponding to a 29% (1–53%) drop and a PaO2/FiO2 increase of 31 (1–80) mm Hg, corresponding to a percentage increment of 39% (1–79%). In the ultimately intubated group, the changes were minimal, with an Aa-O2 decrease of 13 (–30 to 116) mm Hg corresponding to a 3% change (–7 to 27%), and a PaO2/FiO2 increase of 7 (–24 to 16) mm Hg corresponding to a 5% (–38 to 18%) change. In the non-intubated group we observed clinically significant improvement with an Aa-O2 decrease of 132 (84–214) mm Hg, corresponding to a 40% (26–56%) reduction, and PaO2/FiO2 increase of 66 (16–87) mm Hg, corresponding to a percentage rise of 48% (11–93%). When decreases in Aa-O2 and increases in PaO2/FiO2, before and after the first aPP were compared between the patients who subsequently were intubated or not, the p-values were p = 0.043 and p = 0.018 for Aa-O2 and PaO2/FiO2, respectively.
Table 2Physiological parameters before and after the first pronation session in the global patient population with blood gas analyses available for the first aPP (n = 27), in the subgroup of patients who did not later require intubation and in the subgroup of patients who were intubated.
|
All patients (n = 27)
|
Ultimately Intubated (n = 10, 37%)
|
Non intubated (n = 17, 63%)
|
|
Before
|
After
|
p-value |
Before
|
After
|
p-value |
Before
|
After
|
p-value |
| FiO2
|
0.65 (0.60–0.80) |
0.55 (0.36–0.80) |
0.001* |
0.80 (0.80–0.80) |
0.80 (0.60–0.80) |
0.340 |
0.60 (0.60–0.80) |
0.50 (0.36–0.60) |
<0.001* |
| SpO2 (%) |
92 ± 3 |
93 ± 3 |
0.058 |
92 ± 4 |
93 ± 4 |
0.416 |
92 ± 2 |
94 ± 3 |
0.092 |
| PaO2 (mm Hg) |
64 (59–75) |
69 (60–78) |
0.315 |
64 (54–78) |
65 (57–70) |
0.401 |
65 (59–72) |
73 (64–78) |
0.074 |
| Aa-O2 (mm Hg) |
340 (290–422) |
242 (149–351) |
<0.001* |
422 (382–427) |
412 (298–425) |
0.445 |
294 (280–414) |
204 (107–281) |
<0.001* |
| PaO2/FiO2
|
99 (81–115) |
150 (90–193) |
0.001* |
80 (67–115) |
87 (71–111) |
0.646 |
103 (84–116) |
162 (138–195) |
<0.001* |
| pH |
7.46 ±0.04 |
7.44 ±0.04 |
0.011* |
7.44 ±0.04 |
7.43 ±0.04 |
0.054 |
7.47 ±0.03 |
7.45 ±0.03 |
0.042* |
| PaCO2 (mm Hg) |
32.9 ±5.3 |
33.9 ±6.0 |
0.202 |
34.8 ±4.6 |
34.4 ±5.0 |
0.663 |
31.8 ±5.5 |
33.6 ±6.7 |
0.101 |
| RR (breath/min) |
28 ±6 |
26 ±6 |
0.299 |
28 ±5 |
27 ±7 |
0.523 |
28 ±6 |
26 ±6 |
0.406 |
Individual patient data are provided in figure 1.
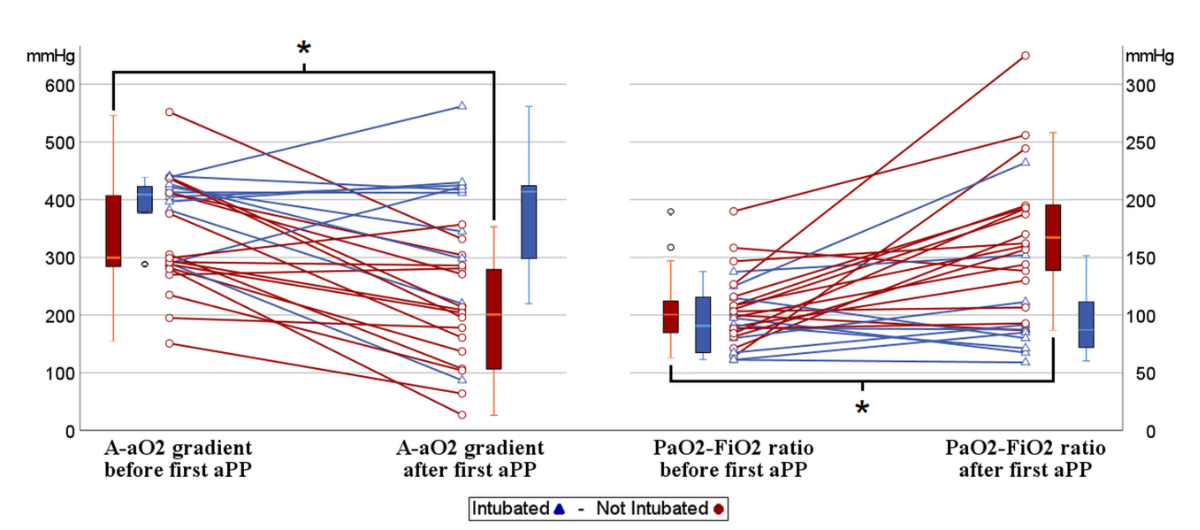
Figure 1 Individual changes in Aa-O2 and PaO2/FiO2 before and after the first aPP for patients who were ultimately intubated (in blue) or not (in red).
Box plots for the groups of data are also reported. Aa-O2: alveolar- arterial gradient of oxygen; PaO2: arterial partial pressure of oxygen; FiO2: Inspired fraction of oxygen.
When all the aPP sessions (n = 80) were considered together, aPP was associated with a significant increase in PaO2/FiO2 from 112 (80–132) to 156 (86–183) mm Hg (p <0.001) and Aa-O2 decreased from 304 (244–418) to 224 (148–361) mm Hg (p = 0.001). Twenty-four patients (77.4%) responded to pronation with an increase in PaO2/FiO2 of at least 20% in at least one session, representing 45 out of 80 sessions (56.2%). For all sessions, PaCO2 and respiratory rate did not differ significantly before and after pronation. pH slightly decreased in the non-intubated group.
Discussion
Our retrospective study reports that aPP in spontaneously breathing patients is feasible, safe and improves oxygenation, as manifested by PaO2/FiO2 increase and alveolar-arterial gradient decrease. Moreover, it seems that the response in term of oxygenation to the first aPP is correlated with intubation rate.
Our study has some limitations, mainly due to the retrospective nature of the design and the absence of a control group. First, FiO2 was estimated according to the oxygen flow delivered using a bedside available conversion table [8, 9] and not measured by oximetry. As patients’ inspiratory flow is unknown, delivered FiO2 is thus only an approximate value. Second, even if the differences were not statistically significant, the patients who subsequently were intubated had slightly lower PaO2/FiO2 ratios and higher Aa-O2 gradients at baseline. These differences could partly explain, independently from the physiological response to the first aPP, why these patients finally required intubation. Nevertheless, it is important to note that criteria for intubation at the time of the study in our ICU was not isolated hypoxaemia but worsening or persistent tachypnoea (respiratory rate >35/min), inability to protect airways, important agitation and haemodynamic reasons. As respiratory rate, pH and PaCO2 were not different between the two groups at baseline, this suggests that patients who subsequently were intubated or not were relatively similar in terms of respiratory distress severity at baseline. For this reason the predictive value of responding to the first aPP in terms of oxygenation probably remains an important element to consider. Regarding intubation rate in our study, 10 patients out of 31 (32.3%) were intubated, which is in line with previously published data [12]. Third, we cannot exclude a selection bias, with longer aPP sessions performed in patients who particularly felt better in the prone position or where considered responders by the clinicians in charge. However, a 20% increase in the PaO2/FiO2 ratio was observed in only 45 sessions out of 80 (56.2%). Conversely, patients with a sudden desaturation are likely to have been encouraged to change position and described as responders even if another reason could explain the increase in oxygen saturation. Transient desaturations that do not reflect a global worsening of the clinical status can be explained by efforts, cough and secretions. In addition, we cannot exclude that the lack of response to pronation may have influenced the clinician in the decision to intubate. However, according to the service protocol, hypoxaemia without respiratory distress was not an intubation criterion and the fact that 33.3% of non-intubated patients (7 out of 21) had FiO2 of 60% or more after the first aPP, tends to confirm good adherence to the protocol.
In conclusion, improvement in alveolar arterial oxygen gradient and PaO2/FiO2 during the first aPP could be indicators of successful prone positioning in awake non-intubated SARS-CoV-2 pneumonia patients. Additional studies are required to confirm our findings and provide additional clinical evidence in favour of this strategy.
Ermes Lupieri
Adult Intensive Care Unit
University Hospital of Lausanne
Rue du Bugnon 46
CH–1011 Lausanne
ermes.lupieri[at]chuv.ch
References
1.
Mauri T
,
Spinelli E
,
Scotti E
,
Colussi G
,
Basile MC
,
Crotti S
, et al.
Potential for Lung Recruitment and Ventilation-Perfusion Mismatch in Patients With the Acute Respiratory Distress Syndrome From Coronavirus Disease 2019. Crit Care Med. 2020 Aug;48(8):1129–34. https://doi.org/10.1097/CCM.0000000000004386
2.
Gattinoni L
,
Chiumello D
,
Caironi P
,
Busana M
,
Romitti F
,
Brazzi L
, et al.
COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 Jun;46(6):1099–102. https://doi.org/10.1007/s00134-020-06033-2
3.
Guérin C
,
Reignier J
,
Richard JC
,
Beuret P
,
Gacouin A
,
Boulain T
, et al.; PROSEVA Study Group
. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 Jun;368(23):2159–68. https://doi.org/10.1056/NEJMoa1214103
4.
Nasa P
,
Azoulay E
,
Khanna AK
,
Jain R
,
Gupta S
,
Javeri Y
, et al.
Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021 Mar;25(1):106. https://doi.org/10.1186/s13054-021-03491-y
5
Ehrmann S
,
Li J
,
Ibarra-Estrada M
, et al.
Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial [published online ahead of print, 2021 Aug 20]. Lancet Respir Med. 2021;S2213-2600(21)00356-8.
6.
Coppo A
,
Bellani G
,
Winterton D
,
Di Pierro M
,
Soria A
,
Faverio P
, et al.
Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020 Aug;8(8):765–74. https://doi.org/10.1016/S2213-2600(20)30268-X
7.
Ferrando C
,
Mellado-Artigas R
,
Gea A
,
Arruti E
,
Aldecoa C
,
Adalia R
, et al.; COVID-19 Spanish ICU Network
. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020 Oct;24(1):597. https://doi.org/10.1186/s13054-020-03314-6
8.
Vincent JL
. Le manuel de réanimation, soins intensifs et médecine d'urgence. France. 5ème edition. Paris. France. Springer, 2017, chapter 2
9
Piraino, Thomas
et al.
“Management of Adult Patients With Oxygen in the Acute Care Setting.”
10 Respiratory care, respcare.09294. 2 Nov. 2022, doi:https://doi.org/10.4187/respcare.09294
11.
Thompson AE
,
Ranard BL
,
Wei Y
,
Jelic S
. Prone Positioning in Awake, Nonintubated Patients With COVID-19 Hypoxemic Respiratory Failure. JAMA Intern Med. 2020 Nov;180(11):1537–9. https://doi.org/10.1001/jamainternmed.2020.3030
12.
Mellemgaard K
. The alveolar-arterial oxygen difference: its size and components in normal man. Acta Physiol Scand. 1966 May;67(1):10–20. https://doi.org/10.1111/j.1748-1716.1966.tb03281.x
13.
Weatherald J
,
Solverson K
,
Zuege DJ
,
Loroff N
,
Fiest KM
,
Parhar KK
. Awake prone positioning for COVID-19 hypoxemic respiratory failure: A rapid review. J Crit Care. 2021 Feb;61:63–70. https://doi.org/10.1016/j.jcrc.2020.08.018
