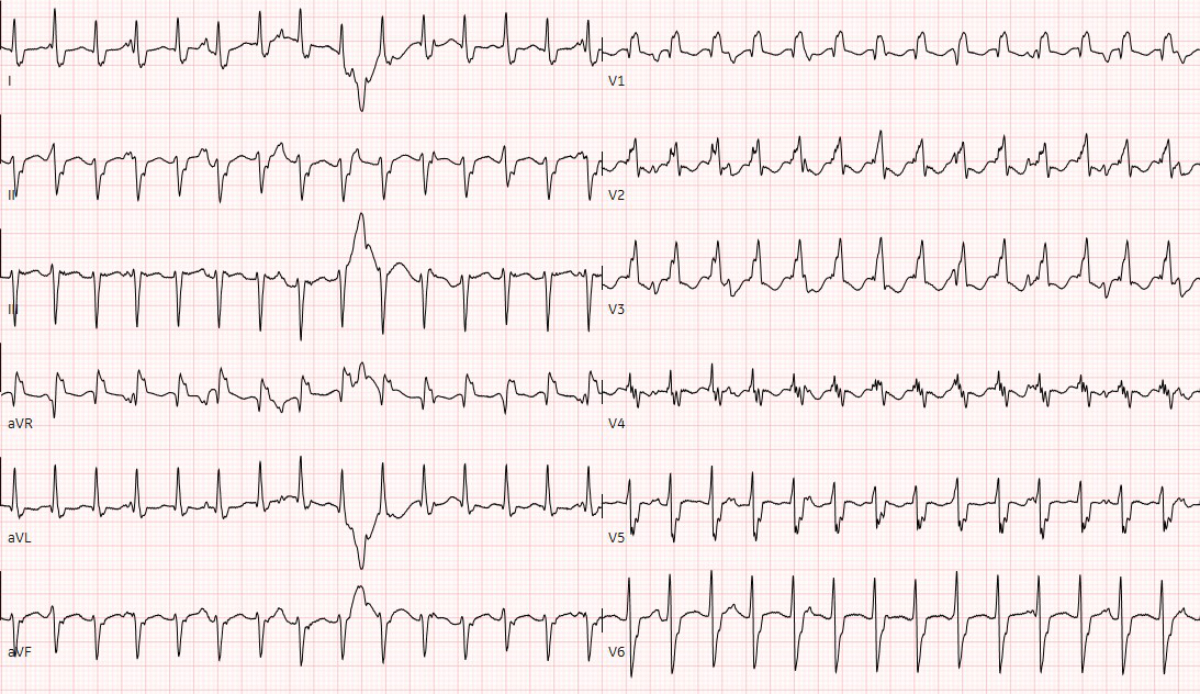
Figure 1 Twelve-lead electrocardiography at admission showed the wide complex ventricular tachycardia with a heart rate of 176 per minute.
DOI: https://doi.org/10.4414/SMW.2022.w30214
Children and adults with a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are at risk for myocarditis [1, 2]. This myocarditis probably results from a combination of direct myocardial damage due to a downregulation of angiotensin II converting-enzyme (ACE-2) receptors caused by the viral spike proteins leading to local cell dysfunction and inflammation [1–4], and from a systemic hyperinflammatory condition leading to a cardiotoxic catecholamine and cytokine storm [5].
Definite diagnosis of myocarditis normally results from histological findings of endomyocardial biopsies [6], but these are rarely performed in suspected cases of COVID-19-associated and other forms of myocarditis potentially triggered by viruses [1, 7]. Nevertheless, particles of SARS-CoV-2 have been found in different types of cells in the heart, supporting the likely cardiotropic nature of COVID-19 [8, 9]. This includes SARS-CoV-2 viral particles in macrophages found in endomyocardial biopsies [8] as well as virus in interstitial cells in the myocardium [9]. This may be associated with the viral entry mechanism of SARS-CoV-2 using ACE-2 receptors, with down-regulated ACE-2 contributing to the cardiac pathology leading to macrophage activation syndrome [10]. A combination of advanced cardiac magnetic resonance (CMR) techniques, clinical history, and serum biomarkers for acute cardiac failure supports the diagnosis [1].
In this case report we discuss the clinical findings, the endomyocardial biopsy and CMR results of a 15-year-old boy with an active SARS-CoV-2 infection and a monomorphic ventricular tachycardia with severely reduced cardiac function.
A Caucasian 15-year-old adolescent with a history of a SARS-CoV-2 infection with mild respiratory symptoms presented to an external paediatric emergency room on the 8th and 10th day after a polymerase chain reaction (PCR) test positive for SARS-CoV-2, with reduced appetite, gastroenteritis, mild dyspnoea and dizziness without fever. On both occasions, the patient was sent home in a haemodynamically stable clinical state without signs of cardiac disease. Prior to the SARS-CoV-2 infection, the boy had been healthy and free from cardiac symptoms. The family history was negative for rhythm disorders or other congenital cardiac diseases.
Thirteen days after the positive PCR test, he was admitted to our cardiac paediatric intensive care unit in decompensated cardiogenic shock due to a monomorphic ventricular tachycardia (fig. 1) and moderately to severely reduced biventricular cardiac function with a biplane left ventricular ejection fraction of 20%, right ventricular fractional area change of 12%, and fractional shortening of 18% (fig. 2). He was afebrile and the second nasophyrangeal swab for SARS-CoV-2 PCR was still positive after admission. On admission troponin-T was 48 ng/l (reference <14 ng/l), creatinine kinase (CK) 201 U/l (<168 U/l), CK-MB 7.3 µg/l (<6.2 µg/l), and N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) 10,738 ng/l (<360 ng/l). There were no signs of thrombocytopenia, increased C-reactive protein, and no elevation or decrease of white blood cells or abnormalities in the white blood cells subsets. The clinical status showed no lymphadenopathy, conjunctivitis, mucus membrane changes, rash, or swollen hands and feet. The ventricular tachycardia was recurrent and required electric cardioversion on three occasions within the first 24 hours, in addition to amiodarone therapy. The severely reduced biventricular myocardial function led to progressive pulmonary oedema, pericardial and pleural effusions, and oxygenation failure requiring intubation, high ventilator pressures, inhaled nitric oxide, and bilateral chest drains. A suspected secondary bacterial pneumonia due to the severe clinical (septic) course was treated empirically for 5 days with antibiotics, although cultures remained negative. He was not given additional antiviral therapy due to limited evidence and spontaneous clinical recovery. Progressive low cardiac output with cardiogenic shock and multi-organ dysfunction (acute renal failure RIFLE-I [11], liver dysfunction with coagulopathy) required high-dose inovasodilators. On day 4, creatinine kinase (maximum 2040 U/l) and myoglobin (maximum 669 ng/ml) were at their highest level. The following ECG revealed altered repolarisation (negative T wave up to V6), but no signs of acute infarction. The myocardial function measured by echocardiography improved rapidly, with a left ventricular ejection fraction of 40–50% within 48 hours of admission. After 5 days of inotropic treatment and mechanical ventilation he was transferred to the cardiac ward under treatment with oral diuretics, enalapril, and amiodarone.

Figure 1 Twelve-lead electrocardiography at admission showed the wide complex ventricular tachycardia with a heart rate of 176 per minute.
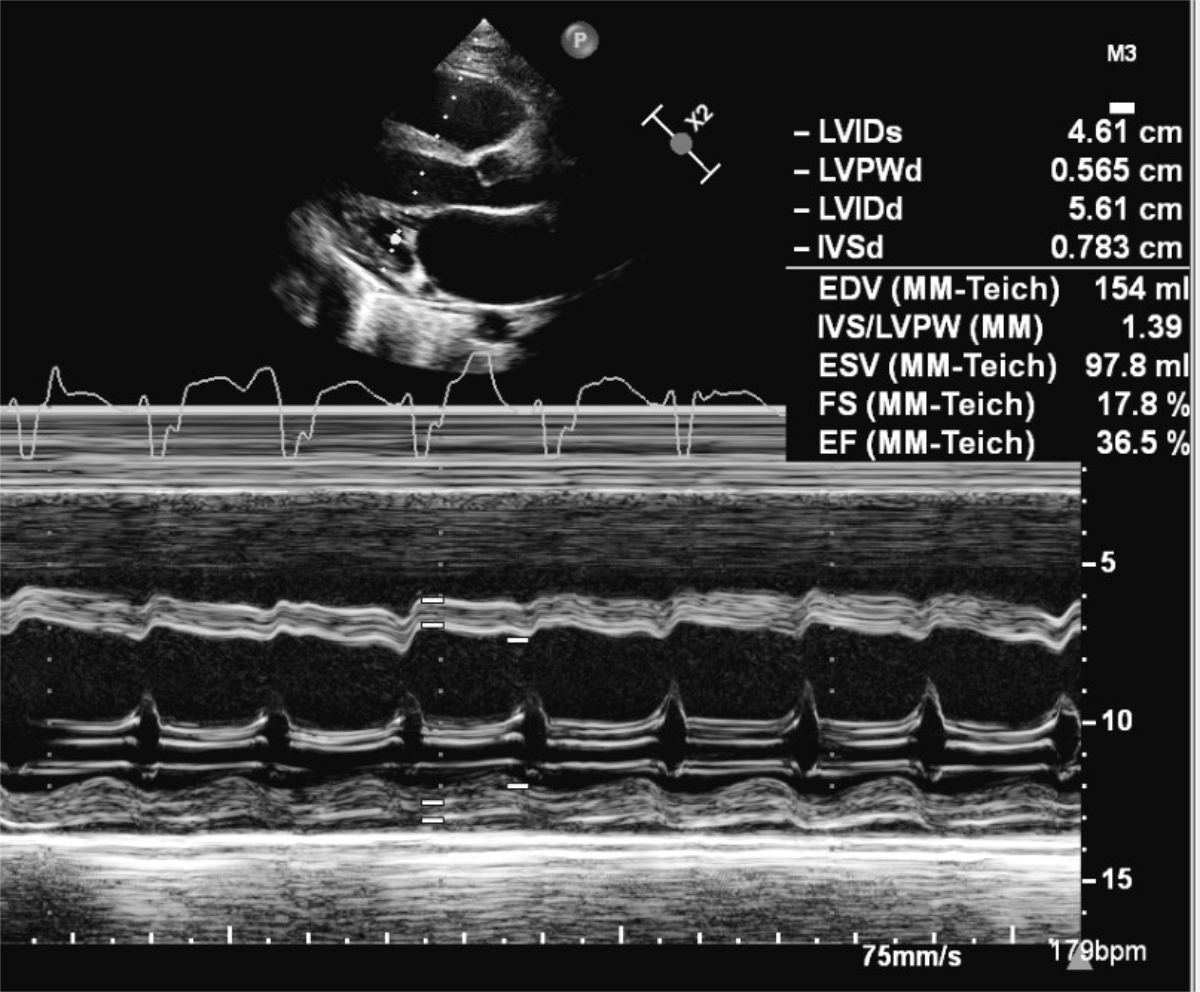
Figure 2 Transthoracic 2D echocardiography at admission showed severe reduced left ventricular function on M-mode with a fractional shortening of 18%.
A broad spectrum of diagnostic tests was performed. A genetic and metabolic cardiomyopathy as well as infection other than SARS-CoV-2 were ruled out by serology, in the biopsy, and with a multiplex PCR for respiratory pathogens (BioFire®). So far, there had been no pathological evidence for genetic and metabolic diseases after genetic counselling and metabolic testing including hereditary storage disease, carnitine deficiency disease and other metabolic diseases affecting energy metabolism. The multiplex PCR includes enterovirus, coxsackievirus A-B, echovirus, parvovirus B19, human herpesvirus 6, Epstein-Barr Virus, adenovirus, human cytomegalovirus, herpes simplex virus type 1 and 2, human herpesvirus 7, Toxoplasma gondii, and Borrelia spp. He received one dose of intravenous immunoglobulin (2 g/kg) as an immunomodulatory treatment for suspected SARS-CoV-2 myocarditis.
Four days after admission, the patient underwent an extensive cardiovascular MRI examination in a 1.5 T scanner for assessment of ventricular function and myocardial tissue characterisation. Both ventricles were dilated, and ejection fractions were markedly reduced (left ventricular ejection fraction 31%, right ventricular ejection fraction 42%). There was a pericardial effusion (fig. 3). High-resolution 3D steady-state free precession (SSFP) sequence did not reveal coronary abnormalities. No myocardial oedema or myocardial contrast enhancement were detected using a short-axis stack of fat-saturated T2 images and late-gadolinium enhancement images acquired with an inversion recovery gradient echo pulse sequence. However, T1 and T2 mapping revealed prolonged relaxation times of 1100 ms and 62 ms in comparison with normal values [12–14], respectively.
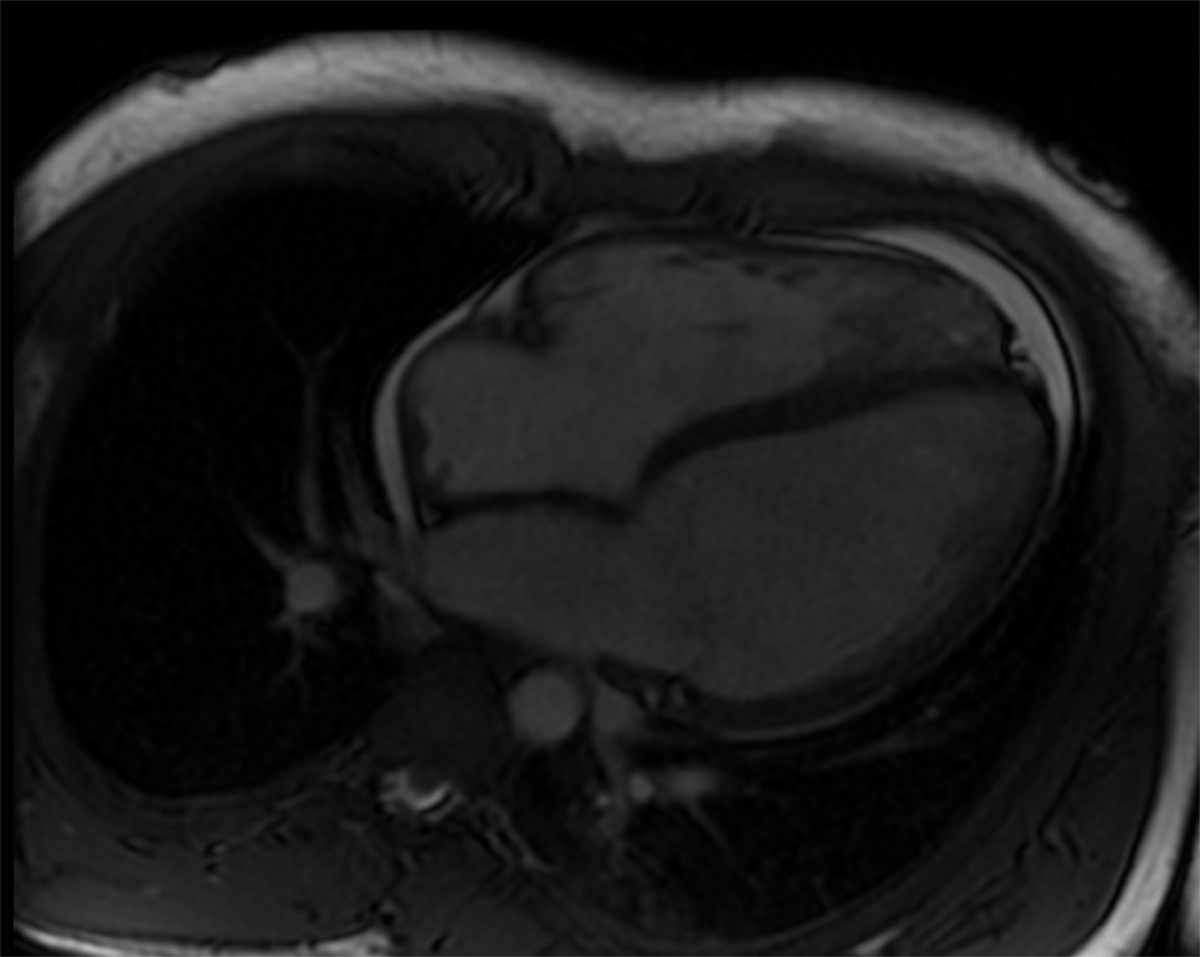
Figure 3 Four-chamber view of the magnetic resonance imaging examination in a 1.5 T scanner. Both ventricles are dilated, and ejection fractions are markedly reduced (left ventricular ejection fraction 31%, right ventricular ejection fraction 42%). There was a pericardial effusion.
Six days after admission, the cardiac haemodynamics was invasively evaluated with a cardiac catheterisation and showed a normal left ventricular end-diastolic pressure without pulmonary hypertension. Coronary anomaly was ruled out. A total of 12 endomyocardial biopsies were taken from the lateral wall and septum of the right ventricle. The histological results (fig. 4) were not consistent with an acute/chronic lymphocytic, eosinophilic, or giant-cell myocarditis, or dilated cardiomyopathy (fig. 4A and B). Morphologically, the numbers of TCD3+ T cells (fig. 4C) were normal, but CD68+ macrophages (fig. 4D) were increased in the myocardium. Nested (RT-) PCR investigations revealed no viral or bacterial DNA/RNA.
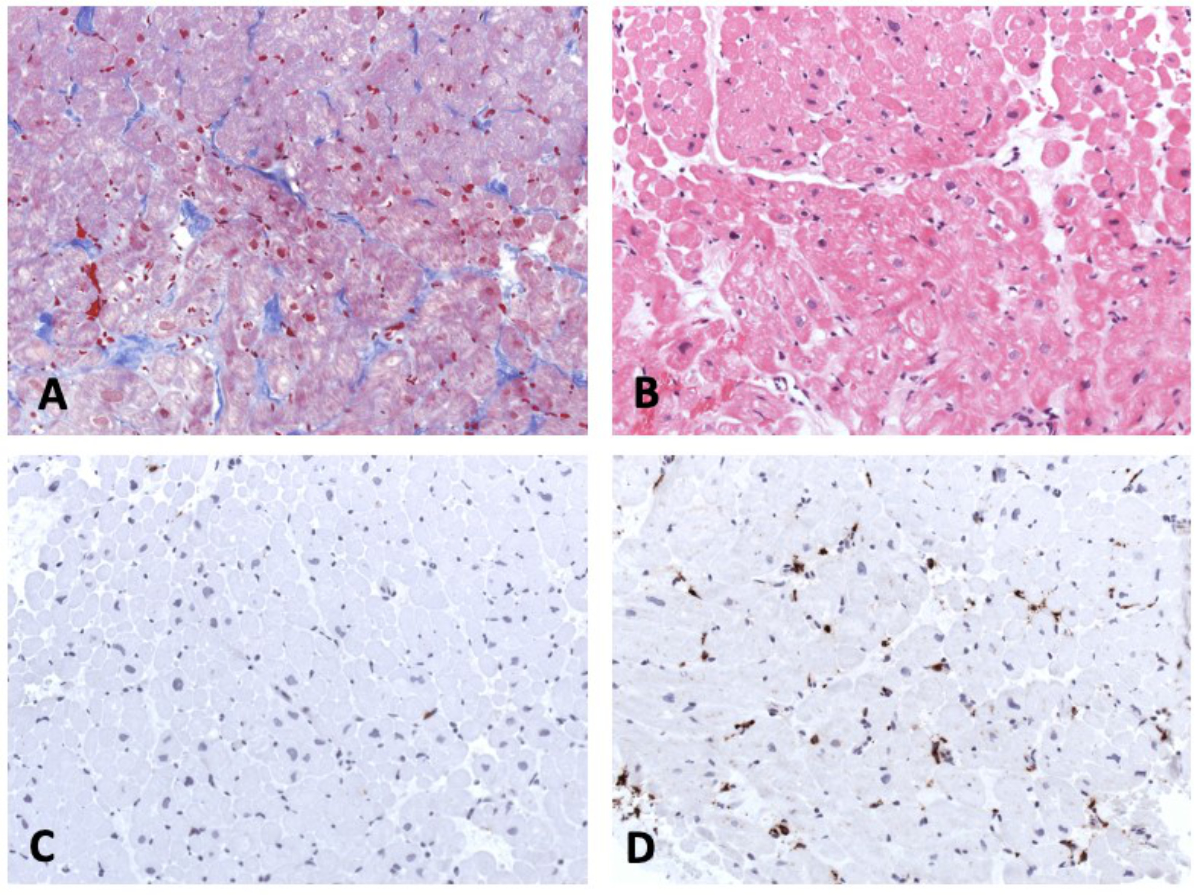
Figure 4 Endomyocardial biopsy. Masson trichrome staining (A),haematoxylin-eosin staining (B), immunohistochemistry for detection of CD3+ T cells (C) and CD68+ macrophages (D). Magnification x200.
Ten days after admission, the patient showed a sinus rhythm with normal de- and repolarisation on repeated 12-lead ECGs and 24-hour ECG. The cardiac biomarkers normalised. The left ventricular ejection fraction (biplane) improved to 55%, and the patient was discharged home in a good clinical status on oral enalapril and amiodarone 17 days after admission.
Taking the previously published literature into account, we interpreted the patients’ ventricular tachycardia and severely reduced cardiac function of our patient as signs of a SARS-CoV-2-induced myocarditis [1, 2], although the biopsy alone did not meet criteria for definitive diagnosis of myocarditis according to the European Society of Cardiology (ESC) criteria [15].
Acute myocarditis and myocardial dysfunction are reported in patients with Paediatric Inflammatory Multisystem Syndrome Temporally related to SARS-CoV-2 (PIMS-TS) [16, 17]. Nevertheless, the absence of pathologies within the coronary arteries, the absent clinical symptoms (e.g., no conjunctivitis, no rash), and the short time interval between the acute infection and ventricular dysfunction were not suggestive for PIMS-TS [17]. In addition, ventricular arrhythmia is not frequent in PIMS-TS patients, being found in <2% of cases [17, 18]. However ventricular arrhythmia has been described in the setting of acute COVID-19 infection in childhood. Gozar et al. described the case of a fetal and neonatal ventricular tachycardia associated with an acute neonatal COVID-19 infection [19]. A further case of a 5-year-old boy with a ventricular tachycardia from acute fulminant myocarditis secondary to acute COVID-19 infection has been described [20]. Both these examples are consistent with our case. Normalisation of the cardiac function in our case was more rapid than would typically be seen in viral myocarditis with moderate-to-severe reduced biventricular function. It seems likely that the prolonged and recurrent ventricular tachycardia induced further myocardial dysfunction in addition to that from the myocarditis alone.
In conclusion, the diagnosis of PIMS remains difficult, especially because so far limited data are available of histological findings in both paediatric and adult patients with PIMS-TS [17, 18, 21].
In our institution, endomyocardial biopsies are part of the standard care in the case of suspected myocarditis or cardiomyopathy. This is a safe procedure, even in the acute phase of myocarditis if performed by experienced paediatric interventionalists [22]. The results of our biopsy, revealing increased numbers of macrophages, are consisted with observations in adult COVID-19 patients with myocarditis [23, 24]. In a series of 21 patients who died from COVID and had cardiac histopathology available, lymphocytic myocarditis was observed only in 3 (14%) patients, although interstitial macrophage infiltration was increased in 18 (86%) patients including 2 of those with lymphocytic myocarditis and 16 with no evidence of myocarditis [23]. In endomyocardial biopsies of patients with SARS-CoV-2 a substantial upregulation of MAPK-associated pathways and of the complement system were observed, representing pathogenetic factors in this disease, although the pathway is not completely understood [24].
Two other case reports of endomyocardial biopsies in children with SARS-CoV-2-associated myocarditis have been described [25, 26]. First, a case of a 17-year-old boy with eosinophilic myocarditis in a post-mortem biopsy [25] and second a case of an 11-year-old girl with acute COVID-19 infection and cardiac dysfunction with interstitial oedema, and increased macrophages and T-lymphocytes [26]. SARS-CoV-2 PCR in the endomyocardial biopsy was negative in both cases [25, 26]. In the second case, myocarditis derived from central cytokine-driven damage (in line with the interstitial oedema of the biopsy) was postulated [26]. The potential therapeutic impact of intravenous immunoglobulins has been described in a young adult [27].
Cardiovascular MRI plays an important role in evaluation of COVID-19-related cardiac involvement including coronary artery alterations, ventricular impairment, late-gadolinium enhancement, and myocardial tissue and pericardial abnormalities [28]. Given the wide range of potential COVID-19-associated cardiac pathologies, it is important to include T1- and T2-mapping sequences in the cardiac MRI protocol to detect subtle myocardial alterations that could be missed otherwise. Our findings of prolonged T1 and T2 relaxation times are in line with the current literature [29, 30]. T1 and T2 mapping abnormalities seem to be frequent findings in COVID-associated myocarditis [30]. Mapping techniques have recently been added to the consensus recommendations (Lake Louise criteria) for improved CMR diagnosis of myocardial inflammation [31].
T1 and T2 mapping as tools for detecting diffuse myocardial fibrosis and oedema may act as imaging biomarkers in COVID-19 myocarditis. The pathological mapping results confirm the cardiac involvement in our patient, in addition to ventricular dilation and impairment, as well as signs of pericarditis.
The cardiac biomarkers do not help in the differential diagnosis between PIMS-TS and acute myocarditis since they are usually high in all tested patients [1, 2].
Our patient recovered well and this is in line with most of the reported SARS-CoV-2- myocarditis cases [1, 2].
We hypothesise that the ventricular tachycardia combined with reduced ventricular function 2 weeks after an acute SARS-CoV-2 infection may be considered as a result of an acute SARS-CoV-2 myocardial affection. Endomyocardial biopsy samples revealed macrophage-rich inflammation of the myocardium. Nevertheless, the definitive diagnosis remains challenging due to limited data on myocardial affection in children. Combined interpretation of clinical findings, EMB results and advanced CMR assessment including T1 and T2 mapping seems reasonable in patients with documented recent SARS-CoV-2 infection and suspected myocarditis.
The authors had no financial support.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Das BB , Sexon Tejtel SK , Deshpande S , Shekerdemian LS . A Review of the Cardiac and Cardiovascular Effects of COVID-19 in Adults and Children. Tex Heart Inst J. 2021 Jul;48(3):e207395. https://doi.org/10.14503/THIJ-20-7395
2. Boehmer TK , Kompaniyets L , Lavery AM , Hsu J , Ko JY , Yusuf H , et al. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data - United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021 Sep;70(35):1228–32. https://doi.org/10.15585/mmwr.mm7035e5
3. Chen L , Li X , Chen M , Feng Y , Xiong C . The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020 May;116(6):1097–100. https://doi.org/10.1093/cvr/cvaa078
4. Li W , Moore MJ , Vasilieva N , Sui J , Wong SK , Berne MA , et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 Nov;426(6965):450–4. https://doi.org/10.1038/nature02145
5. de Cevins C , Luka M , Smith N , Meynier S , Magérus A , Carbone F , et al.; Pediatric-Biocovid Study Group . A monocyte/dendritic cell molecular signature of SARS-CoV-2-related multisystem inflammatory syndrome in children with severe myocarditis. Med (N Y). 2021 Sep;2(9):1072–1092.e7. https://doi.org/10.1016/j.medj.2021.08.002
6. Anderson L , Pennell D . The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Eur Heart J. 2008 Jul;29(13):1696–1696. https://doi.org/10.1093/eurheartj/ehn189
7. Fried JA , Ramasubbu K , Bhatt R , Topkara VK , Clerkin KJ , Horn E , et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation. 2020 Jun;141(23):1930–6. https://doi.org/10.1161/CIRCULATIONAHA.120.047164
8. Tavazzi G , Pellegrini C , Maurelli M , Belliato M , Sciutti F , Bottazzi A , et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020 May;22(5):911–5. https://doi.org/10.1002/ejhf.1828
9. Bojkova D , Wagner JU , Shumliakivska M , Aslan GS , Saleem U , Hansen A , et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020 Dec;116(14):2207–15. https://doi.org/10.1093/cvr/cvaa267
10. Banu N , Panikar SS , Leal LR , Leal AR . Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: therapeutic implications. Life Sci. 2020 Sep;256:117905. https://doi.org/10.1016/j.lfs.2020.117905
11. Abosaif N , Tolba Y . RIFLE classification of acute kidney failure in intensive care. Br J Hosp Med (Lond). 2007 Jun;68(6):304–6. https://doi.org/10.12968/hmed.2007.68.6.23569
12. Burkhardt BE , Menghini C , Rücker B , Kellenberger CJ , Valsangiacomo Buechel ER . Normal myocardial native T1 values in children using single-point saturation recovery and modified look-locker inversion recovery (MOLLI). J Magn Reson Imaging. 2020 Mar;51(3):897–903. https://doi.org/10.1002/jmri.26910
13. Maceira AM , Monmeneu JV , Igual-Muñoz B , Lopez-Lereu PM , Garcia PM , Cosin J . Reference values for regional and global myocardial T2 mapping with cardiovascular magnetic resonance at 1.5T and 3T. J Cardiovasc Magn Reson. 2015 Feb;17(1):12. https://doi.org/10.1186/1532-429X-17-S1-P12
14. Alsaied T , Tseng SY , Siddiqui S , Patel P , Khoury PR , Crotty EJ , et al. Pediatric Myocardial T1 and T2 Value Associations with Age and Heart Rate at 1.5 T. Pediatr Cardiol. 2021 Feb;42(2):269–77. https://doi.org/10.1007/s00246-020-02479-9
15. Caforio ALP , Pankuweit S , Arbustini E , Basso C , Gimeno-Blanes J , Felix SB , et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013 Sep;34(33):2636–48, 2648a–2648d.
16. Toubiana J , Poirault C , Corsia A , Bajolle F , Fourgeaud J , Angoulvant F , et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020 Jun;369:m2094. https://doi.org/10.1136/bmj.m2094
17. Henrina J , Putra IC , Lawrensia S , Marta DS , Wijaya E , Saboe A , et al. Cardiac manifestations, treatment characteristics, and outcomes of paediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2: A systematic review. Prog Pediatr Cardiol. 2021 Dec;63:101365. https://doi.org/10.1016/j.ppedcard.2021.101365
18. Cantarutti N , Battista V , Adorisio R , Cicenia M , Campanello C , Listo E , et al. Cardiac Manifestations in Children with SARS-COV-2 Infection: 1-Year Pediatric Multicenter Experience. Children (Basel). 2021 Aug;8(8):717. https://doi.org/10.3390/children8080717
19. Gozar L , Șuteu CC , Gabor-Miklosi D , Cerghit-Paler A , Făgărășan A . Diagnostic Difficulties in a Case of Fetal Ventricular Tachycardia Associated with Neonatal COVID Infection: case Report. Int J Environ Res Public Health. 2021 Dec;18(23):12796. https://doi.org/10.3390/ijerph182312796
20. Tseng YS , Herron C , Garcia R , Cashen K . Sustained ventricular tachycardia in a paediatric patient with acute COVID-19 myocarditis. Cardiol Young. 2021 Sep;31(9):1510–2. https://doi.org/10.1017/S1047951121000792
21. Bemtgen X , Klingel K , Hufnagel M , Janda A , Bode C , Staudacher DL , et al. Case Report: Lymphohistiocytic Myocarditis With Severe Cardiogenic Shock Requiring Mechanical Cardiocirculatory Support in Multisystem Inflammatory Syndrome Following SARS-CoV-2 Infection. Front Cardiovasc Med. 2021 Sep;8:716198. https://doi.org/10.3389/fcvm.2021.716198
22. Brighenti M , Donti A , Giulia Gagliardi M , Maschietto N , Marini D , Lombardi M , et al.; Italian Society of Pediatric Cardiology . Endomyocardial biopsy safety and clinical yield in pediatric myocarditis: an Italian perspective. Catheter Cardiovasc Interv. 2016 Mar;87(4):762–7. https://doi.org/10.1002/ccd.26319
23. Basso C , Leone O , Rizzo S , De Gaspari M , van der Wal AC , Aubry MC , et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020 Oct;41(39):3827–35. https://doi.org/10.1093/eurheartj/ehaa664
24. Weckbach LT , Schweizer L , Kraechan A , Bieber S , Ishikawa-Ankerhold H , Hausleiter J , et al.; EMB Study Group . Association of Complement and MAPK Activation With SARS-CoV-2-Associated Myocardial Inflammation. JAMA Cardiol. 2022 Mar;7(3):286–97. https://doi.org/10.1001/jamacardio.2021.5133
25. Craver R , Huber S , Sandomirsky M , McKenna D , Schieffelin J , Finger L . Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol. 2020 Jun;39(3):263–8. https://doi.org/10.1080/15513815.2020.1761491
26. Laurence C , Haini M , Thiruchelvam T , Derrick G , Burch M , Yates RW , et al. Endomyocardial Biopsy in a Pediatric Patient With Cardiac Manifestations of COVID-19. Circ Heart Fail. 2020 Nov;13(11):e007384. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007384
27. Hu H , Ma F , Wei X , Fang Y . Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin [Erratum in: Eur Heart J. 2021 Jan 7;42] [2] [:191. PMID: 32176300; PMCID: PMC7184348]. Eur Heart J. 2021 Jan;42(2):206. https://doi.org/10.1093/eurheartj/ehaa190
28. Petersen SE , Friedrich MG , Leiner T , Elias MD , Ferreira VM , Fenski M , et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC cardiovasc imaging (Print) [Internet]. 2021 [cited 2022 Jan 22]; Available from: https://dx.doi.org/https://doi.org/10.1016/j.jcmg.2021.08.021
29. Galea N , Marchitelli L , Pambianchi G , Catapano F , Cundari G , Birtolo LI , et al. T2-mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID-19: a Cardiovascular Magnetic Resonance study. J Cardiovasc Magn Reson. 2021 Jun;23(1):68. https://doi.org/10.1186/s12968-021-00764-x
30. Ojha V , Verma M , Pandey NN , Mani A , Malhi AS , Kumar S , et al. Cardiac Magnetic Resonance Imaging in Coronavirus Disease 2019 (COVID-19): A Systematic Review of Cardiac Magnetic Resonance Imaging Findings in 199 Patients. J Thorac Imaging. 2021 Mar;36(2):73–83. https://doi.org/10.1097/RTI.0000000000000574
31. Ferreira VM , Schulz-Menger J , Holmvang G , Kramer CM , Carbone I , Sechtem U , et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: expert Recommendations. J Am Coll Cardiol. 2018 Dec;72(24):3158–76. https://doi.org/10.1016/j.jacc.2018.09.072