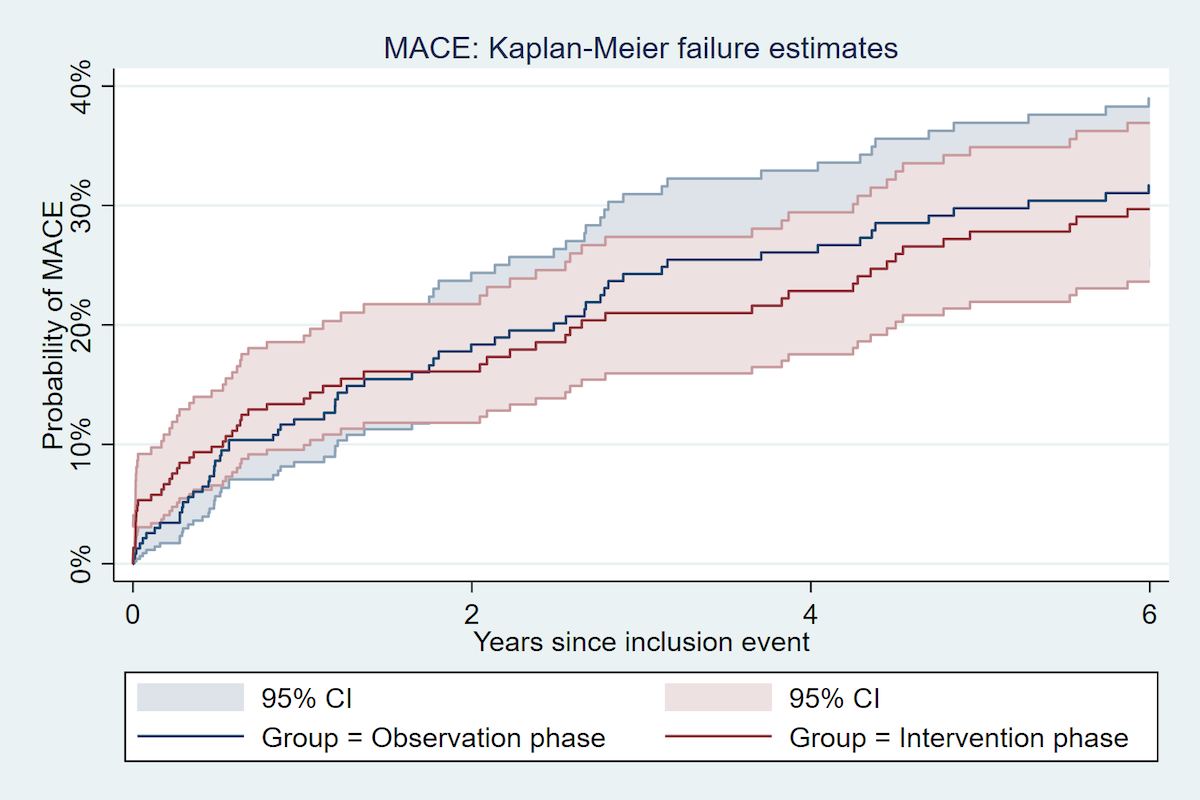
Figure 1 Incidence of Major adverse cardiovascular event 5 years after an acute coronary syndrome, by study phase.
DOI: https://doi.org/10.4414/SMW.2022.w30209
Smoking is an established risk factor for coronary heart disease [1, 2]. Quitting reduces mortality and further cardiac events after acute coronary syndromes (ACS) [3]. Three years after quitting, the risk of recurrent cardiovascular events is decreased to the level of nonsmokers after ACS [4]. It has also been shown that smoking cessation counselling is more effective in hospitalised patients with ACS than in the general population [5]. Thus, smoking cessation counselling for ACS patients is universally recommended by cardiovascular disease prevention guidelines [6].
There are several intensities of smoking cessation counselling interventions for smokers [5, 7–9]. Intensive interventions begun in hospital and sustained after discharge are more likely to increase smoking abstinence than less intense interventions [8, 10]. Furthermore, smoking cessation rates increase when smokers are systematically identified and counselled after ACS [11]. We previously demonstrated an increase in uptake of counselling when smokers admitted for ACS were proactively offered motivational interviewing [12]. Even in smokers not motivated to quit, combining systematic in-hospital and telephone counselling sessions over 2 months seemed to increase smoking abstinence at 12 months, but without reaching statistical significance compared with opportunistic counselling [12]. The long-term benefits of systematic smoking interventions at the time of ACS might, however, be greater because repeated exposure to motivational interviewing shortly after an ACS may enhance the decision to quit, although this has been poorly studied. Therefore, we aimed to study the long-term rate of smoking abstinence and incidence of major adverse cardiovascular event (MACE), comparing a systematic smoking cessation counselling intervention with opportunistic smoking counselling.
We collected data during the 5-year follow-up visit of ACS patients enrolled in the Smoking-ACS study, a clinical trial embedded in the Special Program University Medicine-Acute Coronary Syndromes (SPUM-ACS) observational study (clinical trial registration: NCT01000701) [12, 13]. The SPUM-ACS study is an observational prospective Swiss cohort of patients hospitalised for ACS in four university hospitals in Switzerland. The SPUM-ACS study was designed to assess quality of care after an index ACS and identify new determinants and consequences of coronary heart disease (CHD) [12–15]. The ACS-Smoking study enrolled all smokers hospitalised for ACS between August 2009 and February 2012, at two intervention sites chosen because they had teams providing reactive smoking cessation interventions to hospitalised smokers as a routine standard care [12]. The ACS-Smoking study tested the efficacy on 1-year smoking abstinence of a systematic intensive smoking cessation counselling intervention based on motivational interviewing over opportunistic counselling. In each study phase, participants were followed up over a period of 5 years to assess smoking cessation and recurrence of cardiovascular events.
Inclusion criteria were age ≥18 years, actively smoking at the time of inclusion, a main diagnosis of ST-segment elevation myocardial infarction (STEMI) for patients presenting after pain onset, non-ST-segment elevation myocardial infarction (NSTEMI), or unstable angina [12, 13]. Exclusion criteria were severe physical disability, inability to give consent owing to dementia, and life expectancy of <1 year for non-cardiac reasons. [12]
At time of the index ACS, smoking status was self-reported. Smoking status was categorised into current, former and never-smokers. Active smoking was defined as smoking one cigarette or more per day for the month preceding the hospital stay. At the 5-year follow-up visit, smoking status was assessed during clinical or telephone follow-up visits based on patient’s self-reported 7 days abstinence information.
The ACS-Smoking trial compared an observational phase with an interventional phase. During the observational phase, from August 2009 to October 2010, patients were offered an in-hospital smoking cessation intervention only if their healthcare provider requested it (opportunistic counselling). The counselling session did not include follow-up after discharge. The interventional phase ran from November 2010 to February 2012. During this phase, smokers with ACS were systematically offered intensive smoking cessation counselling during their hospital stay and were followed up with up to four counselling phone calls within a two-month period after discharge. This was an intensive systematic approach to delivering smoking cessation counselling. Nicotine replacement therapy was proposed at each counselling sessions, independently of the study phase [12].
The primary outcome of this study was smoking abstinence at 5 years after the index ACS. The secondary outcomes were incidences of fatal and non-fatal MACE, as well as all-cause mortality over 5 years of follow-up after the index ACS. Incidents clinical events were adjudicated at each study site by medical doctors who reviewed the patient’s file. MACE was a combination of cardiovascular mortality and non-fatal MACE was defined as myocardial infarction, stent thrombosis, repeat unplanned revascularisation, cerebrovascular event, or repeat hospitalisation due to angina.
Pre-existing cardiovascular disease was defined as a previous diagnosis of coronary heart disease, ischaemic cerebrovascular disease or peripheral artery disease. Education status was dichotomised as having graduated from “high school or university” or having a lower-level education. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or use of blood-pressure lowering drugs. Diabetes was either self-reported or diagnosed by the use of anti-hyperglycaemic medication, or a haemoglobin A1c of 6.5% or greater at admission. Hypercholesterolaemia was total cholesterol >5.0 mmol or 190 mg/dl at admission. Data were entered via standardised, web-based case report forms [12, 16]. At the 5-year visit, we cross checked patient self-reports about medical history, medications and use of nicotine replacement therapy with the information in their medical files.
We reported risk ratio (RR) and its 95% confidence interval (CI) for smoking cessation 5 years after index ACS between the cohorts, taking an intention-to-treat approach. In order to take into account the potential risk of confounding bias in a before-after design, we adjusted for sex, age, education level, and type of ACS (STEMI, NSTEMI, unstable angina) with a Poisson regression method. For survival analyses, all patients were analysed. To deal with the high rate of missing data (24.7%) regarding smoking abstinence at the 5-year follow-up visit , we used a multiple imputation by chained equation. By using the distribution of the observed data, the multiple imputation estimates a set of plausible values for the missing data. Random components are incorporated into these estimated values to reflect their uncertainty. Multiple data sets are created and then analysed individually but identically to obtain a set of parameter estimates [17]. In sensitivity analyses, we also reported results of smoking cessation analyses considering lost to follow-up patients as current smokers.
To compare incidence of 5-year MACE and all-cause mortality between the cohorts, we could not assume proportional hazards as Kaplan Meier curves were crossing. Therefore, we used the flexible parametric modelling for survival analyses (Lambert) for hazard ratios (HRs). We adjusted for sex, age, education level, and type of ACS (STEMI, NSTEMI, unstable angina). In the second model, we further adjusted for history of cardiovascular disease, diabetes, hypertension, hypercholesterolaemia and body mass index (BMI). Potential confounders were determined by biological or clinical plausibility. Participants were followedup until the occurrence of fatal or non-fatal MACE or the end of the study. The SPUM-ACS-Smoking study was initially designed to assess 1-year smoking cessation rates. Therefore, the present study was not powered to demonstrate a difference between groups regarding 5-year smoking cessation rates or incidence of MACE. Statistical significance tests were two-tailed; the alpha level was 0.05. We used Stata version 14.2 (StataCorp LP, College Station, TX, USA) for all statistical analyses.
The study was approved by Medical Ethics Committees of each centre (Lausanne, Geneva) and conforms to the ethical guidelines of the 1975 Declaration of Helsinki (Protocol 07-131 for Ethics Geneva and Protocol 106/09 for Ethics Vaud). All patients gave written informed consent to participate.
Overall, 458 smokers hospitalised with ACS were included in the intervention trial. At 5-year follow-up, 51 (11.1%) had died and 113 (24.7%) were lost to follow-up or refused contact; 286 (62.4%) reported their current smoking status. Eight patients had missing information regarding smoking status although they could be contacted. The lost to follow-up rate was similar in both the observation phase cohort (n = 55, 23.6%), and in the intervention phase cohort (n = 58, 25.8%). Because we used multiple imputation by chained equation, 407 smoking status are analysed for the adjusted risk ratio and 458 for the MACE incidence analysis.
We previously described in detail the characteristics of study participants at the time of the smoking cessation intervention (supplementary table S1 in the appendix) [12]. There was no significant difference between the study groups, except that attendance to cardiovascular rehabilitation was more frequent in the intervention phase cohort than in the observation phase cohort (73% vs 58%) [12]. Patients received more in-hospital intensive counselling and had more frequently nicotine replacement therapy at discharge during the intervention phase than during the observation phase (87% vs 22% and 59% vs 18%, respectively) [12]. At the 5-year visit, in the intervention phase cohort, mean age was 61 years; 20% were women, and 96% had attended at least one medical visit within the last year (table 1). Participants did not differ significantly between cohorts regarding age, gender, medical conditions such as diabetes or hypertension, or drug use. In the intervention phase cohort, 44% of the participants had an education level lower than university compared with 27% in the observation phase cohort.
Table 1Characteristics of patients 5 years after index acute coronary syndrome (ACS), by smoking cessation counselling phase (n = 294).
| 5-year follow up | |||
| Intervention phase cohort (n = 146) | Observation phase cohort (n = 148) | p-Value | |
| Age, years, median (Q1; Q3) | 61 (55; 68) | 63 (57; 69) | 0.09 |
| Female, n (%) | 29 (20%) | 27 (18%) | 0.8 |
| Education level: less than university, n (%) | 64 (44%) | 40 (27%) | 0.002 |
| ACS-type: STEMI | 78 (53%) | 76 (51%) | 0.7 |
| BMI (n = 240), kg/m2, median (Q1,Q3) | 26.7 (24.2; 30.0) | 26.5 (23.7; 30.1) | 1.0 |
| Diabetes (n = 246), n (%) | 36 (25%) | 37 (25%) | 0.8 |
| Hypertensiona (n = 218), n (%) | 18 (12%) | 22 (15%) | 0.9 |
| Hypercholesterolaemiab (n = 259), n (%) | 36 (25%) | 30 (20%) | 0.3 |
| At least one medical visit during the last year (n = 287), n (%) | 140 (96%) | 142 (96%) | 0.7 |
| – By primary care physician (n = 275), n (%) | 131 (90%) | 134 (91%) | 0.8 |
| – By cardiologist (n = 279), n (%) | 111 (75%) | 109 (75%) | 1 |
| Use of nicotine replacement therapy to stop smoking since inclusion (n = 143), n (%) | 12 (16%) | 6 (9%) | 0.2 |
| Use of bupropion to stop smoking since inclusion (n = 287), n (%) | 1 (1%) | 0 | NA |
| Use of veraniclin to stop smoking since inclusion (n = 287), n (%) | 0 | 2 (1%) | NA |
| Antiaggregantc (n = 287,) n (%) | 136 (93%) | 136 (92%) | 0.3 |
| Lipid-lowering drugs (n = 287), n (%) | 115 (79%) | 130 (88%) | 0.1 |
| Nitrate drugs (n = 287), n (%) | 14 (10%) | 20 (14%) | 0.4 |
| Anti-hypertensive drugs (n = 287), n (%) | 118 (81%) | 131 (89%) | 0.2 |
BMI: body mass index; STEMI: ST-segment elevation myocardial infarction
a Systolic blood pressure >140 mm Hg in 3 measurements
b Total cholesterol >5.0 mmol or 190 mg/dl.
c Aspirin, clopidogrel, prasugrel or ticagrelor
The 1-year and 5-year smoking cessation rate are reported in the table 2. One year after ACS, 110/214 (51%) quit smoking in the intervention phase cohort, and 94/217 (43%) in the observation phase cohort. After multiple imputation by chained equation, the adjusted risk ratio for stopping was 1.17 (95% CI 0.89–1.54, p = 0.3). At 5 years, 75/140 (54%) quit smoking in the intervention phase cohort, and 68/146 (47%) in the observation phase cohort, with an adjusted risk ratio with multiple imputation for stopping of 1.13, which also did not reach statistical significance (95% CI 0.84–1.51, p = 0.4). Similar results were obtained in sensitivity analyses considering patients lost to follow-up as current smokers (table S3 in the appendix).
Table 2Smoking cessation prevalence comparing participants in the smoking cessation intervention phase cohort with the observation phase cohort at 1 year (n = 431) and 5 years (n = 286) after the index acute coronary syndrome (ACS), with or without multiple imputation for missing data.
| Intervention phase cohort | Observation phase cohort | Risk ratio (95% CI) | Adjusted risk ratio (95% CI) a | Risk ratio with multiple imputation (95% CI) b | Adjusted risk ratio with multiple imputation (95% CI) a,b | ||
| Smoking cessation | 1-year rate | 110/214 (51%) | 94/217 (43%) | 1.19 (0.97–1.45, p = 0.1) | 1.17 (0.89–1.55, p = 0.3) | 1.19 (0.90–1.56, p = 0.2) | 1.17 (0.89–1.54, p = 0.3) |
| 5-years rate | 75/140 (54%) | 68/146 (47%) | 1.15 (0.91–1.45, p = 0.2) | 1.15 (0.82–1.62, p = 0.4) | 1.11 (0.84–1.48, p = 0.5) | 1.13 (0.84–1.51, p = 0.4) | |
CI: confidence interval
a Adjusted for sex, age, education level and type of ACS.
b 12 patients (5 (2%) in intervention phase cohort and 7 (3%) in observation phase cohort) had no smoking status data at 1 year; 114 patients (59 (29%) in intervention phase cohort and 55 (27%) in observation phase cohort) had no smoking status data at 5 years. We used multiple imputation chained equation to estimate a set of plausible values for the missing data. Therefore, 442 datapoints were analysed at 1 year, 407 were analysed at 5 years.
The 1-year and 5-year incidence of fatal and non-fatal MACE are reported in the table 3 and figures 1 and 2.
Table 3Major adverse cardiovascular events (MACE)a and all-cause mortality comparing participants from the intervention phase cohort with the observation phase cohort at 1 year and 5 years after the index ACS using Lambert flexible parametric modelling (n = 458).
| Intervention phase cohort | Observation phase cohort | Unadjusted | Model 1 multivariable adjustement b | Model 2 multivariable adjustement c | |||||||
| Nb events/patients | Incidence rate, per 100 person-years | Nb events/patients | Incidence rate, per 100 person-years | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95%CI) | p-value | ||
| MACE | 1-year | 31/225 (13.8%) | 14.3 | 28/233 (12.0%) | 12.4 | 1.17 (0.70–1.94) | 0.6 | 1.33 (0.79–2.24) | 0.3 | 1.27 (0.75–2.15) | 0.4 |
| 5-years | 60/225 (26.7%) | 6.2 | 66/233 (28.3%) | 6.9 | 0.93 (0.65–1.32) | 0.7 | 1.09 (0.76–1.55) | 0.6 | 1.04 (0.73–1.50) | 0.8 | |
| Non-fatal MACE | 1-year | 27/225 (12.0%) | 12.4 | 25/233 (10.7%) | 11.1 | 1.14 (0.66–1.96) | 0.6 | 1.21 (0.70–2.10) | 0.5 | 1.18 (0.68–2.07) | 0.6 |
| 5-years | 56/225 (24.9%) | 5.8 | 59/233 (25.3%) | 6.1 | 0.97 (0.67–1.40) | 0.9 | 1.10 (0.76–1.59) | 0.6 | 1.06 (0.73–1.55) | 0.8 | |
| All-cause mortality | 1-year | 7/225 (3.1%) | 2.9 | 9/233 (3.8%) | 3.7 | 0.79 (0.29–2.12) | 0.6 | 1.08 (0.39–3.04) | 0.9 | 1.19 (0.39–3.64) | 0.8 |
| 5-years | 18/225 (9.3%) | 1.7 | 29/233 (13.3%) | 2.6 | 0.64 (0.35–1.15) | 0.13 | 0.89 (0.49–1.62) | 0.7 | 0.84 (0.45–1.60) | 0.6 | |
a MACE: cardiovascular mortality, myocardial infarction, stent thrombosis, repeat unplanned revascularisation, cerebrovascular event, or repeat hospitalisation due to angina.
b Model 1: adjusted for age, sex, education level, type of acute coronary syndrome (ACS)
c Model 2: adjusted for age, sex, education level, ACS type, diabetes, body mass index, hypertension, hypercholesterolaemia, previous cardiovascular disease.

Figure 1 Incidence of Major adverse cardiovascular event 5 years after an acute coronary syndrome, by study phase.
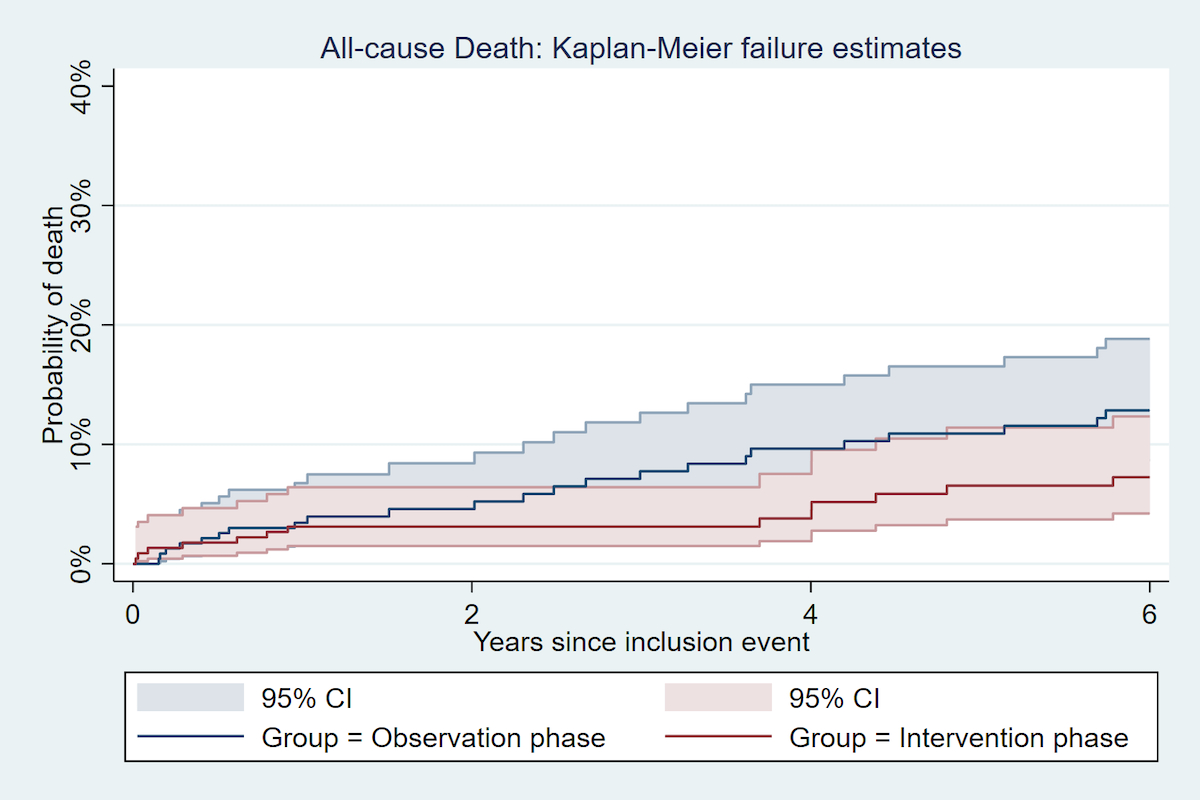
Figure 2 Incidence of all-cause Death 5 years after an acute coronary syndrome, by study phase.
Compared with the observation phase cohort, patients from the intervention phase cohort had a similar risk of MACE at 1 year, with an unadjusted HR of 1.17 (95% CI 0.70–1.94, p = 0.6), and a multivariate adjusted HR of 1.27 (95% CI 0.75–2.15, p = 0.4). At 5 years, compared with the observation phase cohort, patients in the intervention phase cohort had a similar risk of MACE with an unadjusted HR of 0.93 (95% CI 0.65–1.32, p = 0.7), and a multivariate adjusted HR of 1.04 (95% CI 0.73–1.50, p = 0.8). Similar results were found when examining non-fatal MACE only. The multivariate adjusted hazard ratio for all-cause mortality was 0.84 (95% CI: 0.45–1.60, p = 0.6) in the comparison of the intervention phase cohort with the observation phase cohort.
In this 5-year follow-up multicentre clinical study of smokers hospitalised for ACS, we found that smoking cessation counselling provided to all smokers at the time of their ACS, as compared with opportunistic counselling, did not increase long-term smoking cessation rates. We reported that about half of smokers were free from tobacco 5 years after their hospitalisation for ACS, with an absolute difference of abstinence of 7% between study groups, which did not reach statistical significance. We also found that nearly a third of ACS smokers experience a recurrent cardiovascular event over 5 years. This cardiovascular event rate and all-cause mortality were similar when systematic smoking cessation counselling at hospital was compared with opportunistic counselling.
Our findings differ from results of a previous systematic review indicating that smoking cessation counselling interventions delivered during hospitalisation and lasting at least a month after discharge increased the smoking cessation rate [8]. However, our study assessed a different population because our intervention focused on systematic counselling of all ACS smokers, independently of motivation to quit. Therefore, our study addressed a different research question from studies that only included smokers motivated to quit. Among the few other trials that studied the long-term efficacy of smoking cessation counselling over a period of 4 years or more [18–20], most studied patients without previous cardiovascular disease, and none assessed systematic counselling vs opportunistic counselling. Anthonisen et al. offered smoking patients with chronic obstructive pulmonary disease (COPD) an intervention programme of 12 two-hour group sessions during 10 weeks followed by a maintenance programme, and compared this to the usual care. After 5 years, the abstinence rate was higher in the intervention group than the usual care group (21.7% vs 5.4%) [18]. The Nohlert et al. trial compared high versus low-intensity treatment for smoking cessation for 40 minutes counselling during 4 months for healthy patients. After 5–8 years of follow up, the abstinence rate was higher in the high-intensity than in the low-intensity treatment group (31% vs 24%) [19]. In the Lou et al. trial, ambulatory behavioural intervention (home visit 1/week for 1 year then 1/month) was given to COPD patienst for 2 years. After 4 years of follow up, smoking abstinence rates were significantly higher in participants receiving behavioural intervention than those receiving usual care (44.3 vs 5.1%) [20]. As reported previously, our systematic smoking cessation intervention led to an increase in prescriptions for nicotine replacement therapy at discharge compared with the observation phase [12]. After 5 years, the use of nicotine replacement therapy to stop smoking remained slightly higher in the intervention phase than in the observation phase. These differences can be related to the higher proportion of prescriptions for nicotine replacement therapy at hospital discharge during the intervention phase. Overall, these results confirm that the more intensive and the longer the counselling, the higher the long-term smoking cessation rates [18–20].
In addition to the smoking cessation rate, our study assessed the 5-year cardiovascular event rate and mortality with systematic smoking cessation counselling at the time of ACS. Although not statistically significant, we found that all-cause mortality was 4% lower in the intervention phase cohort than in the observational phase cohort. The hazard ratio remained shifted towards benefit even after multivariable adjustment such as for diabetes or pre-existing cardiovascular disease. However, as education level was higher in the intervention phase than in the observation phase, the differences of clinical outcomes observed between study phase could be partially explained by education. After multivariable adjustment including education level, the strength of association of the intervention with mortality was reduced.
In most previous trials of smoking cessation counselling interventions after ACS, a shorter follow-up period for cardiovascular event incidence was reported [10]. Mohiuddin et al. studied the 2-year benefit on smoking cessation, hospitalisation and mortality of a smoking cessation intervention among smokers with cardiovascular disease [10]. After a follow-up of 2 years, they found higher pharmacotherapy utilisation, a higher smoking abstinence rate and lower all-cause mortality and hospitalisation rates in the intervention group. The investigator conducted an intensive smoking cessation counselling intervention with post-discharge counselling sessions. The smoking counselling was approximately 60 min/week for at least 12 weeks and individualised pharmacotherapy was provided at no cost to the intervention group. In comparison with our study, their intervention was not systematically provided to all smokers and their smoking counselling intervention was more intensive than ours. These differences may explain the absence of cardiovascular benefit reported in our study.
Our study has limitations. First, the sample size was not powered to show differences in long-term smoking abstinence nor a significant reduction of the 5-year recurrence of MACE. Therefore, our study results should be interpreted using point estimates instead of confidence intervals. Second, our design was a before-after study. Despite multivariable adjustments, unmeasured differences between participants of the intervention phase cohort and the observation phase cohort may limit comparison of MACE incidence between cohorts. Third, smoking cessation at the 5-year follow-up visit was self-reported. According to a systematic review, smoking abstinence based on self-reports could be overestimated [21]. However, this potential overestimation can be expected to be similar in both the observation phase and intervention phase cohorts and should not affect group comparisons. Fourth, missing information due to death or lost to follow up was very high 5 years after the index ACS. For applying various advanced statistical methods to account for the high lost-to-follow-up rate, we could only relay on our restricted dataset. Observing similar results by including all participants lost to follow-up as smokers or applying multiple imputation strategies for the main outcome and for missing covariables strengthened our confidence in the findings.
In this controlled long-term interventional study, systematic intensive smoking cessation counselling in all hospitalised smokers with ACS did not significantly increase 5-year smoking cessation rates, nor decrease cardiovascular event recurrence, as compared with opportunistic smoking cessation counselling during hospitalisation. Adequately powered studies for long-term smoking abstinence and recurrence of MACE should be performed.
Original data of the SPUM-ACS study are available for secondary analyses upon acceptance of a research protocol by the study steering committee. Original data for this study will be made available upon request based on a signed data transfer agreement. For data access, please first contact the corresponding author.
The SPUM-ACS cohort was supported by the Swiss National Science Foundation (SNSF 33CM30-124112, Inflammation and acute coronary syndromes (ACS) – Novel strategies for prevention and clinical management, andSNSF 32473B_163271, Long-term benefit of the multi-center, multi-dimensional secondary prevention program in patients with acute coronary syndromes) and unrestricted grant from AstraZeneca, Switzerland, Eli Lilly USA, and Medtronic, Switzerland We acknowledge the cooperation of all participating centers, practicing physicians, referring doctors and institutions. This project was also supported by a research grant from the Swiss Tobacco Prevention Fund (FPT 10.000046), the Department of University Medicine and Community Care (DUMSC) of the University of Lausanne, Switzerland and the Swiss Heart Foundation. None of the funding bodies had any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Prof Lüscher reports receiving research grants to the institution from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daichi Sankyo, Eli Lilly, Novartis, Sanofi, Servier and Vifor and consultant or speaker honoraria from Ablative Solutions USA, Acthera, Switzerland, Amgen Europe, Daichi Sankyo Switzerland, DalCor, Switzerland, Novo Nordisk Europe, Sanofi Middle East. Prof Matter reports receiving grants from MSD, Eli Lilly, AstraZeneca, Roche and Bayer; expert testimony from MSD; payment for lectures from MSD, AstraZeneca, and Roche; and having patents from Mabimmune, CH. Prof Windecker reports receiving research contracts to the institution from Abbott, Biotronik, Boston Scientific, Biosensors, Cordis, Medtronic, St. Jude Medical. Prof Mach has received honoraria for advisory boards and conferences on dyslipidaemia from Amgen, AstraZeneca,BMS, Eli Lilly, MSD, Sanofi, and Pfizer. RA’s research on cardiovascular prevention is supported by a grant for prospective researchers from the Swiss National Science Foundation PBLAP3-136774, the Société Académique Vaudoise and the SICPA Foundation. Prof Sudano has received consulting fees, travel grant and honoraria from: Amgen, Astra Zeneca, Daiichi Sankio, Medtronic, MSD, Novartis Recordati, Sanofi und Servier. All unrelated to the topic of the present paper. All other authors report no conflicts of interest.
1. Thun MJ , Carter BD , Feskanich D , Freedman ND , Prentice R , Lopez AD , et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013 Jan;368(4):351–64. https://doi.org/10.1056/NEJMsa1211127
2. Reid DD , Hamilton PJ , McCartney P , Rose G , Jarrett RJ , Keen H . Smoking and other risk factors for coronary heart-disease in British civil servants. Lancet. 1976 Nov;2(7993):979–84. https://doi.org/10.1016/S0140-6736(76)90830-8
3. Yudi MB , Farouque O , Andrianopoulos N , Ajani AE , Kalten K , Brennan AL , et al.; Melbourne Interventional Group . The prognostic significance of smoking cessation after acute coronary syndromes: an observational, multicentre study from the Melbourne interventional group registry. BMJ Open. 2017 Oct;7(10):e016874. https://doi.org/10.1136/bmjopen-2017-016874
4. Rea TD , Heckbert SR , Kaplan RC , Smith NL , Lemaitre RN , Psaty BM . Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002 Sep;137(6):494–500. https://doi.org/10.7326/0003-4819-137-6-200209170-00009
5. Dornelas EA , Sampson RA , Gray JF , Waters D , Thompson PD . A randomized controlled trial of smoking cessation counseling after myocardial infarction. Prev Med. 2000 Apr;30(4):261–8. https://doi.org/10.1006/pmed.2000.0644
6. Piepoli MF , Hoes AW , Agewall S , Albus C , Brotons C , Catapano AL , et al. [2016 European Guidelines on cardiovascular disease prevention in clinical practice]. Kardiol Pol. 2016;74(9):821–936. https://doi.org/10.5603/KP.2016.0120
7. Cabezas C , Advani M , Puente D , Rodriguez-Blanco T , Martin C ; ISTAPS Study Group . Effectiveness of a stepped primary care smoking cessation intervention: cluster randomized clinical trial (ISTAPS study). Addiction. 2011 Sep;106(9):1696–706. https://doi.org/10.1111/j.1360-0443.2011.03491.x
8. Rigotti NA , Clair C , Munafò MR , Stead LF . Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012 May;(5):CD001837. https://doi.org/10.1002/14651858.CD001837.pub3
9. Rovina N , Nikoloutsou I , Katsani G , Dima E , Fransis K , Roussos C , et al. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Ther Adv Respir Dis. 2009 Dec;3(6):279–87. https://doi.org/10.1177/1753465809350653
10. Mohiuddin SM , Mooss AN , Hunter CB , Grollmes TL , Cloutier DA , Hilleman DE . Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007 Feb;131(2):446–52. https://doi.org/10.1378/chest.06-1587
11. Murray RL , Leonardi-Bee J , Marsh J , Jayes L , Li J , Parrott S , et al. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: cluster randomised controlled trial. BMJ. 2013 Jul;347 jul08 1:f4004. https://doi.org/10.1136/bmj.f4004
12. Auer R , Gencer B , Tango R , Nanchen D , Matter CM , Lüscher TF , et al. Uptake and efficacy of a systematic intensive smoking cessation intervention using motivational interviewing for smokers hospitalised for an acute coronary syndrome: a multicentre before-after study with parallel group comparisons. BMJ Open. 2016 Sep;6(9):e011520. https://doi.org/10.1136/bmjopen-2016-011520
13. Nanchen D , Gencer B , Muller O , Auer R , Aghlmandi S , Heg D , et al. Prognosis of Patients With Familial Hypercholesterolemia After Acute Coronary Syndromes. Circulation. 2016 Sep;134(10):698–709. https://doi.org/10.1161/CIRCULATIONAHA.116.023007
14. Gencer B , Montecucco F , Nanchen D , Carbone F , Klingenberg R , Vuilleumier N , et al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur Heart J. 2016 Feb;37(6):546–53. https://doi.org/10.1093/eurheartj/ehv637
15. Selby K , Nanchen D , Auer R , Gencer B , Räber L , Klingenberg R , et al. Low statin use in adults hospitalized with acute coronary syndrome. Prev Med. 2015 Aug;77:131–6. https://doi.org/10.1016/j.ypmed.2015.05.012
16. Butty A , Gencer B , Koskinas KC , Carballo D , Räber L , Klingenberg R , et al. Control of cardiovascular risk factors and health behaviors in patients post acute coronary syndromes eligible for protein convertase subtilisin/kexin-9 inhibitors. Int J Cardiol. 2020 Jan;299:289–95. https://doi.org/10.1016/j.ijcard.2019.10.012
17. White IR , Royston P , Wood AM . Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011 Feb;30(4):377–99. https://doi.org/10.1002/sim.4067
18. Anthonisen NR , Skeans MA , Wise RA , Manfreda J , Kanner RE , Connett JE ; Lung Health Study Research Group . The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005 Feb;142(4):233–9. https://doi.org/10.7326/0003-4819-142-4-200502150-00005
19. Nohlert E , Öhrvik J , Tegelberg Å , Tillgren P , Helgason ÁR . Long-term follow-up of a high- and a low-intensity smoking cessation intervention in a dental setting—a randomized trial. BMC Public Health. 2013 Jun;13(1):592. https://doi.org/10.1186/1471-2458-13-592
20. Lou P , Zhu Y , Chen P , Zhang P , Yu J , Zhang N , et al. Supporting smoking cessation in chronic obstructive pulmonary disease with behavioral intervention: a randomized controlled trial. BMC Fam Pract. 2013 Jun;14(1):91. https://doi.org/10.1186/1471-2296-14-91
21. Connor Gorber S , Schofield-Hurwitz S , Hardt J , Levasseur G , Tremblay M . The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009 Jan;11(1):12–24. https://doi.org/10.1093/ntr/ntn010
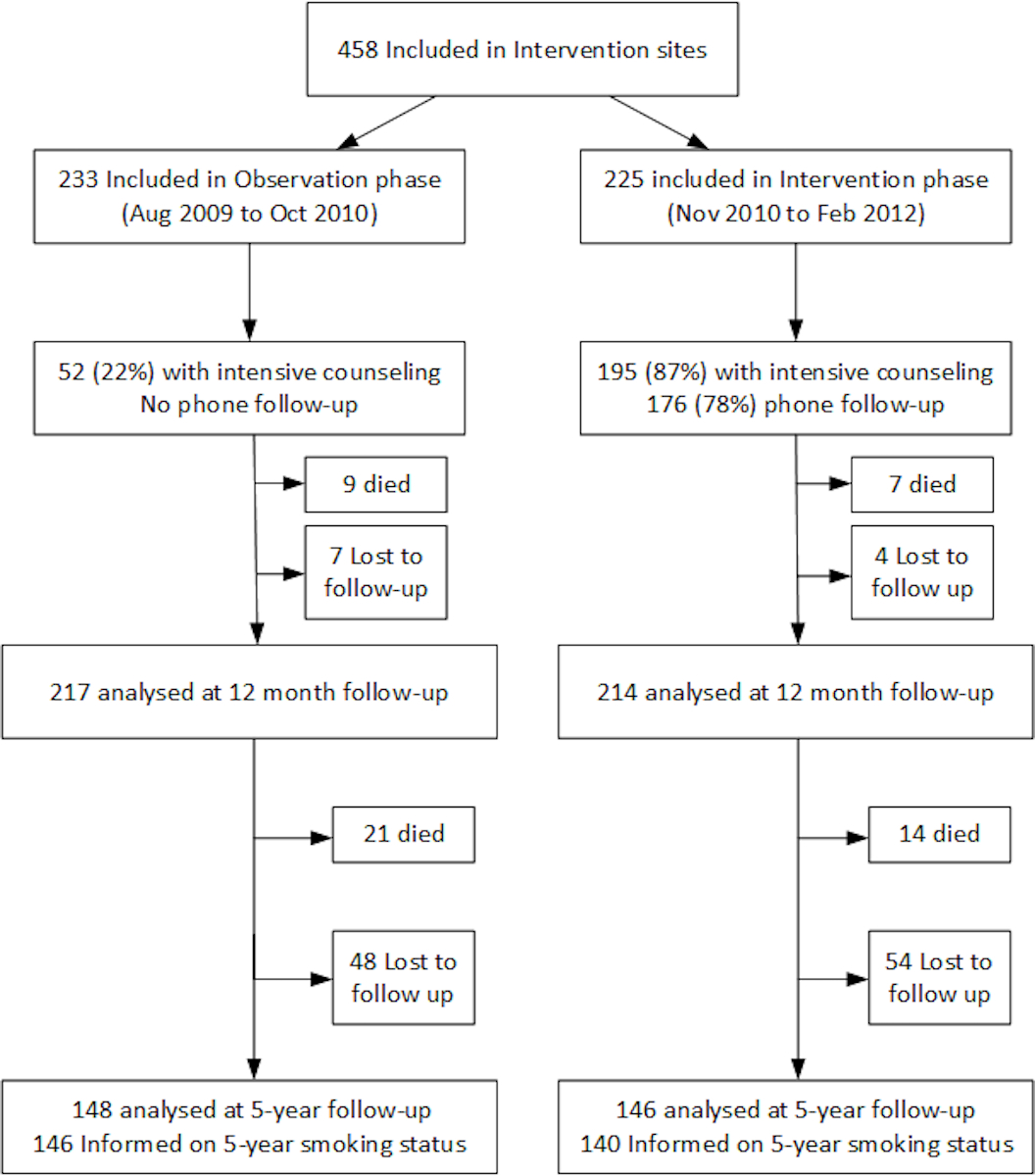
Figure S1 Flow chart.
Table S1Characteristics of patients at baseline, by smoking cessation counselling phase (n = 558).
| Baseline characteristics | |||
| Intervention phase cohort (n = 225) | Observation phase cohort (n = 233) | p-value | |
| Age, years, (mean ± SD) | 55 ± 11 | 57 ± 11 | 0.06 |
| Female, n (%) | 45 (20%) | 46 (20%) | 0.9 |
| Education level: less than university, n (%) | 185 (83%) | 203 (88%) | 0.1 |
| Living alone | 55 (24%) | 68 (29%) | 0.3 |
| Working status: employed, n (%) | 143 (64%) | 136 (59%) | 0.3 |
| Previous CHD, n (%) | 37 (16%) | 46 (20%) | 0.3 |
| ACS-type: STEMI, n (%) | 116 (52%) | 121 (52%) | 0.9 |
| Prescription of all recommanded drug therapy at discharge n (%)a | 216 (96%) | 222 (95%) | 0.6 |
| Attendance to cardiovascular rehabilitation assessed at discharge and 12 months follow-up n (%) | 163 (73%) | 136 (58%) | <0.01 |
ACS: acute coronary syndrome; CHD: coronary heart disease; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction
a Concomitant prescription at discharge unless contraindicated or not indicated for aspirin, clopidogrel/prasugrel or ticagrelor if percutaneous coronary intervention (PCI) - stent treatment, β-blocker, statin, angiotensin-converting-enzyme inhibitor (ACEI) if left ventricular ejection fraction ≤40%. When participants transferred to peripheral hospital, β-blocker and ACEI / angiotensin receptor II antagonist (ATII) coded as not applicable.
Table S2Counselling received by the patients, by smoking cessation counselling phase (n = 558).
| Intervention phase cohort (n = 225) | Observation phase cohort (n = 233) | p-Value | |
| Received intensive counselling during hospital stay, (n, %) | 193 (87)a | 52 (22) | <0.001 |
| Duration of in-hospital counselling per participant in minutes, median (Q1,Q3) | 40 (35, 60) | 45 (45,48) | 0.4 |
| Number of in-hospital counselling sessions, median (min, max) | 1 (1, 3) | 1 (1, 2) | 0.3 |
| Prescribed nicotine replacement therapy at discharge (n %) | 132 (59) | 42 (18) | <0.001 |
a Of the 13% who did not receive an intervention, 24 (11%) were transferred to another facility or discharged home before the counsellor could approach them, 2% (n = 4) completely refused to discuss with counsellor, 1% (n = 2) were in a confused state.
Table S3Smoking cessation prevalence comparing participants in the smoking cessation intervention phase cohort with the observation phase cohort at 1 year (n = 442) and 5 years (n = 407) after the index acute coronary syndrome (ACS), considering lost-to-follow-up patients as current smokersa.
| Intervention phase cohort | Observation phase cohort | Risk ratio (95% CI) | Adjusted Risk Ratio b (95% CI) | ||
| Smoking cessation | 1-year rate | 110/218 (50%) | 94/224 (42%) | 1.20 (0.98–1.47, p = 0.07) | 1.19 (0.90–1.57, p = 0.2) |
| 5-years rate | 75/204 (37%) | 68/203 (33%) | 1.10 (0.84–1.43, p = 0.5) | 1.12 (0.80–1.56, p = 0.5) | |
a 12 patients (5, 2% in intervention phase cohort, 7, 3% in observation phase cohort) had no smoking status data at 1 year;114 patients (59, 29% in intervention phase cohort, 55, 27% in observation phase cohort) had no smoking status data at 5 years. Those lost to follow up were considered as still smoking. Dead participants were excluded. Therefore, 442 datapoints were analysed at 1 year, 407 were analysed at 5 years.
bAdjusted for sex, age, education level and type of ACS
Table S4CONSORT Guideline.
| Item | Description | Reported on page and line number |
| Title | Identification of the study as randomized | p.1 |
| Authors * | Contact details for the corresponding author | p. 1–2 |
| Trial design | Description of the trial design (e.g. parallel, cluster, non-inferiority) | p.3, l.2–6 |
| Methods | ||
| Participants | Eligibility criteria for participants and the settings where the data were collected | p.3, l.9–10 |
| Interventions | Interventions intended for each group | p.3, l.11–15 |
| Objective | Specific objective or hypothesis | p.3, l.16–18 |
| Outcome | Clearly defined primary outcome for this report | p.3, l.9–10 |
| Randomization | How participants were allocated to interventions | p.3, l.11–15 |
| Blinding (masking) | Whether or not participants, care givers, and those assessing the outcomes were blinded to group assignment | p.3, l.15–16 |
| Results | ||
| Numbers randomized | Number of participants randomized to each group | p.4, l.2–3 |
| Recruitment | Trial status | p.3, l.11 and 13 |
| Numbers analysed | Number of participants analysed in each group | p.4, l.3–4 |
| Outcome | For the primary outcome, a result for each group and the estimated effect size and its precision | p.4, l.4–7 |
| Harms | Important adverse events or side effects | NA |
| Conclusions | General interpretation of the results | p.4, l.12–16 |
| Trial registration | Registration number and name of trial register | p.3, l.3–4 |
| Funding | Source of funding | p.16 l. 7–19 |