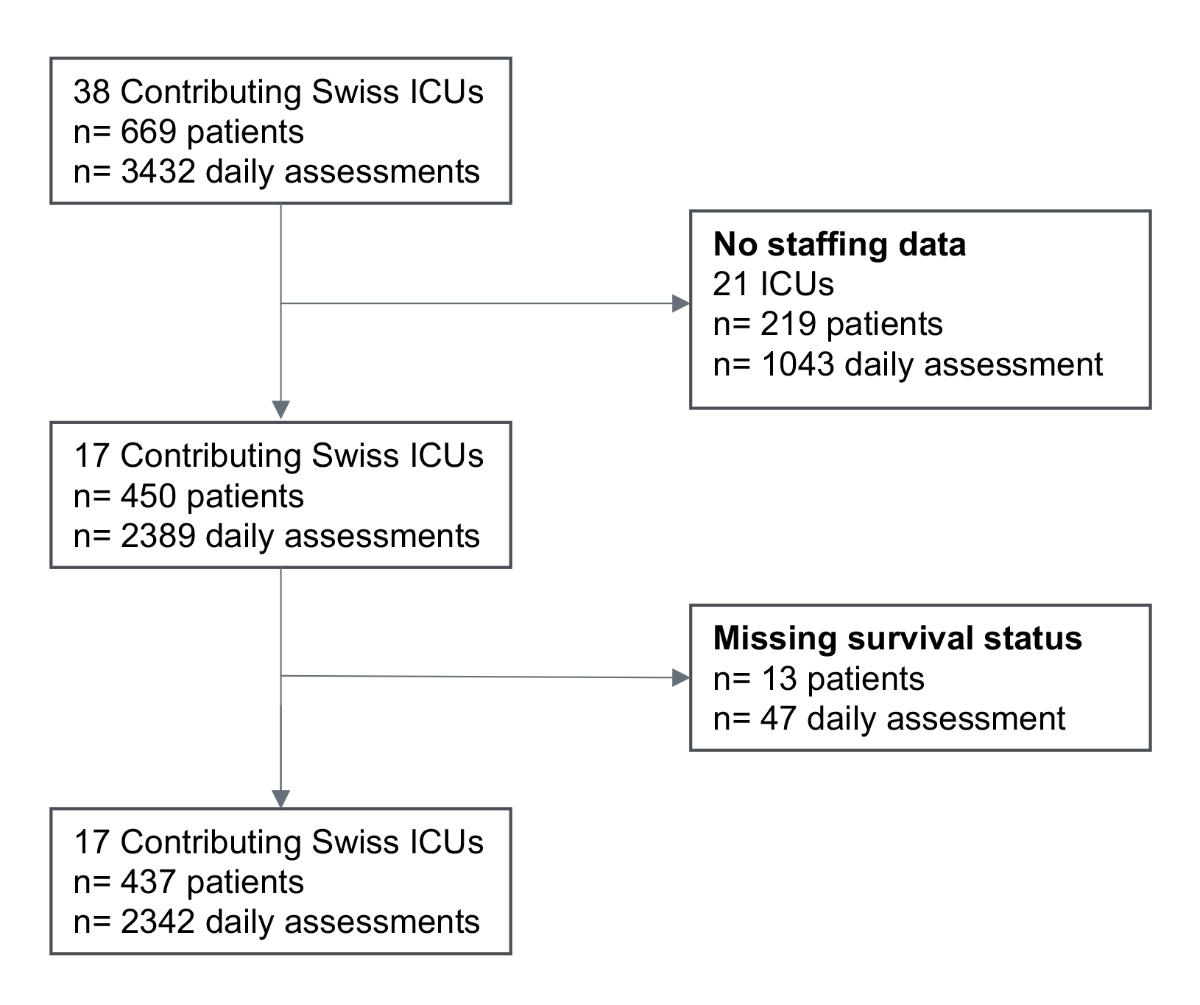
Figure 1 Study Flow Chart. During the first epidemic wave.
ICU = Intensive Care Unit, n = number
DOI: https://doi.org/10.4414/SMW.2022.w30183
Acute Physiology and Chronic Health Evaluation II
C-reactive protein
Guidelines on Good Clinical Practice; Directive
Interquartile ranges
Length of stay
Mean difference
Odds ratios
Rate ratios
Risk Stratification in COVID-19 patients in the Intensive Care Unit
Sequential Organ Failure Assessment
Simplified Acute Physiology Score II
The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the first epidemic wave dramatically stressed healthcare systems in many countries across Europe. In particular, intensive care units (ICUs) were pushed to their limits in terms of critical care staffing resources and bed capacity, and in some cases overwhelming the critical care facilities entirely [1–3]. Patients admitted to the ICU with severe coronavirus disease 2019 (COVID-19) not only required increased resources but sometimes had to be cared for outside of the regular ICU structure [4–6]. Additional non-specialised critical care staff had to be recruited quickly to cope with the increased burden [7].
There were major differences in the numbers of patients infected with SARS-CoV-2 between regions in Switzerland during the first pandemic wave (March 1 to May 31, 2020) [8]. Southern and Western parts of Switzerland experienced higher SARS-CoV-2 incidence than Central and Eastern parts, which resulted in huge differences in ICU occupancy rates [9, 10]. With the increasing demand in ICU beds, the standard of the Swiss Society of Intensive Care Medicine regarding personnel resources, including required training and critical care staffing per bed, could not always be fully satisfied [11].
Before the SARS-CoV-2 pandemic, some studies suggested a relationship between critical care staffing and mortality in critically ill patients [12–14]. An increase of patient-to-critical care staffing ratio was associated with worse patient outcomes such as transmission of infections, postoperative complications, including pulmonary failure and reintubation, and increased mortality [15–18]. Few reports evaluated the impact of critical care staffing on ICU mortality during a pandemic [19]. The goal of the present study was to investigate whether the differences in resource allocation for critical care staffing as well as caseload observed across Swiss ICUs during the first epidemic wave might have affected COVID-19 patient outcomes.
On March 17, 2020 the prospective observational Risk Stratification in COVID-19 patients in the ICU (RISC-19-ICU) registry was launched to capture COVID-19 features and track characteristics and outcome of patients with SARS-CoV-2 infections admitted to ICUs. The registry (ClinicalTrials.gov Identifier: NCT04357275) has been endorsed by the Swiss Society of Intensive Care Medicine (https://www.sgi-ssmi.ch) and was exempt from the need for additional ethics approval and patient informed consent by the ethics committee of the University of Zurich (KEK 2020-00322) [1]. Informed consent for publication was approved by the Ethics committee (KEK 2020-00322, KEK 2020-00375). Collaborating centres have complied with all local legal and ethical requirements. The study complies with the Declaration of Helsinki, the Guidelines on Good Clinical Practice (GCP-Directive) issued by the European Medicines Agency, as well as the Swiss law and Swiss regulatory authority requirements. The registry has been designed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies [28]. Eligibility criteria have been described elsewhere [1, 20]. The current retrospective analysis on the RISC-19-ICU registry (KEK 2020-00375) incorporated an extended dataset consisting of daily patient-to-nurse and patient-to-physician ratios. The analysis has been restricted to the period from March 1, 2020 to May 31, 2020, and to participating ICUs across Switzerland. Due to resource limitations, it was not possible to prospectively obtain comprehensive data on critical care staffing beyond this time window. Data on critical care staffing from March 1 to March 17, 2020 was collected retrospectively.
A standardised core dataset was prospectively collected during the ongoing COVID-19 pandemic for all critically ill COVID-19 patients admitted to the collaborating centres [1, 20]. Data collection was performed through an anonymized electronic case report form managed by the REDCap electronic data capture tool hosted on a secure server by the Swiss Society of Intensive Care Medicine. Data were collected on the day of ICU admission, and on days one, two, three, five and seven, including patient characteristics, treatment modalities and organ support therapies, the use of mechanical ventilation, vital parameters, arterial blood gas analyses, and laboratory values such as inflammatory, coagulation, renal, liver and cardiac parameters.
Critical care staffing, in terms of patient-to-nurse ratio and patient-to-physician ratio per day were prospectively recorded for patients included in the registry as part of the extended dataset. In those participating centres where resource information had not been collected prospectively, critical care staffing and patient assignment data retrieved from the personnel deployment planning (PEP®, staff planning tool, Dübendorf, Switzerland) and local patient assignment tools was matched with the treated patients. Critical care nursing staff consisted of registered nurses and critical care nurses (registered nurses with a postgraduate in critical care nursing).
Primary endpoint was ICU mortality. Secondary endpoints were ICU length of stay (LOS), mechanical ventilation and evolution of disease as assessed by Sequential Organ Failure Assessment (SOFA) score and C-reactive protein (CRP) levels over time during the ICU stay (see below for the calculation formula).
All analyses include the month of ICU admission (March vs April/May) to adjust for time effects. Due to the limited number of deaths in May, we combined the months April and May for analyses. We a priori selected the disease severity scores Acute Physiology and Chronic Health Evaluation II (APACHE II) and SOFA as confounding variables. Both scores reflect relevant domains of disease severity in a composite score. Additionally, we identified weekly caseload as a relevant confounder which might be associated with the outcomes of interest and critical care staffing. All confounding variables are static and measured at admission date.
Calculation of the disease severity scores APACHE II, Simplified Acute Physiology Score II (SAPS II) and SOFA scores was performed using an openly available code library associated with the registry [31].
Maximum differences (Δ) in SOFA and in CRP between days 0 or 1, and 3 or 5, were calculated as follows: Δ = X*{max(Y3,Y5) − min(Y0,Y1)} + (1–X)*{min(Y3,Y5) – max (Y0,Y1)} where Yd is the measured SOFA, respectively CRP, at day dÎ{0,1,3,5}, X = 1 if [(Y3+Y5)/2−(Y0+Y1)/2]>0, and X = 0 otherwise.
We described the study population by counts (n), percentages (%), mean, median, standard deviation (SD) and interquartile range (IQR). Our main variable of interest was the critical care staffing ratio (daily patient-to-nurse and daily patient-to-physician ratio). For each admission, we calculated the median of the daily ‘patient-to-critical care staffing’ ratio over the ICU stay.
We used a hierarchical Gaussian regression model to investigate whether the calendar day of ICU admission is associated with the logarithm of ‘patient-to-critical care staffing’ ratio. Calendar day of ICU admission was used as a restricted cubic spline with 3 knots chosen at the 10th, 50th and 90th percentiles [21]. We used a likelihood ratio test (LRT) to test the non-linear effect of calendar day association on the patient to critical care staffing ratio.
We used multivariable hierarchical regression models to investigate the effect of ‘patient-to-critical care staffing’ ratio on primary and secondary outcomes. We used a hierarchical logistic regression model to investigate the effect of ‘patient-to-critical care staffing’ ratio on ICU mortality and the presence of mechanical ventilation, while we used a hierarchical Poisson regression model for LOS, a hierarchical Gaussian regression model for ΔSOFA/ΔCRP and a hierarchical logistic regression model [22].We report crude and adjusted odds ratios (OR), rate ratios (RR) or mean differences (MD) with 95% confidence intervals. All hierarchical regression models accounted for the fact that admissions are nested within hospitals, that is, for each hospital a random intercept was estimated.
We a priori used the following confounding variables: month of ICU admission (March vs April/May), APACHE II and SOFA severity scores, as well as weekly caseload either time adjusted (adjusting for only month of ICU admission) or fully adjusted (adjusting for all mentioned variables). The ‘patient-to-critical care staffing’ ratio and the weekly caseload was modelled as a linear continuous logarithm-transformed (with respect to basis 2) variable, i.e. the effect on study outcomes is expressed in the doubling of the patient to critical care staffing ratio or the weekly caseload. We used complete case analysis because of a fraction of missing patients and daily assessments smaller than 3% [21]. We analysed the data using the statistical software R Version 3.6.3.
During the first COVID-19 pandemic wave occurring between March 1 and May 31, 2020 in Switzerland, 38 Swiss ICUs collected data from 669 patients representing a total of 3,432 daily assessments (figure 1). Among them, 17 ICUs recorded critical care staffing information and 450 patients with 2,389 daily assessments were eligible for analysis. After the exclusion of 13 (2.9%) patients with missing survival status and their 47 (2.0%) daily assessments, the study population included 17 ICUs, 437 patients and 2,342 daily assessments (figure 1).

Figure 1 Study Flow Chart. During the first epidemic wave.
ICU = Intensive Care Unit, n = number
Demographics and comorbidities of critically ill patients included in the study are presented in Supplemental 1 Table 1. Mean age was 62.6 years (SD 12.3 years) and about three-fourths were male. Patients were severely ill with relatively high severity [mean SAPS-II 57.8 (SD 17.3), mean APACHE II 21.2 (SD 6.8)], and multiple organ dysfunction scores [mean SOFA score 11.4 (SD 4.5)] at the time of admission. Most (84.9%) were on mechanical ventilation, and more than half (55.4%) were put in prone position sometimes during their ICU stay. Continuous renal replacement therapy was administered in 13.0% of the critically ill patients.
ICU mortality reached 20.1% (88 out of 437). Survivors had a median LOS of 13 days (IQR 6.0–22.0 days) whereas non survivors had a median LOS of 10.5 days (IQR 6.0–22.2).
The mean ΔSOFA 0.1 (SD 6.5) and the mean ΔCRP was 6.8 (SD 159) mg/L, which suggests that no clinically meaningful evolution of inflammation or organ failure occurred during the first 5 days in the ICU.
Characteristics of the patients with known discharge status from those 19 Swiss ICUs that did not report critical care staffing had a similar age, gender and ICU mortality distribution [mean age 64.0 (SD 12.8), 74.4% men, 20.0% ICU deaths], but a less severe disease status [mean SAPS II 44.6 (SD 18.4)], [mean APACHE II 16.5 (SD 6.9), mean SOFA 9.2 (SD 4.2)], and were less likely to be mechanically ventilated (62.6%) or to receive a continuous renal replacement therapy (6.2%), as compared to the study population (supplemental table 2).
The daily number of critically ill patients hospitalised in the contributing ICUs mirrored the pandemic wave observed in Switzerland over the study period (March 1 – May 31, 2020, supplemental table 3). This number increased from 3 (calendar week 9) to 134 (calendar week 13) and decreased thereafter to 1 (calendar week 22). The median of the daily patient-to-nurse ratio started at 1.0 (IQR 0.5–1.5; calendar week 9) and peaked at 2.4 (IQR 2.0–2.4; calendar week 16) (supplemental table 3), while the median of the daily patient-to-physician ratio started at 4.0 (IQR 2.1–5.0; calendar week 9) and peaked at 6.8 (IQR 6.3–7.3; calendar week 19) (supplemental table 3). Figure 2 shows the modelled calendar day effect on the critical care staffing using restricted cubic splines. Calendar day was non-linearly associated with the patient-to-nurse ratio (p = 0.007 from LRT) and with the patient-to-physician ratio (p = 0.003 from LRT).
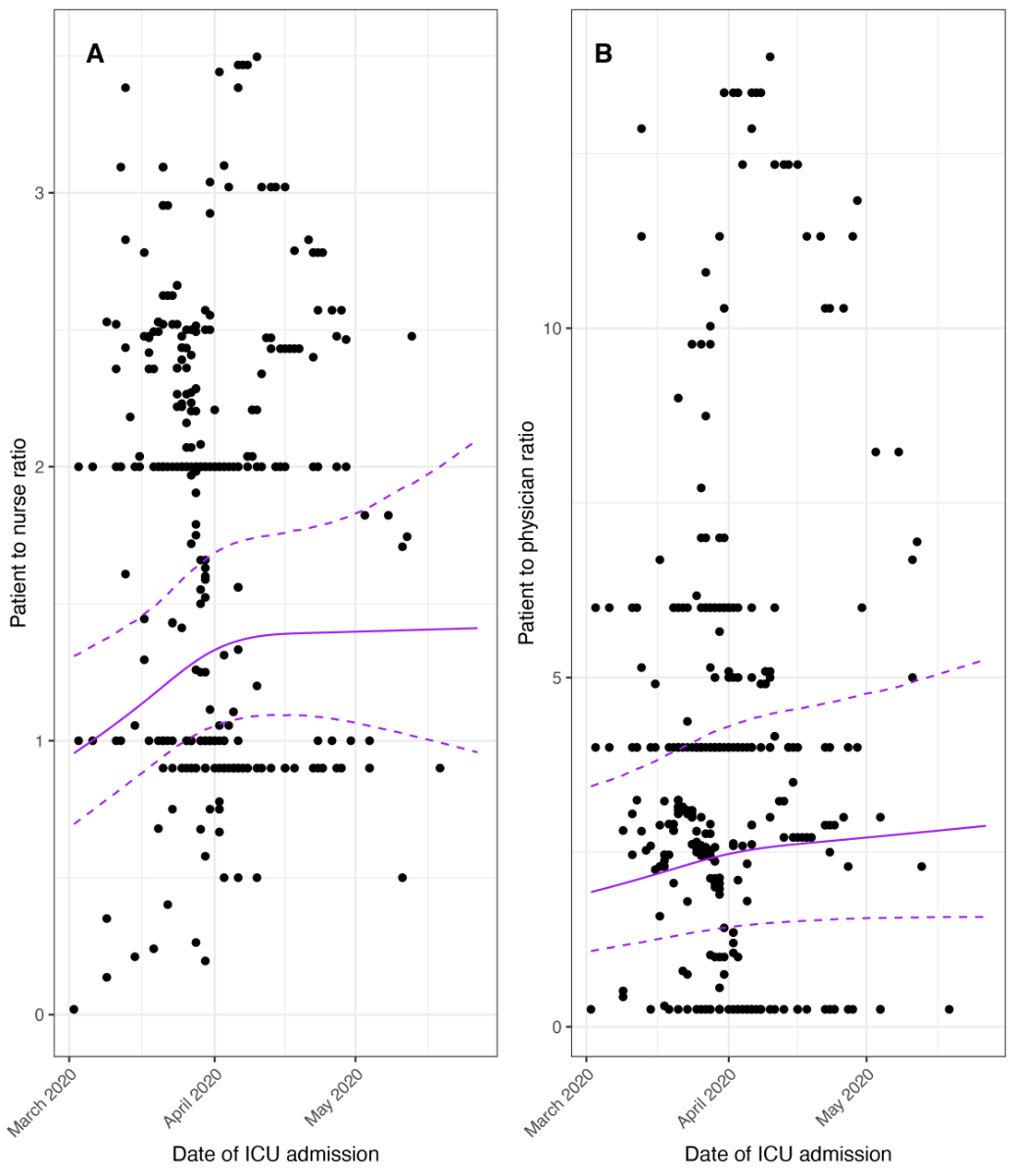
Figure 2 Patient-to-critical care staffing ratio.
ICU = Intensive Care Unit
A doubling of the daily patient-to-nurse ratio did not influence ICU mortality (ORtime adjusted 1.33, 95% CI 0.90–1.96; ORfully adjusted 1.28, 95% CI 0.85–1.93) (fig. 3A), nor any of the secondary study outcomes: LOS [RRtime adjusted 1.01, 95% CI (0.96–1.05); RRfully adjusted 0.98, 95% CI (0.94–1.03)] (fig. 3B), likelihood of being mechanically ventilated (ORtime adjusted 0.97, 95% CI 0.60–1.58; ORfully adjusted 0.78, 95% CI 0.42–1.44), and ΔCRP (MDtime adjusted –8.2, 95% CI –33.8–17.5, MDfully adjusted –3.3, 95% CI –29.4–22.9) (fig. 3C, fig. 4). Disease evolution as measured by ΔSOFA showed an association with ICU mortality in crude models (MDtime adjusted –0.91, 95% CI –1.75– –0.06) but not in adjusted models (MDfully adjusted –0.20, 95% CI –1.00–0.61). For patient-to-physician ratio, similar results were obtained (fig. 5 and 6).
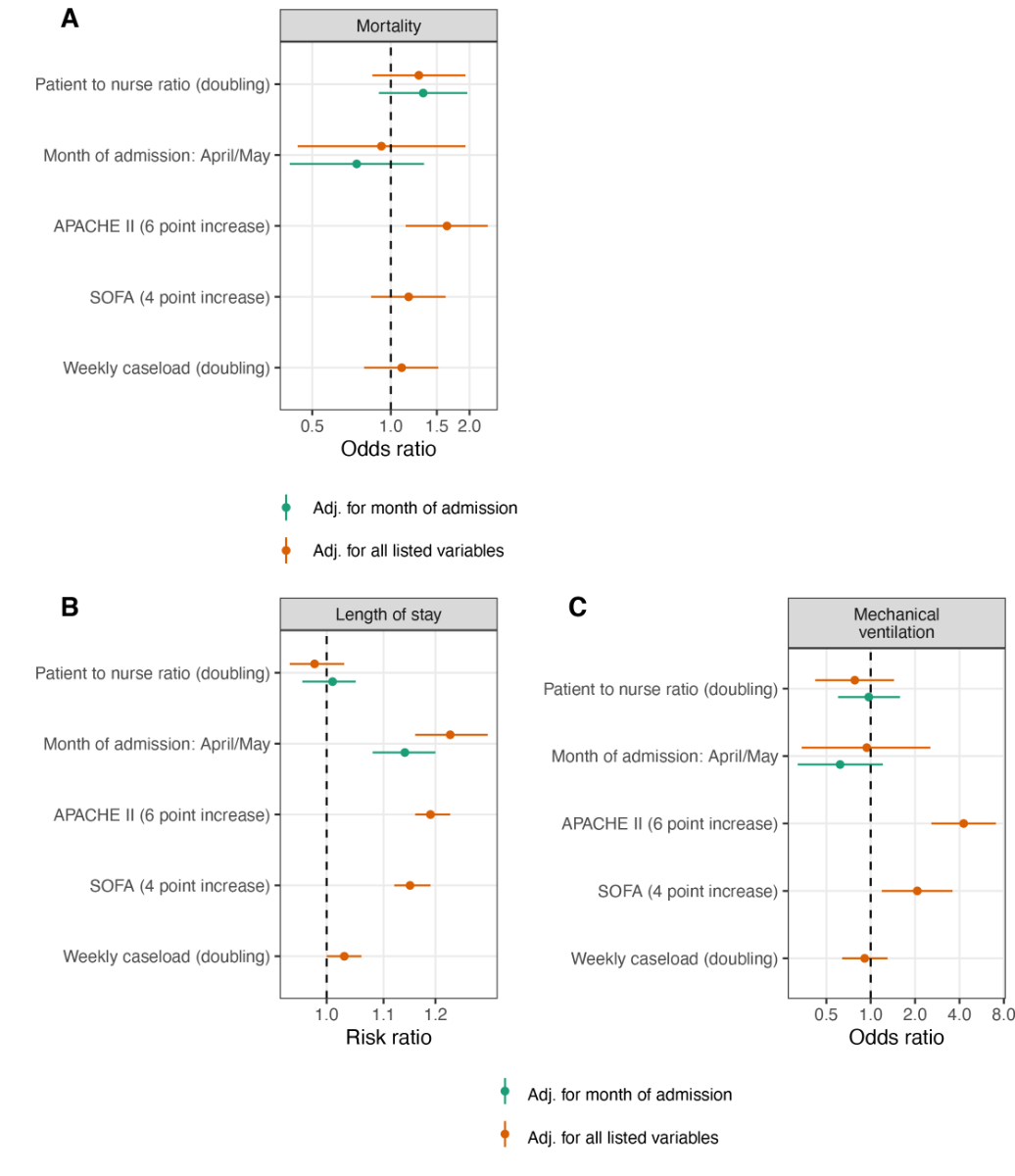
Figure 3 Patient-to-nurse ratio and study outcomes.
APACHE II = Acute Physiology and Chronic Health Evaluation II, SOFA = Sequential Organ Failure Assessment
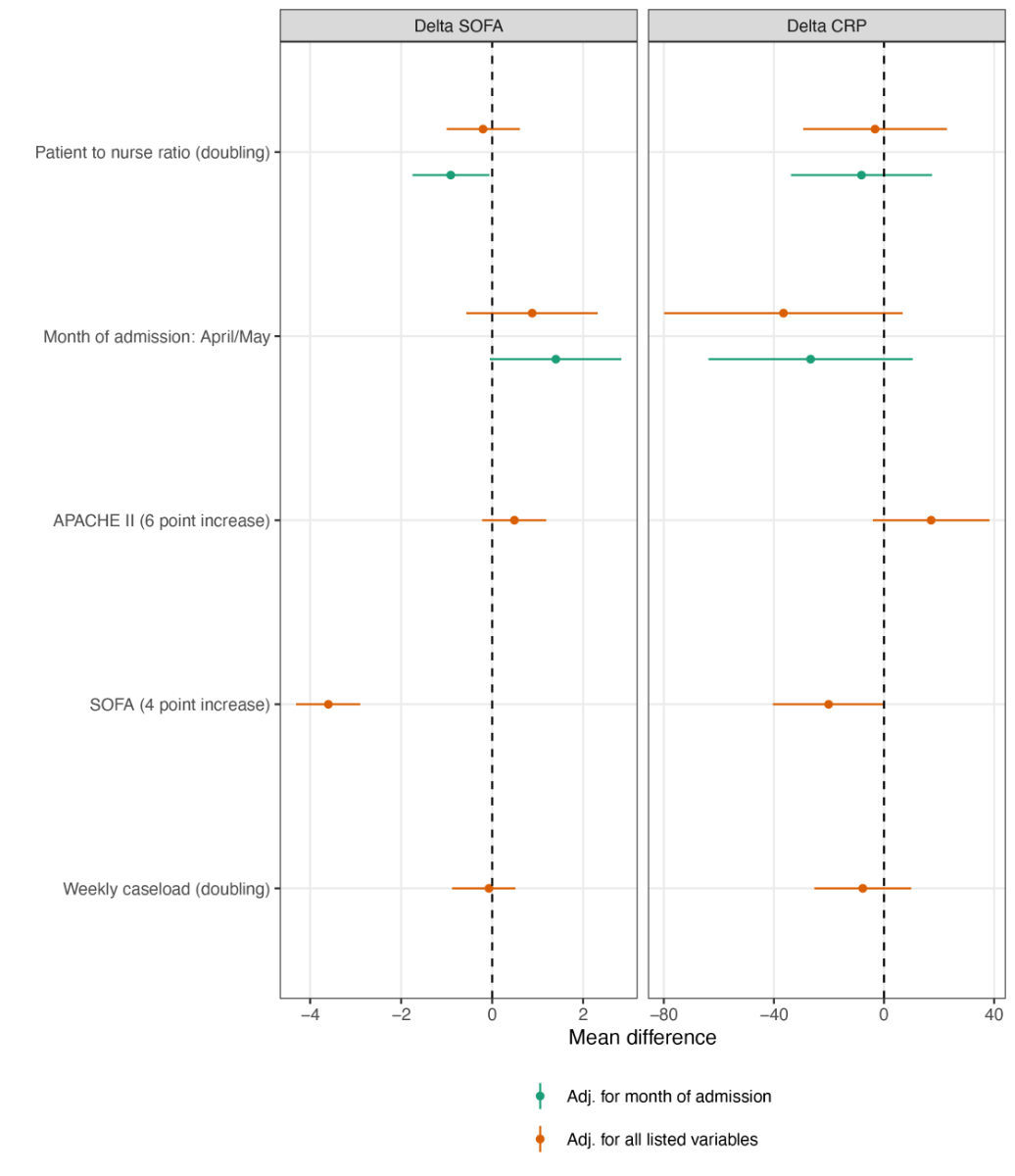
Figure 4 Patient-to-nurse ratio and delta SOFA, delta CRP.
APACHE II = Acute Physiology and Chronic Health Evaluation II, SOFA = Sequential Organ Failure Assessment, CRP = C-reactive protein
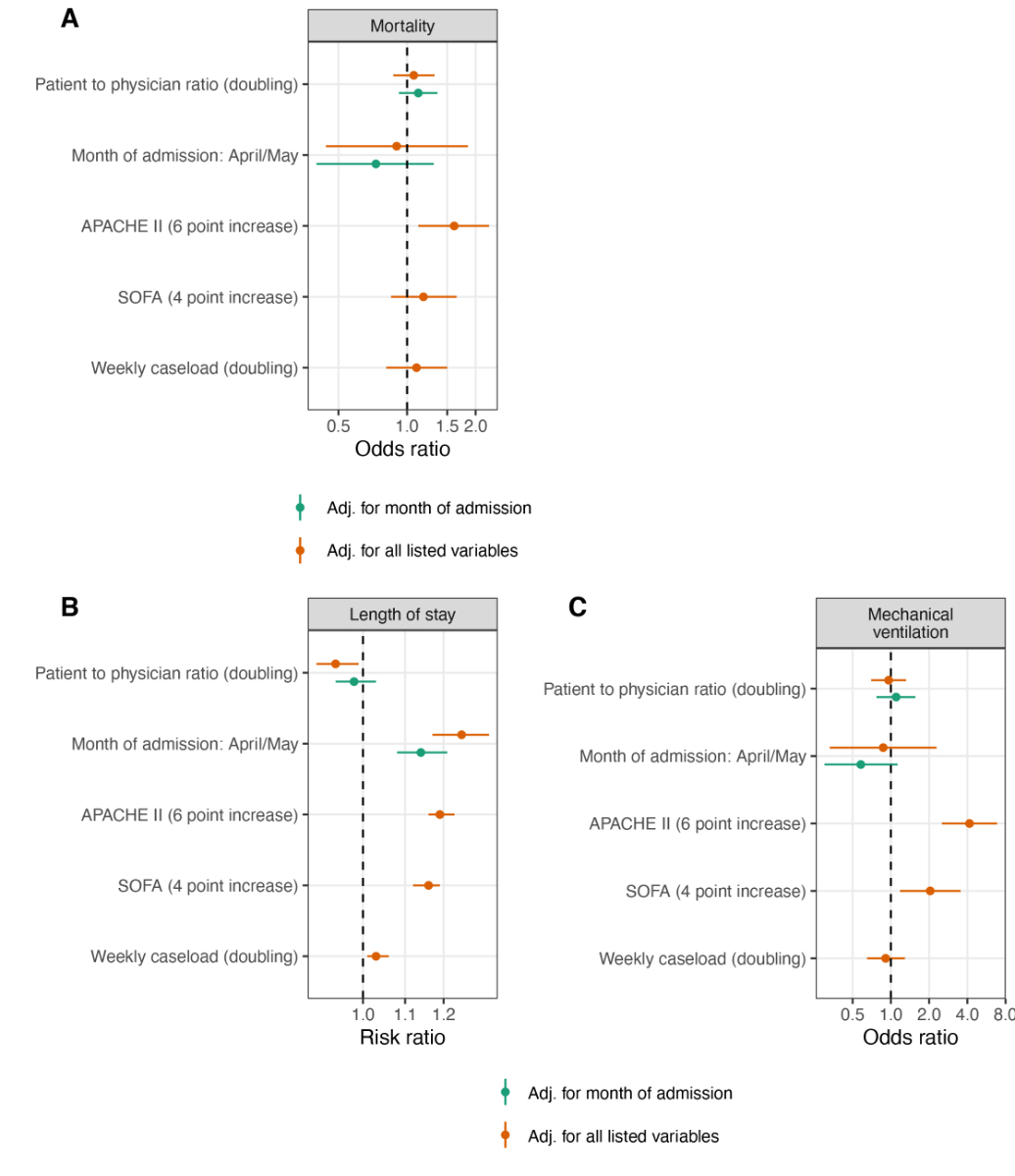
Figure 5 Patient-to-physician ratio and study outcomes.
APACHE II = Acute Physiology and Chronic Health Evaluation II, SOFA = Sequential Organ Failure Assessment
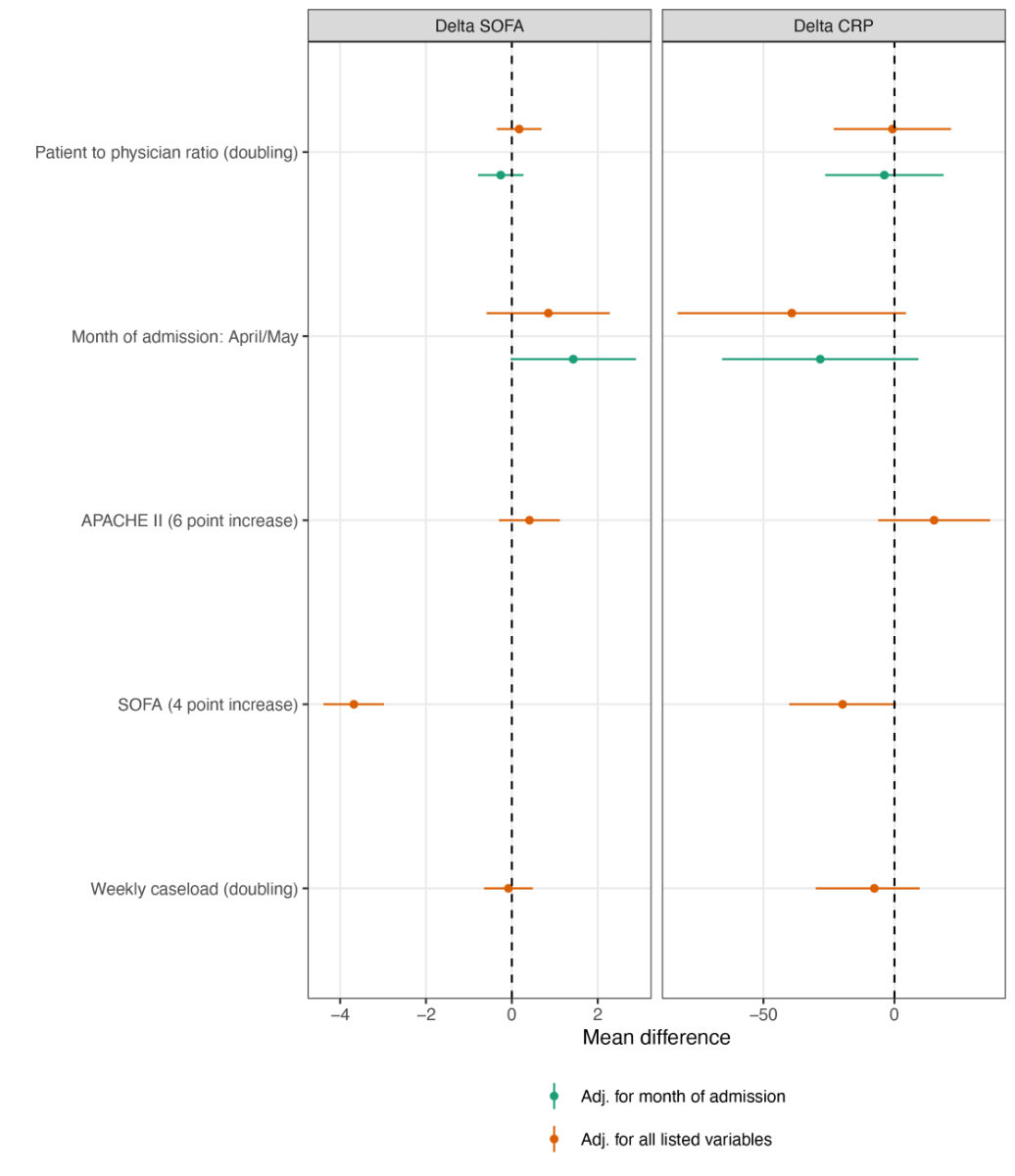
Figure 6 Patient-to-physician ratio and delta SOFA, delta CRP.
APACHE II = Acute Physiology and Chronic Health Evaluation II, SOFA = Sequential Organ Failure Assessment
It has been hypothesised that reduced critical care staffing and increased workload might have influenced mortality and outcomes in critically ill patients with COVID-19 [9, 12–15, 18, 23]. According to the guidelines of the Swiss Society of Intensive Care Medicine, a critically ill patient requiring controlled mechanical ventilation as well as prone positioning should be cared for by at least three ICU-certified nurses per day [11]. This high quality standard often could not be fulfilled during the first pandemic wave in the participating Swiss ICUs.
We observed a significant increase of the daily patient-to-critical care staffing ratio mirroring the increase in the number of patients. This increase remained modest compared to patient-to-critical care staffing ratio that have commonly been reported worldwide before the pandemic, particularly from the USA [15, 23]. This highlights how much flexibility the low pre-pandemic patient-to-critical care staffing ratio gave to Swiss ICUs and might explain why the overall outcome of critically ill patients with COVID-19 hospitalised among Swiss ICUs was not affected by change in patient-to-critical care staffing ratio. It might also reflect that Swiss ICUs had time to prepare themselves for the first wave that first hit in Italy.
Our study is, to the best of our knowledge, among the first to evaluate the impact of critical care staffing on the outcomes of critically ill patients during a pandemic. There have been reports highlighting the importance of the patient-to-critical care staffing ratio on the quality of critical care, but most, if not all of them, had been performed outside pandemic conditions [4, 19, 24–26]. Usually, studies compared patient outcomes across ICU centres that are run with different critical care staffing ratios [27]. The current setting of a pandemic gave us the opportunity to evaluate the effect of critical care staffing changes over time in each participating centre independently.
Organisational characteristics have been recently shown to affect the outcome of critically ill patients during the COVID-19 pandemic: in a study from Belgium, Taccone et al. reported that ICU overflow and the proportion of supplementary beds specially created during the pandemic to care for critically ill patients with COVID-19 were associated with increased in-hospital mortality [28]. Similarly, the US Department of Veteran Affairs Hospital found that strains on critical care capacity—captured by surrogate markers such as the ratio of ICU COVID-19 occupancy to the maximum ICU bed number—were significantly associated with increased COVID-19 ICU mortality [29]. None of these studies investigated patient-to-critical care staffing ratio. However, previous studies reported that better critical care staffing levels as well as higher quality of training of ICU personnel reduced the duration of mechanical ventilation [30]. Also, Hugonnet et al. previously reported that a high nurse-to-patient ratio was associated with a decreased risk for late-onset ventilator-associated pneumonia [31]. Unfortunately, the RISC-19-ICU registry does not collect data to report this outcome.
The increase in critical care staffing during the pandemic could only be reached by hiring healthcare workers without ICU-specific expertise. Thus, the increase in the daily patient-to-nurse and patient-to-physician ratio was linked to a relative decrease in ICU-trained staff. Information on the variation of skill-mix across shifts is unfortunately not recorded in our data set. We could have speculated that the reduction in specialised care could have contributed to a worse outcome for the most severely ill patients [32, 33], which our study, however, did not confirm. Yet, the supervising task for the ICU specialists might have been dramatically higher. This might explain why healthcare workers from Swiss ICUs have increasingly been reporting anxiety, depression, and peri-traumatic distress as well as low well-being [34].
Our study has several strengths that make our observations potentially generalisable. First, the participating centres cover a large spectrum of the existing ICU models of organization: we were able to recruit small low-intensity medical and surgical primary ICUs as well as several large high-intensity interdisciplinary tertiary centres. Second, although all participating ICUs were not equally affected — Eastern Switzerland being much less affected than Western and Southern Switzerland—we could find a consistent effect of patient-to-critical care staffing ratio on ICU mortality and duration of mechanical ventilation across all ICUs after adjustment for heterogeneity based on caseload.
Our study also suffers from some limitations. The primary endpoint was ICU mortality, but the RISC-19-ICU registry does not collect data on hospital mortality. Second, the data was collected before the publication of the Recovery trial results, after which most centres systematically introduced dexamethasone. This may have altered mortality, especially in critically ill patients with high disease severity [35]. Third, not all centres used experimental therapies and we could not exclude a potential bias, as some of these treatments, e.g. chloroquine, have been associated with an increased risk of mortality [36]. Fourth, not all Swiss participating ICUs have been collecting data on critical care staffing which might have introduced a selection bias. We found that patients from centres that did not record critical care staffing information had a less severe diseases status. Fifth, since information on critical care staffing was collected at an aggregated level (i.e., generally for each ICU) and not at an individual level (i.e, for each individual patient), our inferential conclusions on individual outcomes might be affected by a cross-level bias [37], despite the use of hierarchical approaches that include cross-level structure in their analyses [38, 39]. Finally, due to resource limitations, we were not able to collect patient-to-critical care staffing data beyond the study time period.
Providing a sufficient number of highly trained personnel as standard within ICUs is a too often overlooked aspect when it comes to pandemic preparedness. Our study demonstrates that the pre-pandemic low patient-to-critical care staffing ratio that are being enforced by the Swiss Society for Intensive Care Medicine helped the Swiss healthcare system to successfully overcome the first wave of the COVID-19 pandemic. We found no association between reduced critical care staffing resources per patient and overall length of stay or mortality in Swiss ICUs. Future studies should address the effect of reduced availability of critical care staff on long-term outcomes (e.g. post-traumatic stress disorders) of critically ill patients with COVID-19 and the mid-term consequences of the augmented workload on healthcare workers’ health.
Any intensive care unit or other centre treating critically ill COVID‐19 patients is invited to join the RISC‐19‐ICU registry at https://www.risc‐19‐icu.net. While the registry protocol prevents the deposition of the full registry dataset in a third‐party repository, analyses on the full dataset may be requested by any collaborating centre after approval of the study protocol by the registry board. Reproducibility of the results in the present study was ensured by providing code for registry‐specific data transformation and statistical analysis for collaborative development on the GitHub and Zenodo repositories. The registry protocol and data dictionary are publicly accessible at https://www.risc‐19‐icu.net.
RISC-19-ICU Investigators for Switzerland: Institute of Intensive Care Medicine, University Hospital Zurich, Zurich (Reto A. Schüpbach MD; Philipp Bühler, MD; Silvio Brugger, MD, PhD; Jan Bartussek, PhD; Giuliana Capaldo, MD; Sascha David, MD; Stefanie Keiser, PhD; Martina Maibach, PhD; Annelies Zinkernagel, MD, PhD); Soins intensifs, Groupement Hospitalier de l'Ouest Lémanique – Hopital de Nyon, Nyon (Mallory Moret-Bochatay, MD); Interdisziplinaere Intensivstation, Spital Buelach, Buelach (Bernd Yuen, MD; Thomas Hillermann, MD); Soins Intensifs, Hopital cantonal de Fribourg, Fribourg (Hatem Ksouri, MD, PhD; Govind Oliver Sridharan, MD); Departement for intensive care medicine, Kantonsspital Nidwalden, Stans (Anette Ristic, MD; Michael Sepulcri, MD); Departement of Anesthesiology and Intensive Care Medicine, Cantonal Hospital St. Gallen, St. Gallen (Miodrag Filipovic, MD; Urs Pietsch, MD); Intensivstation, Regionalspital Emmental AG, Burgdorf (Petra Salomon, MD; Iris Drvaric, MD); Institut fuer Anesthaesie und Intensivmedizin, Zuger Kantonsspital AG, Baar (Peter Schott, MD; Severin Urech, MD); Intensivmedizin, St. Claraspital, Basel (Adriana Lambert, MD; Lukas Merki, MD); Department Intensive Care Medicine, Spitalzentrum Biel, Biel (Marcus Laube, MD); Intensivmedizin, Kantonsspital Graubünden, Chur (Frank Hillgaertner, MD; Marianne Sieber); Institut fuer Anaesthesie und Intensivmedizin, Spital Thurgau, Frauenfeld (Alexander Dullenkopf, MD; Lina Petersen, MD); Division of Neonatal and Pediatric Intensive Care, Geneva University Hospitals, Geneva (Serge Grazioli, MD; Peter C. Rimensberger, MD); Soins Intensifs, Hirslanden Clinique Cecil, Lausanne (Isabelle Fleisch, MD; Jerome Lavanchy, MD); Pediatric Intensive Care Unit, University Hospital Lausanne, Lausanne (Marie-Helene Perez, MD); Interdisziplinaere Intensivstation, Spital Maennedorf AG, Maennedorf (Katharina Marquardt, MD; Karim Shaikh, MD); Intensivmedizin, Schweizer Paraplegikerzentrum Nottwil, Nottwil (Hermann Redecker, MD); Intensivmedizin, Spital Oberengadin, Samedan (Michael Stephan, MD; Jan Brem, MD); Paediatric Intensive Care Unit, Children’s Hospital of Eastern Switzerland, St. Gallen (Bjarte Rogdo, MD; Andre Birkenmaier, MD); Klinik für Anaesthesie und Intensivmedizin, Spitalzentrum Oberwallis, Visp (Friederike Meyer zu Bentrup, MD, MBA); Interdisziplinaere Intensivstation, Stadtspital Triemli, Zurich (Patricia Fodor, MD; Pascal Locher, MD); Department Intensivmedizin, Universitaetsspital Basel, Basel (Martin Siegemund, MD; Nuria Zellweger); Department of Intensive Care Medicine, University Hospital Bern - Inselspital, Bern (Marie-Madlen Jeitziner, RN, PhD; Beatrice Jenni-Moser, RN, MSc); Interdisziplinaere Intensivmedizin, Lindenhofspital, Bern, Switzerland (Jan Wiegand, MD); Intensivstation, Spital Grabs, Grabs (Christian Bürkle, MD); Medical ICU, Cantonal Hospital St.Gallen, St. Gallen (Gian-Reto Kleger, MD); Service d'Anesthesiologie, EHNV, Yverdon-les-Bains (Marilene Franchitti Laurent, MD; Jean-Christophe Laurent, MD); Abteilung für Anaesthesiologie und Intensivmedizin, Hirslanden Klinik Im Park, Zürich (Tomislav Gaspert, MD; Marija Jovic, MD); Intensivmedizin & Intermediate Care, Kantonsspital Olten, Olten (Michael Studhalter, MD); Institut für Anaesthesiologie und Intensivmedizin, Klinik Hirslanden, Zurich (Christoph Haberthuer, MD; Roger F. Lussman, MD); Anaesthesie Intensivmedizin Schmerzmedizin, Spital Schwyz, Schwyz (Daniela Selz, MD; Didier Naon, MD); Dipartimento Area Critica, Clinica Luganese Moncucco, Lugano (Andrea Glotta, MD; Samuele Ceruti, MD); Institut für Anaesthesiologie Intensivmedizin & Rettungsmedizin, See-Spital Horgen & Kilchberg, Horgen (Julien Marrel, MD; Mirko Brenni, MD); Klinik für Operative Intensivmedizin, Kantonsspital Aarau, Aarau (Rolf Ensner, MD; Marc Michot, MD); Intensivstation, Kantonsspital Schaffhausen, Schaffhausen (Nadine Gehring, MD); Intensivstation, Spital Simmental-Thun-Saanenland AG, Thun (Antje Heise, MD); Klinik für Anaesthesie Intensivmedizin Operationszentrum und Schmerzmedizin, Kantonsspital Muensterlingen, Muensterlingen (Tobias Huebner, MD; Thomas A. Neff, MD); Division of Intensive Care, University Hospitals of Geneva, Geneva (Sara Cereghetti, MD; Filippo Boroli, MD; Jerome Pugin, MD, PhD).
MMJ, AM, YAQ, MPH and SMJ conceived and designed this study. PDWG, MTE, SK, RAS, UP, SC, FB, JM, AAS, HK, PS, AD, IF, AH and JCL acquired the data. AM and MPH performed data validation, statistical analysis and visualisation. MMJ, MTE, SMJ, MPH and YAQ interpreted the data. YAQ and SMJ drafted the manuscript. MMJ, AM, MPH, PDWG, MTE, SK, RAS, UP, SC, FB, JM, AAS, HK, PS, AD, IF, AH and JCL critically revised the manuscript. AM and MPH had full access to the study data and take full responsibility for the accuracy of the data analysis. All authors read and approved the final manuscript.
The RISC‐19‐ICU registry is supported by the Swiss Society of Intensive Care Medicine and funded by internal resources of the Institute of Intensive Care Medicine, of the University Hospital Zurich and by unrestricted grants from CytoSorbents Europe GmbH (Berlin, Germany) and Union Bancaire Privée (Zurich, Switzerland).
MPH, PDWG and RAS declared having received unrestricted grants from CytoSorbents Europe GmbH (Berlin, Germany) and Union Bancaire Privée (Zurich, Switzerland) to maintain the RISC-ICU registry. All other authors declared no conflict of interest related to the present work.
1. Wendel Garcia PD , Fumeaux T , Guerci P , Heuberger DM , Montomoli J , Roche-Campo F , et al.; RISC-19-ICU Investigators . Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020 Aug;25:100449. https://doi.org/10.1016/j.eclinm.2020.100449
2. Kleinpell R , Ferraro DM , Maves RC , Kane Gill SL , Branson R , Greenberg S , et al. Coronavirus Disease 2019 Pandemic Measures: Reports From a National Survey of 9,120 ICU Clinicians. Crit Care Med. 2020 Oct;48(10):e846–55. https://doi.org/10.1097/CCM.0000000000004521
3. Wahlster S , Sharma M , Lewis AK , Patel PV , Hartog CS , Jannotta G , et al. The Coronavirus Disease 2019 Pandemic’s Effect on Critical Care Resources and Health-Care Providers: A Global Survey. Chest. 2021 Feb;159(2):619–33. https://doi.org/10.1016/j.chest.2020.09.070
4. Bruyneel A , Gallani MC , Tack J , d’Hondt A , Canipel S , Franck S , et al. Impact of COVID-19 on nursing time in intensive care units in Belgium. Intensive Crit Care Nurs. 2021 Feb;62:102967. https://doi.org/10.1016/j.iccn.2020.102967
5. Cammarota G , Ragazzoni L , Capuzzi F , Pulvirenti S , De Vita N , Santangelo E , et al. Critical Care Surge Capacity to Respond to the COVID-19 Pandemic in Italy: A Rapid and Affordable Solution in the Novara Hospital. Prehosp Disaster Med. 2020 Aug;35(4):431–3. https://doi.org/10.1017/S1049023X20000692
6. Lefrant JY , Fischer MO , Potier H , Degryse C , Jaber S , Muller L , et al.; French ICU study investigators group . A national healthcare response to intensive care bed requirements during the COVID-19 outbreak in France. Anaesth Crit Care Pain Med. 2020 Dec;39(6):709–15. https://doi.org/10.1016/j.accpm.2020.09.007
7. Uppal A , Silvestri DM , Siegler M , Natsui S , Boudourakis L , Salway RJ , et al. Critical Care And Emergency Department Response At The Epicenter Of The COVID-19 Pandemic: new York City’s public health system response to COVID-19 included increasing the number of in-tensive care units, transferring patients between hospitals, and supplementing critical care staff. Health Aff (Millwood). 2020 Aug;39(8):1443–9. https://doi.org/10.1377/hlthaff.2020.00901
8. Federal Office of Public Helath FOPH . COVID-19 Switzerland [Internet]. 2021 Jul. Available from: www.covid19.admin.ch
9. Jeitziner MM , Jenni-Moser B , Yok-Ai Q , Thurnheer Zrcher MC , Furrer H , Jakob S . Im-portance of critical care staffing and standard intensive care therapy in the COVID-19 era: a de-scriptive study of the first epidemic wave at a Swiss tertiary intensive care unit. Swiss Med Wkly [Internet]. 2021 Jun 25 [cited 2021 Aug 18]; Available from: https://doi.emh.ch/smw.2021.20529
10. Primmaz S , Le Terrier C , Suh N , Ventura F , Boroli F , Bendjelid K , et al. Preparedness and Reorganization of Care for Coronavirus Disease 2019 Patients in a Swiss ICU: Characteristics and Outcomes of 129 Patients. Crit Care Explor. 2020 Jul;2(8):e0173. https://doi.org/10.1097/CCE.0000000000000173
11. Swiss Society of Intensive Care Medicine . 2021. Available from: www.sgi-ssmi.ch/de/coivd19.html
12. Aiken LH , Sloane DM , Bruyneel L , Van den Heede K , Griffiths P , Busse R , et al.; RN4CAST consortium . Nurse staffing and education and hospital mortality in nine European countries: a retrospective observational study. Lancet. 2014 May;383(9931):1824–30. https://doi.org/10.1016/S0140-6736(13)62631-8
13. Needleman J , Buerhaus P , Pankratz VS , Leibson CL , Stevens SR , Harris M . Nurse staffing and inpatient hospital mortality. N Engl J Med. 2011 Mar;364(11):1037–45. https://doi.org/10.1056/NEJMsa1001025
14. Rae PJ , Pearce S , Greaves PJ , Dall’Ora C , Griffiths P , Endacott R . Outcomes sensitive to critical care nurse staffing levels: A systematic review. Intensive Crit Care Nurs. 2021 Dec;67(Jul):103110. https://doi.org/10.1016/j.iccn.2021.103110
15. Neuraz A , Guérin C , Payet C , Polazzi S , Aubrun F , Dailler F , et al. Patient Mortality Is Associated With Staff Resources and Workload in the ICU: A Multicenter Observational Study. Crit Care Med. 2015 Aug;43(8):1587–94. https://doi.org/10.1097/CCM.0000000000001015
16. West E , Barron DN , Harrison D , Rafferty AM , Rowan K , Sanderson C . Nurse staffing, medical staffing and mortality in Intensive Care: an observational study. Int J Nurs Stud. 2014 May;51(5):781–94. https://doi.org/10.1016/j.ijnurstu.2014.02.007
17. Shekelle PG . Nurse-patient ratios as a patient safety strategy: a systematic review. Ann Intern Med. 2013 Mar;158(5 Pt 2 5_Part_2):404–9. https://doi.org/10.7326/0003-4819-158-5-201303051-00007
18. Lasater KB , Sloane DM , McHugh MD , Cimiotti JP , Riman KA , Martin B , et al. Evaluation of hospital nurse-to-patient staffing ratios and sepsis bundles on patient outcomes. Am J Infect Control. 2021 Jul;49(7):868–73. https://doi.org/10.1016/j.ajic.2020.12.002
19. Xi J , Zeng L , Li S , Ai Y , He X , Kang Y , et al. COVID-19 mortality in ICUs associated with critical care staffing. Burns Trauma. 2021 Apr;9:tkab006. https://doi.org/10.1093/burnst/tkab006
20. Wendel Garcia PD , Aguirre-Bermeo H , Buehler PK , Alfaro-Farias M , Yuen B , David S , et al.; RISC-19-ICU Investigators . Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care. 2021 May;25(1):175. https://doi.org/10.1186/s13054-021-03580-y
21. Harrell FE . Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis [Internet]. Cham: Springer International Publishing; 2015 [cited 2021 Aug 18]. (Springer Series in Statistics). Available from: http://link.springer.com/10.1007/978-3-319-19425-7
22. Austin PC , Rothwell DM , Tu JV . A Comparison of Statistical Modeling Strategies for Analyz-ing Length of Stay after CABG Surgery. Health Serv Outcomes Res Methodol. 2002;3(2):107–33. https://doi.org/10.1023/A:1024260023851
23. Lasater KB , Aiken LH , Sloane DM , French R , Martin B , Reneau K , et al. Chronic hospital nurse understaffing meets COVID-19: an observational study. BMJ Qual Saf. 2021 Aug;30(8):639–47. https://doi.org/10.1136/bmjqs-2020-011512
24. Aiken LH , Clarke SP , Sloane DM , Sochalski J , Silber JH . Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002 Oct;288(16):1987–93. https://doi.org/10.1001/jama.288.16.1987
25. Needleman J , Buerhaus P , Mattke S , Stewart M , Zelevinsky K . Nurse-staffing levels and the quality of care in hospitals. N Engl J Med. 2002 May;346(22):1715–22. https://doi.org/10.1056/NEJMsa012247
26. Bergman L , Falk A , Wolf A , Larsson I. Registered nurses’ experiences of working in the inten-sive care unit during the COVID ‐19 pandemic. Nurs Crit Care. 2021 May 10;nicc.12649.
27. Griffiths P , Ball J , Drennan J , Dall’Ora C , Jones J , Maruotti A , et al. Nurse staffing and patient outcomes: strengths and limitations of the evidence to inform policy and practice. A review and discussion paper based on evidence reviewed for the National Institute for Health and Care Excellence Safe Staffing guideline development. Int J Nurs Stud. 2016 Nov;63:213–25. https://doi.org/10.1016/j.ijnurstu.2016.03.012
28. Taccone FS , Goethem NV , De Pauw R , Van Beckhoven D , Meyfroidt G , Blot K . Organization-al characteristics: Effect on outcome of ICU COVID-19 patients in Belgium – Authors’ reply. Lancet Reg Health - Eur. 2021 Apr;3:100070.
29. Bravata DM , Perkins AJ , Myers LJ , Arling G , Zhang Y , Zillich AJ , et al. Association of Intensive Care Unit Patient Load and Demand With Mortality Rates in US Department of Veterans Affairs Hospitals During the COVID-19 Pandemic. JAMA Netw Open. 2021 Jan;4(1):e2034266. https://doi.org/10.1001/jamanetworkopen.2020.34266
30. Blackwood B , Alderdice F , Burns K , Cardwell C , Lavery G , O’Halloran P . Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. BMJ. 2011 Mar 11;342(jan13 2):c7237–c7237.
31. Hugonnet S , Uçkay I , Pittet D . Staffing level: a determinant of late-onset ventilator-associated pneumonia. Crit Care. 2007;11(4):R80. https://doi.org/10.1186/cc5974
32. Musy SN , Endrich O , Leichtle AB , Griffiths P , Nakas CT , Simon M . The association between nurse staffing and inpatient mortality: A shift-level retrospective longitudinal study. Int J Nurs Stud. 2021 Aug;120:103950. https://doi.org/10.1016/j.ijnurstu.2021.103950
33. Musy SN , Endrich O , Leichtle AB , Griffiths P , Nakas CT , Simon M . Longitudinal Study of the Variation in Patient Turnover and Patient-to-Nurse Ratio: Descriptive Analysis of a Swiss University Hospital. J Med Internet Res. 2020 Apr;22(4):e15554. https://doi.org/10.2196/15554
35. Wozniak H , Benzakour L , Moullec G , Buetti N , Nguyen A , Corbaz S , et al. Mental health outcomes of ICU and non-ICU healthcare workers during the COVID-19 outbreak: a cross-sectional study. Ann Intensive Care. 2021 Jul;11(1):106. https://doi.org/10.1186/s13613-021-00900-x
35. Horby P , Lim WS , Emberson JR , Mafham M , Bell JL , Linsell L , et al.; RECOVERY Collaborative Group . Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021 Feb;384(8):693–704. https://doi.org/10.1056/NEJMoa2021436
36. Axfors C , Schmitt AM , Janiaud P , Van’t Hooft J , Abd-Elsalam S , Abdo EF , et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021 Apr;12(1):2349. https://doi.org/10.1038/s41467-021-22446-z
37. Greenland S . Ecologic versus individual-level sources of bias in ecologic estimates of contextual health effects. Int J Epidemiol. 2001 Dec;30(6):1343–50. https://doi.org/10.1093/ije/30.6.1343
38. Wakefield J . Multi-level modelling, the ecologic fallacy, and hybrid study designs. Int J Epidemiol. 2009 Apr;38(2):330–6. https://doi.org/10.1093/ije/dyp179
39. Greenland S . A review of multilevel theory for ecologic analyses. Stat Med. 2002 Feb;21(3):389–95. https://doi.org/10.1002/sim.1024
Table S1Patient characteristics and outcomes, by surviving status.
| Survivor | Non-survivor | Overall | ||
| (n = 349) | (n = 88) | (n = 437) | ||
| Gender | Male | 81 (76.8%) | 25 (71.6%) | 106 (75.7%) |
| Female | 268 (23.2%) | 63 (28.4%) | 331 (24.3%) | |
| Age | Mean (SD) | 61.0 (12.4) | 68.8 (9.63) | 62.6 (12.3) |
| Median [Min, Max] | 62.0 [24.0, 92.0] | 70.0 [31.0, 86.0] | 64.0 [24.0, 92.0] | |
| SAPS II | Mean (SD) | 55.9 (17.5) | 65.5 (14.1) | 57.8 (17.3) |
| Median [Min, Max] | 61.0 [15.0, 90.0] | 69.0 [24.0, 88.0] | 64.0 [15.0, 90.0] | |
| APACHE II | Mean (SD) | 20.5 (6.86) | 24.1 (5.86) | 21.2 (6.82) |
| Median [Min, Max] | 22.0 [3.00, 38.0] | 24.5 [5.00, 35.0] | 23.0 [3.00, 38.0] | |
| SOFA | Mean (SD) | 11.0 (4.40) | 13.0 (4.66) | 11.4 (4.52) |
| Median [Min, Max] | 11.0 [0, 20.0] | 13.5 [0, 21.0] | 11.0 [0, 21.0] | |
| Median patient-to-nurse ratio over ICU stay | Mean (SD) | 1.79 (0.783) | 1.91 (0.674) | 1.81 (0.765) |
| Median [Min, Max] | 2.00 [0.0194, 3.50] | 2.00 [0.667, 3.47] | 2.00 [0.0194, 3.50] | |
| Missing | 80 (22.9%) | 27 (30.7%) | 107 (24.5%) | |
| Median patient-to-physician ratio over ICU stay | Mean (SD) | 4.02 (3.15) | 4.17 (2.98) | 4.05 (3.11) |
| Median [Min, Max] | 3.15 [0.250, 13.9] | 4.00 [0.250, 13.4] | 3.19 [0.250, 13.9] | |
| Missing | 80 (22.9%) | 27 (30.7%) | 107 (24.5%) | |
| Length of stay in ICU (in days) | Mean (SD) | 17.7 (24.5) | 17.7 (29.9) | 17.7 (25.6) |
| Median [Min, Max] | 13.0 [0, 273] | 10.5 [0, 268] | 13.0 [0, 273] | |
| Missing | 0 (0%) | 2 (2.3%) | 2 (0.5%) | |
| Smoking history | Non smoker | 207 (59.3%) | 46 (52.3%) | 253 (57.9%) |
| Past history | 90 (25.8%) | 24 (27.3%) | 114 (26.1%) | |
| Current smoker | 25 (7.2%) | 7 (8.0%) | 32 (7.3%) | |
| Missing | 27 (7.7%) | 11 (12.5%) | 38 (8.7%) | |
| Body mass index (kg/m2) | Mean (SD) | 29.1 (5.24) | 29.0 (6.32) | 29.1 (5.45) |
| Median [Min, Max] | 28.0 [15.6, 50.8] | 27.4 [19.3, 58.4] | 27.8 [15.6, 58.4] | |
| Missing | 6 (1.7%) | 11 (12.5%) | 17 (3.9%) | |
| Steroids used | No | 304 (87.1%) | 68 (77.3%) | 372 (85.1%) |
| Yes | 45 (12.9%) | 20 (22.7%) | 65 (14.9%) | |
| Experimental therapy used | No | 184 (52.7%) | 48 (54.5%) | 232 (53.1%) |
| Yes | 165 (47.3%) | 40 (45.5%) | 205 (46.9%) | |
| Mechanical ventilation | No | 60 (17.2%) | 6 (6.8%) | 66 (15.1%) |
| Yes | 289 (82.8%) | 82 (93.2%) | 371 (84.9%) | |
| Prone positioning | No | 168 (48.1%) | 27 (30.7%) | 195 (44.6%) |
| Yes | 181 (51.9%) | 61 (69.3%) | 242 (55.4%) | |
| ECMO | No | 336 (96.3%) | 78 (88.6%) | 414 (94.7%) |
| Yes | 13 (3.7%) | 10 (11.4%) | 23 (5.3%) | |
| Continuous renal replacement therapy or haemodialysis of any form | No | 308 (88.3%) | 72 (81.8%) | 380 (87.0%) |
| Yes | 41 (11.7%) | 16 (18.2%) | 57 (13.0%) | |
| Chronic arterial hypertension | Not present | 180 (51.6%) | 38 (43.2%) | 218 (49.9%) |
| Present | 169 (48.4%) | 50 (56.8%) | 219 (50.1%) | |
| Ischemic heart disease | Not present | 301 (86.2%) | 69 (78.4%) | 370 (84.7%) |
| Present | 48 (13.8%) | 19 (21.6%) | 67 (15.3%) | |
| Other heart disease | Not present | 310 (88.8%) | 75 (85.2%) | 385 (88.1%) |
| Present | 39 (11.2%) | 13 (14.8%) | 52 (11.9%) | |
| Diabetes mellitus | Not present | 262 (75.1%) | 60 (68.2%) | 322 (73.7%) |
| Present | 87 (24.9%) | 28 (31.8%) | 115 (26.3%) | |
| Chronic pulmonary disease | Not present | 295 (84.5%) | 73 (83.0%) | 368 (84.2%) |
| Present | 54 (15.5%) | 15 (17.0%) | 69 (15.8%) | |
| Immunosuppression | Not present | 294 (84.2%) | 68 (77.3%) | 362 (82.8%) |
| Present | 55 (15.8%) | 20 (22.7%) | 75 (17.2%) | |
| Month of ICU admission | March | 204 (58.5%) | 56 (63.6%) | 260 (59.5%) |
| April/May | 145 (41.5%) | 32 (36.4%) | 177 (40.5%) | |
SAPS II = Simplified Acute Physiology Score II, APACHE II = Acute Physiology and Chronic Health Evaluation II, SOFA = Sequential Organ Failure Assessment, ICU = Intensive Care Unit, n = Number, SD = Standard Deviation
Table S2Characteristics of patients with known discharge status from 19 hospitals not recording critical care staffing*.
| Survivor | Non-survivor | Overall | ||
| (n = 156) | (n = 39) | (n = 195) | ||
| Gender | Male | 115 (73.7%) | 30 (76.9%) | 145 (74.4%) |
| Female | 39 (25.0%) | 9 (23.1%) | 48 (24.6%) | |
| Missing | 2 (1.3%) | 0 (0%) | 2 (1.0%) | |
| Age | Mean (SD) | 62.4 (13.1) | 70.4 (8.96) | 64.0 (12.8) |
| Median [Min, Max] | 63.0 [10.0, 87.0] | 72.0 [50.0, 85.0] | 66.0 [10.0, 87.0] | |
| SAPS II | Mean (SD) | 42.4 (18.4) | 53.6 (15.8) | 44.6 (18.4) |
| Median [Min, Max] | 36.0 [11.0, 80.0] | 51.0 [24.0, 81.0] | 38.0 [11.0, 81.0] | |
| APACHE II | Mean (SD) | 15.8 (6.83) | 19.0 (6.45) | 16.5 (6.86) |
| Median [Min, Max] | 13.0 [5.00, 32.0] | 18.0 [9.00, 30.0] | 14.0 [5.00, 32.0] | |
| SOFA | Mean (SD) | 9.16 (4.02) | 9.23 (4.91) | 9.17 (4.20) |
| Median [Min, Max] | 9.00 [0, 21.0] | 9.00 [0, 19.0] | 9.00 [0, 21.0] | |
| Length of stay in ICU (in days) | Mean (SD) | 11.7 (12.5) | 13.6 (10.6) | 12.1 (12.1) |
| Median [Min, Max] | 8.00 [0, 66.0] | 13.0 [0, 50.0] | 9.00 [0, 66.0] | |
| Smoking history | Non smoker | 100 (64.1%) | 25 (64.1%) | 125 (64.1%) |
| Past history | 21 (13.5%) | 9 (23.1%) | 30 (15.4%) | |
| Current smoker | 14 (9.0%) | 1 (2.6%) | 15 (7.7%) | |
| Missing | 21 (13.5%) | 4 (10.3%) | 25 (12.8%) | |
| Body mass index (kg/m2) | Mean (SD) | 29.2 (6.19) | 29.2 (5.38) | 29.2 (6.01) |
| Median [Min, Max] | 27.8 [20.1, 57.1] | 28.1 [20.8, 49.5] | 27.8 [20.1, 57.1] | |
| Missing | 29 (18.6%) | 4 (10.3%) | 33 (16.9%) | |
| Steroids used | No | 141 (90.4%) | 22 (56.4%) | 163 (83.6%) |
| Yes | 15 (9.6%) | 17 (43.6%) | 32 (16.4%) | |
| Experimental therapy used | No | 99 (63.5%) | 23 (59.0%) | 122 (62.6%) |
| Yes | 57 (36.5%) | 16 (41.0%) | 73 (37.4%) | |
| Mechanical ventilation | No | 70 (44.9%) | 3 (7.7%) | 73 (37.4%) |
| Yes | 86 (55.1%) | 36 (92.3%) | 122 (62.6%) | |
| Prone positioning | No | 110 (70.5%) | 14 (35.9%) | 124 (63.6%) |
| Yes | 46 (29.5%) | 25 (64.1%) | 71 (36.4%) | |
| ECMO | No | 156 (100%) | 39 (100%) | 195 (100%) |
| Yes | 0 (0%) | 0 (0%) | 0 (0%) | |
| Continuous renal replacement therapy or haemodialysis of any form | No | 148 (94.9%) | 35 (89.7%) | 183 (93.8%) |
| Yes | 8 (5.1%) | 4 (10.3%) | 12 (6.2%) | |
| Chronic arterial hypertension | Not present | 88 (56.4%) | 17 (43.6%) | 105 (53.8%) |
| Present | 68 (43.6%) | 22 (56.4%) | 90 (46.2%) | |
| Ischemic heart disease | Not present | 139 (89.1%) | 27 (69.2%) | 166 (85.1%) |
| Present | 17 (10.9%) | 12 (30.8%) | 29 (14.9%) | |
| Other heart disease | Not present | 127 (81.4%) | 30 (76.9%) | 157 (80.5%) |
| Present | 29 (18.6%) | 9 (23.1%) | 38 (19.5%) | |
| Diabetes mellitus | Not present | 124 (79.5%) | 23 (59.0%) | 147 (75.4%) |
| Present | 32 (20.5%) | 16 (41.0%) | 48 (24.6%) | |
| Chronic pulmonary disease | Not present | 137 (87.8%) | 30 (76.9%) | 167 (85.6%) |
| Present | 19 (12.2%) | 9 (23.1%) | 28 (14.4%) | |
| Immunosuppression | Not present | 148 (94.9%) | 34 (87.2%) | 182 (93.3%) |
| Present | 8 (5.1%) | 5 (12.8%) | 13 (6.7%) | |
*From the 19 hospitals 24 patients out of 219 patients had an unknown discharge status.
Notes: SAPS II = Simplified Acute Physiology Score II, APACHE II = Acute Physiology and Chronic Health Evaluation II, SOFA = Sequential Organ Failure Assessment, ICU = Intensive Care Unit, n = Number, SD = Standard Deviation
Table S3Admission characteristics, by week of ICU admission.
| Week of ICU admission | No. of ICU admissions | No. of non-survivors | Median patient-to-nurse ratio | Q1 patient-to-nurse ratio | Q3 patient-to-nurse ratio | Median patient-to-physician ratio | Q1 patient-to-physician ratio | Q3 patient-to-physician ratio |
| 9 | 3 | 1 | 1 | 0.5 | 1.5 | 4 | 2.1 | 5 |
| 10 | 6 | 0 | 1 | 0.4 | 2 | 2.8 | 0.5 | 4 |
| 11 | 31 | 7 | 2 | 1.6 | 2.5 | 3.6 | 2.5 | 5 |
| 12 | 86 | 19 | 2.4 | 2 | 2.6 | 3.1 | 2.8 | 4 |
| 13 | 134 | 29 | 2 | 1 | 2.2 | 4 | 2.4 | 6 |
| 14 | 73 | 14 | 1.1 | 0.9 | 2 | 4 | 1.3 | 5 |
| 15 | 37 | 6 | 2 | 2 | 2.4 | 4.2 | 3.2 | 5.1 |
| 16 | 26 | 7 | 2.4 | 2 | 2.4 | 2.7 | 2.7 | 4 |
| 17 | 24 | 3 | 2 | 0.9 | 2.6 | 2.9 | 0.2 | 4 |
| 18 | 8 | 1 | 1 | 0.9 | 1.9 | 4 | 1.6 | 7.1 |
| 19 | 5 | 0 | 1.7 | 1.4 | 1.8 | 6.8 | 6.3 | 7.3 |
| 20 | 3 | 0 | 1.7 | 1.3 | 2.1 | 1.3 | 0.8 | 1.8 |
| Overall | 437 | 88 | 2 | 1 | 2.4 | 3.2 | 2.4 | 5.1 |