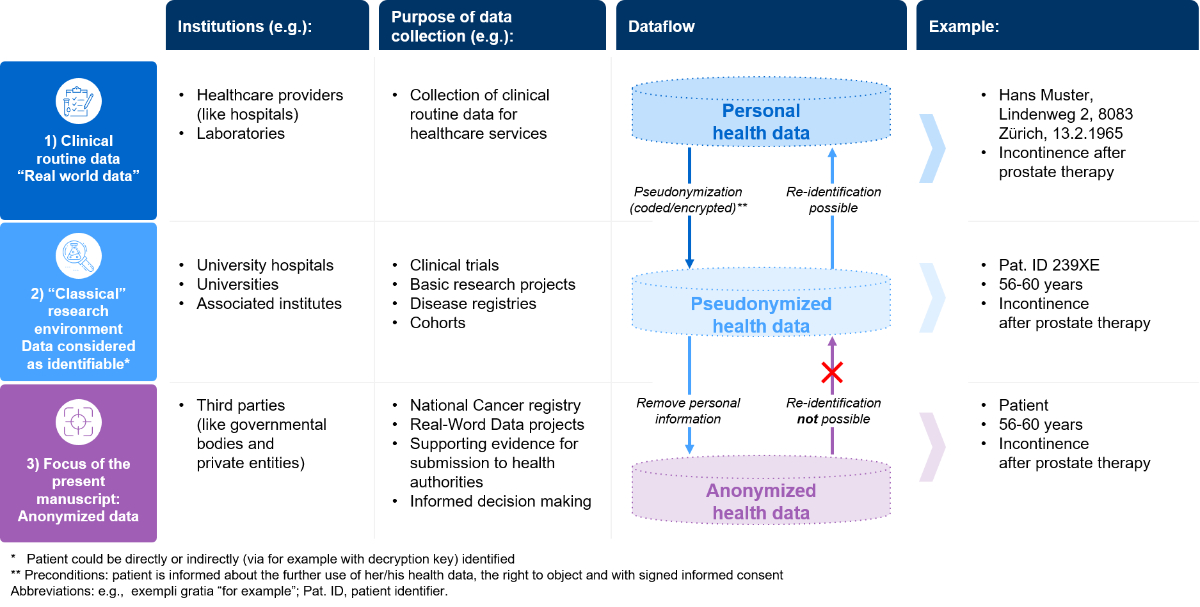
Figure 1 Dataflow: from personal to anonymised health data. Abbreviation: PHC, personalised healthcare.
DOI: https://doi.org/10.4414/SMW.2022.w30182
Routinely collected health data reflect the “real world” and are often referred to as real-world data; yet there is no uniform definition of the term "real-world data". According to the major regulatory bodies US Food and Drug Administration (FDA) and European Medicines Agency (EMA), real-world data are defined as data relating to a patient's health status and deriving from sources reflecting the real-world setting [1, 2]. Real-world data play an important role in driving medical advances and innovation, as well as in the realisation of precision medicine to increase clinical benefit for patients. Additionally, real-world data are pertinent to ascertain efficient and sustainable healthcare systems. Possible sources of real-world data include: electronic health records; health insurance data; disease registries; patient-generated data (e.g. collected through mobile devices such as health trackers) or health insurance data [1, 2]. The present manuscript focuses on routinely collected health data collected in hospitals; hospitals own the richest health data resources to address a large variety of health-related questions. Hence, the present survey focused on the re-use (secondary data use) of these hospital derived data in anonymised form. For the sake of clarity in this manuscript, routinely collected clinical data will be called “health data”.
Substantial progress in the digitalisation of healthcare has resulted in a vast amount of health data, which is continuously generated every day [3]. Nonetheless, most of these data remain “siloed” within the respective healthcare institutions with limited or no access for medical research [4, 5]. Of the numerous healthcare system stakeholders, only a few have access to routinely collected health data. Nevertheless, health data has become increasingly important for third parties such as governmental bodies or stakeholders from the private sector. The reasons why third parties need access to health data are either for research purposes or to make informed decisions. Examples here include addressing epidemiological questions (e.g., National Cancer Registry [6] or the COVID-19 pandemic), advancing personalised medicine, drug development, assessing safety and supporting evidence for submission to health authorities [2]. For these purposes, health data of individual patients (patient-level data) are of substantial importance, and the data should be anonymised as third parties have neither the right nor the interest in re-identifying individual patients.
To bring the role of anonymised health data into the right context within the Swiss setting, it is important to understand the different steps towards anonymisation. In figure 1, we describe a simplified data flow-example from routinely collected health data to anonymized data, which then can be shared with third parties:
The simplified dataflow described in figure 1 does not display technical requirements or any data governance features, but it is in accordance with the Swiss legal framework when anonymising health data. The Swiss data protection regulations and the legal architecture that defines the process of handling health data is complex. However, recent activities by Swissethics and Unimedsuisse (harmonisation of the general consent form [7]), a new Swiss cancer registration act (“Krebsregistrierungsgesetz”; KRG [6]) and the Swiss Personalised Health Network (SPHN) initiative [8, 9] have brought further clarity on how to process routinely collected health data for secondary data use, including anonymisation. The harmonised version of the Swiss general consent covers all requirements (it informs about data collection, right to object, re-use of the data in coded/encrypted or anonymised form), allowing routinely collected data to be re-used for research or to be anonymised for sharing with third parties [7]. The recently introduced Swiss cancer registration act (KRG) has contributed to the importance of the anonymisation process as its accompanying documents define, to some extent, the requirements for anonymisation of health data and the importance of making data from the national registry available for research [10]. For more details about the legal framework in Switzerland, the complexity of the data protection laws and the relevant lex specialis has been summarised comprehensively in a recently published article in Swiss Medical Weekly [11]. From an ethical perspective, health data sharing with private entities is possible, if a list of requirements are met according to the recently published Swiss guidance. If these requirements are fulfilled to address community needs, data sharing between public and private entities is ethical [12]. This is a good starting point to make anonymised data available for third parties.
It is important to understand that the technical requirements for anonymisation are not regulated under Swiss law. Compared with the United States, where according to Health Information Portability and Accountability Act (HIPAA) principles as alternative to an expert determination [13], a list of eighteen predefined variables have to be removed so that the data are considered as anonymised. The situation in Switzerland is not as clear and therefore data users are accountable for ensuring an anonymisation process considering all means reasonably likely to be used to preserve data privacy at all times. Since the technical requirements are not defined in the Swiss law, this room for interpretation remains contentious and leads to ambiguity and controversy. The complexity of the concept of anonymisation has been comprehensively described elsewhere for the Swiss [11] and the European contexts [14, 15].
Establishing a sustainable data ecosystem requires the acceptance and willingness of each individual to share health data [16]. In other words, data sharing in Switzerland starts with the permission of the patient. Whether routinely collected health data can be systematically collected for research and be anonymised depends on the patient’s decision in the first step of the above-mentioned dataflow (figure 1). Therefore, it is important to understand the drivers and potential barriers influencing their willingness to share their health data. A recently published Swiss Survey by Brall et al. found that over 53% of the Swiss citizens would agree to participate in a personalised health research project [17]. This extensive and well-conducted survey generated valuable insight into sharing health data for use in personalised health research projects in the clinical trial setting. Neither the routine care setting nor anonymisation was addressed by Brall et al., and hence it does not cover the above-mentioned third step of the data flow (figure 1)
The willingness to share anonymised health data of the general Swiss population is unclear. Therefore, Roche (Pharma) Switzerland Ltd commissioned an independent research organisation, gfs-zürich, to conduct a survey among the general Swiss population and patients with a chronic disease in Switzerland to assess the current national standing of patient and public opinion towards sharing anonymised patient-level data for medical research with a variety of healthcare system stakeholders.

Figure 1 Dataflow: from personal to anonymised health data. Abbreviation: PHC, personalised healthcare.
This survey focused on two populations in Switzerland, the general, adult (≥18 years) population (sample 1) and patients with a chronic disease (sample 2). For sample 1, the sample size calculations were based on the usual assumptions of gfs-zürich. Based on the typically used quotas gender, age and language region in Switzerland, 1000 interviews were conducted to assure that at least 30 interviews are contained in the smallest cell (in Switzerland: woman 50% X French-speaking 26% X older 64 years 23% = 3%). Above the number of 30 interviews the response behaviour is considered to change to a normal distribution. The resulting theoretical precision of a sample size of n = 1006 corresponds to an error margin of ±3.2% by confidence level of 95%. Eighty percent of sample 1 were randomly drawn from the publicly available telephone directory (landlines) of the German- and French-speaking parts of Switzerland. Another 20% of the sample were contacted on their mobile telephone numbers via random digit dialing. The survey of the sample of the general population was conducted from 14 September to 3 October 2020. The demographic composition of the sample was specifically constructed to be representative of the 18+-year-old French- and German-speaking population of Switzerland according to the predefined quota features: gender, age and language region. This survey was not conducted in the Italian-speaking (around 4% of the total population [19]) and Rhaeto-Romanic (around 0.5% [20]) parts of Switzerland because of resource restraints. The educational level was categorised as high (university or university of applied science), medium (final secondary school examination, higher vocational training) and low (mandatory school).
Sample 2 of chronic disease patients (cancer, multiple sclerosis, arthrosis, eye diseases or chronic back pain) were drawn from a Swiss online panel (Bilendi GmbH, Berlin, Germany). The number of available patients defined the sample size, where 225 patients and a confidence level of 95% resulted in an error margin of ±6.7%. Patients with cancer or multiple sclerosis were contacted first and preferentially included. The survey was conducted online from 15 to 28 September 2020. Subgroup analyses of the survey responses were performed only for the patients with cancer, multiple sclerosis and arthrosis owing to the limited size of the groups with eye diseases or back pain.
The questionnaire consisted of 10 questions about data sharing, covering aspects of potential willingness to share anonymised health data and potential barriers that prevent data being shared, based on the scenario of a hospital visit including a request by the hospital for anonymised data for medical research. Two questions assessed the importance of trust in data collecting institutions and the preferred institution to inform on the topic of handling health data. Seven questions were closed-ended questions (four with five-point Likert scales, two with binary options and one to choose a statement out of four). Information on demographic details included age, gender, education level and purchasing power. Questions about drivers and barriers on sharing anonymised health data were first formulated as open-ended questions to receive an unbiased response on potential drivers and barriers, followed by two closed-ended questions to assess the agreement with preselected answer options describing potential drivers and barriers. The sample of the general population was asked whether they or someone close to them are affected by a chronic disease, and if so how. The questionnaire is available in the pdf version of the article.
The surveys were conducted by gfs-zürich, Markt- und Sozialforschung (Zurich, Switzerland). The questions were designed to take a maximum of 13 minutes, worded in lay language (German and French for the two language regions, respectively), pre-tested with five volunteers and, if needed, adapted prior to starting the survey. The questions were developed in German and translated into French by a professional translation office. The English version was generated to complement this manuscript and it was not used for the survey. The German, French and English versions of the questionnaire are available in the pdf version of the article.
The telephone interviews were conducted by professional and experienced interviewers, trained for interview research. All interviewers received specific instruction for the present survey. They were using a professional software for telephone interviews, ensuring consistent questioning as well as the correct usage of filters and randomisation of questions and items. At least one supervisor was constantly present for quality control. Additionally, randomly selected audio records (10% of all telephone surveys) were checked for correct execution and transcription of the call. When interviewer mistakes were found, all interviews of this interviewer were checked. For quality reasons, in sample 2 online surveys that were completed in less than one third of the median time for completion (so called “speeders”) were discarded.
Answers to open-ended questions were categorised using a verbatim-based codeplan. Results of open-ended and closed-ended questions were summarised descriptively (n, %, mean) and graphically (bar-charts), stratified by predefined subgroups (age group, gender, education level, language region) (IBM SPSS statistics software, IBM, NY, USA). Means of subgroups were compared using an independent t-test or chi-square test, and a Bonferroni correction was applied for multiple testing. Assessment of correlations between the willingness to share anonymised health data and different variables was done by Pearson’s bivariate analysis with r-values of ±0.3 to 0.5 suggesting a moderate correlation.
The underlying survey does not fall under the regulations of the Swiss Human Research Act, hence this survey was not submitted to an ethics committee and no written informed consent was collected. Before the interview was started, the respondents were informed verbally that their participation is voluntary and anonymous, and that the survey is about “handling of personal data”. The respondents of the online panel were informed accordingly in the invitation, which was sent via email. No incentives were offered to the respondents of the telephone interviews for their participation.
In total, 1006 surveys of participants from the general Swiss population and 225 surveys from patients with a chronic disease were evaluable and analysed. The general population interviewed was representative of the French- and German-speaking Swiss population, that is, structurally identical or with a very similar distribution of age groups, gender and regional distribution (table 1, full overview of demographic data in supplementary material 1 in the appendix). Among the patients with a chronic disease, the representativeness for the individual chronic diseases was not investigated. The total number of randomly dialed phone numbers, non-responders, occupied sign, individuals who refused until the final number of participants is reported in supplementary material 2 in the appendix.
Table 1Demographic characterisation of survey participants.
| Population | 18–39 y | 40–64 y | >64 y | Male | Female | DE | FR |
| CH total1 | 35% | 42% | 23% | 50% | 50% | 74% | 26% |
| General Swiss population (N = 1006) | 35% | 43% | 22% | 50% | 50% | 75% | 25% |
| Chronic disease population2 (N = 225) | 21% | 49% | 30% | 43% | 57% | 67% | 33% |
| – Cancer (n = 85) | 8% | 52% | 40% | 41% | 59% | 58% | 42% |
| – MS (n = 43) | 49% | 49% | 2% | 26% | 74% | 74% | 26% |
| – Arthrosis (n = 56) | 9% | 57% | 34% | 48% | 52% | 71% | 29% |
| – Eye disease (n = 24) | 38% | 25% | 38% | 50% | 50% | 71% | 29% |
| – Back pain (n = 17) | 35% | 41% | 24% | 71% | 29% | 71% | 29% |
CH: Switzerland; DE: German-speaking part of Switzerland; FR: French-speaking part of Switzerland; MS: multiple sclerosis.
1 Percentages refer to distribution across the indicated categories and not the entire Swiss population.
2 Representativeness of patients with a chronic disease is unclear.
The first question explored the participants’ willingness to share any data about their person (not limited to personal or anonymised health data). Among the four ranking options that ranged from keeping data as secret as possible to happy sharing data, 73% of the general population tended to be rather cautious when sharing their data about their person (combining the two categories “I don’t like my data being collected and therefore keep it as secret as possible” and “I don’t like it when my data are collected, but sometimes I am forced to give it.” in figure 2A. More detailed results are presented in supplementary material 3 in the appendix). Younger participants (18–39 years) and those from the German-speaking regions were more willing to share their data than the others. Among patients with chronic diseases, 35% tended to be rather cautious when sharing data about their person (supplementary material 3 in the appendix).
The second question assessed the willingness to share health data that were collected during hospital visits in anonymised form for medical research. Seventy-one percent of the general population and 81% of the chronic disease patients expressed a positive attitude to providing their anonymised health data for medical research (fig. 2B). Willingness to share anonymised health data increased with educational level but did not differ substantially between age, gender and purchase power categories (supplementary material 4 in the appendix). Among those who were reluctant or cautiously pragmatic about sharing general personal data, 28% and 40%, respectively, stated full commitment to share anonymised health data for research (supplementary material 5 in the appendix).
The third question assessed the willingness of sharing different types of health data: 74–83% of the general population agreed to potentially share anonymised laboratory values, medical history, biological samples or results of genetic analyses, and of the chronic disease group 81–89% (supplementary material 6 in the appendix).

Figure 2 Willingness to share data. A General personal data. B Anonymised health data for medical research. The different categories are presented in percentages.
The willingness to share anonymised health data for medical research was assessed with an open-ended question. Well-intentioned purpose and guaranteed anonymity were most often mentioned (table 2). Further potential drivers that were mentioned addressed trustworthy/transparent institutions and guaranteed data security.
The following closed-ended question assessed the agreement with potential drivers to share anonymized health data (agreement ranged from “1 agree not at all” to “5 fully agree”, fig. 3). In the general population, highest agreement was reported for the drivers “better treatments for others” (86%), and “data protected from misuse” (85%), followed by “decrease in Swiss healthcare costs” (67%) and “knowing the exact research purpose” (64%). Only 9% of the general population agreed that potential direct financial compensation could be a driver. These findings are very similar in the chronic disease groups (supplementary material 7 in the appendix), except for direct financial compensation, where the agreement was higher (37%) in the chronic disease group than in the general population.
With an open-ended question, the reasons against sharing anonymised health data were investigated. The most frequently mentioned reasons “fear of misuse or unintended use” and “concerns about anonymity” were reported by 26% and 15% of the general population and 13% and 8% of the chronic disease group, respectively (table 2). Other concerns mentioned comprised “general uneasiness”, “data security” and “other reasons”. Thirty percent of the general population were not able to give a reason against sharing anonymised health data for medical research (32% of the chronic disease group; table 2).
Based on the closed-ended questions, the general population reported agreement with the following barriers: “concerns about privacy” (74%), “potential identification despite anonymization” (68%), “risk of higher health insurance premium” (65%) and “financial benefits for other people/companies” (60%) (figure 3). Only 17% agreed with the barrier “no personal health benefit”. The chronic disease group reported similar agreements (supplementary material 7 in the appendix).
Comparisons between gender or age groups did not reveal any noteworthy differences (supplementary material 8 in the appendix).
Table 2Frequency of requirements mentioned (potential drivers or barriers) affecting the attitude to sharing anonymised data for medical research1.
| General Swiss population (N = 1006) | Chronic disease population 2 (N = 225) | |
| Requirements to share anonymised health data | ||
| Well-intentioned purpose | 35% | 25% |
| Anonymity must be guaranteed | 30% | 20% |
| Trusted/transparent institutions | 17% | 9% |
| Data security must be guaranteed | 13% | 8% |
| Personal incentives | 3% | 5% |
| Other | 4% | 6% |
| Nothing, will not share at all | 3% | 1% |
| Nothing, will do anyway | – | 5% |
| Don’t know / no answer | 19% | 41% |
| Reasons against sharing anonymised health data | ||
| No reasons against | 30% | 32% |
| Fear of misuse or unintended use | 26% | 13% |
| Concerns about anonymity | 15% | 8% |
| Concerns about data security | 6% | 5% |
| General uneasiness | 11% | 4% |
| Other | 6% | 7% |
| Don’t know / no answer | 19% | 40% |
1 Open-ended question, multiple selection possible.
2 Representativeness is unclear for Swiss population with chronic diseases.
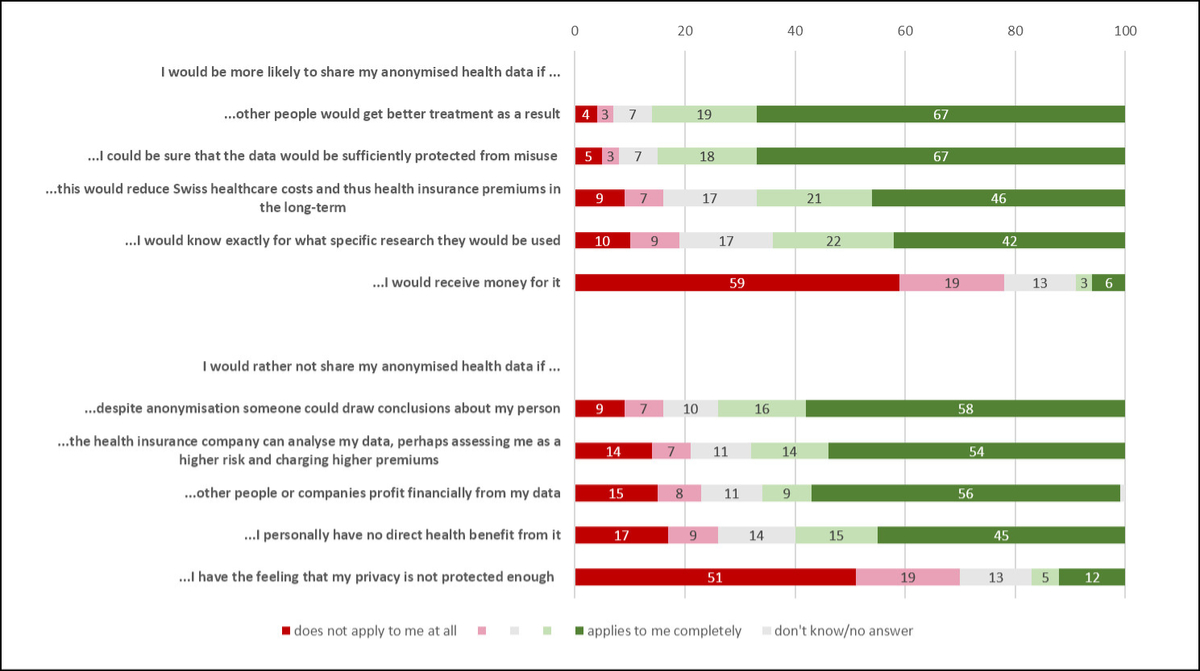
Figure 3 Agreement to potential drivers and barriers affecting the willingness to share health data for medical research (general population, N = 1006). The different categories are presented in percentages.
With a closed-ended question, participants were asked to rate their trust in institutions using anonymised health data for medical research in the interest of patients. In the general population, trust was highest for hospitals (67%) and universities (66%) and lowest for the health insurance (19%) and pharma sectors (19%). Trust in the Swiss Federal Office of Public Health (FOPH) was 56%. The trust in these institutions was rated higher by the chronic diseases population: 72% for hospitals, 72% for universities, 30% for health insurances and 29% the pharmaceutical sector. Only the Swiss Federal Office of Public Health was rated lower (49%). Trust-ratings were stratified by gender and age groups (supplementary material 9 in the appendix).
The participants were asked (closed-ended question) whether they would like to be better informed about the handling of health data. Fifty-six percent of the general population and 78% of the chronic disease patient group would like to be better informed. The last question (open-ended) assessed who the participants see in charge of informing about the topic; 44% of the general population and 69% of the chronic disease population mentioned government bodies (e.g., FOPH) as being responsible for informing about this topic. Physicians, health insurers and hospitals were mentioned by 8%, 9% and 5%, respectively, of the participants of the general population, and by 18%, 12% and 11%, respectively, of the chronic disease group.
The results of this survey show that more than two thirds of the French- and German-speaking Swiss population are willing to share anonymised health data for medical research. The willingness to share anonymised health data was even higher among patients with chronic diseases. The two interviewed populations (the general population and chronic disease patients) responded similarly in most cases and no major deviations were discovered. It is unclear to what extent the chronic disease group is representative for patients with cancer, multiple sclerosis, arthrosis, eye disease or back pain. The tendency of the chronic disease population to marginally higher willingness to share their anonymised health data might be explained by the willingness to participate in online panel surveys, for which the chronic disease patients have subscribed and hence already have a certain openness to share information. Moreover, as a patient, they have already been confronted with this topic in the hospital, be it in relation to a request for study participation or when signing consent forms (such as the Swiss general consent).
From the literature, reported willingness to share health data for research varies from 50% to 90% across western European countries [21–24]. Based on this survey, Switzerland is within this range (71%). Apart from different methodological approaches to conducting the surveys, the willingness to share health data depends on the legal requirements, data protection laws, healthcare system and cultural aspects of the respective countries. Hence, we will focus rather on literature from Switzerland in this discussion. A recent Swiss survey (Brall et al.) assessed the willingness of the general Swiss population to participate in studies on personalised health research with the focus on sharing personal health data (compared with anonymised health data in the present article) [17]. Brall et al. reported that 53.6% of the population were willing to participate in a personalised health research study. Compared with the present survey (71% willing to share their anonymised health data), this is considerably less and cannot be explained by different population characteristics, such as gender, age, education level, etc.. Nevertheless, there are other possible explanations for this discrepancy. First, Brall et al. focused on the clinical research setting and not on the real-world setting like the present survey. Second, the survey by Brall et al. used a different methodological approach (regular mailing with scope and background information, possibility to answer online or on paper, sending out reminders, questions with increased complexity, including the Italian-speaking region) for their investigation. With the letter-based approach, the participants had more time to reflect on their responses compared with telephone interviews, where they had to answer faster and in a more spontaneous way. Third, the survey by Brall et al. was conducted just before the COVID-19 pandemic. Because of the public discussion on lack of digitalisation in healthcare settings or contact tracing during the pandemic, this might have led to an increased acceptance of sharing health data in the present survey. Fourth, the Swiss population differentiates between personal data and anonymised data, wherein the population is more willing to share anonymised health data than personal data (i.e., identifiable data). In this case, anonymisation of data could be considered as a driver to sharing health data. However, the reason for the discrepancy remains unanswered, and future research could assess the potential impact of COVID-19 or anonymisation on the willingness to provide health data. Similarly to Brall et al., the present survey identified population characteristics, such as younger age or higher educational level, to be associated with higher willingness to share data, but in a real world setting.
The present survey showed that the main drivers of the willingness to share anonymised health data are mainly of an altruistic nature, namely “that other people get better treatment”. Only 9% of the participants expected a financial incentive or direct health benefit. The altruistic motivation of the Swiss population is encouraging and supports the current practice of health research where normally no incentives are given to trial participants. A narrative review of publications on attitudes towards the use of health data for research purposes showed similar qualitative results, reporting main drivers for sharing health data such as “sharing for the common good” or “returning own benefits incurred from research” [12]. Although the above-mentioned Swiss survey by Brall et al. did not report on potential drivers, they reported concerns about sharing health data, mainly data protection/security issues and fear of misuse, and hence very similar findings to the present survey. One concern in Brall et al., which stood out, “worried data used to discriminate against me or my family”, is comparable to potential fear from disadvantages by health insurance in the present survey. The concerns identified in the present survey or by Brall et al. have a large overlap with the qualitatively assessed barriers in the narrative review from Kalkman et al. [23]. Kalkman et al. reported the following reasons against sharing anonymised health data: data protection issues, such as data misuse, anonymity and data security. Interestingly, Kalkman reported that lack of public trust in data-sharing activities or lack of trust in data-handling institutions is negatively influencing the willingness to share data, similar to the findings of the present survey.
Data protection and data security concerns of the general Swiss population are understandable, and underline the need for a better public understanding of health data and its usage. This highlights the need for more information or education within the population. Although the legal architecture in Switzerland is complex, the handling of sensitive data (personal health data) and the surrounding accountabilities are sufficiently covered by the different laws and regulations [11]. As mentioned in the introduction, the Swiss legal framework does not define the technical requirement to anonymise data and some people might interpret this as lack of clarity. It is important to understand that anonymisation is a technical instrument and it does not replace data governance or supersede the accountability of the data users [14]. Anonymisation should be considered as part of the data governance. Even if data are anonymised, they should be handled in a securely protected manner to avoid potential re-identification of an individual patient in the future [11, 14, 25].
More than half of the survey participants wished to be better informed about the handling of health data, i.e., to have a better understanding of the appropriate measures taken to protect their personal rights and freedoms as guaranteed by such laws. This knowledge gap could be the reason for the general concerns regarding data protection and security of data. A recent Swiss survey, commissioned by the Swiss Federal Office of Public Health (FOPH), assessed knowledge gaps on the Human Research Act, which became effective in 2014 [21]. This FOPH report revealed that 50–61% of the population would like to know more about legal regulations related to human research. This finding goes in the same direction as our survey assessing whether participants would like to be better informed about the data topic. This raises the question “who is responsible for informing the general public about this topic?"
It was not surprising that in the present survey, the participants clearly see the authorities, i.e., FOPH, to be responsible for educating the Swiss population. This result might be biased because the present survey was conducted during the COVID-19 pandemic (September to October 2020), when the FOPH was proactively informing about current evolutions of the Covid-19 pandemic. After the Swiss authorities, physicians, health insurances and hospitals were frequently mentioned as responsible for informing about health data. As the health data topic becomes increasingly important, the authorities (or other independent bodies) could play a central role to improve the basic knowledge within the general population, which in turn would facilitate the informed consent process of the data-collecting institutions. From the legal perspective, the data-collecting healthcare institutions are obliged to inform the patients if their data is going to be re-used for research [26, 27] and hence the healthcare professionals already play an important role by explaining to their patients consent forms such as the “general consent” for the re-use of health data [28]. To disperse the fears and barriers around data protection issues and potential misuse of health data, informing patients should not be limited to the “data-collecting” institutions, the “data-using” institutions (universities, other third parties, etc) should also inform proactively and transparently about the purpose and their interests in re-using the health data. Consequently, all stakeholders of the healthcare system may play an important role in informing about re-using health data.
The present survey assessed the level of trust in five institutions/stakeholders by asking “how is your trust that this institution uses your anonymized health data for research in the interest of the patients?”. Notably, only two thirds of the general population expressed trust in hospitals or universities, half of the population expressed trust in the Swiss authorities, and less than a fifth trusted in health insurers or the pharmaceutical industry. This low trust in industry has also recently been observed in a survey conducted in The Netherlands and Germany [29]. Trust in an institution correlates with the willingness to share health data (supplementary material 10 in the appendix), hence the lack of trust results in lower willingness to share health data. Transparency is needed to build trust and transparent communication would be an important first step, mainly done by the data-collecting institutions, as explained above, but the other data-using stakeholders of the healthcare system also play an important role. Ideally, the data flow could be displayed from data collection, to coding and anonymisation until final research results. Disclosure of who has access to what data at which time point would increase the trust and decrease the fear of possible data protection issues. Besides the full disclosure of the data flow, the final last step should be to inform the patients about the findings of a research project where they agreed to share their data, as it has been reported by the participants in the survey from Brall et al. [17].
The low level of trust sharing anonymised health data with the pharmaceutical industry shows that the role of the pharmaceutical industry within the healthcare system is not fully understood. Pharmaceutical industry plays an important role in advancing medical research and is the key driver for medical innovation in Switzerland. The pharmaceutical industry needs to raise awareness about its contribution to advancing medical research and how anonymised healthcare data can be used in this context. An open and proactive public engagement is necessary 1) to understand the roles of the different stakeholders in the healthcare data ecosystem and 2) to increase the trust in the general population.
This survey has several strengths. First, the topic of using anonymised health data for medical research has not been investigated yet in Switzerland. Second, the telephone interviews were conducted anonymously, and this was explained to the participants at the beginning of the interview; therefore, it can be expected that the broad concept of anonymisation was understood by most participants. Third, the survey was conducted with short telephone interviews with a simple structure and included open questions allowing spontaneous responses. Fourth, the short and simple structure of the telephone interviews assured the completeness of responses. Fifth, despite their limited representativeness, including a population with chronic diseases provides valuable insights. On the other hand, telephone interviews were time limited, which did not allow to include complex and sophisticated questions. Hence, several topics were not addressed because it was too complicated for the short telephone interviews, including, process of providing informed consent, data governance, exchange of health data between different institutions, willingness to share identifiable (e.g., coded) data vs anonymised data, and different legal theories around anonymisation. Another possible limitation of this survey is that participants who agreed to join a survey might be biased since they tend to be more open to sharing their information than those who refused to participate. This applies also to the sample of chronic disease patients as already mentioned above.
The anonymisation of health data is an important step in unleashing the benefits of a connected healthcare data ecosystem for improved patient care and health system efficiency, and to accelerate data-driven biomedical research and development. Enabling access to health data for third parties, such as the pharmaceutical industry, governmental bodies and others, who usually have no access to routine health data, will be key to ensuring that all stakeholders and ultimately society as a whole can benefit. The present survey showed that the Swiss population is willing to share their anonymised health data, although substantial concerns regarding data protection and security have been raised. To address these concerns, educational efforts to increase understanding and to raise awareness of data sharing and re-use within the general population is needed. Therefore, transparent communication about the use of health data throughout the data journey is crucial. In addition, an ppen dialogue is required to develop a common consent on data governance for Switzerland. This open dialogue should involve all stakeholders of the healthcare system, including patients and third parties such as the pharmaceutical industry. The stakeholders need to declare their interests, needs and preconditions for a fair exchange of value to ultimately foster the trust of the general public towards transparent health data exchange and ultimately towards a more personalised and more sustainable Swiss healthcare system.
We thank the participants in this survey and the only panel for their participation which allowed us to gain this novel insight into the perception of sharing their anonymised health data. We would like to thank the agency SFL Regulatory Affairs & Scientific Communication GmbH for the medical writing support. Finally many thanks to William Archey, Ingo Bühner, Jean-Marc Häusler, Damian Page, Alexandra Schmid and Florian Zabel for their valuable contributions to the present work.
FP and DG are employed by Roche Pharma Schweiz AG and are stakeholders of Roche. KML works as a project manager at gfs-zürich. Roche Pharma Switzerland AG commissioned an independent research organization, gfs-zürich, to conduct this survey. All authors fulfill the ICMJE criteria for authorship by providing substantial contributions to conception and design of the work (DG, FP, KML), data acquisition and analysis (KML), interpretation of the data (DG, FP, KML), drafting the manuscript (DG, FP). DG, FP and KML have approved the final version of the manuscript and are accountable for the final content of the manuscript. The data of this survey was further used by KML for her independently conducted master thesis (Master of Advanced Studies UAS Zurich) at the ZAHW School of Management and Law. KML has no other potential conflict of interest.
1. Cave A , Kurz X , Arlett P . Real-World Data for Regulatory Decision Making: Challenges and Possible Solutions for Europe. Clin Pharmacol Ther. 2019 Jul;106(1):36–9. https://doi.org/10.1002/cpt.1426
2. US Food & Drug Administration. Framework for FDA’s Real-World Evidence Program 2018 [01 October 2021]. Available from: https://www.fda.gov/media/120060/download
3. Vayena E , Dzenowagis J , Brownstein JS , Sheikh A . Policy implications of big data in the health sector. Bull World Health Organ. 2018 Jan;96(1):66–8. https://doi.org/10.2471/BLT.17.197426
4. Geneviève LD , Martani A , Mallet MC , Wangmo T , Elger BS . Factors influencing harmonized health data collection, sharing and linkage in Denmark and Switzerland: A systematic review. PLoS One. 2019 Dec;14(12):e0226015. https://doi.org/10.1371/journal.pone.0226015
5. SWI swissinfo.ch. Digitising clinical data: an uphill road for Switzerland. Published February 12, 2021. Available from: https://www.swissinfo.ch/eng/sanit%C3%A0_digitizing-clinical-data--an-uphill-road-for-switzerland/46363068
6. National Institute for Cancer Epidemiology and Registration . National Agency for Cancer Registration 2020 [01 October 2021]. Available from: https://www.nacr.ch/
7. Universitäre Medizin Schweiz . Generalkonsent für die Forschung 2021 [01 October 2021]. Available from: https://www.unimedsuisse.ch/de/projekte/generalkonsent
8. Swiss Personalised Health Network . Infrastructure building to enable nationwide use and exchange of health data for research. An initiative of the Swiss Government [11 February 2022]. Available from https://sphn.ch/
9. Meier-Abt PJ , Lawrence AK , Selter L , et al. The Swiss approach to precision medicine. Published 2. January 2018. Swiss Medical Weekly. Available from: https://smw.ch/op-eds?tx_swablog_postdetail%5Bpost%5D=65
10. Swiss Federal Council . Konzept für die Auswertung und Veröffentlichung von Krebsdaten - 3. Jährliches Krebsmonitoring 2020 [01 October 2021]. Available from: https://www.bag.admin.ch/bag/de/home/gesetze-und-bewilligungen/gesetzgebung/gesetzgebung-mensch-gesundheit/gesetzgebung-krebsregistrierung/datenbearbeitung_und_datennutzung.html
11. Martani A , Egli P , Widmer M , Elger B . Data protection and biomedical research in Switzerland: setting the record straight. Swiss Med Wkly. 2020 Sep;150:w20332. https://doi.org/10.4414/smw.2020.20332
12. Swissethics. Ethical health data sharing in public-private partnerships - Guidelines. Update published 16. December 2021. Available from: https://swissethics.ch/themen/ethischer-austausch-von-gesundheitsdaten-in-oeffentlich-privaten-partnerschaften
13. Vokinger KN , Stekhoven DJ , Krauthammer M . Lost in Anonymization - A Data Anonymization Reference Classification Merging Legal and Technical Considerations. J Law Med Ethics. 2020 Mar;48(1):228–31. https://doi.org/10.1177/1073110520917025
14. Mourby M . Anonymity in EU Health Law: Not an Alternative to Information Governance. Med Law Rev. 2020 Aug;28(3):478–501. https://doi.org/10.1093/medlaw/fwaa010
15. European Commission - Consumers . Health, Agriculture and Food Executive Agency. Assessment of the EU Member States’ rules on health data in the light of GDPR. [11. February 2022] Available from: https://ec.europa.eu/health/system/files/2021-02/ms_rules_health-data_en_0.pdf
16. Interpharma. Serie Gesundheitsdatenökosystem Teil 1: Mit Massnahmen in sechs Bereichen zum Gesundheitsdatenökosystem 2021 [01 August 2021]. Available from: https://www.interpharma.ch/blog/serie-gesundheitsdatenoekosystem-teil-1-mit-massnahmen-in-sechs-bereichen-zum-gesundheitsdatenoekosystem/
17. Brall C , Berlin C , Zwahlen M , Ormond KE , Egger M , Vayena E . Public willingness to participate in personalized health research and biobanking: A large-scale Swiss survey. PLoS One. 2021 Apr;16(4):e0249141. https://doi.org/10.1371/journal.pone.0249141
18. Bortz J. , Schuster C. Statistik für Human- und Sozialwissenschaftler. Springer Verlage, 7. Auflage. ISBN 978-3-642-12769-4
19. Bundesamt für Statistik . Struktur der ständigen Wohnbevölkerung nach Kanton, 1999-2020. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/stand-entwicklung.assetdetail.18344208.html
20. Bundesamt für Statistik . Hauptsprachen in der Schweiz. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/sprachen-religionen/sprachen.assetdetail.15384655.html
21. Ehrler F , Lebert F . Wissensstand und Haltung der Allgemeinbevölkerung zur Humanforschung und deren Regelungen 2018. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/biomed/forschung-am-menschen/forschung-biomedizin/Schlussbericht-Bevoelkerungsbefragung-Humanforschung.pdf.download.pdf/Schlussbericht-Bevoelkerungsbefragung-Humanforschung.pdf
22. Johnsson L , Helgesson G , Rafnar T , Halldorsdottir I , Chia KS , Eriksson S , et al. Hypothetical and factual willingness to participate in biobank research. Eur J Hum Genet. 2010 Nov;18(11):1261–4. https://doi.org/10.1038/ejhg.2010.106
23. Kalkman S , van Delden J , Banerjee A , et al. Patients’ and public views and attitudes towards the sharing of health data for research: a narrative review of the empirical evidence. J Med Ethics. 2019 Nov.
24. Richter G , Borzikowsky C , Hoyer BF , Laudes M , Krawczak M . Secondary research use of personal medical data: patient attitudes towards data donation. BMC Med Ethics. 2021 Dec;22(1):164. https://doi.org/10.1186/s12910-021-00728-x
25. El Emam K , Rodgers S , Malin B . Anonymising and sharing individual patient data. BMJ. 2015 Mar;350 mar20 1:h1139. https://doi.org/10.1136/bmj.h1139
26. Fedlex. Federal Act on Data Protection (FADP). Art. 4 and Art. 14. [11. February 2022]. Available from: https://www.fedlex.admin.ch/eli/cc/1993/1945_1945_1945/de#art_14
27. Fedlex. Federal Act on Research involving Human Beings. Human Research Act, HRA. Art. 16. [11. February 2022]. Available from: https://www.fedlex.admin.ch/eli/cc/2013/617/en#art_16
28. Griessbach A , Bauer A , Jörger Lebet F , Grossmann R . The concept of General Consent in Switzerland and the implementation at the University Hospital Zurich, a cross-sectional study. Swiss Med Wkly. 2022 Apr;152(15-16):w30159. https://doi.org/10.4414/smw.2022.w30159
29. Richter G , Borzikowsky C , Lesch W , Semler SC , Bunnik EM , Buyx A , et al. Secondary research use of personal medical data: attitudes from patient and population surveys in The Netherlands and Germany. Eur J Hum Genet. 2021 Mar;29(3):495–502. https://doi.org/10.1038/s41431-020-00735-3
The supplementary material is available in the PDF version of this article.