Low total gamma globulin level discovery at diffuse large B-cell lymphoma diagnosis predicts high risk of infection-related death: data from a monocentric retrospective study
DOI: https://doi.org/10.4414/SMW.2022.w30143
Alexandre
Nguyena, Nicolas
Martin-Silvaa, Hubert
de Boyssona, Samuel
Deshayesa, Anne-Claire
Gacb, Emilie
Reboursièreb, Gandhi
Damajb, Achille
Aoubaa
aDepartment of Internal Medicine and Clinical Immunology, Normandie Univ, UNICAEN, CHU de Caen Normandie, Caen, France
bDepartment of Clinical Haematology, Normandie Univ, UNICAEN, CHU de Caen Normandie, Caen, France
*Contributed equally to this work
Summary
OBJECTIVE: Diffuse large B-cell lymphoma can complicate the course of B-cell primary immunodeficiencies or induce lowering of total gamma globulin levels, whose clinical status as an effective secondary immunodeficiency remains unspecified. This study aimed to assess the frequency, and clinical and prognostic relevance of the low total gamma-globulin levels discovered at diagnosis of diffuse large B-cell lymphoma.
RESULTS: In a 2-year monocentric retrospective study, 96 patients diagnosed with diffuse large B-cell lymphoma who had a serum electrophoresis were included. Patients were divided into those with lower (L-TGL and higher (H-TGL) total gamma-globulin levels (total gamma-globulin levels ≤5.5 g/l and >5.5 g/l) and compared for outcomes, including fatal infectious events. Twelve (12.5%; 8 males; age median 68 years, range 55—82 years) exhibited L-TGL. There was no difference between the both groups regarding demographics, Ann Arbor lymphoma stage, inflammatory parameters or chemotherapy regimen. However, overall death rates (10/12, 83.3% versus 22/96, 26.2%; p = 0.03) and infection-related death rates (10/12, 83% versus 6/96, 6.2%; p <0.001) were significantly higher in the L-TGL group.
CONCLUSION: We demonstrate for the first time the strong negative impact of L-TGL on overall and infection-related mortality in diffuse large B-cell lymphoma. Prospective studies should distinguish immunodeficiencies secondary to the lymphoma from pre-existing humoral primary immunodeficiencies, using biomolecular testing and post-treatment total gamma-globulin level monitoring, to determine the best management strategy for infectious risk during diffuse large B-cell lymphoma treatment in the context of L-TGL.
Introduction
Recurrent bacterial, viral, fungal and/or parasitic infections are the hallmarks of primary and secondary immunodeficiencies. The most common forms of primary immunodeficiency are related to inherited adaptive immune system dysfunctions involving B and/or T cells and are not infrequently discovered in adulthood. For secondary immunodeficiencies, the leading causes are related to chemotherapy or immunosuppressant use, or to underlying diseases such as cancers, lymphomas and systemic inflammatory/autoimmune diseases. Other causes and mechanisms of secondary immunodeficiencies result from protein wasting or metabolic disorders, including a severely impaired general state.
In addition to infections, other events, such as autoimmune cytopenias, cancers or lymphoproliferative disorders (including diffuse large B-cell lymphoma), can complicate the natural history of primary immunodeficiencies [1–3]. The most frequent subtype, common variable immunodeficiency, renders affected patients prone to developing lymphomas [4–7] and frequent infections, which is identified as the main feature, responsible for diagnosis and the leading cause of death in this population [2, 8, 9]. The diagnosis of primary immunodeficiencies is often delayed for several years, because of their clinical heterogeneity and higher frequency of minor and neglected infections. In this context, both the onset date and natural history of the different complications of primary immunodeficiencies, such as malignant events, remain little known.
We therefore hypothesised that diffuse large B-cell lymphoma with a datable diagnosis might also reveal primary immunodeficiencies with possible previous infectious events that had not led to the recognition of an immunodeficiency. No study has yet determined the prevalence in adults of primary immunodeficiencies revealed by lymphoma, since the diagnostic criteria of primary immunodeficiencies do not take into account lymphoma [10–15]. On the one hand, humoral primary immunodeficiencies, including common variable immunodeficiency, are frequent forms of immunodeficiency that can be suggested by a low serum total gamma-globulin level identified using serum electrophoresis performed at the time of diagnosis of diffuse large B-cell lymphoma. On the other hand, diffuse large B-cell lymphoma, the most frequent lymphoproliferative disorder, can also induce a decrease in serum total gamma-globulin level owing to clonal selection and proliferation. However, the frequency, risk and prognosis of infectious events related to low total gamma-globulin (L-TGL) discovered at diagnosis of diffuse large B-cell lymphoma are unknown and no specific therapeutic strategy is therefore recommended in this context. In the context of multiple myeloma or chronic lymphoid leukaemia, a L-TGL is well recognised to correspond to a secondary immunodeficiency with a documented increase in infectious events and risk that can require polyvalent immunoglobulin infusions [16–19].
The clinical relevance and utility of serum electrophoresis for identifying L-TGL in diffuse large B-cell lymphoma is unknown, both at diagnosis and during the therapeutic follow-up of the lymphoproliferative disorder. The association of serum electrophoresis and diffuse large B cell lymphoma is of particular interest, as serum electrophoresis can be easily and routinely performed at diagnosis of the lymphoma, in order to assess the functionality of the B-cell compartment. Diffuse large B cell lymphoma onset, unlike the onset of indolent lymphomas, can be easily dated based on B symptoms and rapid tumoral syndrome, allowing follow-up monitoring.
We conducted a retrospective study to assess the clinical and prognostic relevance of L-TGL discovery at the time of diagnosis of diffuse large B-cell lymphoma in Caen University Hospital. Our main judgment criteria were the frequency of L-TGL in comparison with higher (H-TGL) at diffuse large B-cell lymphoma diagnosis, the mortality rate and main causes of death, especially those related to infectious events, in the two subgroups.
Methods
Diffuse large B cell lymphoma patient cohort
Inclusion criteria and definitions
We retrospectively extracted from the monocentric lymphoma database of Caen University Hospital all patients diagnosed with diffuse large B-cell lymphoma between January 2015 and December 2016, and listed those with serum electrophoresis performed at diagnosis before receiving any specific treatment. Included patients were categorised into two groups based on their total gamma globulin level on serum electrophoresis at diagnosis, namely L-TGL or H-TGL. Although the diagnosis of common variable immunodeficiency requires a threshold <5 g/l of IgG isotype in addition to other biological, clinical and anamnestic criteria [20–22], we chose the total serum gamma-globulin level because it is an easily available biological test. We defined L-TGL as a total gamma-globulin level ≤5.5 g/l on serum electrophoresis as it is <50% of the lower limit of our laboratory reference range, is similar to what was used in others studies in haematological malignancies [19] and because IgG represents 75% of the total gamma-globulin level in the absence of monoclonal gammopathy, it would be unlikely to have more than 5 g/l of IgG.
Exclusion criteria
As our hypothesis was based on L-TGL being a possible marker of primary or secondary immunodeficiency related to the aggressive diffuse large B-cell lymphoma, we excluded all patients exhibiting an associated monoclonal peak on serum electrophoresis, unrelated loss of serum protein such as exudative enteropathy, extended burns or nephrotic syndrome, previous treatment with cytotoxic drugs or immunosuppressants, and previous primary or secondary immunodeficiency diagnosed before diffuse large B-cell lymphoma.
Ethics approval and consent
The study was approved by the Research Ethics Committee (CLERS) of Caen (ref: 03/2020/AOU).
Study variables
For all patients, demographic information at diagnosis, disease status, chemotherapy regimen, Ann Arbor staging classification, central nervous system involvement, and international prognostic index and outcome data were collected until the last follow-up date. For biological data, C-reactive protein (CRP), albumin, lymphocyte count, lactate dehydrogenase and ferritin levels were collected at the time of diagnosis in the absence of an infectious process, and, when available, IgG, IgM, and IgA levels were recorded. We considered the albuminaemia/gammaglobulinaemia ratio (AG ratio) to discriminate possible causes for L-TGL: (1) for a nonspecific decrease in total gamma-globulin level related to an impaired general state , a decrease in albumin should be associated with a decrease in total gamma-globulin level, with an unaltered AG ratio; (2) for a specific decrease in total gamma-globulin level directly related to our hypotheses, i.e., L-TGL due to either an undiagnosed primary immunodeficiency or an immunodeficiency secondary to the diffuse large B-cell lymphoma, the albumin level should be close to normal, resulting in an increase in the AG ratio.
Statistical analyses
Data were summarised as the median (range) or number (%). Continuous variables were analysed using the Mann-Whitney test, and Fisher’s test was used to compare categorical variables. A p-value <0.05 was considered statistically significant.
Predictors of death in diffuse large B-cell lymphoma were evaluated using Cox proportional hazard models. All the variables showing a univariate p-value less than 0.20 were entered into a multivariable logistic regression model. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (95% CIs). Two-sided p-values lower than 0.05 were considered significant.
Overall survival in diffuse large B cell lymphoma and subgroups L-TGL and H-TGL were analysed using the Kaplan-Meier method, and these variables were compared using the log-rank test.
All statistical analyses were performed using GraphPad Prism software (7.0) and JMP 9.0.1 (SAS Institute Inc., Cary, NC, USA).
Results
Out of 122 patients diagnosed with diffuse large B-cell lymphoma during the pre-specified period, 96 (74.8%) had a serum electrophoresis test before any chemotherapy, and among these, 12 (12.5%; 8 males;age median 68 years range (55–89 years) had L-TGL. Demographic and biological data for both the L-TGL (n = 12; total gamma-globulin level ≤5.5 g/l) and H-TGL (n = 84; total gamma-globulin level >5.5 g/l) subgroups are summarised in table 1.
Table 1Diffuse large B-cell lymphoma patient characteristics and outcome according to serum total gamma-globulin levels.
|
All patients
|
p-value
|
Deceased patients
|
p-value
|
|
L-TGL (≤ 5.5 g/p) patients
|
H-TGL (> 5.5 g/p) patients
|
L-TGL (≤ 5.5 g/l) patients
|
H-TGL (> 5.5 g/l) patients
|
| No. |
12 |
84 |
NA |
10 |
22 |
|
| Sex (M/F) |
7/5 |
33/51 |
0.12 |
6/4 |
11/11 |
0.71 |
| Age (years) median (range) |
65 (55–80) |
70 (21–89) |
0.89 |
65 (57–80) |
72 (56–85) |
0.41 |
| Lymphoma Ann Arbor Stage |
I |
0 |
16 |
0.69 |
0 |
0 |
1 |
| II |
1 |
15 |
0.69 |
1 |
2 |
1 |
| III |
4 |
15 |
0.29 |
2 |
4 |
1 |
| IV |
7 |
38 |
0.60 |
7 |
16 |
1 |
| Central nervous system involvement (n) |
2 |
5 |
0.24 |
2 |
1 |
1 |
| Richter syndrome |
0 |
2 |
1 |
0 |
2 |
1 |
| International prognostic index risk group (n) |
10 (CNS excluded) |
77 (CNS and RS excluded) |
|
8 (CNS excluded) |
19 (CNS and RS excluded) |
|
|
(0–1) Low |
1 |
7 |
1 |
1 |
0 |
0.31 |
| (2) Low-intermediate |
2 |
28 |
0.72 |
1 |
2 |
1 |
| (3) High-intermediate |
4 |
19 |
0.48 |
2 |
5 |
1 |
| (4–5) High |
4 |
23 |
0.73 |
4 |
12 |
1 |
| Total gamma-globulin level (g/l) median (range) |
4.8 (2.7–5.5) |
9.15 (5.9–19.3) |
<0.01 |
4.78 (2.7–5.5) |
9.25 (5.9–19.3) |
<0.01 |
| Immunoglobulin isotype level (n) |
8 |
46 |
0.80 |
7 |
13 |
1 |
|
IgG level (g/l) median (range) |
5.4 (2.8-5.45) |
9.6 (6.4–15.7) |
<0.01 |
5.45 (2.8–5.45) |
11.4 (6.48–13.8) |
<0.01 |
| IgA level (g/l) median (range) |
0.7 (0.35–5.91) |
2.3 (0.3–5.0) |
0.31 |
0.61 (0.35–5.91) |
1.49 (0.3–2.16) |
0.31 |
| IgM level (g/l) median (range) |
0.4 (0.2–3.7) |
1.0 (0.2–3.3) |
0.32 |
0.46 (0.2–3.7) |
1.26 (0.25–1.35) |
0.32 |
| CRP level (g/l) median (range) |
31 (3–116) |
15 (3–237) |
0.31 |
26.5 (3–116) |
37 (3–119) |
0.31 |
| Ferritin level (µg/l) median (range) |
433 (73–1628) |
170 (4–3472) |
0.02 |
538.5 (73–1628) |
217.5 (93–1496) |
0.02 |
| Albumin level (g/l) median (range) |
32.5 (24–40.7) |
39 (16–50) |
0.15 |
33.8 (24–40.7) |
36 (23–46) |
0.15 |
| Serum total protein level (g/l) median (range) |
58.17 (46–90) |
64.5 (46–72) |
<0.01 |
58 (46–90) |
63.5 (52–72) |
0.24 |
| AG ratio median (range) |
6.79 (4.62–12.22) |
4.39 (1.7–7.13) |
<0.01 |
6.79 (4.62–12.22) |
4.15 (1.71–7.13) |
<0.01 |
| LDH (U/l) median (range) |
341.5 (156–2784) |
278 (132–10091) |
0.93 |
285 (156–2784) |
366 (162–2793) |
0.77 |
| Lymphocyte count (G/l) median (range) |
0.8 (0.2–2.58) |
1.26 (0.18–7.56) |
0.02 |
0.8 (0.2–2.58) |
0.92 (0.27–7.56) |
0.27 |
| Follow-up (months), median (range) |
15.2 (1.23–57.77) |
55.53 (1.17–68.6) |
<0.001 |
11.7 (1.23–18.16) |
12.18 (1.17–64.23) |
0.02 |
| Deaths |
10 (83.3%) |
22 (26.2%) |
0.03 |
10 (83.3%) |
22 (26.2%) |
0.03 |
| Causes of death |
Infections |
10 (100%) |
6 (27.3%) |
<0.001 |
10 (100%) |
6 (27.3%) |
<0.001 |
| Diffuse large B-cell lymphoma progression |
0 (0%) |
12 (54.5%) |
0.35 |
0 (0%) |
12 (54.5%) |
0.35 |
| Other causes |
0 (0%) |
4 (18%) |
1 |
0 (0%) |
4 (18%) |
1 |
Ann Arbor staging and International prognostic index were comparable between the L-TGL and H-TGL groups. No Richter syndrome was reported in the L-TGL group, but two cases were described in the H-TGL group.
IgG level was lower in L-TGL patients regardless of albumin and inflammatory status
Immunoglobulin isotype levels were available only for two thirds (8/12) of L-TGL and approximately half (46/84; 55%) of H-TGL patients: the median levels of IgG were significantly lower (p <0.001) in the L-TGL subgroup than in the H-TGL subgroup and in parallel with total gamma-globulin level levels, whereas the IgA and IgM levels were not significantly different (p = 0.31 and 0.32, respectively). No patient had detectable viral load for Epstein-Barr virus or cytomegalovirus or active bacterial infection at diagnosis.
The serum total protein level was lower (p <0.01) and AG ratio was higher (p <0.01) in L-TGL than in H-TGL patients. Even though higher serum ferritin levels were found in L-TGL patients than in H-TGL patients (p = 0.02), which could indicate higher biological inflammatory status or specific organ involvement, the levels of CRP were comparable in both subgroups.
Lymphocyte levels were significantly lower in L-TGL patients (p = 0.02), but comparable between the two groups for deceased patients (table 1).
Death and infection-related death rates were higher in the L-TGL subgroup
The mortality rate was higher in the L-TGL group 10/12, 83% versus 22/84, 26.2%; p = 0.03) and median follow-up was shorter (duration 15.2 months versus 55.53 months; p <0.001) than in H-TGL subgroup (table 1). Similarly, the rate of death caused by an infection was significantly higher in L-TGL than in H-TGL patients (10/10, 100% versus 6/22, 27.3%; p <0.001), as shown in table 1. In H-TGL patients, deaths were caused by diffuse large B-cell lymphoma progression for most patients (54.5%; 12/22 deceased patients), whereas the remaining patients (18.2%; 4/22 deceased patients) died from other causes that were independent of the background disease or related treatments.
among the infection-related deaths, no opportunistic infection was identified. All 10 deaths in the L-TGL subgroup and 3 out of 6 infection-related deaths in H-TGL subgroup were related to pleuro-pneumopathy, either associated or not with ear, nose and throat infections and/or Streptococcus pneumoniae. The remaining three H-TGL infection-related deaths were caused by septic shock complicating pyelonephritis, staphylococcal bacteraemia and cutaneous cellulitis of a diabetic foot.
As seen on Kaplan-Meier curves shown in figure 1, the survival of L-TGL patients was significantly lower (p <0.001) than all the other groups in the whole patient cohort, including H-TGL patients (n = 84), all patients who had a serum electrophoresis (i.e., L-TGL and H-TGL patients considered together; n = 96) and patients who did not have serum electrophoresis at diagnosis (n = 26).
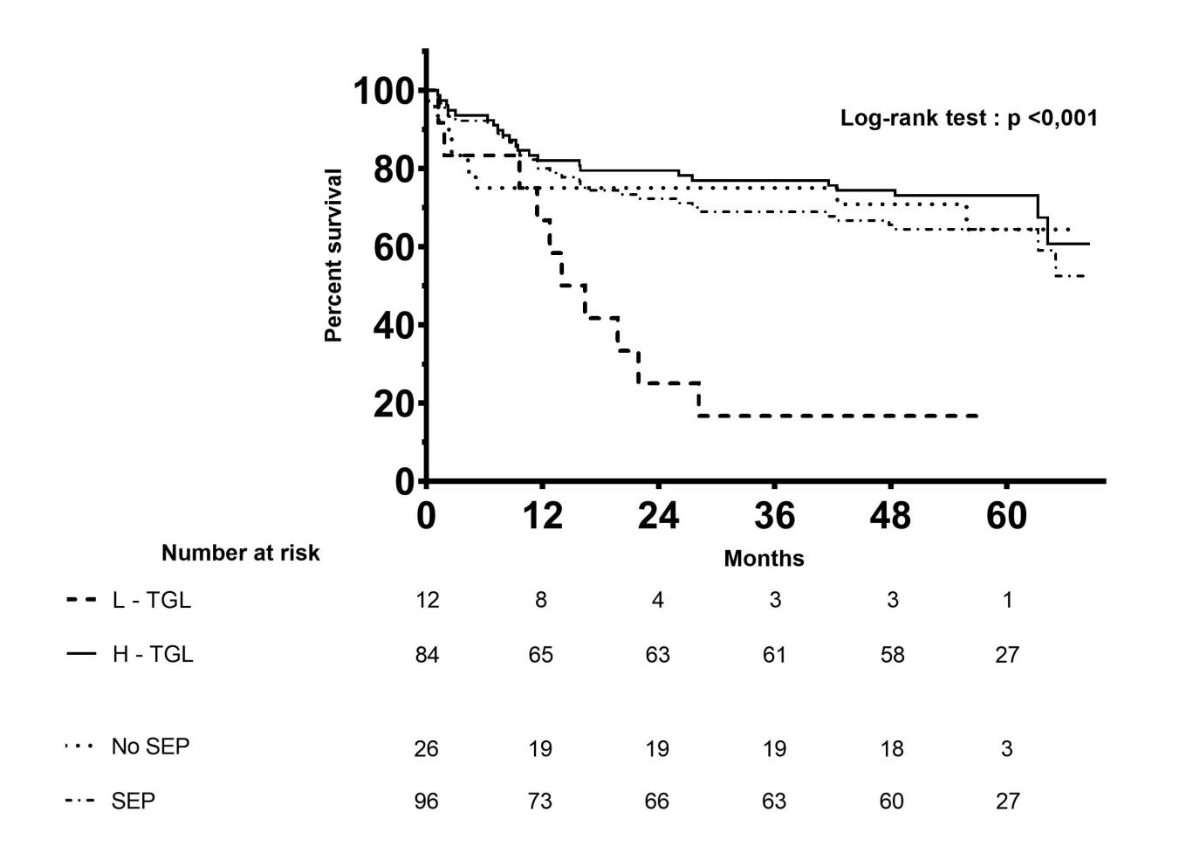
Figure 1 Kaplan-Meier survival curves of diffuse large B-cell lymphoma patients according to serum total gamma-globulin levels.
TGL: total gamma-globulin levels; L-TGL: lower-TGL; H-TGL: higher-TGL; SEP: patients with serum electrophoresis; No SEP: patients without serum electrophoresis at diagnosis. Time is expressed in months on the x-axis.
No concordance between total gamma-globulin and albumin levels in L-TGL and H-TGL subgroups
Both subgroups were similar for all clinical and usual biological parameters including albumin levels, except for the median total gamma-globulin (p <0.01) and IgG levels (p <0.01), which were lower in L-TGL patients than in H-TGL patients, as expected. Moreover, the AG ratio was higher in L-TGL than in H-TGL patients (p <0.01), indicating that the decrease in total gamma-globulin level was more pronounced than that of albumin, suggesting that albumin serum level was unrelated to total gamma-globulin level in our diffuse large B-cell lymphoma patients subgroups.
Chemotherapy regimens were comparable between L-TGL and H-TGL subgroups
The distribution of chemotherapy regimens is shown in table 2 for deceased and living patients in both the L-TGL and H-TGL subgroups. First-line chemotherapy was the R-CHOP regimen (21-day interval; rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine and oral prednisone) for 85 (88.5%) patients in total, whereas 3 others, all in the H-TGL subgroup, received ACBVP (2-week interval; daunorubicin, cyclophosphamide, bleomycin, vindesine, and oral prednisone) followed by sequential consolidation therapy consisting of two cycles of methotrexate because of their younger age; 7 others (2 and 5 in the L-TGL and H-TGL subgroups, respectively) received the RMPV regimen (rituximab, methotrexate, procarbazine and vincristine) because of cerebral involvement; the remaining patient was an elderly patient in the H-TGL subgroup who refused any chemotherapy.
A second-line chemotherapy regimen was chosen for 37 of the 96 patients (9 in the L-TGL and 28 in the H-TGL subgroups), whereas third- and fourth-line chemotherapy regimens were proposed for 10 patients and 2 patients in L-TGL and H-TGL subgroups. As shown in table 2, the characteristics of patients who died and those who remained alive at last follow-up in each subgroup appeared comparable, except for lymphoma stage: stage I lymphomas were significantly overrepresented in H-TGL patients who remained alive (p = 0.02; table 2). Of note, stage IV lymphoma and central nervous system involvement were not significantly associated with death among H-TGL patients, although the low number of events precluded any firm conclusion (table 2).
Table 2Demographic, clinical and main biological characteristics in diffuse large B-Cell lymphoma deceased and sruviving patients, according to serum total gamma-globulin levels.
|
|
L-TGL (TGL ≤5.5 g/l) patients
|
H-TGL (TGL >5.5 g/l) patients
|
|
|
Deceased patients
|
Patients alive
|
p-value
|
Deceased patients
|
Patients alive
|
p-value
|
| No. |
10 |
2 |
NA |
22 |
62 |
|
| Sex (M/F) |
6/4 |
1/1 |
NA |
11/11 |
22/40 |
0.31 |
| Age (years) median [range] |
65 (57–80) |
66 (55-78) |
NA |
72 (56–85) |
69 (21–89) |
0.07 |
| Lymphoma Ann Arbor Stage |
I |
0 |
0 |
NA |
0 |
16 |
0.02 |
| II |
1 |
0 |
NA |
2 |
13 |
0.35 |
| III |
2 |
2 |
NA |
4 |
11 |
1 |
| IV |
7 |
0 |
NA |
16 |
22 |
0.09 |
| Central nervous system involvement |
2 |
0 |
NA |
1 |
4 |
1 |
| Richter syndrome |
0 |
0 |
NA |
2 |
0 |
0.07 |
| IPI risk group (n) |
8 (CNS excluded) |
2 |
|
19 (CNS and RS excluded) |
58 (CNS excluded) |
|
|
(0–1) Low |
1 |
0 |
NA |
0 |
7 |
0.4 |
| (2) Low-intermediate |
1 |
0 |
NA |
2 |
26 |
0.06 |
| (3) High-intermediate |
2 |
2 |
NA |
5 |
14 |
1 |
| (4–5) High |
4 |
0 |
NA |
12 |
11 |
0.02 |
| Chemotherapy regimen |
| – First-line |
R-CHOP |
8 (80%) |
2 (100%) |
0.11 |
21 (95%) |
54 (87%) |
0.85 |
| RMPV |
2 (20%) |
0 |
NA |
1 (5%) |
4 (6%) |
1 |
| ACBVP |
0 |
0 |
NA |
0 |
3 (5%) |
0.56 |
| – Second-line |
7 (70%) |
2 (100%) |
NA |
11 (50%) |
17 (27%) |
0.55 |
| – Third-line |
3 (30%) |
0 |
NA |
4 (18%) |
3 (5%) |
0.09 |
| – Fourth-line |
1 (10%) |
0 |
NA |
1 (5%) |
0 |
0.27 |
| Total gamma-globulin level (g/l) median (range) |
5.2 (2.7–5.5) |
4.15 (3.8–4.5) |
NA |
9.25 (5.9–18.3) |
9.15 (5.9–17.9) |
0.82 |
| Immunoglobulin isotype levels (n) |
7 |
1 |
NA |
13 |
33 |
1 |
|
IgG level (g/l) median (range) |
5.45 (2.8–5.45) |
4.44 |
NA |
11.4 (6.48–13.8) |
9.06 (6.38–14.6) |
0.16 |
| IgA level (g/l) median (range) |
0.61 (0.35–5.91) |
1.11 |
NA |
1.49 (0.28–2.16) |
1.42 (1.12–2.47) |
0.43 |
| IgM level (g/l) median (range) |
0.46 (0.2–3.7) |
0.2 |
NA |
1.26 (0.25–1.35) |
0.96 (0.18–1.44) |
0.57 |
| CRP level (g/L) median (range) |
26.5 (3–116) |
65 |
NA |
37 (3–119) |
11 (3–237) |
0.19 |
| Ferritin level (µg/l) median (range) |
538.5 (73–1628) |
112 |
NA |
217.5 (93–1496) |
151 (4–3472) |
0.47 |
| Albumin level (g/l) median (range) |
33.8 (24–40.7) |
32 |
|
36 (23–46) |
39 (16–50) |
0.20 |
| AG ratio median (range) |
6.79 (4.62–12.22) |
7.63 |
NA |
4.15 (1.85–7.13) |
4.3 (1.7–6.61) |
0.64 |
| LDH (t/l) median (range) |
285 (156–2784) |
776.5 (537–1016) |
NA |
373 (162–2793) |
278 (132–10091) |
0.69 |
| Lymphocyte count (G/l) median (range) |
0.8 (0.2–2.58) |
0.62 (0.29–0.95) |
NA |
0.85 (0.27–7.56) |
1.26 (0.18–7.56) |
0.83 |
Hypogammaglobulinaemia at diagnosis is associated with a higher risk of death
Table 3 presents hazard ratios for the different variables studied using a Cox proportional hazards model. Ann Arbor stage IV was positively associated with the occurrence of death (HR 3.47, 95% CI 1.61–7.47; p <0.01). More interestingly, L-TGL was also found to be independently associated with a higher risk of death (HR 12.8, 95% CI 4.93–34.31; p <0.001), whereas H-TGL was associated with a lower risk of death (HR 0.21, 95% CI 0.09–0.46; p <0.01).
Table 3Univariate and multivariate Cox proportional hazards model analysis examining factors associated with the occurrence of death in diffuse large-B cell lymphoma
|
Hazard ratio (95% CI)
|
Univariate p-value
|
Multivariate p-value
|
| Sex |
1.4 (0.56–3.7) |
0.45 |
|
| L-TGL |
12.8 (4.93–34.31) |
<0.001 |
<0.01 |
| H-TGL |
0.21 (0.09–0.46) |
<0.01 |
– |
| Inflammation (CRP >10 mg/l) |
5.5 (1.54–35.2) |
<0.01 |
NS |
| Hyperferritinaemia (Ferritin >300 ug/l) |
6.22 (1.5–41.9) |
0.01 |
NS |
| Ann Arbor stage IV |
4.5 (1.6–16.02) |
<0.01 |
NS |
| Cerebral involvement |
1.5 (0.24–5.42) |
0.59 |
|
| Richter syndrome |
4.16 (0.23–20.34) |
0.25 |
|
| Second-line treatment |
4.32 (1.6–13.5) |
<0.01 |
NS |
| Third-line treatment |
1.76 (0.41–5.35) |
0.40 |
|
Discussion
This study is the first to estimate the frequency (12.5%) and emphasise the clinical utility of L-TGL determination using a systematic serum electrophoresis at diffuse large B cell lymphoma diagnosis . We demonstrated that L-TGL has a strong negative impact on the overall and infection-related mortality rates. The higher AG ratio in L-TGL subgroup suggests that the severe decrease in total gamma-globulin level and the associated clinical outcomes in the L-TGL subgroup may be (1) independent of other possible associated nonspecific causes and (2) mostly and directly related to one of our two initial hypotheses: either a pre-existent undiagnosed primary immunodeficiency on which diffuse large B cell lymphoma occurred or a secondary immunodeficiency directly due to the lymphoma. For both hypotheses, considered alone or in combination, the secondary immunodeficiency induced by chemotherapy can worsen, or reveal a greater susceptibility to infections in L-TGL patients, but is unquantifiable in the design of our study. Our population was close to that described in France during this period of time, with a median age of 69 years, representing 35 to 40% of patients with lymphoma [23, 24]. In another study, 39% of infection-related death was also observed in multiple myeloma before the first line of treatment. Regarding overall cause of death in this disease, a rate of 17% was reported for infections, representing the third cause of death after disease progression and renal failure [25]. In chronic lymphocytic leukaemia (CLL), which is another lymphoproliferative malignancy that frequently leads to lower immunoglobulin levels, infections were the main cause and the second cause of death, respectively, in an intermediate risk group and in all CLL patients [26]. Moreover, infections leading to death accounted from 8 to 60% of deaths in CLL patients [27, 28]. Finally, the proportion of 100% of infection-related death in L-TGL group in our study is higher than in these other conditions, but must take into account the stronger chemotherapy regimen in DLCBL.
At diffuse large B-cell lymphoma diagnosis, serum electrophoresis is not unanimously recommended according to the international work-group guidelines for malignant lymphoma [13–15]. However, in our study we advocated for serum electrophoresis at diagnosis. There were no significant differences between the two subgroups regarding inflammatory parameters, assessed using CRP level, and albuminaemia, suggesting that the decrease in total gamma-globulin level may not be secondary to metabolic/inflammatory causes or increased loss of immunoglobulins, but probably and directly related to low gamma-globulin production. Since neither the Ann Arbor staging classification, international prognostic index, nor the chemotherapy regimen were significantly different between the two groups, the increase in the overall and specific infection-related death rates may be attributed to the decrease in total gamma-globulin level in the L-TGL subgroup. We did not exclude Richter syndrome nor associated central nervous system involvement that could exhibit a worse malignant prognosis, because these patients were identified only in the H-TGL subgroup and neither subtype showed overall high mortality rate in this study. There was no stage I lymphoma in the L-TGL group, whereas the majority of in the L-TGL group were stage IV, and this group had a poorer outcome. This could mean that L-TGL is linked to the severity of lymphoma, but the fact that stage IV lymphoma represented about half of the H-TGL group would go against this hypothesis. Hence, T-LGL appears to be a credible independent and deleterious prognosis factor, especially for infection-related death in diffuse large B-cell lymphoma patients. Therefore, we may consider that this study drew up an overall picture of all subtypes of diffuse large B cell lymphoma. Moreover, L-TGL patients exhibited a lower survival rate than all other possible subgroups of diffuse large B cell lymphoma, including patients who did not have serum electrophoresis at diagnosis, confirming the importance of total gamma-globulin level assessment.
The main limitations of this study include its retrospective design with some missing data, including mainly immunoglobulin isotype levels, absence of systematic and reliable assessment of previous infections, and monitoring of total gamma-globulin level in follow-up for the entire cohort. Consequently, two main secondary objectives could not be reached in this study: determination of an approach for eventual common variable immunodeficiency diagnosis criteria and the leading cause of L-TGL considered herein, namely, primary or diffuse large B cell lymphoma-related secondary immunodeficiencies. We assumed that combining analyses of both diffuse large B cell lymphoma outcome and total gamma-globulin level after chemotherapy should partially help to differentiate between causes of decrease in total gamma-globulin level for each patient. Indeed, complete or partial remission of diffuse large B cell lymphoma and concomitant increase in total gamma-globulin level after chemotherapy would support the hypothesis of a diffuse large B cell lymphoma-related secondary immunodeficiency . Conversely, in cases of a lack of response or early death, no conclusion could be drawn, since chemotherapy would induce a further decrease in total gamma-globulin level for an unknown duration.
To conclude, epidemiological data on lymphoma treatment strategies are optimistic and have seen improvements in survival throughout the years. This study, using a simple, widely and easily available biological test, shows that the L-TGL patient subgroup exhibits a worse prognosis with higher overall and infection-related mortality than the H-TGL subgroup. Therefore, this study advocates for the need to systematically perform at least serum electrophoresis at diffuse large B-cell lymphoma diagnosis to improve our practice and emphasises the need to conduct prospective studies to confirm these results in order to: (1) determine nosological and prognostic distinctions between previously undiagnosed primary immunodeficiencies and diffuse large B-cell lymphoma-related secondary immunodeficiencies using, for example, next-generation sequencing methods to uncover molecular defects of humoral primary immunodeficiencies [22, 25] and to assess the timing of a possible total gamma-globulin level recovery after chemotherapy in relation to lymphoma outcomes; (2) assess the possible clinical benefit of immunoglobulin supplementation for secondary immunodeficiencies, or prophylactic antibiotics, taking into account advances in chemotherapy and management strategies in onco-haematology.
Availability of data and materials
The datasets analysed during the current study are available from Pr Gandhi Damaj on reasonable request.
Acknowledgements
Author contributions: AN A A performed the research and wrote the paper; NM-S designed the research study; HdB, SD, AA-V performed statistical analyses; A-CG, ER, GD entered patients into the registry.
Alexandre Nguyen, MD
Department of Internal Medicine and Clinical Immunology
CHU Côte de Nacre
Université Basse Normandie
Avenue de la Côte de Nacre
FR-14000 Caen
nguyen-a[at]chu-caen.fr
References
1.
Cunningham-Rundles C
,
Siegal FP
,
Cunningham-Rundles S
,
Lieberman P
. Incidence of cancer in 98 patients with common varied immunodeficiency. J Clin Immunol. 1987 Jul;7(4):294–9. https://doi.org/10.1007/BF00915550
2.
Rezaei N
,
Hedayat M
,
Aghamohammadi A
,
Nichols KE
. Primary immunodeficiency diseases associated with increased susceptibility to viral infections and malignancies. J Allergy Clin Immunol. 2011 Jun;127(6):1329–41.e2. https://doi.org/10.1016/j.jaci.2011.02.047
3.
Baldovino S
,
Montin D
,
Martino S
,
Sciascia S
,
Menegatti E
,
Roccatello D
. Common variable immunodeficiency: crossroads between infections, inflammation and autoimmunity. Autoimmun Rev. 2013 Jun;12(8):796–801. https://doi.org/10.1016/j.autrev.2012.11.003
4.
Vajdic CM
,
Mao L
,
van Leeuwen MT
,
Kirkpatrick P
,
Grulich AE
,
Riminton S
. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood. 2010 Aug;116(8):1228–34. https://doi.org/10.1182/blood-2010-03-272351
5.
Shapiro RS
. Malignancies in the setting of primary immunodeficiency: implications for hematologists/oncologists. Am J Hematol. 2011 Jan;86(1):48–55. https://doi.org/10.1002/ajh.21903
6.
Tran H
,
Nourse J
,
Hall S
,
Green M
,
Griffiths L
,
Gandhi MK
. Immunodeficiency-associated lymphomas. Blood Rev. 2008 Sep;22(5):261–81. https://doi.org/10.1016/j.blre.2008.03.009
7.
Mellemkjaer L
,
Hammarström L
,
Andersen V
,
Yuen J
,
Heilmann C
,
Barington T
, et al.
Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002 Dec;130(3):495–500. https://doi.org/10.1046/j.1365-2249.2002.02004.x
8.
Oksenhendler E
,
Gérard L
,
Fieschi C
,
Malphettes M
,
Mouillot G
,
Jaussaud R
, et al.; DEFI Study Group
. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008 May;46(10):1547–54. https://doi.org/10.1086/587669
9.
Cunningham-Rundles C
. The many faces of common variable immunodeficiency. Hematology (Am Soc Hematol Educ Program). 2012;2012(1):301–5. https://doi.org/10.1182/asheducation.V2012.1.301.3798316
10.
CEREDIH: The French PID study group
. The French national registry of primary immunodeficiency diseases. Clin Immunol. 2010 May;135(2):264–72. https://doi.org/10.1016/j.clim.2010.02.021
11.
Picard C
,
Al-Herz W
,
Bousfiha A
,
Casanova JL
,
Chatila T
,
Conley ME
, et al.
Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015 Nov;35(8):696–726. https://doi.org/10.1007/s10875-015-0201-1
12.
Chan HY
,
Yang YH
,
Yu HH
,
Chien YH
,
Chiang LL
,
Chiang BL
. Clinical characteristics and outcomes of primary antibody deficiency: a 20-year follow-up study. J Formos Med Assoc. 2014 Jun;113(6):340–8. https://doi.org/10.1016/j.jfma.2012.07.005
13.
Dabaja BS
,
Advani R
,
Hodgson DC
,
Dhakal S
,
Flowers CR
,
Ha CS
, et al.
ACR Appropriateness Criteria® Diffuse Large B-Cell Lymphoma. Am J Clin Oncol. 2015 Dec;38(6):610–20. https://doi.org/10.1097/COC.0000000000000215
14.
Horwitz SM
,
Zelenetz AD
,
Gordon LI
,
Wierda WG
,
Abramson JS
,
Advani RH
, et al.
NCCN Guidelines Insights: Non-Hodgkin’s Lymphomas, Version 3.2016. J Natl Compr Canc Netw. 2016 Sep;14(9):1067–79. https://doi.org/10.6004/jnccn.2016.0117
15.
Tilly H
,
Gomes da Silva M
,
Vitolo U
,
Jack A
,
Meignan M
,
Lopez-Guillermo A
, et al.; ESMO Guidelines Committee
. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015 Sep;26 Suppl 5:v116–25. https://doi.org/10.1093/annonc/mdv304
16.
Raanani P
,
Gafter-Gvili A
,
Paul M
,
Ben-Bassat I
,
Leibovici L
,
Shpilberg O
. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis. Leuk Lymphoma. 2009 May;50(5):764–72. https://doi.org/10.1080/10428190902856824
17.
Jolles S
,
Chapel H
,
Litzman J
. When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clin Exp Immunol. 2017 Jun;188(3):333–41. https://doi.org/10.1111/cei.12915
18.
Patel SY
,
Carbone J
,
Jolles S
. The Expanding Field of Secondary Antibody Deficiency: Causes, Diagnosis, and Management [Internet]. Front Immunol. 2019 Feb;10:33. [cited 2020 Aug 24] Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.00033/full https://doi.org/10.3389/fimmu.2019.00033
19.
Benbrahim O
,
Viallard JF
,
Choquet S
,
Royer B
,
Bauduer F
,
Decaux O
, et al.
A French observational study describing the use of human polyvalent immunoglobulins in hematological malignancy-associated secondary immunodeficiency. Eur J Haematol. 2018 Jul;101(1):48–56. https://doi.org/10.1111/ejh.13078
20.
Seidel MG
,
Kindle G
,
Gathmann B
,
Quinti I
,
Buckland M
,
van Montfrans J
, et al.; ESID Registry Working Party and collaborators
. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract. 2019 Jul - Aug;7(6):1763–70. https://doi.org/10.1016/j.jaip.2019.02.004
21.
Ameratunga R
,
Woon ST
,
Gillis D
,
Koopmans W
,
Steele R
. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin Exp Immunol. 2013 Nov;174(2):203–11. https://doi.org/10.1111/cei.12178
22.
Ameratunga R
,
Woon ST
. Perspective: Evolving Concepts in the Diagnosis and Understanding of Common Variable Immunodeficiency Disorders (CVID). Clin Rev Allergy Immunol. 2020 Aug;59(1):109–21. https://doi.org/10.1007/s12016-019-08765-6
23.
Perry AM
,
Diebold J
,
Nathwani BN
,
MacLennan KA
,
Müller-Hermelink HK
,
Bast M
, et al.
Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016 Oct;101(10):1244–50. https://doi.org/10.3324/haematol.2016.148809
24.
Desandes E
,
Lacour B
,
Clavel J
. le Réseau français des registres de cancers [Cancer in adolescents and young adults in France: Epidemiology and pathways of care]. Bull Cancer (Paris). 2016 Dec;103(12):957–65. https://doi.org/10.1016/j.bulcan.2016.09.020
25.
Cowan AJ
,
Allen C
,
Barac A
,
Basaleem H
,
Bensenor I
,
Curado MP
, et al.
Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018 Sep;4(9):1221–7. https://doi.org/10.1001/jamaoncol.2018.2128
26.
Wang Y
,
Achenbach SJ
,
Rabe KG
,
Shanafelt TD
,
Call TG
,
Ding W
, et al.
Cause of death in patients with newly diagnosed chronic lymphocytic leukemia (CLL) stratified by the CLL-International Prognostic Index. Blood Cancer J. 2021 Aug;11(8):140. https://doi.org/10.1038/s41408-021-00532-1
27.
Strati P
,
Parikh SA
,
Chaffee KG
,
Kay NE
,
Call TG
,
Achenbach SJ
, et al.
Relationship between co-morbidities at diagnosis, survival and ultimate cause of death in patients with chronic lymphocytic leukaemia (CLL): a prospective cohort study. Br J Haematol. 2017 Aug;178(3):394–402. https://doi.org/10.1111/bjh.14785
28.
Tadmor T
,
Welslau M
,
Hus I
. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev Hematol. 2018 Jan;11(1):57–70. https://doi.org/10.1080/17474086.2018.1407645
30.
Mahlaoui N
,
Picard C
,
Bach P
,
Costes L
,
Courteille V
,
Ranohavimparany A
, et al.; CEREDIH French PID study group
. Genetic diagnosis of primary immunodeficiencies: A survey of the French national registry. J Allergy Clin Immunol. 2019 Apr;143(4):1646–1649.e10. https://doi.org/10.1016/j.jaci.2018.12.994
