Multifocal lymphadenopathies with polyclonal reactions primed after EBV infection in a mRNA-1273 vaccine recipient
DOI: https://doi.org/10.4414/SMW.2022.w30188
François R.
Girardina, Alexandar
Tzankovb, Giuseppe
Pantaleoc, Françoise
Livioa, Gilbert
Greubde
aService of Clinical Pharmacology, Department of Laboratory Medicine and Pathology, Lausanne University Hospital and University of Lausanne Switzerland
bInstitute of Medical Genetics and Pathology, University Hospital Basel, Switzerland
cService of Immunology and Allergy, Departments of Medicine and Laboratory Medicine and Pathology, Lausanne University Hospital, University of Lausanne, Switzerland
dService of Infectious Diseases, University Hospital Centre (CHUV), Lausanne, Switzerland
eInstitute of Microbiology, University of Lausanne and University Hospital Centre (CHUV), Lausanne, Switzerland
Summary
We report a case of recurrent tender, multifocal lymphadenopathies associated with B-symptoms, clinically mimicking lymphoma in a mRNA-1273 vaccine recipient after a recent Epstein-Barr virus (EBV) infection.
In the lymph node biopsy, monocytoid B-cell hyperplasia, TH2 (GATA3+) predominance, and hyperplasia of interferon-gamma-producing plasmacytoid dendritic cells were observed along with sustained neutralising antibody production against SARS-CoV-2 wild-type and five variants. High titres of anti-S antibodies and neutralising antibodies were observed, excepted for variant B.1.529** (omicron) and B.1.351** (beta), due to several mutations in the spike protein, including the E484K mutation.
We postulated that EBV acted as an immunological enhancer with the mRNA-1273 vaccine, inducing a sustained inflammatory response over several weeks. However, the polyclonal nature of the lymphadenopathy with polytypic plasmacytosis and pseudo-tumoural reaction cell hyperplasia were associated with failure to mount acute phase responses.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is causing coronavirus disease 2019 (COVID-19) with a wide range of polymorphic clinical manifestations and outcomes. Messenger RNA (mRNA)-based vaccines against SARS-CoV-2 have an overall excellent safety profile, but can be associated with unusual adverse reactions, such as diffuse adenopathy that may be mistaken for malignancy [1]. Most adverse reactions following immunisation are mild, local and self-limiting. Systemic adverse reactions include fatigue, lymphadenopathies, fever, myalgia, appendicitis, myocarditis, Guillain-Barré syndrome or Bell’s palsy. Cases of herpes simplex and zoster have been reported after mRNA COVID-19 vaccines, but no evidence of oropharyngeal shedding of herpesviruses was observed within one week after immunisation [2, 3].
Case description
A 44-year-old woman, indoor cat owner (5-year-old), suffered in mid-February 2021 from acute fatigue, palpable spleen and tonsillitis with white spots, diagnosed as mononucleosis based on Epstein-Barr virus (EBV) serology. She took no medication, had no relevant medical history, and had tested negative for EBV with a previous hospitalisation for pneumonia and dysaesthesia with spontaneous resolution.
Early in March 2021, the patient received her first mRNA-1273 SARS-CoV-2 vaccine (left deltoid) and after 48–72 hours, she presented with new left-sided supraclavicular lymphadenopathy, fever, night sweats and weight loss (–4% of total body weight in 15 days).
On examination, the patient presented with fever (up to 39 °C), multiple tender cervical, supraclavicular and axillary lymph nodes. Initial investigations found a white blood cell count of 7.1 × 109/l with reactive lymphocytes and stable C-reactive protein levels between 6.0 and 7.9 mg/l (reference range 0–8 mg/l). Routine biochemistry including kidney and liver function tests were within normal limits.
Ultrasonography revealed large supraclavicular lymh nodes, one 17 × 13 × 10 mm (fig. 1A). A contrast-enhanced computed tomography (CT) scan confirmed enlarged axillar and supraclavicular lymphadenopathies. Smear from a fine needle aspiration revealed a florid reactive lymphadenopathy pattern with tingible body macrophages and lymphocytes at different stages of maturation.
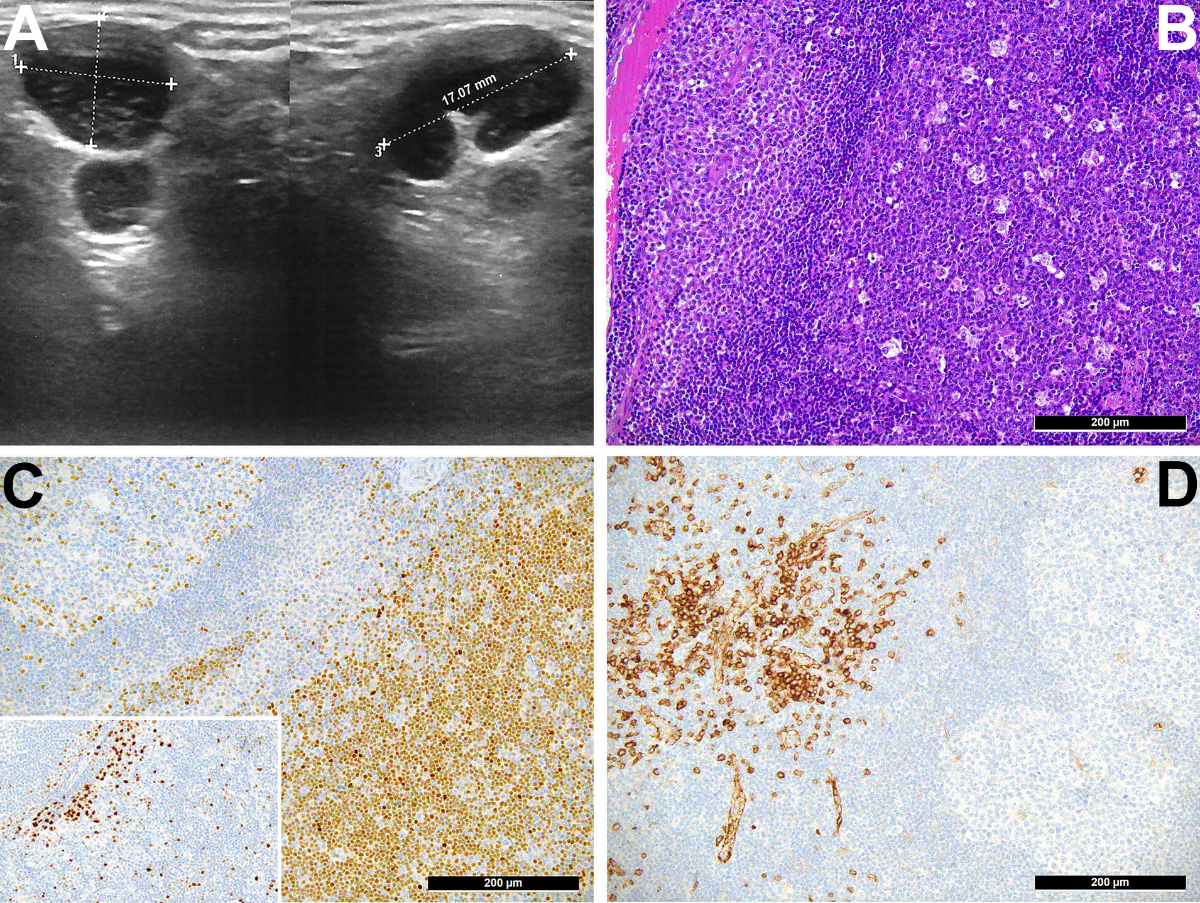
Figure 1 A. Ultrasonography revealed large supraclavicular lymph nodes of 17 × 13 × 10 mm.
B. Medium power view of the enlarged lymph node showing follicular hyperplasia (dark purples cells intermingled with starry sky-appearing tingible body macrophages on right part) and monocytoid/plasmacytoid parafollicular hyperplasia (paler cell collection on the left side); Haematoxylin eosin stain, original magnification 200×.
C. Significant T-helper 2 cell-skewing in the paracortex shown by the positive staining of the respective cellular compartment for GATA3, compared with the lower presence of T-helper 1-equivalents in the same area in the staining for T-bet/TBX21 (insert); immunoperoxidase staining, original magnification 200×.
D. Significant increase of CD123-positive interferon-gamma-producing plasmacytoid dendritic cells in the parafollicular compartment; immunoperoxidase staining, original magnification 200×.
One month after the first vaccination, the second mRNA-1273 vaccine dose was administered, followed within 24 hours by a more pronounced reaction with extension to contralateral lymph nodes associated with B-symptoms that resolved after 6 weeks. Given the progression of symptoms and to rule out a malignant lymphoproliferative disease, excisional biopsies of the supraclavicular and cervical nodes were performed. Histopathology showed follicular and monocytoid B-cell hyperplasia, a TH2 (GATA3+) predominance with hyperplasia of interferon-gamma-producing plasmacytoid dendritic cells (fig. 1B-D), and a moderate polyclonal plasmacytosis (figure 2).

Figure 2 Polyclonal plasmacytosis (kappa brown; lambda red).
The clinical picture was consistent with an acute inflammatory state and reflects abnormal reaction in the background of a primed adaptive immune system. Immunohistochemical staining for SARS-CoV-2 N-antigen, adenovirus, cytomegalovirus (CMV), herpes simplex virus 1 and 2, human herpes virus 8, parvovirus B-19, varicella zoster virus, toxoplasma- and spirochaete-antigens were negative. In situ hybridisation for EBV small RNA (EBER) showed scattered (maximum 2‰) positive parafollicular immunoblastoid cells.
Bartonella henselae, Toxoplasma gondii, CMV, adenovirus IgG and IgM were all negative. There was no serological evidence of previous SARS-CoV-2 infection (absence of anti-nucleocapsid antibodies), but high-titre neutralising antibodies and anti-spike antibodies were detected (Luminex® assay), as expected after immunisation. The patient developed sustained SARS-CoV-2 antibody production for wild type and five main variants: 18 and 57 days after the first vaccination, the neutralising activities were 9-fold and >11-fold higher than the threshold. Eight months after the first vaccination, the neutralising activities against wild type SARS-CoV-2 and the main variants decreased significantly, but they still remained above the neutralising threshold, except for the variant B.1.131** (table 1).
Table 1IgG antibodies directed against the spike protein (S) or the nucleocapsid protein (N) of the SARS-CoV-2 virus, assessed using a Luminex® assay (10) and neutralising antibodies towards the spike protein of the wild type virus and of various SARS-CoV-2 variants. Note the expected high titres of anti-S antibodies and heterogeneously high titres of neutralising antibodies after SARS-CoV-2 vaccination. These neutralising antibodies appear, however, to exhibit reduced efficacy on variant B.1.529** (omicron) and B.1.351** (beta), as expected owing to several mutations in the spike protein, including the E484K mutation.
|
IgG antibodies against the SARS-CoV-2 virus
|
Neutralising antibodies against the spike protein of the wild type SARS-CoV-2 wild type (WT) and five variants
|
|
Day after vaccination
|
Spike
|
Nucleocapsid
|
WT
|
B.1.1.7**
(alpha)
|
B.1.351**
(beta)
|
P.1**
(gamma)
|
B.1.617.2**
(delta)
|
B.1.529**
(omicron)
|
| D0 (March 2021) |
First vaccine (standard dose) |
| D18 |
113 |
NEG* |
448 |
303 |
46 |
159 |
99 |
39 |
| D28 |
Second vaccine (standard dose) |
| D57 |
115 |
NEG* |
564 |
397 |
121 |
280 |
200 |
73 |
| D240 |
55.1 |
NEG* |
103 |
79 |
44 |
67 |
57 |
– |
| D290 |
Third vaccine (half dose) |
|
D365 (March 2022)
|
81.7 |
NEG* |
1806 |
1136 |
552 |
1269 |
833 |
513 |
In mid-December 2021, reckoning the increasing need for protection from more contagious SARS-CoV-2 variants, the patient decided to receive a booster vaccine, but with only the half dose of the standard mRNA BNT162b2. Despite a previous positive rechallenge (second immunisation) after the acute EBV infection, she had neither clinical signs, nor reported symptoms, excepted a mild local reaction 12 hours following the injection.
Discussion
The patient presented with severe relapsing systemic post-vaccination lymphoma-mimicking reactions lasting for several weeks and leading to invasive diagnostic procedures. In a nationwide cohort study, BNT162b2 mRNA COVID-19 vaccine was associated with an increased risk of lymphadenopathy (risk ratio 2.43, 95% confidence interval [CI] 2.05–2.78; risk difference 78.4 events per 100,000 individuals, 95% CI 64.1–89.3), as well as myocarditis (risk ratio 3.24) and appendicitis (risk ratio 1.40) [2]. Generally, lymphadenopathies are considered as an indicator of successful immunisation as they are intrinsically linked to vaccine-induced activation of the immune system [4]. In the present case, the patient developed sustained antibody production against SARS-CoV-2 wild type and variants. The recent EBV infection likely contributed to these reactions, serving as a primer: systemic reactions associated with EBV, polytypic B-cell proliferation and lymphoid hyperplasias are reported in several situations, such as in the context of injuries, sun exposure, exposure to certain drugs, mosquito bites and other vaccinations, which may all serve as triggers [5]. Together with fever, lymphadenopathy is a well-known adverse reaction following immunisation with various vaccines, including measles-mumps-rubella, human papilloma virus, influenza, hepatitis B virus, smallpox or Calmette-Guérin bacilli [5–7]. EBV is a carcinogenic virus, associated with various lymphomas, as well as immunostimulation [8]. EBV nuclear antigen 1 (EBNA1) is able to recruit pro-inflammatory and immune-stimulating macrophages, histiocytes and TH2 cells, which secret a wide range of interferons, interleukins and other cytokines that contribute to the development of lymphadenopathies with B-symptoms. Unfortunately, EDTA blood samples were not available from the time of the major post-vaccination syndrome, which precluded our study of the EBV-DNA load dynamics during this post-vaccination illness. Further, since a small proportion of adults (1–5%) fail to mount anti-EBNA IgG, we cannot formally exclude the possibility that the present EBV serology represents a reactivation or presence of persisting irrelevant cross-reactive IgM. At least, the anti-EBV IgM were not cross-reactive in the setting of a syphilis or human immunodeficiency virus (HIV) primary infection. Bartonella henselae and Toxoplasma were included in the differential diagnosis since both may be acquired by exposure to cats and both cause lymphadenopathy, but the negative serology and the negative immunohistochemistry excluded these two hypothetical causative agents.
The histopathological appearance of the patient's lymphadenectomy specimen indicated a predominance of TH2, polyclonal plasmacytosis, hyperplasia of B cells, and plasmacytoid dendritic cells that is consistent with the clinical picture of a protracted evolution of an abnormal immune response over several weeks.
We postulate that recent EBV infections primed the lymph node microenvironment of lymphocytes and macrophages, providing high levels of cytokines and/or growth factors, and decisively contributed to enhanced immune-stimulation after application of the mRNA-1273 vaccine. As for EBV, the conjunction of recent infections with other lymphotropic viruses (e.g., CMV, HHV6, SARS-CoV-2), medications (e.g., drug rash with eosinophilia and systemic symptoms-DRESS syndrome) or mRNA-1273 SARS-CoV-2 vaccination are likely to predispose to major lymphoma-mimicking reactions [9]. Thus, since there was no systemic reaction after the third immunisation with another mRNA COVID-19 vaccine 9 months later, EBV involvement as primer seems likely in the first and second systemic reactions following vaccination. Failure to mount acute phase responses (e.g., relatively low CRP level) is not uncommon, even though there is a rise of multifocal lymphadenopathies associated with B-symptoms. This discrepancy might be explained by the polyclonal nature of the lymphadenopathy and pseudo-tumoural B-cell reaction with interferon-gamma production.
In our patient, adenopathy completely resolved with no sign of malignancy. One year after vaccination, IgG and neutralising antibodies against the spike protein were still above the half-maximum effective concentration threshold (EC50) for neutralising activity of SARS-CoV-2 wild type and four variants (variant alpha B.1.1.7**; variant gamma P.1**; variant delta B.1.617.2**; variant omicron B.1.529**) [10].
The causal relationship between the mRNA COVID-19 vaccine and the development of lymphadenopathies with B-symptoms was estimated as certain, considering the mechanistic and temporal relationship plausibility, as well as the positive rechallenge. The absence of systemic reaction after the third immunisation (ie, 10 months after the primary infection) strengthens the role of viral factors as causative agent. In conclusion, EBV could act as an immunological primer, sustaining a cascade of inflammatory processes with polytypic B-cell proliferations and hyperplasia of interferon-gamma-producing plasmacytoid dendritic cells.
Acknowledgments
AT is supported by the Botnar Research Centre for Child Health. We thank the laboratory technicians and researchers of the Institute of Microbiology and Service of Immunology at CHUV, Lausanne, for performing the PCR tests, the serology and the measurements of the neutralising antibodies, especially Dr Céline Pellaton, PhD and Mrs Nina Plumey, Laboratoire Risch, Delémont, Jura. We also thank Dr Philippe Geissbühler (Delémont) for providing ultrasonography and Dr Laurent Tschopp who performed the lymph node resection (Delémont).
Contributors: We were all involved in caring for the patient, performing the analyses and revising the manuscript. FG, AT, FL, GP, and GG verified the data and produced the first draft. Written consent for publication was obtained from the patient.
Prof. François R. Girardin, MD
Division of clinical Pharmacology
University Hospital Centre (CHUV)
Bugnon 17
CH-1011 Lausanne
francois.girardin[at]chuv.ch
References
1.
Hagen C
,
Nowack M
,
Messerli M
,
Saro F
,
Mangold F
,
Bode PK
. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly. 2021 Jul;151:w20557. https://doi.org/10.4414/smw.2021.20557
2.
Barda N
,
Dagan N
,
Ben-Shlomo Y
,
Kepten E
,
Waxman J
,
Ohana R
, et al.
Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021 Sep;385(12):1078–90. https://doi.org/10.1056/NEJMoa2110475
3.
Brosh-Nissimov T
,
Sorek N
,
Yeshayahu M
,
Zherebovich I
,
Elmaliach M
,
Cahan A
, et al.
Oropharyngeal shedding of herpesviruses before and after BNT162b2 mRNA vaccination against COVID-19. Vaccine. 2021 Sep;39(40):5729–31. https://doi.org/10.1016/j.vaccine.2021.08.088
4.
Brewer KD
,
DeBay DR
,
Dude I
,
Davis C
,
Lake K
,
Parsons C
, et al.
Using lymph node swelling as a potential biomarker for successful vaccination. Oncotarget. 2016 Jun;7(24):35655–69. https://doi.org/10.18632/oncotarget.9580
5.
Hartsock RJ
. Postvaccinial lymphadenitis. Hyperplasia of lymphoid tissue that simulates malignant lymphomas. Cancer. 1968 Apr;21(4):632–49. https://doi.org/10.1002/1097-0142(196804)21:4<632::AID-CNCR2820210415>3.0.CO;2-O
6.
Dorfman RF
,
Herweg JC
. Live, attenuated measles virus vaccine. Inguinal lymphadenopathy complicating administration. JAMA. 1966 Oct;198(3):320–1. https://doi.org/10.1001/jama.1966.03110160148051
7.
Hendry AJ
,
Dey A
,
Beard FH
,
Khandaker G
,
Hill R
,
Macartney KK
. Adverse events following immunisation with bacille Calmette-Guérin vaccination: baseline data to inform monitoring in Australia following introduction of new unregistered BCG vaccine. Commun Dis Intell Q Rep. 2016 Dec;40(4):E470–4.
8.
Kanda T
,
Yajima M
,
Ikuta K
. Epstein-Barr virus strain variation and cancer. Cancer Sci. 2019 Apr;110(4):1132–9. https://doi.org/10.1111/cas.13954
9.
Steeper TA
,
Horwitz CA
,
Ablashi DV
,
Salahuddin SZ
,
Saxinger C
,
Saltzman R
, et al.
The spectrum of clinical and laboratory findings resulting from human herpesvirus-6 (HHV-6) in patients with mononucleosis-like illnesses not resulting from Epstein-Barr virus or cytomegalovirus. Am J Clin Pathol. 1990 Jun;93(6):776–83. https://doi.org/10.1093/ajcp/93.6.776
10.
Fenwick C
,
Croxatto A
,
Coste AT
,
Pojer F
,
André C
,
Pellaton C
, et al.
Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol. 2021 Jan;95(3):e01828–20. https://doi.org/10.1128/JVI.01828-20

