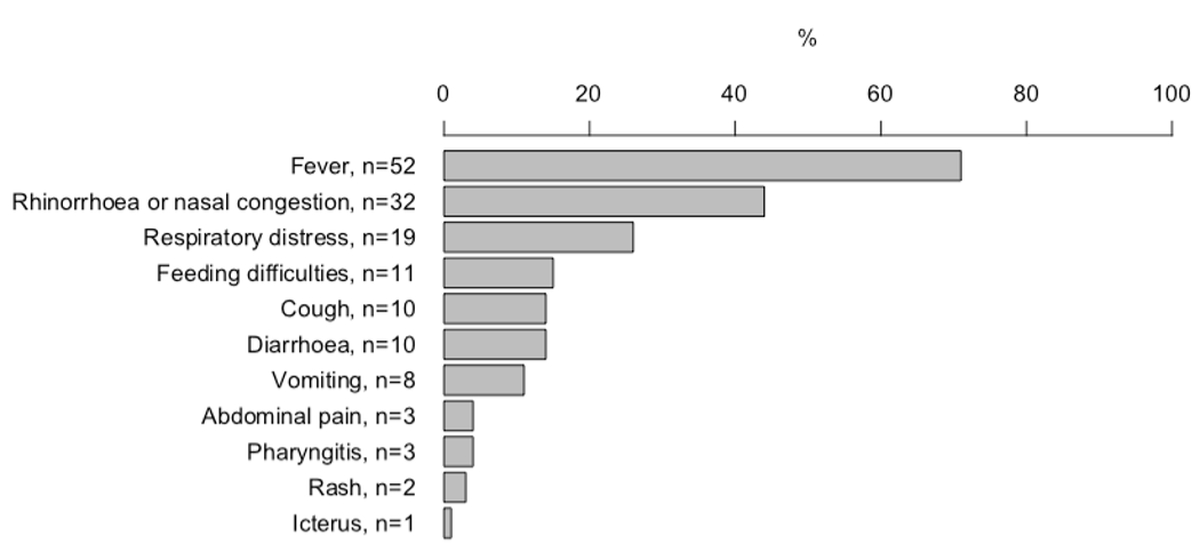
Figure 1 Distribution of symptoms in neonates with SARS-CoV-2 infection.
DOI: https://doi.org/10.4414/SMW.2022.w30185
Coronavirus disease (COVID-19) is generally a mild disease in children [1–10]. Although, it has been reported that infants infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) get more severely ill compared with older children [11–16], there are, however, only a few case series summarising the presentation and outcomes of neonates suffering from COVID-19. Most reports to date include mainly asymptomatic neonates who were tested because of exposure to maternal SARS-CoV-2 infection [6, 11, 17–23]. Approximately 2 to 5% of neonates born to women with COVID-19 during pregnancy are infected with SARS-CoV-2 [24–27]. Up to 60% of neonates infected in the first days after birth remain asymptomatic [18, 21–23]. If symptoms are reported, they include fever, respiratory distress, feeding difficulties and vomiting [18, 21–23]. Data on neonates infected with SARS-CoV-2 after the perinatal period is sparse. As fever and other infectious signs and symptoms in the first month of life trigger extensive investigations and empirical antibacterial treatment, it is important to define the clinical characteristics and burden of neonatal COVID-19.
In this study, we summarised epidemiological data, clinical characteristics, treatment and outcomes of hospitalised neonates with symptomatic COVID-19 from a nationwide active surveillance system of SARS-CoV-2 infections in children. The inclusion only symptomatic neonates in our study clearly distinguishes our data from previous studies, which mostly included asymptomatic neonates who were exposed to infected mothers.
The study was designed as a prospective nationwide observational cohort study recording detailed epidemiological, clinical, therapeutic and outcome data of hospitalised neonates ≤28 days of age with laboratory-confirmed SARS-CoV-2 infection (a positive SARS-CoV-2 polymerase chain reaction [PCR] test on a respiratory sample). Neonates without symptoms were excluded from detailed analysis. Data were collected anonymously through the Swiss Paediatric Surveillance Unit (SPSU, http://www.spsu.ch) from 1 March 2020 to 31 September 2021. All 29 hospitals in Switzerland that have a paediatric and/or neonatology department participated and submitted cases monthly. Upon notification the investigators sent the reporting centres an electronic clinical report form through REDCap or in paper form (the questionnaire is provided in the appendix) [15]. If data were not submitted within one month a reminder was sent. Data were collected at one timepoint either during or after the hospitalisation. There was no outpatient follow-up. All data were reviewed by the investigators and further clarified with the reporting physician when needed. All missing data are indicated were applicable. The study received ethics approval by the ethics committee (Ethikkommission Nordwest- und Zentralschweiz (EKNZ 2020-01130). All collected data were anonymous, therefore according to the ethics approval no informed consent was necessary.
Continuous data are summarised using median, interquartile range (IQR), mean, standard deviation and range. Categorical data are presented as percentage. R (Version 1.2.5019) was used for designing figures.
In total, 80 neonates were reported in our database. Four neonates were excluded from the analysis because they were entered in duplicate, two because they were asymptomatic and one because he was not hospitalised. Of the 73 neonates included, 7 (10%) were preterm (table 1). Forty (55%) were female. The median age of the neonates at presentation was 17 days (IQR 11–23) days. Most of the neonates were Caucasian (54, 74%), followed by Arabic (3, 4%), Black (3, 4%), Asian (2, 3%) and Hispanic (1, 1%). Ethnicity was not reported for 10 (14%) neonates. The majority of neonates (64, 88%) were admitted from home, 8 (11%) were transferred from another hospital and 1 (1%) preterm neonate was admitted from the delivery room. Nine (12%) neonates had a pre-existing medical condition (prematurity 7 [10%], trisomy 21 1 [1%], intrauterine growth restriction 1 [1%]).
Table 1Baseline characteristics of neonates with SARS-CoV-2 infection (n = 73).
| Sex | Female, n (%) | 40 (55) |
| Age at presentation in days | Median (IQR) | 17 (11–23) |
| Mean (SD; range) | 17 (7; 1–28) | |
| Gestational age at birth in weeks* | Median (IQR) | 40+0 (38+4–40+6) |
| Mean (SD; range) | 39+1 (2+4; 29+1–41+4) | |
| Birth weight in g** | Median (IQR) | 3475 (3110–3625) |
| Mean (SD; range) | 3335 (610; 1300–4560) | |
| Weight on admission in g | Median (IQR) | 3700 (3400–4000) |
| Mean (SD; range) | 3680 (680; 1370–5200) | |
| Ethnicity | Caucasian, n (%) | 54 (74) |
| Arabic, n (%) | 3 (4) | |
| Black, n (%) | 3 (4) | |
| Asian, n (%) | 2 (3) | |
| Hispanic, n (%) | 1 (1) | |
| Unknown, n (%) | 11 (15) | |
| Pre-existing medical condition | Total | 9 (12) |
| Prematurity, n (%) | 7 (10) | |
| Trisomy 21, n (%) | 1 (1) | |
| Intrauterine growth restriction, n (%) | 1 (1) | |
| Admitted from | Home, n (%) | 64 (88) |
| Other hospital, n (%) | 8 (11) | |
| Delivery room, n (%) | 1 (1) |
* Data missing for one neonate
** Data missing for two neonates
IQR: interquartile range; SD: standard deviation
Overall, the most common symptom reported was fever, in 52 (71%), followed by rhinorrhoea or nasal congestion in 32 (44%) and respiratory distress in 19 (26%) (fig. 1). Further symptoms included feeding difficulties in 11 (15%), cough in 10 (14%), diarrhoea in 10 (14%), vomiting in 8 (11%), abdominal pain in 3 (4%), pharyngitis in 3 (4%), rash in 2 (3%) and jaundice in 1 (1 %) neonate.

Figure 1 Distribution of symptoms in neonates with SARS-CoV-2 infection.
Fever was the only symptom in 20 (27%) neonates; all of these were admitted for fever of unknown source. A total of 20 (27%) neonates did not have fever, of these 13 presented with respiratory symptoms, 5 with gastrointestinal symptoms, and one each with feeding difficulties and rash.
Seventy-two (99%) neonates had a positive SARS-CoV-2 PCR test on nasopharyngeal swab. One neonate did not have a nasopharyngeal swab and was diagnosed with positive PCR on tracheal aspiration. Two neonates additionally had a SARS-CoV-2 PCR test on tracheal aspirations, which was positive. In one neonate, a SARS-CoV-2 PCR test was done on stool, which was negative. No serology test was done.
A total of 44 (60%) neonates had further viruses tested on their nasopharyngeal swab; 4 tested positive for picornavirus. Ten (14%) had chest radiography (4 normal, 4 bilateral changes, 1 unilateral changes and 1 preterm neonate with hyaline membrane disease). Six (8%) neonates had echocardiography, all of them were normal, apart from a patent foramen ovale in one neonate. All of the remaining tests were normal (lumbar puncture in 7 [10%], abdominal sonography in 2 [3%], cranial sonography in 1 [1%], cranial magnetic resonance imaging in 1 [1%]) (table 2).
Table 2Investigations in neonates with SARS-CoV-2 infection.
| n (%) | |
| Nasopharyngeal swab for other viruses | 43 (59) |
| – Negative | 39 |
| – Picornavirus | 4 |
| Chest radiography | 10 (14) |
| – Normal | 4 |
| – Bilateral changes | 4 |
| – Unilateral changes | 1 |
| – Hyaline membrane disease | 1 |
| Echocardiography | 6 (8) |
| – Normal | 5 |
| – Patent foramen ovale | 1 |
| Lumbar puncture | 7 (10) |
| – Normal | 6 |
| – Mononuclear-predominate pleocytosis, SARS-CoV-2 PCR negative | 1 |
| Abdominal sonography | 2 (3) |
| – Normal | 2 |
| Cranial sonography | 1 (1) |
| – Normal | 1 |
| Cranial magnetic resonance imaging | 1 (1) |
| – Normal | 1 |
Seven neonates (10%, 5 term and 2 preterm) needed admission to an intensive care unit, 5 for respiratory failure and 2 for intensive monitoring. One (1%) neonate required inotropic support. The median length of hospital stay in term neonates was 4 days (IQR 3–5) and in preterm neonates 6 days (IQR 3–25) (table 3).
Table 3Admission, treatment and complications in neonates with SARS-CoV-2 infection.
| Term, n = 66 | Preterm, n = 7 | |
| Length of hospital stay in days | ||
| – Median (IQR) | 4 (3–5) | 6 (3–25)* |
| – Mean (SD; range) | 5 (5; 2–35) | 12 (6; 2–35)* |
| Admission to intensive care unit, n (%) | 5 (8) | 2 (29) |
| – Respiratory failure | 3 | 2 |
| – Monitoring | 2 | 0 |
| Oxygen, n (%) | 14 (21) | 3 (43) |
| – Median duration in days (IQR) | 2 (1–3) | nc |
| – Mean duration in days (SD; range) | 4 (6; 1–18) | nc (nc; 1–9) |
| High-flow oxygen, n (%) | 4 (6) | 0 |
| Noninvasive ventilation, n (%) | 3 (5) | 2 (29) |
| Mechanical ventilation, n (%) | 1 (2) | 2 (29) |
| Inotropic support, n (%) | 1 (2) | 0 |
| Corticosteroids, n (%) | 2 (3) | 0 |
| Remdesivir, n (%) | 1 (2) | 0 |
| Complications | 2 (3) | 0 |
| – Central apnoea, n (%) | 1 (2) | 0 |
| – Blood stream infection with Strep. pneumoniae and Strep. salivarius, n (%) | 1 (2) | 0 |
IQR: interquartile range; nc: not calculable; SD: standard deviation
* 2 preterm neonates not included as they were still admitted at the time of data analysis.
Of the 73 neonates, 17 (23%) needed supplementary oxygen, 4 (5%) high-flow oxygen, 5 (7%) noninvasive ventilation and 3 (4%) mechanical ventilation (table 3). Two (3%) neonates were treated with corticosteroids and 1 (1%) with remdesivir.
Two neonates were reported to have complications. One neonate suffered from central apnoea and one from a blood stream infection with Streptococcus pneumoniae and Streptococcus salivarius on admission (table 3). Two preterm neonates were still admitted at the time of data analysis, all other neonates were discharged to their homes without symptoms.
In total, 60 neonates (73%) had contact with a person known or suspected to have a SARS-CoV-2 infection. The distribution is detailed in figure 2.

Figure 2 Exposure to known or suspected SARS-CoV-2-infected cases in 73 neonates.
This nationwide study presents data from active surveillance of symptomatic SARS-CoV-2 infection in neonates in Switzerland during periods when the original virus and alpha and delta variants were circulating. The inclusion of only symptomatic neonates in our study clearly distinguishes our data from previous studies, which mostly included asymptomatic neonates who were exposed to infected mothers [6, 11, 17–23]. Our cohort mainly presented with fever and symptoms of upper and lower respiratory tract infection. One quarter presented with fever without a source. Most neonates were exposed to a known index case. Strain-specific analysis of the data was not possible.
The findings from our study are in line with those from the currently largest review summarising 176 case reports of neonates with SARS-CoV-2 infection [28]. In this review, neonates also mainly presented with fever and respiratory symptoms. However, compared with our cohort, gastrointestinal symptoms were reported more frequently. This may be explained by the larger proportion of preterm-born neonates included , who often have feeding difficulties, even in the absence of an infection. In contrast to our cohort of symptomatic neonates, the review included neonates who were tested because of exposure to maternal SARS-CoV-2 infection and almost half of them were asymptomatic. This likely explains the age difference at presentation, with a mean age of 5 days versus 17 days in our cohort. In the review, an estimated 30% of infections were vertical. In our study, we could not differentiate between vertically transmitted [29] or post-partum infection, but in 40% of neonates the mother was deemed to be the index case.
Fever and other infectious symptoms in the first month of life usually triggers careful clinical evaluation, extensive investigations, empirical antibacterial treatment and hospital admission. Based on our findings, the differential diagnosis of COVID-19 needs to be added as a possible cause of fever without source for this young age group. It has recently been reported that approximately 15% of infants less than 90 days of age admitted for investigations because of fever are diagnosed with a SARS-CoV-2 infection [30]. However, the detection of SARS-CoV-2 should not preclude further investigations for possible bacterial infection, as shown in our study where one neonate with SARS-CoV-2 infection was bacteraemic with Streptococcus pneumoniae on admission. Similarly, three other studies including infants up to the age of 90 days, although not frequently, also reported bacteraemia and urinary tract infections in infants who tested positive for SARS-CoV-2 [30–32]. Of note, although four neonates with COVID-19 also tested positive for picornavirus, we did not find any co-infections with respiratory syncytial virus or influenza.
The rate of admission to an intensive care unit in term neonates with SARS-CoV-2 infection found in our study is similar to the rates reported in older children (2 to 13%) [1, 7, 8, 33, 34]. In the above mentioned review of case reports of neonates with SARS-CoV-2 infection a larger proportion of neonates were admitted to intensive care; this is likely due to the higher proportion of preterm neonates included [28]. Antiviral or anti-inflammatory treatment was rarely required and complications were rare.
The strengths of our study are the multicentre nationwide study design and the availability of detailed epidemiological and clinical data on symptomatic preterm and term neonates. The limitations include the fact that for infants born preterm it is difficult to distinguish between symptoms attributable to COVID-19 or to prematurity, as well as the lack of laboratory values and long-term follow-up data. Furthermore, we did not have strain-specific data.
In neonates, COVID-19 mainly presents with fever, and symptoms of upper and lower respiratory tract infection. The clinical course is mostly mild, requiring a short duration of hospitalisation. COVID-19 needs to be added as a differential diagnosis in neonates who present with fever without a source. However, detecting SARS-CoV-2 infection should not deter from the search for a serious bacterial infection in a neonate. A small proportion of hospitalised neonates requires admission to an intensive care unit.
Further data from surveillance studies are needed to better understand COVID-19 in neonates, guide therapy and to evaluate whether the clinical spectrum is changing with new SARS-CoV-2 variants.
Data collected for the study and the study protocol will be made available to others on request.
We thank the representatives of the paediatric units in Switzerland: M. Albisetti; V. Bernet; C. Betti; F. Cachat; P. Caplazi; M-L. Decker; E. Durrer; S. Fluri; M. Gebauer; M. Gehri; E. Giannoni; S. Grupe; M. Horn; A. L’Huiller; T. Karen; E. Kellner; L. Kottanattu; G. Laube; B. Laubscher; J. Llor; F. Luterbacher; H. Madlon; A. Malzacher; M. Martins; J. McDougall; A. Merglen; S. Minocchieri; V. Muehlethaler; T. Neuhaus; A. Niederer; S. Nikorelou; M. Plebani; Ratnasabapathy; T. Riedel; M. Russo; H. Schmid; K. Staudacher; M. Torres Escobar; J. Wildhaber; A. Wörner; A. Zemmouri
We would also like to acknowledge the administrative support by Daniela Beeli, Mirjam Mäusezahl and Fabien Tschagellar, from the Federal Office of Public Health Switzerland.
Authors’ contributions: PZ, AU and NR designed the study, did the data analysis and wrote the first draft. All authors revised the manuscript and approved the final draft.
The study was supported by the Swiss Federal Office of Public Health and has received grants from the Swiss Society of Paediatrics and the Paediatric Infectious Disease Group of Switzerland.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed..
1. Götzinger F , Santiago-García B , Noguera-Julián A , Lanaspa M , Lancella L , Calò Carducci FI , et al.; ptbnet COVID-19 Study Group . COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020 Sep;4(9):653–61. https://doi.org/10.1016/S2352-4642(20)30177-2
2. Zimmermann P , Curtis N . Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020 May;39(5):355–68. https://doi.org/10.1097/INF.0000000000002660
3. Castagnoli R , Votto M , Licari A , Brambilla I , Bruno R , Perlini S , et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. 2020 Sep;174(9):882–9. https://doi.org/10.1001/jamapediatrics.2020.1467
4. Ludvigsson JF . Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020 Jun;109(6):1088–95. https://doi.org/10.1111/apa.15270
5. Zimmermann P , Curtis N . Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020 Dec;archdischild-2020-320338.
6. Zimmermann P , Curtis N . COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr Infect Dis J. 2020 Jun;39(6):469–77. https://doi.org/10.1097/INF.0000000000002700
7. Uka A , Buettcher M , Bernhard-Stirnemann S , et al. Factors associated with hospital and intensive care admission in paediatric SARS-CoV-2 infection: a prospective nationwide observational cohort study. Eur J Pediatr. Forthcoming 2021.
8. Ward JL , Harwood R , Smith C , et al. Risk factors for intensive care admission and death amongst children and young people admitted to hospital with COVID-19 and PIMS-TS in England during the first pandemic year. medRxiv 2021:2021.07.01.21259785. https://doi.org/10.1101/2021.07.01.21259785
9. WHO . COVID-19 detailed surveillance data dashboard. (accessed 27 July 2021 at https://covid19.who.int)
10. Stokes EK , Zambrano LD , Anderson KN , Marder EP , Raz KM , El Burai Felix S , et al. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020 Jun;69(24):759–65. https://doi.org/10.15585/mmwr.mm6924e2
11. Liguoro I , Pilotto C , Bonanni M , Ferrari ME , Pusiol A , Nocerino A , et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020 Jul;179(7):1029–46. https://doi.org/10.1007/s00431-020-03684-7
12. Dong Y , Mo X , Hu Y , Qi X , Jiang F , Jiang Z , et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020 Jun;145(6):e20200702. https://doi.org/10.1542/peds.2020-0702
13. Parri N , Lenge M , Buonsenso D ; Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group . Children with Covid-19 in Pediatric Emergency Departments in Italy. N Engl J Med. 2020 Jul;383(2):187–90. https://doi.org/10.1056/NEJMc2007617
14. Tagarro A , Epalza C , Santos M , et al. Screening and Severity of Coronavirus Disease 2019 (COVID-19) in Children in Madrid, Spain. JAMA Pediatr 2020.
15. Liu W , Zhang Q , Chen J , Xiang R , Song H , Shu S , et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Apr;382(14):1370–1. https://doi.org/10.1056/NEJMc2003717
16. Bialek S , Gierke R , Hughes M , McNamara LA , Pilishvili T , Skoff T ; CDC COVID-19 Response Team . Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr;69(14):422–6. https://doi.org/10.15585/mmwr.mm6914e4
17. Mullins E , Hudak ML , Banerjee J , Getzlaff T , Townson J , Barnette K , et al.; PAN-COVID investigators and the National Perinatal COVID-19 Registry Study Group . Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021 Apr;57(4):573–81. https://doi.org/10.1002/uog.23619
18. Bellos I , Pandita A , Panza R . Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021 Jan;256:194–204. https://doi.org/10.1016/j.ejogrb.2020.11.038
19. Alipour Z , Samadi P , Eskandari N , Ghaedrahmati M , Vahedian M , Khalajinia Z , et al. Relationship between coronavirus disease 2019 in pregnancy and maternal and fetal outcomes: retrospective analytical cohort study. Midwifery. 2021 Nov;102:103128. https://doi.org/10.1016/j.midw.2021.103128
20. Al-Lawama M , Badran E , Ghanim N , Irsheid A , Qtaishat H , Al-Ammouri I , et al. Perinatal Transmission and Clinical Outcomes of Neonates Born to SARS-CoV-2-Positive Mothers. J Clin Med Res. 2021 Aug;13(8):420–4. https://doi.org/10.14740/jocmr4578
21. Juan J , Gil MM , Rong Z , Zhang Y , Yang H , Poon LC . Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020 Jul;56(1):15–27. https://doi.org/10.1002/uog.22088
22. Sánchez-Luna M , Fernández Colomer B , de Alba Romero C , Alarcón Allen A , Baña Souto A , Camba Longueira F , et al.; SENEO COVID-19 Registry Study Group . Neonates Born to Mothers With COVID-19: Data From the Spanish Society of Neonatology Registry. Pediatrics. 2021 Feb;147(2):147. https://doi.org/10.1542/peds.2020-015065
23. Farghaly MA , Kupferman F , Castillo F , Kim RM . Characteristics of Newborns Born to SARS-CoV-2-Positive Mothers: A Retrospective Cohort Study. Am J Perinatol. 2020 Nov;37(13):1310–6. https://doi.org/10.1055/s-0040-1715862
24. Shalish W , Lakshminrusimha S , Manzoni P , Keszler M , Sant’Anna GM . COVID-19 and Neonatal Respiratory Care: Current Evidence and Practical Approach. Am J Perinatol. 2020 Jun;37(8):780–91. https://doi.org/10.1055/s-0040-1710522
25. Barrero-Castillero A , Beam KS , Bernardini LB , Ramos EG , Davenport PE , Duncan AR , et al.; Harvard Neonatal-Perinatal Fellowship COVID-19 Working Group . COVID-19: neonatal-perinatal perspectives. J Perinatol. 2021 May;41(5):940–51. https://doi.org/10.1038/s41372-020-00874-x
26. Angelidou A , Sullivan K , Melvin PR , Shui JE , Goldfarb IT , Bartolome R , et al. Association of Maternal Perinatal SARS-CoV-2 Infection With Neonatal Outcomes During the COVID-19 Pandemic in Massachusetts. JAMA Netw Open. 2021 Apr;4(4):e217523. https://doi.org/10.1001/jamanetworkopen.2021.7523
27. Allotey J , Stallings E , Bonet M , Yap M , Chatterjee S , Kew T , et al.; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020 Sep;370:m3320. https://doi.org/10.1136/bmj.m3320
28. Raschetti R , Vivanti AJ , Vauloup-Fellous C , Loi B , Benachi A , De Luca D . Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020 Oct;11(1):5164. https://doi.org/10.1038/s41467-020-18982-9
29. World Health Association . Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2. (accessed 22 Feburary 2021 at https://urldefense.com/v3/__https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1__!!Dc8iu7o!hcW8vbYo89O-c-KELcz2o5Gd4aPcI74tcij7brMBoa7QaU7Kah76lBGCGUrtOhzSiKFmpg$)
30. Paret M , Lalani K , Hedari C , Jaffer A , Narayanan N , Noor A , et al. SARS-CoV-2 Among Infants <90 Days of Age Admitted for Serious Bacterial Infection Evaluation. Pediatrics. 2021 Oct;148(4):148. https://doi.org/10.1542/peds.2020-044685
31. Blázquez-Gamero D , Epalza C , Cadenas JA , Gero LC , Calvo C , Rodríguez-Molino P , et al. Fever without source as the first manifestation of SARS-CoV-2 infection in infants less than 90 days old. Eur J Pediatr. 2021 Jul;180(7):2099–106. https://doi.org/10.1007/s00431-021-03973-9
32. Mithal LB , Machut KZ , Muller WJ , Kociolek LK . SARS-CoV-2 Infection in Infants Less than 90 Days Old. J Pediatr. 2020 Sep;224:150–2. https://doi.org/10.1016/j.jpeds.2020.06.047
33. Lu X , Zhang L , Du H , Zhang J , Li YY , Qu J , et al.; Chinese Pediatric Novel Coronavirus Study Team . SARS-CoV-2 Infection in Children. N Engl J Med. 2020 Apr;382(17):1663–5. https://doi.org/10.1056/NEJMc2005073
34. Bhuiyan MU , Stiboy E , Hassan MZ , Chan M , Islam MS , Haider N , et al. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine. 2021 Jan;39(4):667–77. https://doi.org/10.1016/j.vaccine.2020.11.078
The appendix is available in the pdf verion of the article.