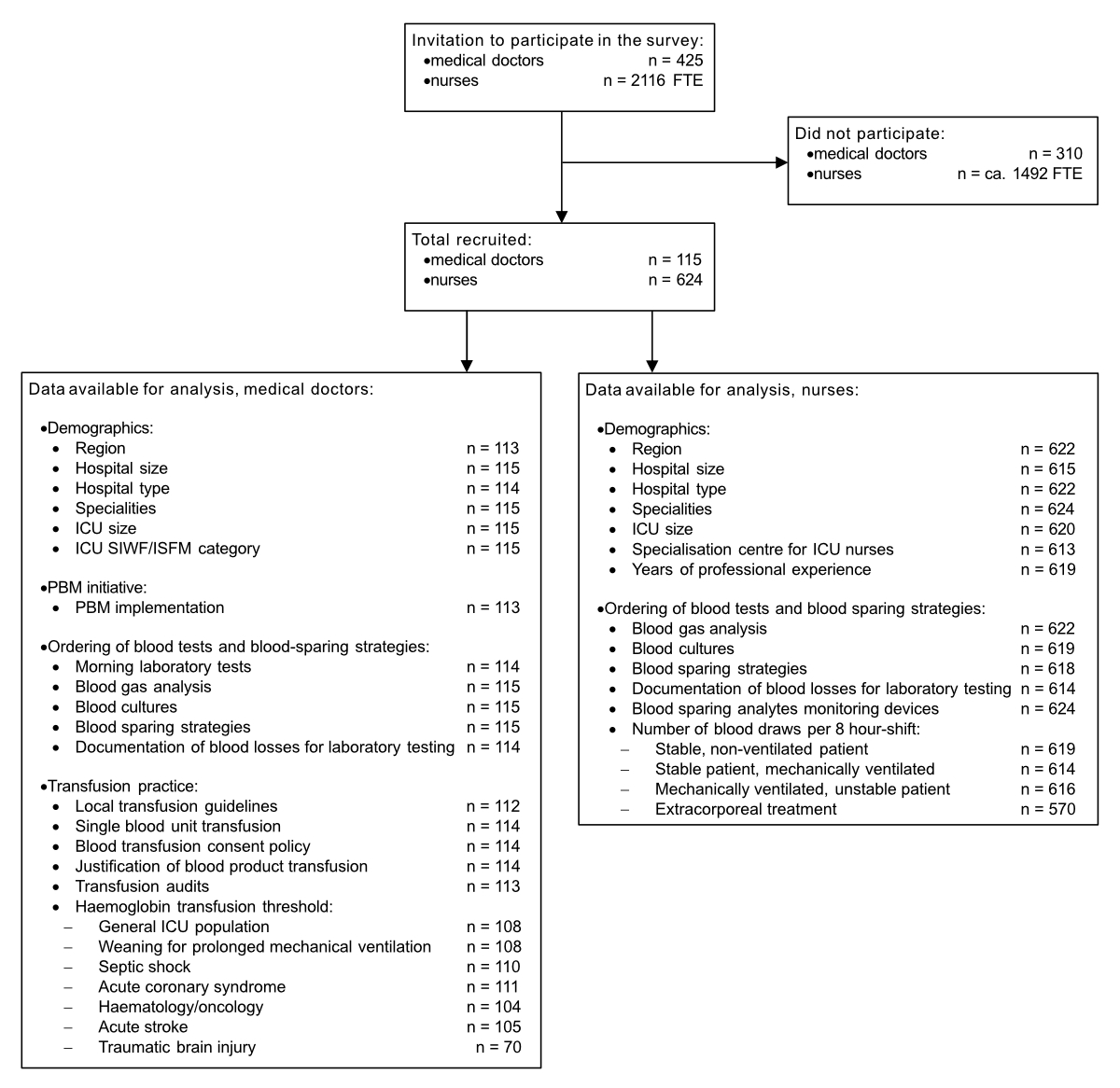
Figure 1 Data flow.
DOI: https://doi.org/10.4414/SMW.2022.w30184
A substantial number of patients are either anaemic at admission or become anaemic during their hospitalisation. Hospital stays lengthen, mortality rises and hospital expenses increase with anaemia severity [1, 2]. Allogeneic blood transfusion is considered an essential therapeutic measure. However, it bears the risk of increased morbidity and mortality in a dose-dependent relationship [3]. Large observational studies have shown that transfusion is specifically associated with increased infection rates [4], lung injury [5], acute kidney injury [6], cardiac overload [7] and thromboembolic events [8]. In intensive care unit (ICU) patients, allogeneic blood transfusion is associated in a dose-dependent relationship with nosocomial infections [9], acute respiratory distress syndrome [10], ICU and hospital length of stay and death [11]. In recent decades, following the landmark Transfusion Requirements in Critical Care trial results [12], a restrictive transfusion strategy (seeking to maintain the haemoglobin level above 70 g/l) has proven to be as safe as or, in specific subpopulations, even superior to a liberal transfusion strategy (aiming at haemoglobin levels above 100 g/l) [13].
Patient blood management (PBM) is a multimodal, multidisciplinary approach based on three main pillars: improving red cell mass, for example by the early detection and treatment of preoperative anaemia, minimising blood loss, for example by optimising surgical and anaesthetic techniques, and harnessing and maximising the physiological tolerance of anaemia [14]. In a recent meta-analysis, the systematic application of a PBM programme improved essential clinical outcomes in a predominantly surgical patient population [15]. More recently, some institutions have extended patient blood management programmes to nonsurgical patients, including ICU patients, who are among the leading recipients of allogeneic blood transfusions [16].
Although several authors have long considered patient blood management as a standard of care [17–19], there is no register of hospitals participating in the patient blood management initiative, and, consequently, we know little about the patient blood management programme in a whole country. The present survey explored the dissemination of the patient blood management initiative in Swiss adult ICUs, investigated the efforts to limit iatrogenic blood loss in ICUs with and without a patient blood management programme, and evaluated the policies adopted for transfusion practice.
This survey was submitted to the Ethics Committee of the Canton Ticino (Bellinzona, Switzerland) and was deemed exempt from ethical review.
We used an online platform (EvaSys 6.1, Electric Paper Evaluationssysteme Gmbh, Lüneburg, Germany) to set up two questionnaires addressed to ICU doctors and ICU nurses. Three intensivists (MP, JC, AP) designed the questionnaires, which were then reviewed by an international patient blood management key opinion leader (AH) and a statistician (BC). The reference materials included a review [16], an article providing comprehensive bundles of patient blood management [20], and the recommendations for survey methodology [21, 22]. The survey aimed to characterise the participants, their hospitals and their ICUs while ensuring their anonymity; therefore, the hospitals’ and ICUs’ identities remained unknown. We selected the investigation’s elements, aware that ICU clinicians may not be aware of all the details related to the management of preoperative anaemia. Instead, we developed more specific constructs about practices within the ICU concerning blood draws, blood-sparing techniques and transfusion strategies.
The doctors’ questionnaire was created in Italian and translated into English (made available for the survey) and then back into Italian to confirm proper translation. It included 37 questions and addressed the following domains: hospital characteristics (hospital type and size, geographical region, medical specialities), in-hospital blood management programme and protocols (availability of patient blood management [yes, no, unknown] including its three pillars [preoperative anaemia detection and treatment, blood loss reduction, and optimisation of anaemia tolerance]; management of preoperative blood product requests; implementation of the “single blood unit transfusion” policy; transfusion consent form policy; involvement of blood bank physicians, blood products concerned and requested information; transfusion audits performance), ICU characteristics (number of ICU beds, postgraduate training category) including blood test ordering policies, blood-sparing strategies and red blood cell-related transfusion practice, focusing on the preferred haemoglobin transfusion threshold for patients in seven clinically relevant subpopulations with adequate O2 delivery. The hospitals were differentiated by type (regional hospital, i.e., primary and secondary level; cantonal hospital, i.e., tertiary level; university hospital, i.e., quaternary level; private clinic) and size (fewer than 200 beds; from 200 to 500 beds; more than 500 beds). The ICUs were divided by size (small, fewer than 7 beds; medium-small, 7 to 10 beds; medium-large, 11 to 15 beds; large, more than 15 beds) and postgraduate training category (Au, almost complete spectrum of critically ill patients, part of a university centre; A, similar to Au, but without academic affiliation and the requirement of a research programme; B, wide range of critically ill patients; C, limited range of critically ill patients; none, not recognised as a training centre) according to the Swiss Institute of Medical Education (SIWF/ISFM; see appendix for static version).
The nurses' questionnaire included twenty questions. It was created in Italian and translated into French and German and then back into Italian by bilingual people to confirm proper translation. The participants could select one of the three versions according to their language preference. The questionnaire addressed demographic, hospital and ICU characteristics (hospital type and size, geographical region, medical specialities in the hospital, nurses’ work experience [less than two years; two to five years; six to ten years; and more than ten years], and ICU size) and blood sampling in the ICU (blood gas analysis ordering policy, number of samplings per work shift in four different clinical situations, blood culture collection practice, availability and type of blood-sparing strategies, documentation policy regarding blood loss for laboratory testing, and use of blood-sparing analyte monitoring devices; see English version for publication in the appendix). To gather details, we asked participants to select information from predefined lists and gave them the option of providing other information or more detailed answers if needed. After pilot testing by intensivists and specialised nurses, we developed the final versions to ensure content validity and optimise the response process.
We conducted a cross-sectional survey on patient blood management using online self-administered anonymous questionnaires. The survey was open from 10 October 2018, to 13 March 2019, and offered no incentives. To be eligible, participants had to be among the ordinary medical members (physicians with a Swiss speciality degree in intensive care medicine or recognised equivalent) of the Swiss Society of Intensive Care Medicine or the registered ICU nurses from the 77 certified adult Swiss ICUs. The survey was entitled Swiss VAMPIRES: Variability among modalities, practices, indications, requests of blood components and samplings in Swiss intensive care units. Completion of the survey took approximately 10 minutes. We invited doctors to participate in the survey via email and sent a reminder once. To contact the nurses, we emailed the department heads twice and asked them to distribute the invitation to participate in the inquiry and the survey platform’s link to their collaborators. Additionally, the Swiss Society of Intensive Care Medicine, who endorsed the study, promoted it at its annual congress and through its periodical newsletter and invited participants to access the survey on its webpage.
Our exploratory survey aimed to analyse PBM-related interventions adopted in Swiss ICUs, relating them to the spread of patient blood management in Swiss hospitals.
To this end, we established the proportion of intensivists who worked in hospitals participating in the patient blood management initiative, characterising them according to the region, the hospital size and type, the presence of specialities and the ICU size and postgraduate training category.
The main objectives of our analysis were divided into two groups: (1) to investigate the efforts to reduce iatrogenic blood loss, including (i) blood draw decision practices, (ii) availability of blood-sparing strategies during blood draw, (iii) the number of samples per work-shift in four different clinical settings, (iv) the documentation policy of blood loss for laboratory testing, and (v) the use of blood-sparing analyte monitoring devices; (2) to analyse transfusion practices, including (i) the availability of local ICU transfusion guidelines, (ii) the haemoglobin transfusion thresholds used for seven critically ill patient categories, (iii) the implementation of the “single blood unit transfusion” policy, (iv) the transfusion consent form policy, (v) the involvement of blood bank physicians (need to justify the transfusion, blood products concerned and requested information), (vi) the practice of ordering blood products before operations; and finally, (vii) the implementation of transfusion audits.
Statistical analysis was performed in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [23]. The sample size was determined by the number of ordinary medical members of the Swiss Society of Intensive Care Medicine and the full-time equivalents of registered ICU nurses during the study period. All fully and partially completed surveys were analysed. As 98.2% of the received questionnaires were complete (predefined lists), we performed a complete case analysis and used no imputation method to estimate potential missing data. We used descriptive statistics to analyse the demographic, hospital and ICU characteristics. We presented categorical data using relative frequencies (percentage) or observations and relative frequencies. We summarised the main results regarding patient blood management programmes (yes vs no and unknown PBM categories) as a function of both the linguistic region and the hospital size using a logistic regression model with both these covariates. The transfusion triggers in different situations were ordinal categorical data with the following categories: inferior to 60 g/l (±5 g/l); 60 g/l (± 5 g/l); 70 g/l (± 5 g/l); 80 g/L (± 5 g/l); 90 g/l (± 5 g/l); 100 g/l (± 5 g/l). We reported the preferred transfusion triggers by indicating the median class and the percentage of observations in the classes below and above this median class.
We investigated the association between two categorical variables with Fisher's exact or chi-square tests when they met the use conditions. The type I error rate was 0.05. All computations were performed with R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria), using the following additional libraries: descr, knitr, and MASS.
Among the 425 ordinary medical members of the Swiss Society of Intensive Care Medicine, 115 doctors participated in the survey (response rate 27.1%). We received 624 questionnaires from nurses, which roughly corresponds to a 30% response rate calculated on the 2116 full-time equivalents employed in the 77 certified Swiss ICUs. One hundred and thirteen medical (98.2%) and 613 (98.2%) nurse inquiries were complete. The data flow is shown in figure 1. Participating doctors practised mainly in regional (33/115, 28.7%) and cantonal hospitals (41/115, 35.7%; missing: 1, 0.9%), nurses mainly in university (251/624, 40.2%) and cantonal hospitals (186/624, 29.8%); missing: 2, 0.3%). The number of professionals per category who participated in the survey per major Swiss region was uneven (chi-square test of independence between profession and region, p = 0.001). For example, Northwestern Switzerland contributed with 7.8% (9/115) of the doctors’ survey participants but only with 2.7% (17/624, missing: 2, 0.3%) of the nurses’ survey participants. Table 1 shows other demographics.

Figure 1 Data flow.
Table 1Respondents’ demographics.
| Demographics | Doctors, n = 115 (%) | Nurses, n = 624 (%) | |
| Number of hospital beds | <200 | 28 (24.3) | 81 (13.0) |
| 200–500 | 54 (46.9) | 223 (35.7) | |
| >500 | 33 (28.7)) | 311 (49.8) | |
| Missing | 0 | 9 (1.4) | |
| Type of institution | Regional hospital | 33 (28.7) | 129 (20.7) |
| Cantonal hospital | 41 (35.7) | 186 (29.8) | |
| University hospital | 23 (20.0) | 251 (40.2) | |
| Private hospital | 17 (14.8) | 56 (9.0) | |
| Missing | 1 (0.9) | 2 (0.3) | |
| Major region of Switzerland (No. of ICUs) | Lake Geneva (13) | 24 (20.8) | 77 (12.3) |
| Midland (14) | 23 (20.0) | 133 (21.3) | |
| Northwestern Switzerland (11) | 9 (7.8) | 17 (2.7) | |
| Zurich (13) | 25 (21.7) | 231 (37.0) | |
| Eastern Switzerland (10) | 13 (11.3) | 46 (7.4) | |
| Central Switzerland (10) | 11 (9.6) | 57 (9.1) | |
| Ticino (6) | 8 (7.0) | 61 (9.8) | |
| Missing | 2 (1.7) | 2 (0.3) | |
| Medical specialities available in the structure | Cardiac surgery | 50 (43.5) | 357 (57.2) |
| Trauma centre | 58 (50.4) | 364 (58.3) | |
| Haematology/oncology | 93 (80.9) | 464 (74.4) | |
| Organ transplant surgery | 29 (25.2) | 250 (40.0) | |
| None of the above | 20 (17.4) | 99 (15.9)) | |
| Missing | 0 | 0 | |
| Number of ICU beds | <7 (small ICU) | 14 (12.2) | 52 (8.3) |
| 7–10 (medium-small ICU) | 36 (31.3) | 188 (30.1) | |
| 11–15 (medium-large ICU) | 20 (17.4) | 173 (27.7) | |
| >15 (large ICU) | 45 (39.1) | 207 (33.2) | |
| Missing | 0 | 4 (0.6) | |
| SIWF/ISFM category of the ICU | Au | 19 (16.5) | |
| A | 41 (35.7) | ||
| B | 23 (20.0) | ||
| C | 15 (13.0) | ||
| None | 17 (14.8) | ||
| Missing | 0 | ||
| Daily presence of an intensivist in the ICU (h/d) | <12 | 26 (22.6) | 128 (20.5) |
| 12–18 | 37 (32.2) | 115 (18.4) | |
| >18 | 51 (44.3) | 377 (60.4) | |
| Missing | 1 (0.9) | 4 (0.6) | |
| Specialisation centre for ICU nurses | Yes | 569 (91.2) | |
| No | 37 (5.9) | ||
| Unknown | 7 (1.1) | ||
| Missing | 11 (1.8) | ||
| Professional experience (yr) | <2 | 94 (15.0) | |
| 2–5 | 113 (18.1) | ||
| 5–10 | 121 (19.4) | ||
| >10 | 291 (46.6) | ||
| Missing | 5 (0.8) | ||
ICU: intensive care unit; SIWF/ISFM: Swiss Institute for Medical Education.
Hospitals had implemented a patient blood management programme according to 48 out of 115 doctors (41.7%; missing: 2, 1.7%); twenty doctors (17.4%) did not know if their hospital had joined the patient blood management initiative (unknown PBM). Figure 2 shows physicians' answers by region, hospital size and type, and ICU size. Responses regarding PBM in ICUs of different training categories were distributed as follows: Au, 10/18 (55.6%); A, 18/40 (45.0%); B, 7/23 (30.4%); C, 3/15 (20.0%); none, 10/17 (58.8%); p = 0.054 for chi-square test of independence between PBM and the training categories; missing: 2, 1.7%. Overall, doctors from the German-speaking regions of Switzerland (odds ratio [OR] of implementation of PBM at 3.39 for German-speaking regions when compared with non-German-speaking regions, 95% CI 1.23–9.35; associated p of the logistic regression coefficient = 0.018) and hospitals with more than 500 beds (OR of implementation of PBM at 3.91 for hospitals with more than 500 beds when compared with hospitals with less than 500 beds, 95% CI 1.48–10.4; associated p of the logistic regression coefficient = 0.006) more commonly reported working in a PBM adherent facility.
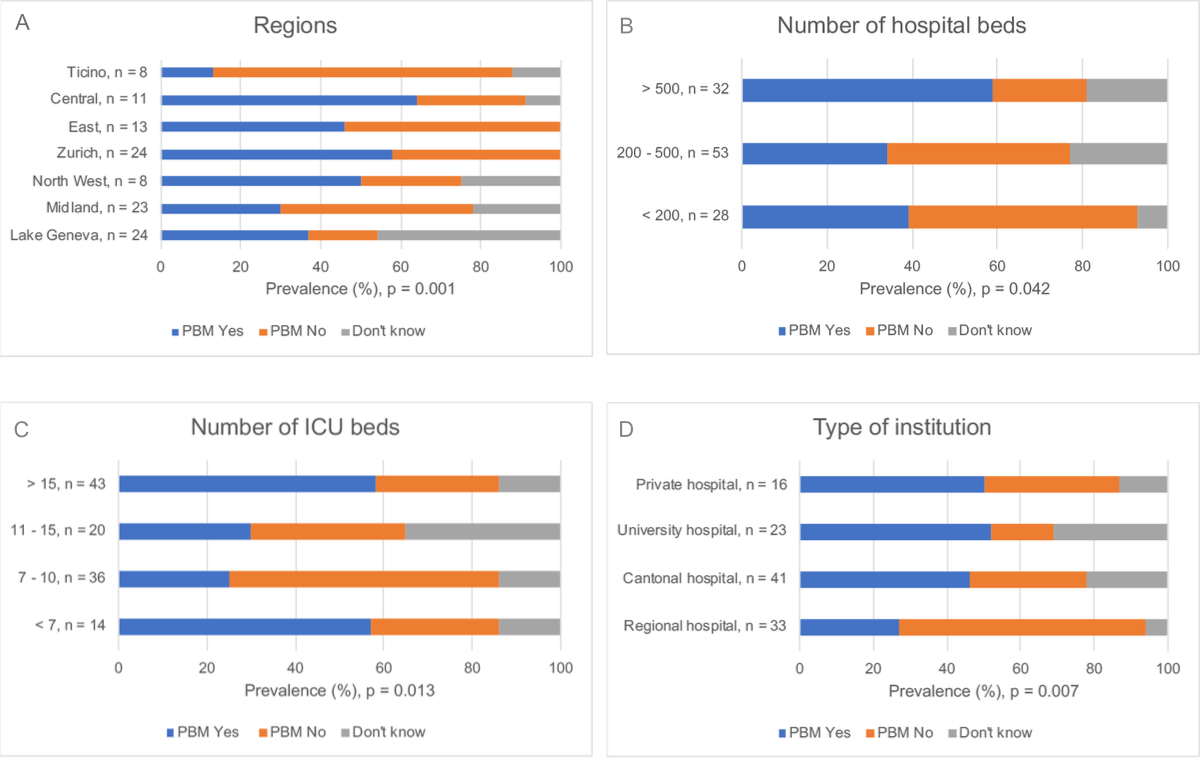
Figure 2 Bar graph showing the percentage of physicians working in hospitals that joined the patient blood management initiative according to different institutional characteristics.
Panel A shows significant differences in the PBM prevalence between the seven Swiss major regions (p = 0.001, (Fisher's exact test of independence between PBM and the Swiss region).
Panel B shows the degree of implementation according to the hospital size. Compared with other respondents, those from hospitals with more than 500 beds more commonly reported the presence of the PBM initiative (OR 3.91, 95% CI 1.48–10.4; p = 0.006, logistic regression coefficient).
Panel C shows the degree of PBM implementation according to the number of ICU beds. Participants from large and small ICUs reported more commonly than those from medium-small or medium-large units that their ICU had implemented PBM (p = 0.013, Fisher's exact test of independence between PBM and number of beds in the ICU).
Finally, Panel D depicts PBM dissemination according to the type of institution. Regional hospitals less often adhered to the PBM initiative (p = 0.007, Fisher's exact test of independence between PBM and type of hospital). PBM indicates Patient Blood Management, ICU indicates intensive care unit.
Physicians from hospitals with specialities reported limited patient blood management implementation with no significant difference compared with those working in non-speciality hospitals (PBM available, 41/93, 44.1% vs 7/20, 35.0%; no PBM, 33/93, 35.5% vs 12/20, 60.0%; unknown PBM, 19/93, 20.4% vs 1/20, 5.0%). PBM positive answers were distributed as follows: trauma centre, 25/56 (44.6%); haematology/oncology, 41/91 (45.1%); organ transplant surgery, 15/28 (53.6%); cardiac surgery, 24/48, (50.0%).
Among the medical doctors reporting some patient blood management activity in their hospital, 39 (81.3%) mentioned the detection and correction of anaemia before surgery, 45 (93.8%) cited the minimisation of perioperative blood loss and 47 (97.9%) the optimisation of anaemia tolerance.
The laboratory tests were ordered in the following way: by the intensivist during morning rounds, 82/115 (71.3%); by a trainee, 15/115 (13.0%); according to routine procedures, 9/115 (7.8%) and specific protocols, 8/115 (7.0%); missing: 1 (0.9%). The larger the ICU, the less often the intensivist took direct care of this task (small ICUs, 13/14, 92.9%; small-medium ICUs, 29/36, 80.6%; medium-large ICUs, 15/20, 75.0%; large ICUs, 25/44, 56.8%).
According to the doctors, blood gas analyses were carried out in the following way: on medical indication, 88/115 (76.5%); according to a protocol, 8/115 (7.0%); on nurses’ initiative, 19/115 (16.5%). Nurses reported autonomy in deciding on blood gas analysis according to 302 of 624 responses (48.4%; missing: 2, 0.3%; autonomy according to physicians compared with nurses, p <0.001, chi-square test of independence between profession and the reporting that blood gas analyses are carried out on medical indication). Blood cultures were obtained upon explicit medical request according to 114 of 115 doctors (99.1%) and 560 of 624 nurses (89.7%); missing nurses’ answers: 5 (0.8%). ICUs had strategies to reduce blood loss from laboratory tests according to 263 of 624 nurses (42.1%; missing: 6, 1%) and 54 of 115 doctors (47.0%). As reported by the latter, the availability of strategies correlated with the presence of PBM (26/48, 54.2%; no PBM, 13/45, 28.9%; unknown PBM, 15/20, 75%; p = 0.006, Fisher's exact test of independence between PBM and the presence of strategies to reduce blood loss; missing: 2, 1.7%). According to nurses, measures applied were to return the blood withdrawn to clear the line before sampling, use of small volume laboratory tubes and closed blood sampling devices for 74 of 263 (27.8%), 27 of 263 (10.3%), and 200 of 263 (74.5%), respectively. Figure 3 shows the nurse-reported blood draws per 8-hour shift from patients in four different clinical conditions. In a clinically stable, nonventilated patient, 296 of 624 (47.4%, missing: 5, 0.8%) would perform no sampling. In stable, mechanically ventilated patients, 392 of 624 (62.8%, missing: 10, 1.6%) would draw blood once or twice, and in mechanically ventilated unstable patients, 372 of 624 participants (59.6%, missing: 8, 1.3%) would draw blood between three and five times. Finally, 299 of 624 (47.9%, missing: 54, 8.7%) would draw blood between three and five times from patients undergoing extracorporeal treatment. We found no association between the number of blood draws from patients and the interviewees' work experience or the presence of an intensive care physician in hours per day. Only 2.6% of doctors (3/115, missing: 1, 0.9%) and 3.0% of nurses (19/624, missing: 10, 1.6%) documented daily blood losses for laboratory testing. More than three quarters (484/624, 77.6%) of the nurses used some blood-sparing analyte monitoring devices: end-tidal-CO2 (430/624, 68.9%); noninvasive haemoglobin monitoring by pulse co-oximetry (50/624, 8.0%); and continuous intravascular glucose monitoring (2/624, 0.4%).
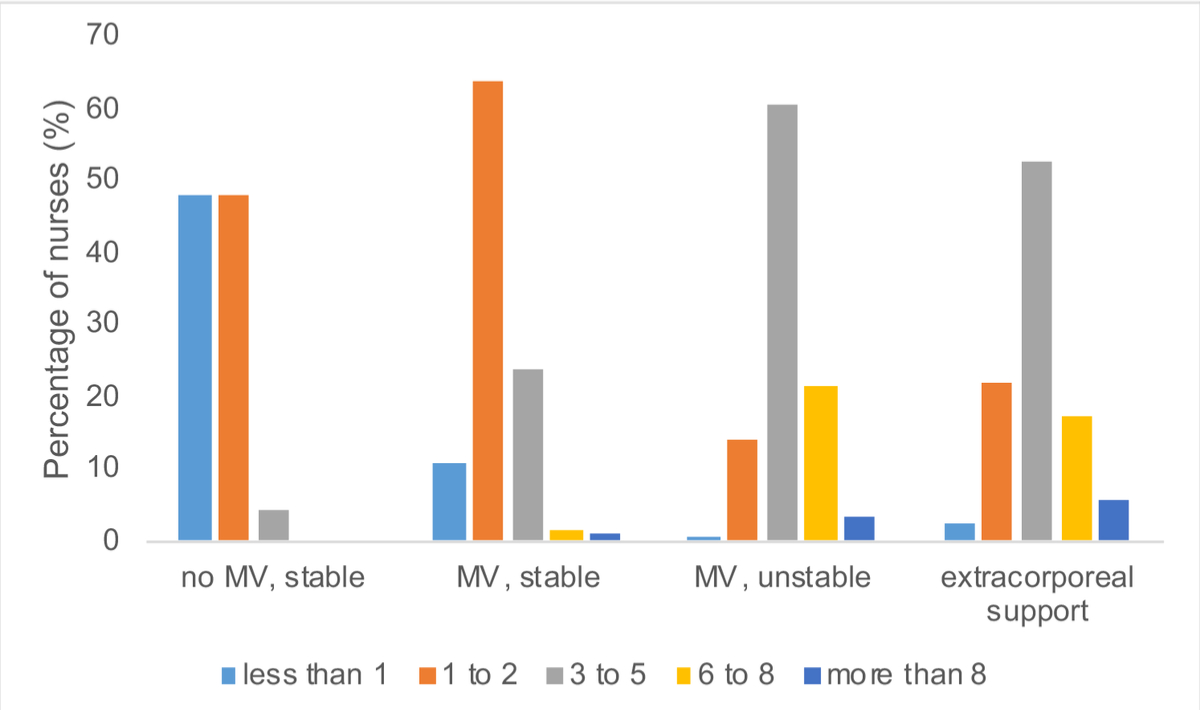
Figure 3 Number of blood draws per 8-hour shift in four clinical settings. MV: mechanical ventilation
Local ICU transfusion guidelines were available according to 67 of 115 intensivists (58.3%; missing: 3, 2.6%), mainly in hospitals adhering to the patient blood management initiative (PBM available, 38/47, 80.9%; no PBM, 23/45, 51.1%; unknown PBM, 6/20, 30.0%; p <0.001, Fisher's exact test of independence between PBM and the presence of ICU transfusion guidelines). The median reported haemoglobin transfusion threshold in nonbleeding ICU patients was 70 g/l in 82/115 (71.3%), below 70 g/l in 15/115 (13.0%), and above 70 g/l in 11/115 (9.6%); (missing: 7,6.1%). Participants applied the same threshold to patients with prolonged weaning from mechanical ventilation (91/115, 79.1%; below 70 g/l, 2/115, 1.7%; and above 70 g/l, 15/115, 13.0%; missing: 7, 6.1%) and to haematology/oncology patients (79/115, 68.7%; below 70 g/l, 13/115, 11.3%; and above 70 g/l, 12/115, 10.4%; missing: 11, 9.6%). Respondents reported higher haemoglobin transfusion thresholds for patients with an acute coronary syndrome (80 g/l, 65/115, 56.5%; below 80 g/l, 5/115, 4.3%; and above 80 g/l, 41/115, 35.7%; missing: 4, 3.5%). We observed the most substantial variation in transfusion thresholds for patients with septic shock (70 g/l, 48/115, 41.7%; below 70 g/l, 2/115, 1.7%; above 70 g/l, 60/115, 52.2%; missing: 5, 4.3%), acute ischaemic stroke (80 g/l, 45/115, 39.1%; below 80 g/l, 32/115, 27.8%; above 80 g/l, 28/115, 24.3%; missing: 10, 8.7%), and traumatic brain injury (80 g/l, 21/115, 18.2%; below 80 g/l, 23/115, 20.0%; above 80 g/l, 26/115, 22.6%; missing: 45, 39.1%).
The single-unit blood transfusion policy was associated with a PBM programme (22/48, 45.8%; no PBM, 6/45, 13.3%; unknown PBM, 2/20, 10%; p <0.001, Fisher's exact test of independence between PBM and the presence of a single-unit blood transfusion policy; missing: 2, 1.7%), but only 30 of the 115 intensivists (26.1%; missing: 1, 0.9%) operated it as a standard measure in clinically stable patients. Just 34 of 115 intensivists (29.6%; missing: 1, 0.9%) were able to obtain informed consent before blood transfusions. Among the 115 interviewees, 24 (20.9%; missing: 1, 0.9%) had to provide justifications to blood bank physicians prior to administering allogeneic blood products. This requirement applied to packed red blood cells (20/24, 83.3%), platelet concentrates (24/24, 100%) and fresh frozen plasma (17/24, 70.8%), and regarded underlying clinical conditions (23/24, 95.8%), laboratory values (17/24, 70.8%) and the planned procedure (9/24, 37.5%). Approximately one third of responding physicians (41/115, 35.7%; missing: 2, 1.7%) participated in transfusion audits, mainly if a PBM programme was in place (24/48, 50.0%; no PBM, 13/45, 28.9%; unknown PBM, 4/20, 20.0%; p = 0.005, Fisher's exact test of independence between PBM and presence of transfusion audits; missing: 2, 1.7%).
The main findings of our survey are: (1) moderate and variable dissemination of patient blood management strategies in hospitals of different regions and sizes; (2) liberal blood sample collection practice with extensive decision-making autonomy for ICU nurses; (3) lack of blood-sparing strategies regardless of a high number of daily blood draws; and (4) firm adherence to international guidelines on transfusion thresholds.
The World Health Organization (WHO) has been urging member countries to implement patient blood management since 2010 [24]. Nevertheless, its prevalence in Swiss adult ICUs and hospitals is still low, with significant variability between regions and hospital types. The fragmented Swiss federal healthcare system, where each of the twenty-six cantons is responsible for organising hospital services in its territory, might explain why only half of the participants from large hospitals and private institutions and a quarter from regional hospitals had a well-structured patient blood management programme. In approximately two thirds of European countries, several hospitals, professional societies and medical associations currently endorse patient blood management. Italy has even imposed its widespread introduction by law [25]. However, patient blood management implementation in Europe is still limited, and considerable variations exist [18, 26].
The WHO defines anaemia as a condition in which the number of red blood cells is insufficient to meet the body’s physiological needs, with a haemoglobin level of less than 130 g/l in men and 120 g/l in women at sea level [27,28]. It is considered an epidemic that affects a large proportion of hospitalised patients. Accordingly, upon admission to the ICU, mean haemoglobin levels vary between 100 and 115 g/l, depending on the origin and age of the patients, with approximately two thirds having haemoglobin less than 120 g/l [29]. Over time, the haemoglobin levels converge towards 90–100 g/l irrespective of the admitting value [30–32]. Critically ill patients are among the primary recipients of allogeneic blood transfusions, with ICU transfusion rates ranging between 33% and 75% [33, 34], which is of concern, both in terms of safety and efficacy and for economic reasons [35–37]. Since low haemoglobin and anaemia have a central role in increasing the risk of transfusion and adverse outcomes [38], their prevention and optimal management are cornerstones of patient blood management. A routine application of this strategy can benefit patients admitted to surgical ICUs and generate fewer transfusions, better outcomes and reduced costs [39]. Probably other patients admitted to ICU can also take advantage of careful management of all the factors favouring anaemia.
One potentially modifiable risk factor for developing hospital-acquired anaemia is unnecessary diagnostic blood draws. In our survey, blood sample collection was handled rather liberally and seemed poorly regulated by protocols. Nurses managed independently about half of arterial blood gas tests, which doctors might not be fully aware of, given the difference on this point recorded in the nurses' and medical doctors' investigation. Of note, the number of necessary samples is unclear and depends on many factors, such as the clinical picture, the therapies applied (e.g., anticoagulation) and, potentially, the availability of noninvasive methods for monitoring specific parameters. In line with the literature [30, 40], our investigation revealed increasing diagnostic blood draws with the severity of patients' disease. Several approaches have shown the potential to avoid excessive laboratory tests. A set of changes encompassing physician and nursing staff education and several structural modifications (elimination of standing orders, laboratory order review during daily rounds, limitation of gasometer cartridges to those for blood gas analysis) reduced blood test orders by one third [41]. Additionally, group assessments of the potential of each arterial blood gas test to change patient management achieved a 60% assay reduction with no negative impact on the patient outcome [42].
According to the survey results, there was virtually no documentation of the daily iatrogenic blood loss, even in centres participating in the patient blood management initiative, suggesting it is still considered unavoidable or negligible. Only one third of survey participants had closed blood sampling devices available, and less than 5% used small laboratory tubes, a valuable way to reduce blood waste and the need for blood transfusion [40, 43, 44]. The discarded first millilitres of the infusate-blood mixture obtained when collecting blood from a fluid-infusing catheter represent avoidable waste that causes significant blood loss [45]. In addition, most blood collected in standard volume laboratory tubes will be discarded as current laboratory instruments require only a small amount of dead volume and process only a few microlitres.
Local ICU transfusion guidelines were available for only approximately 60% of respondents, mainly in centres with patient blood management. These crucial recommendations promote implementation and process and enhance sustainability. They should emphasise the need to individualise procedures, including not only haemoglobin-based transfusion triggers but also the patient's risk profile, laboratory values (e.g., platelet count, coagulation testing, lactate), the presence of active bleeding, and physiological factors.
General transfusion guidelines may not apply to critically ill patients for many reasons, such as frequent imbalances between tissue oxygen supply and requirements during critical illness, impaired erythropoiesis secondary to inflammation and iron sequestration, risk of iatrogenic anaemia, and increased risk of transfusion-related morbidity and mortality [46]. Furthermore, uncertainties remain regarding optimal transfusion thresholds for some everyday situations in the ICU, such as acute neurological injury, acute coronary syndrome, critically ill adults with malignancies, elderly patients or those undergoing extracorporeal membrane oxygenation (ECMO). According to our respondents, the median haemoglobin transfusion threshold for the nonbleeding ICU population was 70 g/l and, for the other investigated subcategories, it was consistently at the lower limit of those proposed by current ICU-specific guidelines [47]. This shows that staff training and education may lead to tangible results. Furthermore, it might partially explain the year-long decline in demand for packed red blood cells observed by the Swiss Red Cross [48].
Our survey indicated poor adherence to the single-unit transfusion policy. Nevertheless, the nonemergent administration of single units consistently reduced the consumption of allogeneic blood products in several observational studies, proving to be of greater health and economic interest [49–51] and finding a place in transfusion guidelines as a crucial element of the Optimal Blood Use concept [52, 53].
According to our investigation, transfusion medicine personnel only marginally supervised blood transfusions, and only a minority of institutions performed transfusion audits. However, the collaboration between clinicians and blood bank physicians could help optimise transfusion practice and set up medical logic and best practice alerts in the electronic medical records to audit blood products. In addition, it might be a prerequisite for developing a PBM-related metrics programme to identify potential areas for improvement and allow continuous benchmarking between PBM centres.
This survey's strengths are the broad but careful look at many aspects of patient blood management in Swiss ICUs and hospitals, and the substantial number of respondents from the medical and nursing categories. However, some limitations must be listed. First, the survey response rate among doctors was approximately 27%, and that of nurses can only be estimated based on full-time equivalents (30%). Although in line with other incentive-free surveys directly involving the hospital staff [54–58], our survey’s response rate shows that patient blood management has not yet gained the attention of the broad medical establishment and limits the representativeness of our results. Second, the anonymous nature of the survey precludes determination of the number of participants per hospital or how many hospitals have implemented a patient blood management programme. The survey was open to all ordinary medical members of the Swiss Society of Intensive Care Medicine and all registered ICU nurses, and we did not attempt to limit the number of responses from any one institution. Therefore, the results might overly represent certain hospitals. A top-down survey approach addressed to unit heads might have avoided sampling bias and obtained more comprehensive data about patient blood management dissemination in the hospitals, but compromised the participants’ anonymity. Additionally, our inquiry also aimed to investigate the actual practice in the departments, where the management can vary at least in part depending on the people involved. Third, an excess of professional caregivers with a particular interest in patient blood management may have participated in the survey, potentially influencing some conclusions, such as a more frequent implementation of patient blood management in the German-speaking regions of Switzerland. Fourth, the interviewees might be unaware of aspects unrelated to the ICU, such as detecting and treating preoperative anaemia before hospitalisation. Therefore, some survey results may only illustrate the participants’ level of knowledge on some protocols and not the objective situation per se. Fifth, we evaluated the preferred transfusion trigger by asking for the haemoglobin threshold in patients without inadequate oxygen delivery. We did not further investigate the role of comorbidities or other clinical markers (e.g., hypotension, tachycardia, elevated lactate level, significant ECG changes), which most intensivists consider crucial, together with haemoglobin levels, to guide transfusion [59]. Sixth, due to the study design, multivariate analysis was not appropriate. As a result, it was impossible to exclude confounding variables from the observed associations. Finally, as with any clinical practice survey, the participants' responses might differ from actual practices.
The reported prevalence of patient blood management in Swiss ICUs and hospitals is limited and displays significant variability between regions and hospital types. The risk of iatrogenic anaemia is relevant because of liberal blood sample collection practices and the underuse of blood-sparing techniques. The reported transfusion thresholds suggest excellent adherence to current international ICU-specific transfusion guidelines.
The Swiss Society for Intensive Care Medicine should coordinate a national multidisciplinary collaborative to identify patient blood management practice gaps and critical organisational issues to implement a Swiss patient blood management programme, overcoming the difficulties caused by a fragmented health system. The group's main working area should be developing patient blood management guidelines covering the appropriate management of preoperative anaemia and haemostasis, implementing blood-sparing strategies, creating PBM-specific protocols and updating transfusion guidelines on optimal blood use.
Individual hospitals should incorporate the collaborative group's advice and invest in employee training.
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
MP helped conceptualise and design the study, acquire and analyse the data, draft and revise the manuscript and approved the final version to be published. JC helped conceptualise and design the study, acquire the data, draft and revise the manuscript and approved the final version to be published. BC helped design the study, acquire and analyse the data, draft and revise the manuscript and approved the final version to be published. BG helped draft the manuscript and approved the final version to be published. AH helped conceptualise the study, draft the manuscript and approved the final version to be published. AFC helped draft the manuscript and approved the final version to be published. AS helped draft the manuscript and approved the final version to be published. DLR helped draft the manuscript and approved the final version to be published. SC helped draft the manuscript and approved the final version to be published. MPa helped draft the manuscript and approved the final version to be published. AP helped conceptualise and design the study, acquire and analyse the data, draft and revise the manuscript and approved the final version to be published.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. BC reports consulting fees paid to his institution from the Swiss Society of Intensive Care Medicine and having stocks from Air Liquide. BG reports grants (unrestricted research grant) and personal fees from Pfizer; personal fees and funding for accredited continuing medical education from Sanofi, Alnylam and Thermo Fisher Scientific; during the conduct of the study, funding for an accredited continuing medical education programme from Axonlab, Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Janssen, Mitsubishi Tanabe Pharma, NovoNordisk, Octapharma, Takeda, Sanofi, SOBI; nonfinancial support from Axonlab and Thermo Fisher from outside the submitted work. AH reports personal fees and financial support during the past 36 months from Celgene, G1 Therapeutics, PBMe Solutions, Takeda SA, Vifor Pharma AG, Vision Plus, Werfen; personal honoraria for lectures from Heart Team Education Association Switzerland, Korean Surgical Society, Med Ed Global Solutions France; Takeda SA, Vifor Pharma AG, Vision Plus and Werfen. AS reports that Alliance Rouge currently supports implementing a PBM programme in his institution. No potential conflict of interest was disclosed.
1. Koch CG , Li L , Sun Z , Hixson ED , Tang A , Phillips SC , et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med. 2013 Sep;8(9):506–12. https://doi.org/10.1002/jhm.2061
2. Corwin HL , Gettinger A , Pearl RG , Fink MP , Levy MM , Abraham E , et al. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004 Jan;32(1):39–52. https://doi.org/10.1097/01.CCM.0000104112.34142.79
3. van Straten AH , Bekker MW , Soliman Hamad MA , van Zundert AA , Martens EJ , Schönberger JP , et al. Transfusion of red blood cells: the impact on short-term and long-term survival after coronary artery bypass grafting, a ten-year follow-up. Interact Cardiovasc Thorac Surg. 2010 Jan;10(1):37–42. https://doi.org/10.1510/icvts.2009.214551
4. Gulack BC , Kirkwood KA , Shi W , Smith PK , Alexander JH , Burks SG , et al.; Cardiothoracic Surgical Trials Network (CTSN) . Secondary surgical-site infection after coronary artery bypass grafting: A multi-institutional prospective cohort study. J Thorac Cardiovasc Surg. 2018 Apr;155(4):1555–1562.e1. https://doi.org/10.1016/j.jtcvs.2017.10.078
5. Vossoughi S , Gorlin J , Kessler DA , Hillyer CD , Van Buren NL , Jimenez A , et al. Ten years of TRALI mitigation: measuring our progress. Transfusion. 2019 Aug;59(8):2567–74. https://doi.org/10.1111/trf.15387
6. Koch CG , Li L , Duncan AI , Mihaljevic T , Cosgrove DM , Loop FD , et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006 Jun;34(6):1608–16. https://doi.org/10.1097/01.CCM.0000217920.48559.D8
7. Menis M , Anderson SA , Forshee RA , McKean S , Johnson C , Holness L , et al. Transfusion-associated circulatory overload (TACO) and potential risk factors among the inpatient US elderly as recorded in Medicare administrative databases during 2011. Vox Sang. 2014 Feb;106(2):144–52. https://doi.org/10.1111/vox.12070
8. Goel R , Patel EU , Cushing MM , Frank SM , Ness PM , Takemoto CM , et al. Association of Perioperative Red Blood Cell Transfusions With Venous Thromboembolism in a North American Registry. JAMA Surg. 2018 Sep;153(9):826–33. https://doi.org/10.1001/jamasurg.2018.1565
9. Shorr AF , Jackson WL , Kelly KM , Fu M , Kollef MH . Transfusion practice and blood stream infections in critically ill patients. Chest. 2005 May;127(5):1722–8. https://doi.org/10.1378/chest.127.5.1722
10. Gong MN , Thompson BT , Williams P , Pothier L , Boyce PD , Christiani DC . Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005 Jun;33(6):1191–8. https://doi.org/10.1097/01.CCM.0000165566.82925.14
11. Kneyber MC , Hersi MI , Twisk JW , Markhorst DG , Plötz FB . Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007 Aug;33(8):1414–22. https://doi.org/10.1007/s00134-007-0741-9
12. Hébert PC , Wells G , Blajchman MA , Marshall J , Martin C , Pagliarello G , et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999 Feb;340(6):409–17. https://doi.org/10.1056/NEJM199902113400601
13. Trentino KM , Farmer SL , Leahy MF , Sanfilippo FM , Isbister JP , Mayberry R , et al. Systematic reviews and meta-analyses comparing mortality in restrictive and liberal haemoglobin thresholds for red cell transfusion: an overview of systematic reviews. BMC Med. 2020 Jun;18(1):154. https://doi.org/10.1186/s12916-020-01614-w
14. Leahy MF , Hofmann A , Towler S , Trentino KM , Burrows SA , Swain SG , et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017 Jun;57(6):1347–58. https://doi.org/10.1111/trf.14006
15. Althoff FC , Neb H , Herrmann E , Trentino KM , Vernich L , Füllenbach C , et al. Multimodal Patient Blood Management Program Based on a Three-pillar Strategy: A Systematic Review and Meta-analysis. Ann Surg. 2019 May;269(5):794–804. https://doi.org/10.1097/SLA.0000000000003095
16. Shander A , Javidroozi M , Lobel G . Patient Blood Management in the Intensive Care Unit. Transfus Med Rev. 2017 Oct;31(4):264–71. https://doi.org/10.1016/j.tmrv.2017.07.007
17. Shander A , Bracey AW Jr , Goodnough LT , Gross I , Hassan NE , Ozawa S , et al. Patient Blood Management as Standard of Care. Anesth Analg. 2016 Oct;123(4):1051–3. https://doi.org/10.1213/ANE.0000000000001496
18. Shander A , Van Aken H , Colomina MJ , Gombotz H , Hofmann A , Krauspe R , et al. Patient blood management in Europe. Br J Anaesth. 2012 Jul;109(1):55–68. https://doi.org/10.1093/bja/aes139
19. Bruun MT , Pendry K , Georgsen J , Manzini P , Lorenzi M , Wikman A , et al. Patient Blood Management in Europe: surveys on top indications for red blood cell use and Patient Blood Management organization and activities in seven European university hospitals. Vox Sang. 2016 Nov;111(4):391–8. https://doi.org/10.1111/vox.12435
20. Meybohm P , Richards T , Isbister J , Hofmann A , Shander A , Goodnough LT , et al. Patient Blood Management Bundles to Facilitate Implementation. Transfus Med Rev. 2017 Jan;31(1):62–71. https://doi.org/10.1016/j.tmrv.2016.05.012
21. Burns KEA , Burns KEA , Duffett M , Duffett M , Kho ME , Kho ME , et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2008 Jul 29;179(3):245–52.
22. Kelley K , Clark B , Brown V , Sitzia J . Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003 Jun;15(3):261–6. https://doi.org/10.1093/intqhc/mzg031
23. von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007 Oct;4(10):e296. https://doi.org/10.1371/journal.pmed.0040296
24. World Health Assembly Resolution WHA . 63.12. Availability, safety and quality of blood products [Internet]. Geneva: World Health Organization; 2010 May p. 1–4. Available from: https://apps.who.int/iris/handle/10665/3086
25. Vaglio S , Gentili S , Marano G , Pupella S , Rafanelli D , Biancofiore G , et al. The Italian Regulatory Guidelines for the implementation of Patient Blood Management. Blood Transfus. 2017 Jul;15(4):325–8.
26. Van der Linden P , Hardy JF . Implementation of patient blood management remains extremely variable in Europe and Canada: the NATA benchmark project: An observational study. Eur J Anaesthesiol. 2016 Dec;33(12):913–21. https://doi.org/10.1097/EJA.0000000000000519
27. WHO . Anaemia [Internet]. 2008 [cited 2022 Jan 31]. Available from: https://www.who.int/data/nutrition/nlis/info/anaemia
28. WHO . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity [Internet]. 2011 [cited 2022 Jan 31]. Available from: https://www.who.int/vmnis/indicators/haemoglobin.pdf
29. Vincent JL , Jaschinski U , Wittebole X , Lefrant JY , Jakob SM , Almekhlafi GA , et al.; ICON Investigators . Worldwide audit of blood transfusion practice in critically ill patients. Crit Care. 2018 Apr;22(1):102. https://doi.org/10.1186/s13054-018-2018-9
30. Vincent JL , Baron JF , Reinhart K , Gattinoni L , Thijs L , Webb A , et al.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators . Anemia and blood transfusion in critically ill patients. JAMA. 2002 Sep;288(12):1499–507. https://doi.org/10.1001/jama.288.12.1499
31. Warner MA , Kor DJ , Frank RD , Dinglas VD , Mendez-Tellez P , Himmelfarb CR , et al. Anemia in Critically Ill Patients With Acute Respiratory Distress Syndrome and Posthospitalization Physical Outcomes. J Intensive Care Med. 2021 May;36(5):557–65. https://doi.org/10.1177/0885066620913262
32. Thomas J , Jensen L , Nahirniak S , Gibney RT . Anemia and blood transfusion practices in the critically ill: a prospective cohort review. Heart Lung. 2010 May-Jun;39(3):217–25. https://doi.org/10.1016/j.hrtlng.2009.07.002
33. Vincent JL , Sakr Y , Sprung C , Harboe S , Damas P , Investigators SO . in AIP (SOAP). Are Blood Transfusions Associated with Greater Mortality Rates? Anesthesiology. 2008;108(1):31–9. https://doi.org/10.1097/01.anes.0000296070.75956.40
34. Palmieri TL , Caruso DM , Foster KN , Cairns BA , Peck MD , Gamelli RL , et al.; American Burn Association Burn Multicenter Trials Group . Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med. 2006 Jun;34(6):1602–7. https://doi.org/10.1097/01.CCM.0000217472.97524.0E
35. Isbister JP , Shander A , Spahn DR , Erhard J , Farmer SL , Hofmann A . Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev. 2011 Apr;25(2):89–101. https://doi.org/10.1016/j.tmrv.2010.11.001
36. Marik PE , Corwin HL . Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008 Sep;36(9):2667–74. https://doi.org/10.1097/CCM.0b013e3181844677
37. Shander A , Hofmann A , Ozawa S , Theusinger OM , Gombotz H , Spahn DR . Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010 Apr;50(4):753–65. https://doi.org/10.1111/j.1537-2995.2009.02518.x
38. Shander A , Javidroozi M , Ozawa S , Hare GM . What is really dangerous: anaemia or transfusion? Br J Anaesth. 2011 Dec;107 Suppl 1:i41–59. https://doi.org/10.1093/bja/aer350
39. Gross I , Seifert B , Hofmann A , Spahn DR . Patient blood management in cardiac surgery results in fewer transfusions and better outcome. Transfusion. 2015 May;55(5):1075–81. https://doi.org/10.1111/trf.12946
40. Smoller BR , Kruskall MS . Phlebotomy for diagnostic laboratory tests in adults. Pattern of use and effect on transfusion requirements. N Engl J Med. 1986 May;314(19):1233–5. https://doi.org/10.1056/NEJM198605083141906
41. Raad S , Elliott R , Dickerson E , Khan B , Diab K . Reduction of Laboratory Utilization in the Intensive Care Unit. J Intensive Care Med. 2017 Sep;32(8):500–7. https://doi.org/10.1177/0885066616651806
42. Blum FE , Lund ET , Hall HA , Tachauer AD , Chedrawy EG , Zilberstein J . Reevaluation of the utilization of arterial blood gas analysis in the Intensive Care Unit: effects on patient safety and patient outcome. J Crit Care. 2015 Apr;30(2):438.e1–5. https://doi.org/10.1016/j.jcrc.2014.10.025
43. Sanchez-Giron F , Alvarez-Mora F . Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med. 2008 Dec;132(12):1916–9. https://doi.org/10.5858/132.12.1916
44. Mukhopadhyay A , Yip HS , Prabhuswamy D , Chan YH , Phua J , Lim TK , et al. The use of a blood conservation device to reduce red blood cell transfusion requirements: a before and after study. Crit Care. 2010;14(1):R7. https://doi.org/10.1186/cc8859
45. Henry ML , Garner WL , Fabri PJ . Iatrogenic anemia. Am J Surg. 1986 Mar;151(3):362–3. https://doi.org/10.1016/0002-9610(86)90468-X
46. Vlaar AP , Juffermans NP . Transfusion-related acute lung injury: a clinical review. Lancet. 2013 Sep;382(9896):984–94. https://doi.org/10.1016/S0140-6736(12)62197-7
47. Vlaar AP , Oczkowski S , de Bruin S , Wijnberge M , Antonelli M , Aubron C , et al. Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020 Apr;46(4):673–96. https://doi.org/10.1007/s00134-019-05884-8
48. Schweiz BS . Stabile Blutversorgung, Blutstammzellspende im Wachstum [Internet]. 2020 [cited 2020 Oct 13]. Available from: https://www.blutspende-srk.ch/de/Medien/Pressemitteilungen/stabile-blutversorgung-blutstammzellspende-im-wachstum
49. Berger MD , Gerber B , Arn K , Senn O , Schanz U , Stussi G . Significant reduction of red blood cell transfusion requirements by changing from a double-unit to a single-unit transfusion policy in patients receiving intensive chemotherapy or stem cell transplantation. Haematologica. 2012 Jan;97(1):116–22. https://doi.org/10.3324/haematol.2011.047035
50. Warner MA , Schaefer KK , Madde N , Burt JM , Higgins AA , Kor DJ . Improvements in red blood cell transfusion utilization following implementation of a single-unit default for electronic ordering. Transfusion. 2019 Jul;59(7):2218–22. https://doi.org/10.1111/trf.15316
51. Yang WW , Thakkar RN , Gehrie EA , Chen W , Frank SM . Single-unit transfusions and hemoglobin trigger: relative impact on red cell utilization. Transfusion. 2017 May;57(5):1163–70. https://doi.org/10.1111/trf.14000
52. Shih AW , Liu A , Elsharawi R , Crowther MA , Cook RJ , Heddle NM . Systematic reviews of guidelines and studies for single versus multiple unit transfusion strategies. Transfusion. 2018 Dec;58(12):2841–60. https://doi.org/10.1111/trf.14952
53. Lasocki S , Pène F , Ait-Oufella H , Aubron C , Ausset S , Buffet P , et al. Management and prevention of anemia (acute bleeding excluded) in adult critical care patients. Ann Intensive Care. 2020 Jul;10(1):97. https://doi.org/10.1186/s13613-020-00711-6
54. Zha N , Alabousi M , Katz DS , Su J , Patlas M . Factors Affecting Response Rates in Medical Imaging Survey Studies. Acad Radiol. 2020 Mar;27(3):421–7. https://doi.org/10.1016/j.acra.2019.06.005
55. Fontela PC Jr , Forgiarini LA Jr , Friedman G . Clinical attitudes and perceived barriers to early mobilization of critically ill patients in adult intensive care units. Rev Bras Ter Intensiva. 2018 Apr-Jun;30(2):187–94.
56. Eastwood GM , Peck L , Bellomo R , Baldwin I , Reade MC . A questionnaire survey of critical care nurses’ attitudes to delirium assessment before and after introduction of the CAM-ICU. Aust Crit Care. 2012 Aug;25(3):162–9. https://doi.org/10.1016/j.aucc.2012.01.005
57. LeBlanc JM , Kane-Gill SL , Pohlman AS , Herr DL . Multiprofessional survey of protocol use in the intensive care unit. J Crit Care. 2012 Dec;27(6):738.e9–17. https://doi.org/10.1016/j.jcrc.2012.07.012
58. Zhou JJ , Patel SJ , Jia H , Weisenberg SA , Furuya EY , Kubin CJ , et al. Clinicians’ knowledge, attitudes, and practices regarding infections with multidrug-resistant gram-negative bacilli in intensive care units. Infect Control Hosp Epidemiol. 2013 Mar;34(3):274–83. https://doi.org/10.1086/669524
59. de Bruin S , Scheeren TW , Bakker J , van Bruggen R , Vlaar AP , Antonelli M , et al.; Cardiovascular Dynamics Section and Transfusion Guideline Task Force of the ESICM . Transfusion practice in the non-bleeding critically ill: an international online survey-the TRACE survey. Crit Care. 2019 Sep;23(1):309. https://doi.org/10.1186/s13054-019-2591-6
The appendix is available in the pdf version of this article.