New perspectives of biological therapy for severe asthma in adults and adolescents
DOI: https://doi.org/10.4414/SMW.2022.w30176
Chenda
Chheanga, Stéphane
Guinandb, Christophe
von Garnierc, Claudio
Sartoria
aDepartment of Internal Medicine, Lausanne University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
bPediatric pulmonologist, Clinique Générale-Beaulieu, Geneva, Switzerland
cDivision of Pulmonary Medicine, Lausanne University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
Summary
Severe asthma is associated with increased morbidity, mortality, healthcare costs and impaired quality of life. Asthma is no longer considered as a single entity but as a heterogeneous disease with different clinical presentations (phenotypes) and variable underlying mechanistic biological pathways (endotypes). Two different endotypes are based on the inflammatory Type 2 T-helper response: T2-high and T2-low. The understanding of these endotypes has revolutionised the management of severe asthma. Recent guidelines from the 2019 European Respiratory Society/American Thoracic Society (ERS/ATS) and Global Initiative for Asthma (GINA) 2021 specifically address the diagnosis and the management of severe asthma in adults, but less evidence exists for the paediatric population. Presently, five biologics for the treatment of severe asthma are approved, i.e., omalizumab (anti-IgE antibody), mepolizumab and reslizumab (anti-IL-5 antibody), benralizumab (anti-IL-5 receptor antibody) and dupilumab (anti-IL-4 receptor alpha antibody). This article reviews the pathological mechanisms of severe asthma, clinical biomarkers related to the T2-high endotype, and their use for the prediction of the severity of the disease and response to biological therapy. Furthermore, future developments of biologics for severe asthma are presented.
Introduction
Asthma is one of the most common chronic respiratory diseases [1], affecting an estimated 250 million persons worldwide [2]. Despite optimal treatment, severe asthma remains uncontrolled in a minority of asthmatic patients, about 4–10% of adults [3] and 5% of children [4]. The underlying immunopathological mechanisms of asthma cause a heterogeneous chronic airway inflammation, leading to long-term airway remodelling, a process of irreversible structural changes in the bronchial architecture [5]. Modern management of asthma is increasingly taking into account its heterogeneity and complexity, including different phenotypes and endotypes [6]. Biological therapies represent a new era that revolutionises the treatment of severe asthma. Since the introduction of the first monoclonal antibody, omalizumab, an anti-IgE antibody first approved by U.S. Food and Drugs Administration in 2003, an increasing number of new generation of monoclonal antibodies is now available.
Asthma definitions, phenotypes and endotypes
Asthma is characterised by heterogeneous chronic airway inflammation and the presence of respiratory symptoms such as wheezing, shortness of breath, chest tightness and cough, all of which fluctuate both in time and intensity, combined with a variable limitation of the expiratory air flow [7].
According to the Global Initiative for Asthma (GINA), difficult-to-treat asthma is defined as asthma that is uncontrolled despite prescription of medium or high-dose inhaled corticosteroids (ICS) combined with long-acting beta-agonists (LABA), or that requires maintaining oral corticosteroids for symptom control and to reduce the risk of exacerbations.
An estimated 17% of asthmatics suffer from difficult-to-treat asthma, in which poor control is due to factors other than asthma itself, including suboptimal adherence to treatment, incorrect inhaler technique, smoking or comorbidities (gastro-oesophageal reflux, chronic rhino-sinusitis, obesity, obstructive sleep apnoea) [7].
Severe asthma is a subset of difficult-to-treat asthma which fails to improve despite confirmation of the diagnosis and adequate treatment of comorbidities and confounding factors, such as inhaler technique, adherence, risk factors, and triggers [7].
Phenotypes and endotypes of asthma
The classical view of asthma as a single disease entity was recently challenged by an expanded understanding of its underlying pathophysiological mechanisms [8]. The recognition of an intricate biological network of distinct and interrelating inflammatory pathways has led to the identification of different asthma phenotypes and endotypes, in particular the Type 2 T-helper response [6].
Clinical phenotypes are defined as observable characteristics resulting from the combination of genetic and environmental influences [6]. For asthma, the so called “T2-high phenotypes” are classified into three different groups: early-onset eosinophilic allergic asthma, late-onset eosinophilic allergic or non-allergic asthma and aspirin-exacerbated respiratory disease (AERD). On the other hand, “T2-low phenotypes” are associated with clinical characteristics such as obesity, smoking and advanced age [6].
Two major asthma endotypes describe these distinct phenotypes at the cellular and molecular level [6] (figure 1).
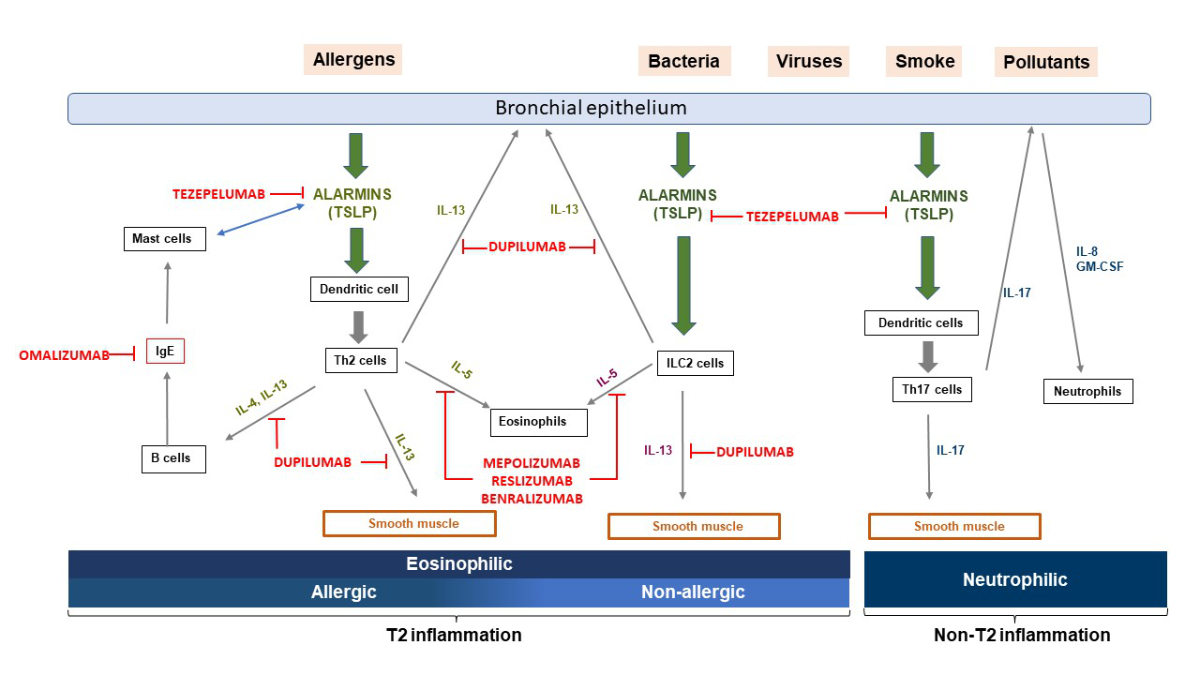
Figure 1 Pathophysiological mechanisms of T2-high asthma.
T-helper cells type 2 (Th2) and group 2 innate lymphoid cells (ILC2) when stimulated by alarmins (IL-25, IL-33, TSLP) released by bronchial epithelial cells in response to stressors, produce T2 inflammatory cytokines such as IL-4, IL-5 and IL-13, leading to IgE switching in B cells, degranulation of mast cells, airway eosinophilia, mucus hypersecretion from goblet cells, and smooth muscle contraction resulting in airway hyper-responsiveness. Alarmins can also directly induce mast cells to produce T2 cytokines, and mast cells themselves can produce significant amounts of TSLP following IgE cross-linking.
Pathophysiological mechanisms of T2-high asthma.
T-helper cells type 2 (Th2) and group 2 innate lymphoid cells (ILC2) when stimulated by alarmins (IL-25, IL-33, TSLP) released by bronchial epithelial cells in response to stressors, produce T2 inflammatory cytokines such as IL-4, IL-5 and IL-13, leading to IgE switching in B cells, degranulation of mast cells, airway eosinophilia, mucus hypersecretion from goblet cells, and smooth muscle contraction resulting in airway hyper-responsiveness. Alarmins can also directly induce mast cells to produce T2 cytokines, and mast cells themselves can produce significant amounts of TSLP following IgE cross-linking.
T2-High asthma endotype (allergic or non-allergic eosinophilic asthma)
T2-high asthma is orchestrated by T-helper cells type 2 (Th2) and group 2 innate lymphoid cells (ILC2). When stimulated by alarmins, epithelial cytokines released from bronchial epithelial cells in response to stressors such as infection or inflammation, these cells generate abundant amounts of cytokines that lead to IgE antibody production with blood and sputum eosinophilia [6].
IL-4 is the predominant cytokine that drives Th2 differentiation and production of downstream cytokines, including IL-5 and IL-13, as well as B cell activation inducing the class switching and secretion of IgE isotype antibodies [6]. IL-4 and IL-13 bind to a common IL-4Rɑ chain, promoting goblet cell overexpression, increased mucus secretion, and airway hyper-responsiveness.
In addition to mediating the immediate hypersensitivity response in allergic asthma via activation and degranulation of mast cells, specific IgE antibodies generate a delayed phase reaction in response to allergen exposure, characterised by influx of eosinophils and other inflammatory cells.
IL-5 plays a central role in promoting the differentiation and maturation of eosinophilic progenitors in the bone marrow, as well as their subsequent migration and survival.
Eosinophils can also activate bronchial fibroblasts through the production of profibrotic factors and are thus associated with remodelling characteristics, including the thickening of the basement membrane [6].
T2-Low or non-T2 asthma endotype (neutrophilic asthma)
T2-low or non-T2 asthma endotype is less frequent than T2-high asthma endotype [9] and includes various asthma phenotypes, related to obesity, smoking, occupational exposures, or advanced age/late onset, defined as >50 years or >65 years, depending on the literature source [10, 11]. Such non-eosinophilic asthma refers to the inflammatory endotype of asthma in which non-T2 cytokines play a role in the physiopathology of the disorder. Importantly, this endotype tends to be more resistant to inhaled corticosteroids and presents with a degree of either neutrophilic or pauci-granulocytic inflammation, orchestrated by a range of immune mechanisms such as Th1 and Th17 cytokines (IL-8, IL-17, IL-22), and the alarmins (thymic stromal lymphopoietin (TSLP), IL-25 and IL-33) [6].
For patients with severe asthma, the understanding of their inflammatory endotypes may help to predict severity of asthma and to select the optimal biological therapy [7, 12].
Use of T2 biomarkers to predict severity of asthma and/or response to therapy
Table 1Clinical features and type 2 inflammatory biomarkers (adapted from Medrek et al. [12]).
|
Biomarker
|
Advantages
|
Disadvantages
|
| Lung function |
Routinely use |
|
| Provide a window into disease control |
| Inverse correlation with blood eosinophils |
| Sputum eosinophils |
Use for guiding inhaled corticosteroid therapy |
Difficult to obtain adequate samples |
| Analysis can be challenging and not universally available |
| Blood eosinophils |
Easy to measure |
Varying cut-offs are used |
| Correlate with sputum eosinophilia and poor asthma control |
Can be elevated due to others causes, such as parasitic infection |
| Predict the recurrence of severe exacerbations |
| FeNO |
Predictive for presence of asthma |
Requires specialised equipment |
| Directly correlated to airway hyper-responsiveness and risk for exacerbations |
Several confounding factors including smoking, atopic, use of anti-inflammatory medications |
| Serum IgE |
Easy to measure |
Various cut-offs are used |
| High levels correlate with severity of asthma |
Not useful for assessment of response to anti-IgE |
| Serum periostin |
Higher level associated with decline in FEV1 |
Can be elevated due to other diseases such as atopic dermatitis, allergic rhinitis, scleroderma, cancer and cardiovascular disease |
| Levels correlate with blood eosinophil counts, |
Not utilised in clinical routine |
| FeNO and serum IgE |
An optimal biomarker should be sensitive, specific, straightforward to assess, and provide relevant information. Several biomarkers have been introduced to identify patients with T2 asthma endotypes, predict the severity of the disease, and evaluate response to specific therapies targeting this pathway [13, 14].
Sputum eosinophils: Differential cell counts in sputum is useful to determine profiles of inflammatory cells, i.e. eosinophilic, neutrophilic, pauci-granulocytic or mixed granulocytic [15]. Sputum eosinophils are considered the “gold standard” T2 biomarker (with threshold value >2%) [6, 7]. Nonetheless, considering the risk to perform induced sputum in uncontrolled asthma and requirement of a skilled cytologist able to prepare samples, this method is only performed in specialised centres [6].
Blood eosinophils represent the most commonly used predictive biomarker for T2-high asthma [16]. High levels (>150/µL) predict the risk of more recurrent severe exacerbations, poor asthma control and response to biologics [17].
Exhaled nitric oxide: Nitric oxide is produced by the nitric oxide synthase 2 (iNOS) in the respiratory epithelial cells where it plays a role as an intracellular messenger, inflammatory mediator and vasodilator [18]. FeNO levels >20 parts per billion (ppb) are predictive of the presence of eosinophilic asthma and its response to biologics [9]. Furthermore, elevated FeNO correlate with airway hyper-responsiveness as well as the risk of exacerbations [19, 20].
Serum IgE are used as a marker of atopy and their serum concentrations correlate with the severity of asthma in both adults and children [21].
Serum Periostin: Periostin is an extracellular matrix protein belonging to the fasciclin family that can be upregulated by the type-2 cytokines IL-4 and IL-13 in human bronchial epithelial cells [22]. Serum periostin is associated with eosinophilic airway inflammation and airway remodelling [23]. Levels ≥95 ng/ml are associated with a decline in forced expiratory volume in one second (FEV1) ≥30 ml per year [24]. Furthermore, serum periostin levels correlate with blood eosinophil counts, FeNO and serum total IgE [25]. Of note, serum periostin is not employed as a biomarker in routine clinical practice.
In addition to clinically validated biomarkers of T2 inflammation in asthmatic patients, recent evidence suggests that RNA transcriptomics, and genetic profiling may improve the assessment of asthma, enabling the prediction of severity and response to treatment [26]. MicroRNAs are small noncoding RNAs that can be measured in peripheral blood and can regulate gene expression at the post-transcriptional level. The expression of selected microRNA appears to be higher in children with severe asthma [27]. MicroRNA are currently being evaluated as possible biomarkers of outcome as well as exacerbation predictors in severe asthma [28].
Similarly, gene expression [29], exhaled breath analysis [30], and lung microbiome [31] are currently being explored as alternative sources of novel biomarkers.
New options based on T2-high endotypes to treat severe asthma
Based on improved understanding of mechanisms underlying T2 asthma phenotypes and endotypes, the treatment of severe asthma has evolved over the past decade with the development of targeted biologic therapies aiming to downregulate the T2 inflammatory cascade. The 2019 European Respiratory Society/American Thoracic Society (ERS/ATS) and GINA 2021 guidelines recommend using of these new biological agents as add-on therapy for severe uncontrolled asthma [7,12]. However, physicians still face challenges to identify patients who are most likely to respond to a specific targeted therapy and to select the best biological molecules in the absence of direct comparisons between them [32]. Furthermore, the choice of biotherapy is not only based on biomarkers and endotypes but also on clinical features of the patients such as the presence of chronic rhinosinusitis with nasal polyposis or eosinophilic granulomatosis with polyangiitis [33].
To date, five monoclonal antibodies are available for treatment of severe T2 asthma [32, 34].
Table 2Main characteristics and effects of existing biologic therapies against severe asthma based on Global Initiative for Asthma 2021 [7] and Swissmedic recommendations.
|
Drug name
|
Omalizumab, XOLAIR
®
|
Mepolizumab, NUCALA
®
|
Reslizumab, CINQAERO
®
|
Benralizumab, FASENRA
®
|
Dupilumab, DUPIXENT
®
|
Tezepelumab, TEZSPIRE
®
|
|
Target
|
IgE |
IL-5 |
IL-5 |
IL-5 receptor a |
IL-4 receptor alpha |
TSLP |
|
Mode of administration
|
Subcutaneous injection |
Subcutaneous injection |
Intravenous injection |
Subcutaneous injection |
Subcutaneous injection |
Subcutaneous injection |
|
Eligibility criteria
|
Sensitization to inhaled allergens on skin prick test or specific IgE |
Blood eosinophil >300 cells/µL or >150 cells/µL at treatment initiation |
Blood eosinophil >400 cells/µL |
Blood eosinophil >300 cells/µL |
Blood eosinophil >150 cells/µL |
Independent from blood eosinophil |
| Exacerbations within last year |
Exacerbations within last year |
Exacerbations within last year |
Exacerbations within last year |
Exacerbations within last year |
T2-low phenotypes? |
| FeNO >25 ppb |
| Maintenance OCS |
|
Other indications
|
Nasal polyposis |
Nasal polyposis |
|
|
Nasal polyposis |
|
| Chronic idiopathic urticaria |
Eosinophilic granulomatosis with polyangiitis |
Moderate-severe atopic dermatitis |
| Hypereosinophilic syndrome |
|
Age indication
|
≥6 years |
≥6 years |
≥18 years |
≥18 years |
≥12 years |
≥12 years |
|
Dosage
|
75–600 mg (based on weight and tot IgE) every 2 or 4 weeks |
100 mg every 4 weeks |
3 mg/kg every 4 weeks |
30 mg every 4 weeks for 3 doses then every 8 weeks |
2 × 300 mg loading dose then 1 × 300 mg every 2 weeks |
210 mg every 4 weeks |
|
Expected outcomes
|
Decreased number of exacerbations |
Decreased number of exacerbations |
Decreased number of exacerbations |
Decreased number of exacerbations |
Decreased number of exacerbations |
Decreased number of exacerbations |
| Improved quality of life |
Improved quality of life |
Improved lung function and quality of life |
Improved lung function and quality of live |
Improved lung function ad quality of live |
Improved lung function and quality of life |
| Minimal effect on FEV1 |
Decrease oral corticosteroid |
Decrease oral corticosteroid |
Decrease oral corticosteroid |
| Minimal to moderate effect on FEV1 |
|
Duration of treatment
|
The GINA 2021 severe asthma guidelines suggest an initial trial of biologic therapy for at least 4 months |
|
Follow up
|
Response to add-on biologic therapy should be assessed after 3-4 months and then every 3-6 months, based on: asthma symptom control, frequency and severity of exacerbations, lung function, type 2 comorbidities, side effects, dose of OCS and patient satisfaction |
Omalizumab
Omalizumab, a humanised recombinant monoclonal anti-IgE antibody, inhibits binding of free serum IgE to the high affinity surface receptor (FcεRI) on mast cells and basophils [35], which reduces the inflammatory response caused by the activation of such effector cells when interacting with the allergen. Omalizumab has also been shown to have a preventative effect on viral-induced exacerbations in children with allergic asthma by reducing susceptibility to rhinovirus infections [36]. Dendritic cells express the FcεRI receptor on their surface and their antiviral activity is inhibited when IgE binds to surface FcεRI [37]. Omalizumab-dependent reduction of FcεRI expression on dendritic cells enhances antiviral immune responses, reducing the frequency of asthma exacerbations [37].
Serum IgE titer is used to calculate the dose of omalizumab [38], but does not allow prediction of efficacy nor monitoring of treatment response [39]. Peripheral blood eosinophil levels ≥300 cells/µL are associated with an improved response to omalizumab by reducing exacerbations [40].
Several trials have demonstrated that omalizumab significantly decreases the number of severe exacerbations, the dosage of inhaled and oral corticosteroid, and improves patients’ quality of life [41,42]. The current 2019 ERS/ATS and GINA guidelines 2021 recommend this monoclonal antibody as an add-on therapy in adolescents and adults with severe allergic asthma with high blood eosinophil counts ≥260 cells/µL or elevated FeNO ≥20 ppb [7,12].
Mepolizumab
Mepolizumab, a human monoclonal antibody directed against IL-5 (anti-IL5), reduces eosinophils and eosinophils precursors in the bone marrow and bronchial mucosa [43]. Several randomised controlled trials in adults and adolescents with severe asthma have shown the efficacy of mepolizumab in reducing blood eosinophilia. This, in turn, lowers the rate of severe exacerbations and the usage of oral corticosteroid while improving asthma controls and increasing lung function [41, 44]. Inclusion in such trials was allowed for patients who met one of the following criteria: patients with blood eosinophil levels ≥150 cells/µL at the time of inclusion, patients on oral corticosteroid, those with blood eosinophils ≥300 cells/µL in the preceding year, or who had had severe recurrent exacerbations in the previous year despite regular use of high-dose inhaled corticosteroid associated with another controller (ICS-LABA). High blood eosinophil count is used as a predictive biomarker of response to mepolizumab [44, 45].
Reslizumab
Reslizumab is a humanised monoclonal antibody anti-IL5, resulting in a reduction of sputum eosinophils and blood eosinophils and, in turn, reduction of exacerbations and asthma symptoms and improved lung function [41,46]. Reslizumab is recommended by the 2019 ERS/ATS and GINA 2021 as an add-on therapy in adults with severe eosinophilic asthma, ≥400 blood eosinophils/µL and a history of asthma exacerbations in previous year [7,12].
Benralizumab
Benralizumab, a monoclonal antibody of murine origin that binds the alpha chain of the IL-5 receptor (anti-IL5R) leading to antibody-dependent cell-mediated cytotoxicity and almost complete depletion of eosinophils in the bone marrow, blood and peripheral tissues [47]. Benralizumab reduces administration of oral corticoids, decreases the number of exacerbation and improves both asthma-related quality of life and lung function [41, 48, 49]. Benralizumab is recommended as an add-on therapy in adults and adolescents with severe uncontrolled eosinophilic asthma who have ≥300 blood eosinophils/µL and for those with severe corticosteroid-dependent asthma with at least 2 severe exacerbations in previous year [7, 12].
Dupilumab
The most recently approved biologic, dupilumab, is a fully human monoclonal antibody that binds to the alpha subunit of the IL-4 receptor (mutual to IL-4 and IL-13 receptors), thereby inhibiting both the IL-4 and IL-13 pathway [50]. In patients with severe asthma, dupilumab reduces severe exacerbations and use of oral corticosteroids. In addition, it significantly improves quality of life, symptom controls and lung function [41, 51, 52]. The GINA 2021 guidelines recommend dupilumab as an add-on option for patients with T2-high severe uncontrolled asthma with blood eosinophil level ≥150 cells/µL or FeNO ≥25 ppb or requiring maintenance oral corticosteroids [7]. In contrast, the 2019 ERS/ATS proposes dupilumab as add-on therapy for adult patients with severe eosinophilic asthma and for those severe corticosteroid-dependent asthma regardless of blood eosinophilic counts [12].
The two concerns regarding long term use of biologic therapies are cost and safety. Despite being relatively expensive, the use of these biologics appear to be cost-effective [48]. Efficacy and safety of long term use of biologic treatments are extended up to 148 weeks in dupilumab [53], 156 weeks in mepolizumab [54] and up to 5 years in benralizumab [55].
Future developments of biologics for severe asthma management
Currently, research for next-generation biologics is underway. TSLP, IL-33 and IL-25 are alarmins expressed by airway epithelial cells that have been associated with pathogenesis of asthma and disease severity. These may, therefore, present novel targets for biological therapies in severe asthma [56].
Thymic stromal lymphopoietin (TSLP)
TSLP is produced upstream in the inflammatory cascade by activated lung epithelial cells in response to various environmental insults including viruses, bacteria, fungal products, allergens, chemical irritants and physical injury [57]. In contrast to IL-4, IL-5 or IL-13, TSLP may affect disease activity more widely than a single cytokine acting downstream in the inflammatory cascade [58]. It has been demonstrated that TSLP acts predominantly on dendritic cells, leading to an increase in the T2 inflammatory response (IL-5, IL-4 and IL-13) [59].
Tezepelumab is a fully human monoclonal antibody that binds directly to TSLP receptor (TSLP-R), reducing its stimulating activity on dendritic cells in response to TSLP [60].
Recent double-blind, randomised trials demonstrated a reduction in asthma exacerbations, improved lung function and asthma-related quality of life in patients receiving tezepelumab versus placebo [61, 62] with an adequate safety and tolerability profile in adults with severe, uncontrolled asthma [63, 64]. This molecule has been approved on 17 December 2021 by the FDA for clinical use in the United States [65].
Other targeted therapy
Several trials are underway to evaluate other biologic drugs targeting IL-33 or its ST2 receptor, which synergises with TSLP in promoting type-2 immune/inflammatory responses and induces airway hyper-responsiveness via the release of IL-13 from ILC2 and mast cells.
Itepekimab, a new human monoclonal antibody against interleukin-33, shows the efficacy in improving asthma control, quality of life and lung function in patients with moderate-to-severe asthma in a phase 2 randomised controlled trial [66].
Interleukin-23 has been associated with pathogenesis of asthma by promoting Th2 cytokine production and eosinophil infiltration [67]. Risankizumab, an anti-interleukine-23p19 antibody, failed to show efficacy in reducing asthma exacerbation compared to placebo in adults with severe asthma [68].
In asthma, expression of the prostaglandin D2 receptor 2 (DP2 receptor) is increased in the bronchial submucosa, and its ligand, prostaglandin D2 is elevated in bronchoalveolar lavage. DP2 receptor stimulation by prostaglandin D2 mediates the activation and migration of some of the key inflammatory cell types in asthma, including T-helper-2 (Th2) cells, type 2 innate lymphoid cells, basophils, and eosinophils. It also stimulates type 2 cytokine release from these cells making it a potential new target for the treatment of asthma.
Fevipiprant, an oral antagonist of the prostaglandin D2 receptor 2, did not show results superior to placebo in the reduction of asthma exacerbations, improvement in lung function or other asthma-related clinical outcomes in recent randomized controlled trials [69].
Management of T2-Low and/or non-T2 asthma endotype
Compared to T2-high asthma, the underlying pathophysiological mechanisms in T2-low and/or non-T2 asthma are not completely understood. Presently, the suggested management involves lifestyle modifications such as smoking cessation and weight loss, as well as cessation of short-acting B-agonist use and utilisation of low-dose corticosteroid, long-acting muscarinic antagonists (LAMA), macrolides, and possibly bronchial thermoplasty [70].
Currently, no biological is approved for T2-low and/or non-T2 asthma, but several biological agents targeting IL-17 and other pathways are under investigation [70–75]. Recent RCT studies [76,77] suggested the efficacy of tezepelumab in reducing asthma exacerbations, independently of baseline bloods eosinophil counts (<250 cells/µL vs >250 cells/µL) and Th2 status (IgE >100 IU ml or less). It is therefore possible to speculate that, based on its wide upstream anti-inflammatory effects, tezepelumab may reduce asthma exacerbations equally in patients with different asthma phenotypes, including the T2-low asthma [60].
Management of severe asthma in children and adolescents: differences from adult guidelines
Asthma is still the most common chronic inflammatory airways disease in children and severe childhood asthma is associated with high morbidity and mortality. It thus represents a challenge for these children and adolescents, their families and the health care system [4].
Current guidelines provide appropriate management for mild to moderate asthma in the paediatric population but there is still a significant lack of research concerning the management in children and adolescent with severe asthma [7, 12].
Macrolides decrease the requirement for corticosteroids, improve asthma symptoms and reduce the rate of exacerbations in adults with severe asthma [78]. However, very few RCTs studied the role of macrolides in children and adolescents with asthma and failed to prove efficacy, most likely because of being insufficiently powered [79]. Therefore, thecurrent guidelines recommend against the utilisation of macrolides in the paediatric population with severe asthma [7, 12].
Children and adolescents present higher level of periostin than adults due to bone growth during puberty. Clinicians should therefore be cautious in interpreting serum periostin as a marker of asthma in children because normal ranges fluctuate among different age groups [80].
Presently, no evidence-based guidelines exist for the use of biologic therapy in children with severe uncontrolled asthma. Omalizumab and mepolizumab are approved for children ≥6 years old with moderate to severe asthma [7, 12].
Several randomised trials focused on the efficacy of benralizumab, reslizumab or dupilumab in severe T2-high asthma in 12 to 17 year olds. However, larger randomised controlled trials evaluating the safety and efficacy in children and adolescents with severe uncontrolled asthma are required to support specific evidence-based guidelines.
Conclusions
Severe asthma encompasses a small percentage of all patients with asthma, but represents an important disease burden with high mortality, morbidity, and costs. In the recent years, a major improvement in our understanding of underlying pathophysiological mechanisms, phenotypes and endotypes has revolutionised management of severe asthma with the development of specific biologic therapies.
T2-high asthma represents the majority of cases of severe uncontrolled asthma in adults and adolescents. It involves the activation of type 2 cytokines IL-4, IL-5, IL-13 and epithelial alarmins, such as TSLP, cytokines released by bronchial epithelial cells when exposed to various stimuli such as virus, bacteria, fungal products, smoke, and chemical environment.
Several randomised controlled trials have shown the efficacy and safety of new specific biological drugs targeting this T2-mediated inflammation in reducing asthma exacerbations, improving asthma related quality of life, symptom control and lung function. Based on the results of the NAVIGATOR study [63], tezepelumab is undergoing the approval process in Switzerland for treating T2-high and T2-low severe asthma. Moreover, other biotherapies against alarmins are in phase II trials [77, 81]. To date, there have been no head-to-head comparison of different biologics and trials have primarily focused on adult populations with insufficient data available in paediatric populations.
While it appears important to consider patients’ characteristics, predictive biomarkers, phenotypes and endotypes to improve management of severe asthma, future research is crucial to understand pathophysiological mechanisms, particularly in T2-low and non-T2 asthma. More research is also needed to provide evidence-based strategies for novel therapeutic approaches in all age groups.
Chenda Chheang
Eden-Roc 11
CH-1073 Savigny
chenda.chheang[at]hotmail.com
References
1.
To T
,
Stanojevic S
,
Moores G
,
Gershon AS
,
Bateman ED
,
Cruz AA
, et al.
Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012 Mar;12(1):204. https://doi.org/10.1186/1471-2458-12-204
2.
Vos T
,
Lim SS
,
Abbafati C
,
Abbas KM
,
Abbasi M
,
Abbasifard M
, et al.; GBD 2019 Diseases and Injuries Collaborators
. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct;396(10258):1204–22. https://doi.org/10.1016/S0140-6736(20)30925-9
3.
Hekking PW
,
Wener RR
,
Amelink M
,
Zwinderman AH
,
Bouvy ML
,
Bel EH
. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015 Apr;135(4):896–902. https://doi.org/10.1016/j.jaci.2014.08.042
4.
Ahmed H
,
Turner S
. Severe asthma in children-a review of definitions, epidemiology, and treatment options in 2019. Pediatr Pulmonol. 2019 Jun;54(6):778–87. https://doi.org/10.1002/ppul.24317
5.
Boulet LP
. Airway remodeling in asthma: update on mechanisms and therapeutic approaches. Curr Opin Pulm Med. 2018 Jan;24(1):56–62. https://doi.org/10.1097/MCP.0000000000000441
6.
Kuruvilla ME
,
Lee FE
,
Lee GB
. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin Rev Allergy Immunol. 2019 Apr;56(2):219–33. https://doi.org/10.1007/s12016-018-8712-1
7.
Global Initiative for Asthma
. 2021 GINA Main Report, global strategy for asthma management and prevention (2021 update). (https://ginasthma.org/gina-reports)
8.
Agache I
,
Eguiluz-Gracia I
,
Cojanu C
,
Laculiceanu A
,
Del Giacco S
,
Zemelka-Wiacek M
, et al.
Advances and highlights in asthma in 2021. Allergy. 2021 Nov;76(11):3390–407. https://doi.org/10.1111/all.15054
9.
Carr TF
,
Zeki AA
,
Kraft M
. Eosinophilic and Noneosinophilic Asthma. Am J Respir Crit Care Med. 2018 Jan;197(1):22–37. https://doi.org/10.1164/rccm.201611-2232PP
10.
Pite H
,
Pereira AM
,
Morais-Almeida M
,
Nunes C
,
Bousquet J
,
Fonseca JA
. Prevalence of asthma and its association with rhinitis in the elderly. Respir Med. 2014 Aug;108(8):1117–26. https://doi.org/10.1016/j.rmed.2014.05.002
11.
Nanda A
,
Baptist AP
,
Divekar R
,
Parikh N
,
Seggev JS
,
Yusin JS
, et al.
Asthma in the older adult. J Asthma. 2020 Mar;57(3):241–52. https://doi.org/10.1080/02770903.2019.1565828
12.
Holguin F
,
Cardet JC
,
Chung KF
,
Diver S
,
Ferreira DS
,
Fitzpatrick A
, et al.
Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020 Jan;55(1):1900588. https://doi.org/10.1183/13993003.00588-2019
13.
Parulekar AD
,
Diamant Z
,
Hanania NA
. Role of T2 inflammation biomarkers in severe asthma. Curr Opin Pulm Med. 2016 Jan;22(1):59–68. https://doi.org/10.1097/MCP.0000000000000231
14.
Medrek SK
,
Parulekar AD
,
Hanania NA
. Predictive Biomarkers for Asthma Therapy. Curr Allergy Asthma Rep. 2017 Sep;17(10):69. https://doi.org/10.1007/s11882-017-0739-5
15.
Simpson JL
,
Scott R
,
Boyle MJ
,
Gibson PG
. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006 Jan;11(1):54–61. https://doi.org/10.1111/j.1440-1843.2006.00784.x
16.
Breiteneder H
,
Peng YQ
,
Agache I
,
Diamant Z
,
Eiwegger T
,
Fokkens WJ
, et al.
Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. 2020 Dec;75(12):3039–68. https://doi.org/10.1111/all.14582
17.
Nair P
,
O’Byrne PM
. Measuring Eosinophils to Make Treatment Decisions in Asthma. Chest. 2016 Sep;150(3):485–7. https://doi.org/10.1016/j.chest.2016.07.009
18.
Lane C
,
Knight D
,
Burgess S
,
Franklin P
,
Horak F
,
Legg J
, et al.
Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004 Sep;59(9):757–60. https://doi.org/10.1136/thx.2003.014894
19.
Petsky HL
,
Kew KM
,
Turner C
,
Chang AB
. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016 Sep;9(9):CD011440. https://doi.org/10.1002/14651858.CD011440.pub2
20.
Busse WW
,
Wenzel SE
,
Casale TB
,
FitzGerald JM
,
Rice MS
,
Daizadeh N
, et al.
Baseline FeNO as a prognostic biomarker for subsequent severe asthma exacerbations in patients with uncontrolled, moderate-to-severe asthma receiving placebo in the LIBERTY ASTHMA QUEST study: a post-hoc analysis. Lancet Respir Med. 2021 Oct;9(10):1165–73. https://doi.org/10.1016/S2213-2600(21)00124-7
21.
Gevaert P
,
Wong K
,
Millette LA
,
Carr TF
. The Role of IgE in Upper and Lower Airway Disease: More Than Just Allergy! Clin Rev Allergy Immunol. 2022 Feb;62(1):200–15. https://doi.org/10.1007/s12016-021-08901-1
22.
Izuhara K
,
Conway SJ
,
Moore BB
,
Matsumoto H
,
Holweg CT
,
Matthews JG
, et al.
Roles of Periostin in Respiratory Disorders. Am J Respir Crit Care Med. 2016 May;193(9):949–56. https://doi.org/10.1164/rccm.201510-2032PP
23.
Sidhu SS
,
Yuan S
,
Innes AL
,
Kerr S
,
Woodruff PG
,
Hou L
, et al.
Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010 Aug;107(32):14170–5. https://doi.org/10.1073/pnas.1009426107
24.
Kanemitsu Y
,
Matsumoto H
,
Izuhara K
,
Tohda Y
,
Kita H
,
Horiguchi T
, et al.
Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013 Aug;132(2):305–12.e3. https://doi.org/10.1016/j.jaci.2013.04.050
25.
Izuhara K
,
Nunomura S
,
Nanri Y
,
Ono J
,
Takai M
,
Kawaguchi A
. Periostin: an emerging biomarker for allergic diseases. Allergy. 2019 Nov;74(11):2116–28. https://doi.org/10.1111/all.13814
26.
Kuo CS
,
Pavlidis S
,
Loza M
,
Baribaud F
,
Rowe A
,
Pandis I
, et al.; U-BIOPRED Project Team ‡
. A Transcriptome-driven Analysis of Epithelial Brushings and Bronchial Biopsies to Define Asthma Phenotypes in U-BIOPRED. Am J Respir Crit Care Med. 2017 Feb;195(4):443–55. https://doi.org/10.1164/rccm.201512-2452OC
27.
Midyat L
,
Gulen F
,
Karaca E
,
Ozkinay F
,
Tanac R
,
Demir E
, et al.
MicroRNA expression profiling in children with different asthma phenotypes. Pediatr Pulmonol. 2016 Jun;51(6):582–7. https://doi.org/10.1002/ppul.23331
28.
Heffler E
,
Allegra A
,
Pioggia G
,
Picardi G
,
Musolino C
,
Gangemi S
. MicroRNA Profiling in Asthma: Potential Biomarkers and Therapeutic Targets. Am J Respir Cell Mol Biol. 2017 Dec;57(6):642–50. https://doi.org/10.1165/rcmb.2016-0231TR
29.
Frøssing L
,
Kjærsgaard Klein D
,
Backer V
,
Baines KJ
,
Porsbjerg C
. The six-gene expression signature in whole sampled sputum provides clinically feasible inflammatory phenotyping of asthma. ERJ Open Res. 2020 Mar;6(1):00280–02019. https://doi.org/10.1183/23120541.00280-2019
30.
Aldakheel FM
,
Thomas PS
,
Bourke JE
,
Matheson MC
,
Dharmage SC
,
Lowe AJ
. Relationships between adult asthma and oxidative stress markers and pH in exhaled breath condensate: a systematic review. Allergy. 2016 Jun;71(6):741–57. https://doi.org/10.1111/all.12865
31.
Shukla SD
,
Shastri MD
,
Chong WC
,
Dua K
,
Budden KF
,
Mahmood MQ
, et al.
Microbiome-focused asthma management strategies. Curr Opin Pharmacol. 2019 Jun;46:143–9. https://doi.org/10.1016/j.coph.2019.06.003
32.
Dragonieri S
,
Carpagnano GE
. Biological therapy for severe asthma. Asthma Res Pract. 2021 Aug;7(1):12. https://doi.org/10.1186/s40733-021-00078-w
33.
Porsbjerg C
,
Menzies-Gow A
. Co-morbidities in severe asthma: clinical impact and management. Respirology. 2017 May;22(4):651–61. https://doi.org/10.1111/resp.13026
34.
Brusselle GG
,
Koppelman GH
. Biologic Therapies for Severe Asthma. N Engl J Med. 2022 Jan;386(2):157–71. https://doi.org/10.1056/NEJMra2032506
35.
Kawakami T
,
Blank U.
From IgE to Omalizumab. J Immunol Baltim Md 1950. 2016 Dec 1;197(11):4187–92.
36.
Esquivel A
,
Busse WW
,
Calatroni A
,
Togias AG
,
Grindle KG
,
Bochkov YA
, et al.
Effects of Omalizumab on Rhinovirus Infections, Illnesses, and Exacerbations of Asthma. Am J Respir Crit Care Med. 2017 Oct;196(8):985–92. https://doi.org/10.1164/rccm.201701-0120OC
37.
Avila PC
. Does anti-IgE therapy help in asthma? Efficacy and controversies. Annu Rev Med. 2007;58(1):185–203. https://doi.org/10.1146/annurev.med.58.061705.145252
38.
Licari A
,
Marseglia A
,
Caimmi S
,
Castagnoli R
,
Foiadelli T
,
Barberi S
, et al.
Omalizumab in children. Paediatr Drugs. 2014 Dec;16(6):491–502. https://doi.org/10.1007/s40272-014-0107-z
39.
Tabatabaian F
,
Ledford DK
. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy. 2018 Apr;11:53–61. https://doi.org/10.2147/JAA.S107982
40.
Casale TB
,
Chipps BE
,
Rosén K
,
Trzaskoma B
,
Haselkorn T
,
Omachi TA
, et al.
Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018 Feb;73(2):490–7. https://doi.org/10.1111/all.13302
41.
Agache I
,
Beltran J
,
Akdis C
,
Akdis M
,
Canelo-Aybar C
,
Canonica GW
, et al.
Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020 May;75(5):1023–42. https://doi.org/10.1111/all.14221
42.
Bousquet J
,
Humbert M
,
Gibson PG
,
Kostikas K
,
Jaumont X
,
Pfister P
, et al.
Real-World Effectiveness of Omalizumab in Severe Allergic Asthma: A Meta-Analysis of Observational Studies. J Allergy Clin Immunol Pract. 2021 Jul;9(7):2702–14. https://doi.org/10.1016/j.jaip.2021.01.011
43.
Menzies-Gow A
,
Flood-Page P
,
Sehmi R
,
Burman J
,
Hamid Q
,
Robinson DS
, et al.
Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003 Apr;111(4):714–9. https://doi.org/10.1067/mai.2003.1382
44.
Harrison T
,
Canonica GW
,
Chupp G
,
Lee J
,
Schleich F
,
Welte T
, et al.
Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J. 2020 Oct;56(4):2000151. https://doi.org/10.1183/13993003.00151-2020
45.
Ortega HG
,
Yancey SW
,
Mayer B
,
Gunsoy NB
,
Keene ON
,
Bleecker ER
, et al.
Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016 Jul;4(7):549–56. https://doi.org/10.1016/S2213-2600(16)30031-5
46.
Ibrahim H
,
O’Sullivan R
,
Casey D
,
Murphy J
,
MacSharry J
,
Plant BJ
, et al.
The effectiveness of Reslizumab in severe asthma treatment: a real-world experience. Respir Res. 2019 Dec;20(1):289. https://doi.org/10.1186/s12931-019-1251-3
47.
FitzGerald JM
,
Bleecker ER
,
Nair P
,
Korn S
,
Ohta K
,
Lommatzsch M
, et al.; CALIMA study investigators
. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016 Oct;388(10056):2128–41. https://doi.org/10.1016/S0140-6736(16)31322-8
48.
Padilla-Galo A
,
García-Ruiz AJ
,
Levy Abitbol RC
,
Olveira C
,
Rivas-Ruiz F
,
García-Agua Soler N
, et al.
Real-life cost-effectiveness of benralizumab in patients with severe asthma. Respir Res. 2021 May;22(1):163. https://doi.org/10.1186/s12931-021-01758-0
49.
Kavanagh JE
,
Hearn AP
,
Dhariwal J
,
d’Ancona G
,
Douiri A
,
Roxas C
, et al.
Real-World Effectiveness of Benralizumab in Severe Eosinophilic Asthma. Chest. 2021 Feb;159(2):496–506. https://doi.org/10.1016/j.chest.2020.08.2083
50.
Santini G
,
Mores N
,
Malerba M
,
Mondino C
,
Anzivino R
,
Macis G
, et al.
Dupilumab for the treatment of asthma. Expert Opin Investig Drugs. 2017 Mar;26(3):357–66. https://doi.org/10.1080/13543784.2017.1282458
51.
Campisi R
,
Crimi C
,
Nolasco S
,
Beghè B
,
Antonicelli L
,
Guarnieri G
, et al.
Real-World Experience with Dupilumab in Severe Asthma: One-Year Data from an Italian Named Patient Program. J Asthma Allergy. 2021 May;14:575–83. https://doi.org/10.2147/JAA.S312123
52.
Castro M
,
Corren J
,
Pavord ID
,
Maspero J
,
Wenzel S
,
Rabe KF
, et al.
Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018 Jun;378(26):2486–96. https://doi.org/10.1056/NEJMoa1804092
53.
Wechsler ME
,
Ford LB
,
Maspero JF
,
Pavord ID
,
Papi A
,
Bourdin A
, et al.
Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med. 2022 Jan;10(1):11–25. https://doi.org/10.1016/S2213-2600(21)00322-2
54.
Khatri S
,
Moore W
,
Gibson PG
,
Leigh R
,
Bourdin A
,
Maspero J
, et al.
Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019 May;143(5):1742–1751.e7. https://doi.org/10.1016/j.jaci.2018.09.033
55.
Korn S
,
Bourdin A
,
Chupp G
,
Cosio BG
,
Arbetter D
,
Shah M
, et al.
Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021 Dec;9(12):4381–4392.e4. https://doi.org/10.1016/j.jaip.2021.07.058
56.
Gauvreau GM
,
Sehmi R
,
Ambrose CS
,
Griffiths JM
. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020 Aug;24(8):777–92. https://doi.org/10.1080/14728222.2020.1783242
57.
West EE
,
Kashyap M
,
Leonard WJ
. TSLP: A Key Regulator of Asthma Pathogenesis. Drug Discov Today Dis Mech. 2012 Dec;9(3-4):e83–8. https://doi.org/10.1016/j.ddmec.2012.09.003
58.
Varricchi G
,
Pecoraro A
,
Marone G
,
Criscuolo G
,
Spadaro G
,
Genovese A
, et al.
Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front Immunol. 2018 Jul;9:1595. https://doi.org/10.3389/fimmu.2018.01595
59.
Watanabe N
,
Hanabuchi S
,
Soumelis V
,
Yuan W
,
Ho S
,
de Waal Malefyt R
, et al.
Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004 Apr;5(4):426–34. https://doi.org/10.1038/ni1048
60.
Pelaia C
,
Pelaia G
,
Crimi C
,
Maglio A
,
Gallelli L
,
Terracciano R
, et al.
Tezepelumab: A Potential New Biological Therapy for Severe Refractory Asthma. Int J Mol Sci. 2021 Apr;22(9):4369. https://doi.org/10.3390/ijms22094369
61.
Corren J
,
Garcia Gil E
,
Griffiths JM
,
Parnes JR
,
van der Merwe R
,
Sałapa K
, et al.
Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol. 2021 Feb;126(2):187–93. https://doi.org/10.1016/j.anai.2020.10.008
62.
Corren J
,
Karpefors M
,
Hellqvist Å
,
Parnes JR
,
Colice G
. Tezepelumab Reduces Exacerbations Across All Seasons in Patients with Severe, Uncontrolled Asthma: A Post Hoc Analysis of the PATHWAY Phase 2b Study. J Asthma Allergy. 2021 Jan;14:1–11. https://doi.org/10.2147/JAA.S286036
63.
Menzies-Gow A
,
Colice G
,
Griffiths JM
,
Almqvist G
,
Ponnarambil S
,
Kaur P
, et al.
NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020 Oct;21(1):266. https://doi.org/10.1186/s12931-020-01526-6
64.
Menzies-Gow A
,
Ponnarambil S
,
Downie J
,
Bowen K
,
Hellqvist Å
,
Colice G
. DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020 Oct;21(1):279. https://doi.org/10.1186/s12931-020-01541-7
65. FDA approves maintenance treatment for severe asthma. FDA. 2021 Dec 20. (https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-maintenance-treatment-severe-asthma)
66.
Wechsler ME
,
Ruddy MK
,
Pavord ID
,
Israel E
,
Rabe KF
,
Ford LB
, et al.
Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N Engl J Med. 2021 Oct;385(18):1656–68. https://doi.org/10.1056/NEJMoa2024257
67.
Li Y
,
Hua S
. Mechanisms of pathogenesis in allergic asthma: role of interleukin-23. Respirology. 2014 Jul;19(5):663–9. https://doi.org/10.1111/resp.12299
68.
Brightling CE
,
Nair P
,
Cousins DJ
,
Louis R
,
Singh D
. Risankizumab in Severe Asthma - A Phase 2a, Placebo-Controlled Trial. N Engl J Med. 2021 Oct;385(18):1669–79. https://doi.org/10.1056/NEJMoa2030880
69.
Brightling CE
,
Gaga M
,
Inoue H
,
Li J
,
Maspero J
,
Wenzel S
, et al.
Effectiveness of fevipiprant in reducing exacerbations in patients with severe asthma (LUSTER-1 and LUSTER-2): two phase 3 randomised controlled trials. Lancet Respir Med. 2021 Jan;9(1):43–56. https://doi.org/10.1016/S2213-2600(20)30412-4
70.
Hinks TS
,
Levine SJ
,
Brusselle GG
. Treatment options in type-2 low asthma. Eur Respir J. 2021 Jan;57(1):2000528. https://doi.org/10.1183/13993003.00528-2020
71. Teva Branded Pharmaceutical Products R&D, Inc. A 16-Week, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Proof-of-Concept Study to Evaluate the Efficacy and Safety of TEV-48574 in Adults With T2-low/Non-T2 Severe Uncontrolled Asthma. clinicaltrials.gov; 2021 Dec. Report No.: NCT04545385. (https://clinicaltrials.gov/ct2/show/NCT04545385)
72.
University of California
. Davis. Phosphodiesterase 4 Inhibitor, Roflumilast, Improves Beta Agonist Responsiveness Compared to Placebo in Low T2 Asthma Patients. clinicaltrials.gov; 2021 May. Report No.: NCT04108377. (https://clinicaltrials.gov/ct2/show/NCT04108377)
73.
Novartis Pharmaceuticals
. A Randomized, Subject- and Investigator-blinded, Placebo Controlled, Multi-center, Multiple Dose Study to Assess the Efficacy and Safety of CJM112 in Patients With Inadequately Controlled Moderate to Severe Asthma. clinicaltrials.gov; 2021 Oct. Report No.: NCT03299686. (https://clinicaltrials.gov/ct2/show/NCT03299686)
74.
Kyriakopoulos C
,
Gogali A
,
Bartziokas K
,
Kostikas K
. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021 Jun;7(2):00309–02020. https://doi.org/10.1183/23120541.00309-2020
75.
Samitas K
,
Zervas E
,
Gaga M
. T2-low asthma: current approach to diagnosis and therapy. Curr Opin Pulm Med. 2017 Jan;23(1):48–55. https://doi.org/10.1097/MCP.0000000000000342
76.
Corren J
,
Parnes JR
,
Wang L
,
Mo M
,
Roseti SL
,
Griffiths JM
, et al.
Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med. 2017 Sep;377(10):936–46. https://doi.org/10.1056/NEJMoa1704064
77.
Menzies-Gow A
,
Corren J
,
Bourdin A
,
Chupp G
,
Israel E
,
Wechsler ME
, et al.
Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med. 2021 May;384(19):1800–9. https://doi.org/10.1056/NEJMoa2034975
78.
Wong EH
,
Porter JD
,
Edwards MR
,
Johnston SL
. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014 Aug;2(8):657–70. https://doi.org/10.1016/S2213-2600(14)70107-9
79.
Pincheira MA
,
Bacharier LB
,
Castro-Rodriguez JA
. Efficacy of Macrolides on Acute Asthma or Wheezing Exacerbations in Children with Recurrent Wheezing: A Systematic Review and Meta-analysis. Paediatr Drugs. 2020 Apr;22(2):217–28. https://doi.org/10.1007/s40272-019-00371-5
80.
Inoue T
,
Akashi K
,
Watanabe M
,
Ikeda Y
,
Ashizuka S
,
Motoki T
, et al.
Periostin as a biomarker for the diagnosis of pediatric asthma. Pediatr Allergy Immunol. 2016 Aug;27(5):521–6. https://doi.org/10.1111/pai.12575
81.
Porsbjerg CM
,
Sverrild A
,
Lloyd CM
,
Menzies-Gow AN
,
Bel EH
. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J. 2020 Nov;56(5):2000260. https://doi.org/10.1183/13993003.00260-2020
