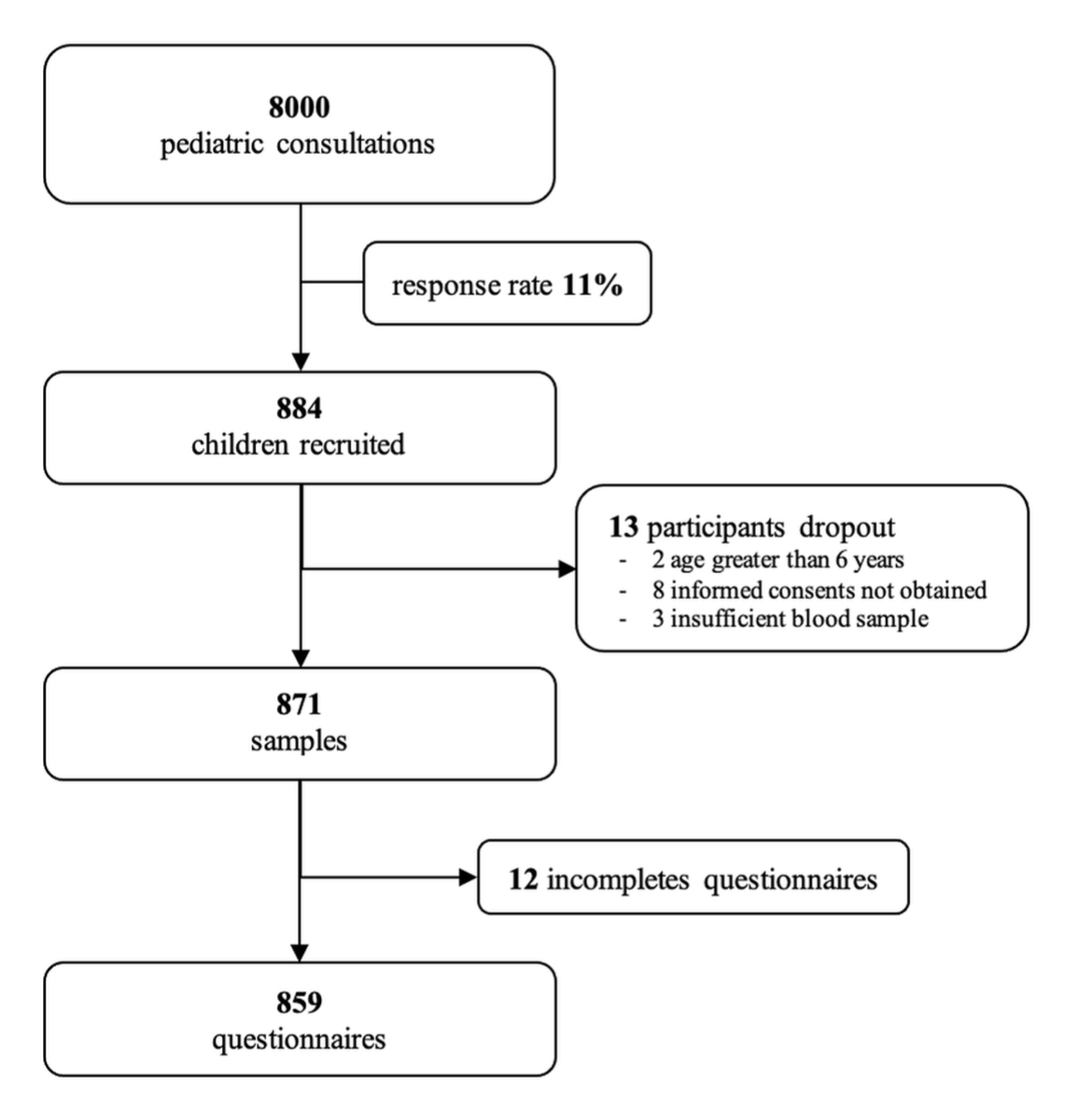
Figure 1 Recruitment of participants.
DOI: https://doi.org/10.4414/SMW.2022.w30173
During the second wave of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, Switzerland had one of the highest incidences of infections in Europe. This created an ideal environment to study SARS-CoV-2 infections in children. In March 2021, approximately one year after the first coronavirus disease 19 (COVID-19) case in Switzerland, only 10% of the total polymerase chain reaction (PCR)-confirmed infections occurred among children and adolescents less than 19 years of age and 1% among children less than 9 years of age [1]. However, since a large proportion of children infected with SARS-CoV-2 remain asymptomatic or mildly symptomatic and are therefore not tested [2–4], this likely underestimates the true rate of infection in this age group. Few data are available about the rate of infections in young children, as they are rarely studied separately from older children.
Recent studies suggest that children might be infected with SARS-CoV-2 as frequently as adults [3, 5] and have similar viral loads [6, 7]. Nonetheless, children seem to transmit the virus less and respiratory tract viral shedding is shorter [5]. The role of children in transmission of SARS-CoV-2 is an important discussion point in determining if day-care facilities and schools can remain open. In Switzerland, schools were closed from 16 March to 11 May 2020 [8]. During the same time period, childcare facilities were open only to children of parents with essential jobs [9, 10]. On 11May 2020 schools and childcare facilities re-opened with protective measures [11, 12]. This decision was based on data that showed that schools had a limited role in the transmission of SARS-CoV-2 [5, 13–17]. However, data on transmission in childcare facilities are lacking.
Household transmission plays a major role in the spread of SARS-CoV-2 [2, 16, 18, 19]. Within households, children are less frequently the index case, but there are few data comparing the risk of transmission between children less than 6 years, as they are rarely studied separately from older children [20–23].
The aim of the COVPED study was to assess the SARS-CoV-2 seroprevalence in children less than 6 years of age in the canton of Fribourg, Switzerland and to identify risk factors associated with seropositivity.
The COVPED study was designed as a population-based cross-sectional study in children less than 6 years of age living in the canton of Fribourg, Switzerland. Children were recruited during a 9-week period from 11 January to 14 March 2021, during the second wave of the pandemic.
Of 42 private paediatricians working in the canton, 34 recruited children for the study in 13 different towns. Additionally, children were recruited from the paediatric emergency department of the Fribourg Hospital. Inclusion criteria were (a) consultation at any of the participating sites regardless of the purpose or complaint, (b) age less than 6 years, (c) primary residence in the canton of Fribourg, (d) no previous participation in the study. The exclusion criterion was no language knowledge in German or French. All children full filling the inclusion criteria were offered participation, including healthy and sick children (e.g., consultation for developmental check-ups, medical and surgical conditions).
Epidemiological data, including childcare arrangements, was collected through a standardised online questionnaire via the secure web platform REDCap (version 10.0.6 – Vanderbilt University) filled in by parents / legal guardians in either French or German. Childcare arrangements were defined as "intra-familial care" when children were being looked after by parents and as "extra-familial care" when children were being looked after by grandparents, other family members, friends, neighbours, a daytime mom, at school or attending a day-care for at least one day per week. Day-care was defined as a care structure with more than two adults supervising children. Daytime mom was defined as a private care structure with one adult supervising children. Data on family size, the number of children present in extra-familial care and exposure to SARS-CoV-2-positive individuals (defined as a self-reported positive SARS-CoV-2 PCR on a respiratory sample since the beginning of the pandemic) were collected. Children above 12 years of age and adults were grouped into one category since the same clinical criteria for SARS-CoV-2 testing were recommended by the Swiss Federal Office of Public Health for both.
Capillary blood samples of a minimum of 400 µl were collected in SSTTM tubes with silica micro-particles and serum separator (BD Switzerland). Samples were immediately refrigerated locally and then transported to the laboratory of the Fribourg Hospital. Upon arrival samples were centrifuged and plasma was frozen at –80° within 48 hours after collection. Samples were grouped by batch and sent to the laboratory of the Lausanne University Hospital under standardised storage conditions. IgG antibodies against SARS-CoV-2 trimeric spike (S) protein were measured by an in-house Luminex assay developed by the immunology-allergology laboratory of the Lausanne University Hospital, the Federal Polytechnic School of Lausanne and the Swiss Vaccine Centre [24]. The same assay is used in large national study investigating the population SARS-CoV-2 seroprevalence in Switzerland [25]. The sensitivity of the assay depends on the time between onset of symptoms of a SARS-CoV-2 infection and blood sampling: for 0 to 5 days the sensitivity is 12.5%; 6 to 10 days 42.1%; 11 to 15 days 91.7%; and 16 to 33 days 96.6%. The specificity is 99.2% regardless of the time between symptoms and sampling [24]. A blood sample was considered as positive when the ratio between the mean fluorescence intensity and the mean fluorescence intensity of a pre-pandemic blood pool sample (negative control) was equal or greater than 6. A ratio lower than 3.9 was considered as negative, and between 4 to 5.9 as indeterminate. Negative and indeterminate results were grouped together as ‘negative/indeterminate anti-SARS-CoV-2 IgG antibodies’.
The sample size was calculated according to the desired precision of the prevalence confidence interval (CI). The total number of children under 6 years of age in the canton of Fribourg is estimated at 17,343 [26]. Of these, 3400 children attended a day-care facility [27]. The expected seropositivity of SARS-CoV-2 in children less than 6 years of age was assumed to be between 2% and 10%. We calculated a sample size of 750 participants to provide sufficient precision. Assuming a prevalence of 2%, the 95% Wilson CI will range from 1.2% to 3.3%. For a prevalence of 5%, the 95% Wilson CI will range from 3.7% to 6.9 % and for a prevalence of 10%, the 95% Wilson CI will range from 8% to 12.3%.
Written informed consent was obtained from participants’ parents or legal guardians. The study was approved by the Cantonal Research Ethics Commission of Vaud (CER-VD), Switzerland (Project-ID: 2020-01276). The study was done according to the Declaration of Helsinki.
The data of this study are available from the corresponding author, upon request.
Data were summarised by group (seropositive vs seronegative). Continuous variables were summarised using median and the interquartile range (IQR). Categorical variables were summarised with numbers and percentages. The associations between the outcome and covariables were tested using a logistic regression model. The functional relationships between the outcome and continuous variables were tested using fractional polynomials. Factors associated with the outcome with a p-value of less than 10% from the univariable analysis were considered in a backward procedure to estimate a multivariable model. Goodness of fit of the multivariable model was tested with the Hosmer-Lemeshow goodness-of-fit test and standards diagnostic tools for a logistic regression model. A p-value of less than 0.05 was considered as significant. Stata 16 software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StatCorp LLC) was used for statistical analyses.
Through patient lists from private paediatricians and the paediatric emergency department the number of eligible children was estimated at approximately 8000. A total of 884 children were enrolled in the study, which represents a participation rate of 11% (fig. 1).

Figure 1 Recruitment of participants.
Of the 884 children enrolled, 2 were excluded because they were above 6 years of age, 8 because informed consent was not obtained and 3 because of an insufficient blood sample. Blood samples from 871 children were analysed to determine the overall seroprevalence and to investigate the association of age and sex on the seroprevalence. To investigate the association of childcare arrangement on the seroprevalence, 12 participants had to be excluded because of incomplete questionnaires.
The median age of the included children was 33 months (range 6 days to 5 years 11 months). Of these, 180 (21%, 95% CI 18–24%) tested positive for IgG antibodies against the SARS-CoV-2 S protein, 677 (78%, 95% CI 75–81%) negative and 14 (1%, 95% CI 0.9–3) indeterminate.
The distribution of children by age group (seven groups) can be found in table 1 and figure 2. The seropositivity rate was higher in the group less than 3 months of age but similar in the other age groups (p = 0.66).
Table 1SARS-CoV-2 seroprevalence and age groups comparison for children aged 0 to 71 months, January–March 2021 in Fribourg, Switzerland.
| Children, n (%) | Seroprevalence % (95% CI) | OR (95% CI) | |||
| Overall | Seropositive | ||||
| Total | 871 | 180 | 21 (18–23) | NA | |
| Age (months) | ≤3 | 22 (3) | 8 (4) | 36 (18–60) | 2.17 (0.805.89) |
| 3.1–11 | 96 (11) | 20 (11) | 21 (13–29) | ref. | |
| 12–23 | 192 (22) | 46 (26) | 24 (18–30) | 1.20 (0.662.17) | |
| 24–35 | 146 (17) | 27 (15) | 18 (1225) | 0.86 (0.451.64) | |
| 36–47 | 159 (18) | 34 (19) | 21 (1528) | 1.03 (0.561.92) | |
| 48–59 | 130 (15) | 22 (12) | 17 (1023) | 0.77 (0.391.52) | |
| 60–71 | 126 (14) | 23 (13) | 18 (1225) | 0.85 (0.431.66) | |
ref.: reference; 95% CI: 95% confidence interval; OR: unadjusted odds ratio; NA: not attributed

Figure 2 SARS-CoV-2 seroprevalence as a function of age.
Of the 871 participants, 412 (47%) were female. Females were less likely to be seropositive (72/412 (17%, 95% CI 14–22%) than males 108/459 (24%, 95% CI 2028%), OR 0.69, 95% CI 0.490.96; p = 0.03 (table 2).
Table 2Data summary and logistic regression analysis comparing seropositive versus seronegative SARS-CoV-2 in children less than 6 years of age.
| Variables | Overall | Seropositive children | Seronegative children | Univariable analysis OR (p-value) | Multivariable Analysis aOR (p-value) | |
| Total of participants, n (%) | 871 (100) | 180 (21) | 691 (79) | |||
| Age in month (as continuous | Median (IQR) | -- | 27 (949) | 34 (974) | 1.0 (0.08)1 | |
| Age (as binary) | >3 months, n (%) | 849 (97) | 172 (20) | 677 (80) | ref. | |
| ≤3 months, n (%) | 22 (3) | 8 (36) | 14 (64) | 2.25 (0.07)2 | ||
| Gender, n (%) | Male | 459 (53) | 108 (24) | 351 (76) | ref. | ref. |
| Female | 412 (47) | 72 (17) | 340 (83) | 0.69 (0.03) | 0.58 (0.01) | |
| Extra-familial vs. Intra-familial care, n (%) | Extra-familial care | 654 (76) | 136 (21) | 518 (79) | ref. | |
| Intra-familial care | 205 (24) | 43 (21) | 162 (79) | 0.99 (0.96) | ||
| Type of childcare arrangement, n (%) | No day-care | 538 (63) | 125 (23) | 413 (77) | ref. | ref. |
| Day-care | 321 (37) | 54 (17) | 267 (83) | 0.67 (0.03) | 0.60 (0.02) | |
| No daytime mom | 709 (83) | 147 (21) | 562 (79) | ref. | ||
| Daytime mom | 150 (17) | 32 (21) | 118 (79) | 1.04 (0.87) | ||
| No grandparents | 480 (56) | 92 (19) | 388 (81) | ref. | ||
| Grandparents | 379 (44) | 87 (23) | 292 (77) | 1.26 (0.18) | ||
| No other childcare arrangment | 764 (89) | 161 (21) | 603 (79) | ref. | ||
| Other childcare arrangement | 95 (11) | 18 (19) | 77 (81) | 0.88 (0.63) | ||
| Children group size in childcare arrangements, median (IQR) | Day-care | -- | 10.0 (6.0) | 10.0 (5.00) | 0.98 (0.41) | |
| Daytime mom | -- | 3.0 (3.0) | 4.0 (3.0) | 0.84 (0.06) | ||
| Grandparents | -- | 2.0 (1.0) | 2.0 (2.0) | 0.94 (0.58) | ||
| Day-care, number of adults and children ≥12 years with a positive COVID PCR test, n (%) | 0 positive PCR test | 174 (54) | 34 (20) | 140 (80) | ref. | |
| 1 positive PCR test | 58 (18) | 8 (14) | 50 (86) | 0.66 (0.33) | ||
| 2 positive PCR tests | 88 (28) | 12 (14) | 76 (86) | 0.65 (0.24) | ||
| Daytime mom, number of adults and children ≥12 years with a positive COVID PCR test, n (%) | 0 positive PCR test | 30 (20) | 6 (20) | 24 (80) | ref. | |
| 1 positive PCR test | 30 (20) | 8 (27) | 22 (73) | 1.45 (0.54) | ||
| 2 positive PCR tests | 89 (60) | 18 (20) | 71 (80) | 1.01 (0.98) | ||
| Grandparents, number of adults and children ≥12 years with a positive COVID PCR test, n (%) | 0 positive PCR test | 12 (3) | 3 (25) | 9 (75) | ref. | |
| 1 positive PCR test | 114 (30) | 48 (42) | 66 (58) | 2.18 (0.26) | ||
| 2 positive PCR tests | 251 (67) | 35 (14) | 216 (86) | 0.49 (0.30) | ||
| School, n (%) | School | 190 (22) | 34 (18) | 156 (82) | ref. | |
| No school | 681 (78) | 146 (21) | 535 (79) | 1.25 (0.29) | ||
| Number of household members (family size), n (%) | ≤3 members | 205 (25) | 50 (24) | 155 (76) | ref. | ref. |
| 4 members | 393 (47) | 88 (22) | 305 (78) | 0.89 (0.58) | 0.73 (0.19) | |
| ≥5 members | 236 (28) | 36 (15) | 200 (85) | 0.56 (0.02) | 0.35 (<0.001) | |
| Household, number of adults and children ≥12 years with a positive COVID PCR test, n (%) | 0 positive PCR test | 629 (72) | 50 (8) | 579 (92) | ref. | ref. |
| 1 positive PCR test | 101 (12) | 39 (39) | 62 (61) | 7.28 (<0.001) | 7.80 (<0.001) | |
| 2 positive PCR tests | 126 (14) | 81 (64) | 45 (36) | 20.84 (<0.001) | 22.07 (<0.001) | |
| ≥3 positive PCR tests | 15 (2) | 10 (67) | 5 (33) | 23.16 (<0.001) | 32.20 (<0.001) | |
IQR: interquartile range; OR: unadjusted odds ratio; aOR: adjusted odds ratio; PCR: polymerase chain reaction; ref. – reference
Column-1 (variable name), columns-2, -3, -4 the overall and by group summary, column-5 the odds-ratio and associated p-value from univariable analysis (unadjusted OR), column-6 the odds-ratio and associated p-value from multivariable analysis (adjusted OR = aOR).
1 the OR obtained from the continuous analysis correspond to the ratio of the odds when the age is incremented by one month.
2 the OR obtained from the binary analysis correspond to the ratio of the odds between a chid aged ≤3 months compared to a child aged >3 months (=ref. group).
The seropositivity risk in children in extra-familial care was similar to the risk of children in intra-familial care, with 136/654 (21%, 95% CI 18–24%) and 43/205 (21%, 95% CI 16–27%) being seropositive, respectively, OR 0.99, 95% CI 0.67–1.45, p = 0.97.
Children in day-care had a lower seropositivity risk with 54/321 (17%, 95% CI 13-21%) compared with children not in day-care 125/538 (23%, 95% CI 20–27%), OR 0.67, 95% CI 0.47–0.95, p = 0.03, table 2. Children cared for by a daytime mum showed no increased risk of exposure 147/709 (21%, 95% CI 18–24%) compared with 32/150 (21%, 95% CI 15–29%) with no daytime mum, OR 1.04, 95% CI 0.67–1.60, p = 0.87. We found the same result for children cared for by grandparents, 87/379 (23%, 95% CI 19–28%) compared with children not cared for by grandparents 92/480 (19%, 95% CI 16–23%), OR 1.26, 95% CI 0.90–1.75, p = 0.18, and in other childcare arrangements 18/95 (19%, 95% CI 11–27%) compared to children not cared for in other childcare arrangements 161/764 (21%, 95% CI 18–24%), OR 0.88, 95% CI 0.51–1.51, p = 0.63.
For children in day-care and those cared for by a daytime mom, neither the number of children, nor the number of children or adults with a positive PCR test was associated with the seropositivity risk (table 2).
In the children looked after by grandparents, the number of children present was also not associated with the seropositivity risk. When one grandparent had a positive PCR test the risk for children to be seropositive increased (OR 2.18, 95% CI 0.56–8.49, p = 0.26). However, when both grandparents had a positive PCR test the risk decreased (OR 0.49, 95% CI 0.13–1.88 p = 0.30).
School attendance was not associated with the seropositivity risk: 34/190 (18%, 95% CI 13–25%) children who attended school were seropositive compared with 146/681 (21%, 95% CI 18–25%) who did not (OR 1.25, 95% CI 0.83–1.89, p = 0.29) (table 2).
In the univariable analyses, an increase in the number of household members above the age of 12 years being positive for SARS-CoV-2 by PCR test increased the risk of seropositivity in children less than 6 years of age (OR 7.28, 95% CI 4.45–11.93, p- <0.001 for one, OR 20.84, 95% CI 13.09–33.18, p <0.001 for two and OR 23.16, 95% CI 7.62-70.40, <0.001 for three or more household members) (table 2).
In the multivariable analysis number of household members and number of adults and children above the age of 12 years with a positive SARS-CoV-2 PCR were identified as independent risk factors for seropositivity. An increase in the number of household members above the age of 12 years being positive for SARS-CoV-2 PCR test increased the risk of seropositivity in children (aOR 7.80, 95% CI 4.65-13.07, p-value <0.001 for 1 household, aOR 22.07, 95% CI 13.49-36.11, p-value <0.001 for 2 household and aOR 32.20, 95% CI 9.30-111.55, p-value <0.001 for 3 or more household members).
An increase in the number of household members (family size) was associated with lower risk of seropositivity, as soon as the family consists of 5 or more members (including children), table 2.
Model diagnosis and the Hosmer-Lemshow goodness of fit test shows that the multivariable model fitted reasonably well the data. The power of the multivariable model to discriminate between seropositive and seronegative individuals was calculated using the area under the ROC curve AUC = 82.74.
This study is one of the first studies to investigate associations of childcare arrangements and other factors on SARS-CoV-2 seroprevalence in children under less than 6 years of age. The seropositivity rate of 21% found in this age group is similar to the rate of 19% found in adults living in the canton of Fribourg at the same time [28]. The seropositivity rate is also similar to that of children living in other Swiss cantons, which have been reported to be between 16% and 21% during the same time period [28–30]. None of these studies reported separate results for young children. We did not find differences in seroprevalence across different age groups, except in children less than 3 months of age, which likely reflects maternal antibodies. In previous studies, age also did not influence the seroprevalence; however, these studies did not compared subgroups of children less than 6 years of age [31–33].
In our study, male children were more often seropositive. Another study in Switzerland also reported a higher seroprevalence in male children [29]. This is in line with the higher rate of SARS-CoV-2 infection and more severe COVID-19 found in male adults [2, 34–36]. A male predominance for infection and increased severity of disease has already been reported with other new viruses, such as the Spanish influenza or the Middle Eastern respiratory syndrome-CoV outbreak [37]. One hypothesis to explain this sex difference is that certain genes involved in the immune response are located on the X chromosome, thus females carryng two X chromosomes with different allelic options and mosaicism caused by X chromosome inactivation can have an immune advantage [36, 37].
One of the main findings of our study was that childcare facilities and school are not places of increased exposure to SARS-CoV-2 for young children. Even more strikingly, attending day-care decreases the risk of children being seropositive. This might be due to the mitigation measures implemented in day-care. Our results are in line with those from a study from the UK, which also showed that the risk of seropositivity is not associated with school attendance [32]. When children are in day-care or being looked after by a daytime mom or grandparents, neither the size of the group of children nor the number of adults who have had a positive COVID PCR is associated with the seropositivity rate. This reinforces the hypothesis that contact between young children has little impact on the transmission of SARS-CoV-2.
As previously reported [31], our study also showed that SARS-CoV-2 transmission for children under 6 years of age mainly occurs at home. This can be explained by the fact that at home there are usually fewer mitigation measures in place. We also found a similar trend with grandparents: if one grandparent has SARS-CoV-2 PCR positive, the risk for a young child to become seropositive is doubled. However, if both grandparents are SARS-CoV-2 positive, the risk for the child to become positive decreases. This might be explained by grandparents limiting their contact with children when more than one grandparent is sick. It has been showed that transmission is reduced significantly by distancing and mask wearing [23].
We found that for children less than 3 months of age, the risk of being seropositive is positively associated with an increase in the number of family members, and this is reversed for older children. It is possible that the risk of transmission within the family is greater for younger children since they can be infected by both parents and older siblings. Moreover, children less than 3 months usually have much closer and longer physical contact with parents than older children. A decreasing contact with parents might protect children living in large families. Also, mitigation measures are easier to follow when children are older.
As the majority of adults and children will soon be vaccinated and new virus variants will be circulating, the risk of exposure for young children will likely change and needs further monitoring. Further studies are required to determine the role of long-term humoral responses in children and their association with protection against reinfection.
The strengths of the study are the large sample size, recruitment from both primary and tertiary paediatric care and availability of detailed data about childcare arrangements. The limitations are a possible selection bias through an increased likelihood of parents enrolling their children if they had experienced COVID-19 symptoms, a possible misclassification bias due to self-reporting of results from SARS-CoV-2 PCR tests and a limited sensitivity of serology. Furthermore, there is the possibility of an attribution bias due only measuring IgG antibodies against the trimeric S protein. Moreover, there is potential selection bias as we included children who sought medical care. However, since this included regular scheduled developmental check-ups, the sample also included healthy children.
The seroprevalence of SARS-CoV-2 antibodies is similar in young children compared with older children and adults. In young children, extra-familial care does not increase the risk of becoming SARS-CoV-2 seropositive, neither does the number of contacts present in extra-familial care. The number of household members tested positive for SARS-CoV-2 (PCR test) is the main exposure risk for seropositivity for children less than 6 years of age. But the family size is not associated with an increased risk of infection. As adults and children will be vaccinated and new virus variants will be circulating the risk of exposure for young children will likely change and needs further monitoring.
We thank the participants and their families for their participation. We also acknowledge all paediatricians who helped with the recruitment of participants: Dr K. Morel-Gotzos, Dr M. Schmutz, Dr S. Pitchon, Dr S. Rouvenaz, De V. Thonney, Dr K. Murray, Dr V. Dénervaud, Dr C. Chavonnet, Dr S. Gateau, Dr A. De Lucia, Dre S. Gachet, Dr V. Genoud, Dr S. Goumaz, Dr M. Zürcher, Dr Jica, Dr Lendenmann-Cordula, Dre V. Ischi, Dre A. Stritt, Dre C. Gehring, Dr M. Rais, Dr M. Yerli, Dr D. Barbey, Dr M. Veste, Dr E. Bloch, De M. Corminboeuf, Dr M. Rossé, Dr A. Bertoni, Dr R. Burmeister, Dr A. Combelles, Dr M. Burkhalter, Dr A. Gysler, Dr M. Borer, Dr A. Jäggi, Dr N. Vilimanovic, Dr N. Kuhn and all their medical assistants, as well as to all the doctors and nurses of the paediatric service of the Fribourg Hospital .
Thanks to our volunteers Bernice Fagan, Marie-Claire Pharisa and Robert Schmitt for their unfailing support during the 9 weeks of our sampling.
All the collaborators of the laboratory of the Fribourg Hospital as well as their director, Dr Jean-Luc Magnin.
Financial support from the paediatric department of the Fribourg Hospital, in particular Professor J. Wildhaber.
Thanks to Dr Olivier Duperrex for his advice at the beginning of the project.
To thank the companies Villars© and Matel© who provided each child with a small reward.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Open database : COVID-19 Switzerland [Internet] Federal Office of Public Health (OFSP). c2021 [cited 2021 Aug 11]. Available from : https://www.covid19.admin.ch/fr/overview
2. Guan WJ , Ni ZY , Hu Y , Liang WH , Ou CQ , He JX , et al.; China Medical Treatment Expert Group for Covid-19 . Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr;382(18):1708–20. https://doi.org/10.1056/NEJMoa2002032
3. Qifang Bi, Yongsheng Wu, Shujiang Mei, Chenfei Y, Xuan Zou, Zhen Zhang, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. 2020;(January):19–21.
4. Zimmermann P , Curtis N . Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020 May;39(5):355–68. https://doi.org/10.1097/INF.0000000000002660
5. European Centre for Disease Prevention and Control . COVID-19 in children and the role of school settings in transmission - second update. [Internet]. 2021 Jul [cited 2021 Oct 7]. Available from : https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-in-children-and-the-role-of-school-settings-in-transmission-second-update.pdf
6. L’Huillier AG , Torriani G , Pigny F , Kaiser L , Eckerle I . Culture-Competent SARS-CoV-2 in Nasopharynx of Symptomatic Neonates, Children, and Adolescents. Emerg Infect Dis. 2020 Oct;26(10):2494–7. https://doi.org/10.3201/eid2610.202403
7. Baggio S , L’Huillier AG , Yerly S , Bellon M , Wagner N , Rohr M , et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Viral Load in the Upper Respiratory Tract of Children and Adults With Early Acute Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2021 Jul;73(1):148–50. https://doi.org/10.1093/cid/ciaa1157
8. graphics.reuters.com . [Internet]. Reuters COVID-19 Tracker. c2021 [cited 2021 Oct 7]. Available from : https://graphics.reuters.com/world-coronavirus-tracker-and-maps/fr/countries-and-territories/switzerland/
9. ffaes.ch . [Internet]. Switzerland : Protection concept for day care centers and after-school care facilities - Kibesuisse. c2020 [cited 2021 Oct 25]. Available from: http://ffaes.ch/fileadmin/user_upload/Documents/FFAES/WWW/Editors/CORONAVIRUS/2020.05.01_200430_Modele_conceptprotection_creches_parascolaire.pdf
10. bdlf.fr.ch . [Internet]. Switzerland : Ordinance defining measures for the control of the coronavirus (COVID-19) - Canton of Fribourg. c2020 [cited 2021 Oct 25]. Available from: https://bdlf.fr.ch/app/fr/texts_of_law/821.40.21
11. fr.ch . [Internet]. Switzerland : Protection concept for compulsory education from 1H-11H - Canton of Fribourg. c2020 [cited 2021 Oct 25]. Available from: https://www.fr.ch/sites/default/files/2020-05/20200506_Ecole_obligatoire_OCC_COVID_Plan_de_protection.pdf
12. kibesuisse.ch . [Internet]. Switzerland : 8 Golden Rule - Kibesuisse. c2021 [cited 2021 Oct 25]. Available from: https://www.kibesuisse.ch/fileadmin/Dateiablage/kibesuisse_Dokumente/Corona/8_Golden_Rules_FR_A4.pdf
13. Ulyte A , Radtke T , Abela IA , Haile SR , Braun J , Jung R , et al. Seroprevalence and immunity of SARS-CoV-2 infection in children and adolescents in schools in Switzerland: design for a longitudinal, school-based prospective cohort study. Int J Public Health. 2020 Dec;65(9):1549–57. https://doi.org/10.1007/s00038-020-01495-z
14. Fontanet A , Tondeur L , Grant R , Temmam S , Madec Y , Bigot T , et al. SARS-CoV-2 infection in schools in a northern French city: a retrospective serological cohort study in an area of high transmission, France, January to April 2020. Euro Surveill. 2021 Apr;26(15):1–12. https://doi.org/10.2807/1560-7917.ES.2021.26.15.2001695
15. Ludvigsson JF . Children are unlikely to be the main drivers of the COVID-19 pandemic - A systematic review. Acta Paediatr. 2020 Aug;109(8):1525–30. https://doi.org/10.1111/apa.15371
16. Barcellini L , Forlanini F , Sangiorgio A , Gambacorta G , Alberti L , Meta A , et al. Does school reopening affect SARS-CoV-2 seroprevalence among school-age children in Milan? Folgori L, editor. PLoS One. 2021 Sep 2;16(9):1–6.
17. Goldstein E , Lipsitch M , Cevik M . On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J Infect Dis. 2021 Feb;223(3):362–9. https://doi.org/10.1093/infdis/jiaa691
18. Esposito S , Marchetti F , Lanari M , Caramelli F , De Fanti A , Vergine G , et al.; Working Group on COVID-19 in Pediatrics of the Emilia-Romagna Region (RE-CO-Ped) . COVID-19 Management in the Pediatric Age: Consensus Document of the COVID-19 Working Group in Paediatrics of the Emilia-Romagna Region (RE-CO-Ped), Italy. Int J Environ Res Public Health. 2021 Apr;18(8):1–29. https://doi.org/10.3390/ijerph18083919
19. Götzinger F , Santiago-García B , Noguera-Julián A , Lanaspa M , Lancella L , Calò Carducci FI , et al.; ptbnet COVID-19 Study Group . COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020 Sep;4(9):653–61. https://doi.org/10.1016/S2352-4642(20)30177-2
20. Zhu Y , Bloxham CJ , Hulme KD , Sinclair JE , Tong ZW , Steele LE , et al. A Meta-analysis on the Role of Children in Severe Acute Respiratory Syndrome Coronavirus 2 in Household Transmission Clusters. Clin Infect Dis. 2021 Jun;72(12):e1146–53. https://doi.org/10.1093/cid/ciaa1825
21. Li W , Zhang B , Lu J , Liu S , Chang Z , Peng C , et al. Characteristics of Household Transmission of COVID-19. Clin Infect Dis. 2020 Nov;71(8):1943–6. https://doi.org/10.1093/cid/ciaa450
22. Maltezou HC , Vorou R , Papadima K , Kossyvakis A , Spanakis N , Gioula G , et al. Transmission dynamics of SARS-CoV-2 within families with children in Greece: A study of 23 clusters. J Med Virol. 2021 Mar;93(3):1414–20. https://doi.org/10.1002/jmv.26394
23. Galow L , Haag L , Kahre E , Blankenburg J , Dalpke AH , Lück C , et al. Lower household transmission rates of SARS-CoV-2 from children compared to adults. J Infect. 2021 Jul;83(1):e34–6. https://doi.org/10.1016/j.jinf.2021.04.022
24. Fenwick C , Croxatto A , Coste AT , Pojer F , André C , Pellaton C , et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. Subbarao K, editor. J Virol. 2021 Jan 13;95(3):1–12.
25. corona-immunitas.ch . [Internet]. Switzerland : Corona Immunitas - choosing test. c2021 [cited 2021 Aug 11]. Available from: https://www.corona-immunitas.ch/aktuell/choosing-a-common-test/
26. Open database : Canton of Fribourg [Internet]. Statistics on the population of the canton of Fribourg. c2020 [cited 2021 Dec 1]. Available from : http://appl.fr.ch/stat_statonline/portraitif/etape2.asp?Niveau=2&langue=fr&initMenu=1
27. Crechesfribourg.ch . [Internet]. Fédération des crèches et garderies fribourgeoises [cited 2021 January 7]. Available from : p. https://www.crechesfribourg.ch/fr/contact
28. corona-immunitas.ch . [Internet]. Switzerland : Corona Immunitas - results. c2021 [cited 2021 Oct 7]. Available from: https://www.corona-immunitas.ch/fr/programme/resultats/
29. Ulyte A , Radtke T , Abela IA , Haile SR , Ammann P , Berger C , et al. Evolution of SARS-CoV-2 seroprevalence and clusters in school children from June 2020 to April 2021: prospective cohort study Ciao Corona. Swiss Med Wkly. 2021 Nov;151:w30092.
30. Ulyte A , Radtke T , Abela IA , Haile SR , Berger C , Huber M , et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ. 2021 Mar;372(616):n616. https://doi.org/10.1136/bmj.n616
31. Zinszer K , Mckinnon B , Bourque N , Pierce L , Saucier A , Otis A , et al. Seroprevalence of SARS-CoV-2 Antibodies Among Children in School and Day Care in Montreal, Canada. 2021;4(11):1–12.
32. Ladhani SN , Baawuah F , Beckmann J , Okike IO , Ahmad S , Garstang J , et al. SARS-CoV-2 infection and transmission in primary schools in England in June-December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021 Jun;5(6):417–27. https://doi.org/10.1016/S2352-4642(21)00061-4
33. Waterfield T , Watson C , Moore R , Ferris K , Tonry C , Watt A , et al. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child. 2021 Jul;106(7):680–6. https://doi.org/10.1136/archdischild-2020-320558
34. Gudbjartsson DF , Helgason A , Jonsson H , Magnusson OT , Melsted P , Norddahl GL , et al. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 2020 Jun;382(24):2302–15. https://doi.org/10.1056/NEJMoa2006100
35. Jutzeler CR , Bourguignon L , Weis CV , Tong B , Wong C , Rieck B , et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 Sep - Oct;37:101825. https://doi.org/10.1016/j.tmaid.2020.101825
36. Abate BB , Kassie AM , Kassaw MW , Aragie TG , Masresha SA . Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020 Oct;10(10):e040129. https://doi.org/10.1136/bmjopen-2020-040129
37. Bienvenu LA , Noonan J , Wang X , Peter K . Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020 Dec;116(14):2197–206. https://doi.org/10.1093/cvr/cvaa284