Epidemiology of Kawasaki Disease in children in Switzerland: a national prospective cohort study
DOI: https://doi.org/10.4414/SMW.2022.w30171
Eugénie
Gradoux a, Stefano
Di Bernardob, Sabrina
Bressieux-Degueldreb, Yvan
Mivelazb, Tatiana
Boulos Ksontinib, Milan
Prsab, Nicole
Sekarskib
a
Department of women-mother-child, Lausanne University Hospital, Lausanne, Switzerland
b
Division of Pediatric Cardiology, Department of women-mother-child, Lausanne University Hospital, Lausanne, Switzerland
Summary
AIM OF THE STUDY: Kawasaki disease is a febrile illness which can lead to significant coronary artery lesions. Its incidence varies among countries and is highest in Japan (330.2 children under 5 years old/100,000 per year). Since the epidemiology of Kawasaki disease in Switzerland is unknown, we conducted a national prospective data collection between 2013 and 2017 to describe its incidence, diagnosis, and treatment.
METHODS: We collected demographic and clinical data of the children under 17 years old hospitalised with Kawasaki disease in Switzerland between March 2013 and February 2017 using anonymous data collection forms with the help of the Swiss Paediatric Surveillance Unit (SPSU). We defined Kawasaki disease per the 2004 American Heart Association criteria: patients with ≥5 days of fever and ≥4 of the 5 main clinical features were included as complete Kawasaki disease and patients with ≥5 days of fever and <4 of the 5 main clinical features were included as incomplete Kawasaki disease. The incidence was calculated with the data of the Federal Statistical Office of Switzerland, considering permanent residents of the country. The different groups were compared by the unpaired student t-test for continuous variables and Pearson’s chi squared test for categorical variables, respectively.
RESULTS: We included 175 patients: 60% were boys, with a mean age of 38.2 months. The incidence of Kawasaki disease was 3.1/100,000 [95% CI 2.6–3.7] per year in children under 17 years of age and 8.4/100,000 [95% CI 6.7–10.2] per year in children under 5 years of age. The most frequent clinical signs were a rash (85.4%) and changes of the lips and oral/pharyngeal mucosa (83.4%). The diagnosis of Kawasaki disease was made at a mean of 7.3 days after the first symptom. Echocardiography was abnormal in 52.3%. The treatment with intravenous immunoglobulins (IVIG) and acetylsalicylic acid was administered in accordance with international guidelines. Subgroup analysis showed that children older than 5 years old had significantly more complete Kawasaki disease than the younger ones (78.8% vs 57.4%, p = 0.021). Children with “extreme ages” (<1 year old and >8 years old) were diagnosed later (8.6 (±0.9) vs 7.0 (±0.3) days, p = 0.0129), had longer duration of fever (9.8 (±0.9) vs 8.1 (±0.3) days, p = 0.013) and had more echocardiographic abnormalities (n = 26 (70.3%) vs n = 65 (47.5%), p = 0.014) at diagnosis. One child died during the acute phase of the illness.
CONCLUSIONS: The incidence of Kawasaki disease in Switzerland is in the lower range of other European countries.
Introduction
In 1967, Dr Tomisaku Kawasaki publihed in Japan the first description of an acute inflammatory vasculitis of medium sized arteries now called Kawasaki disease. It is an acute febrile illness of unknown aetiology occurring predominantly in infants and young children that can sometimes affect the myocardium, pericardium, and the coronary arteries, with dilatations, ectasia, and aneurysms. Because of the lack of a pathognomonic test, the diagnosis of the disease is based on a constellation of internationally agreed clinical signs, which may appear sequentially.
Children usually present with high-spiking fever and some, if not all, of the following clinical manifestations: bilateral non-exudative conjunctivitis, erythema of the oropharynx, red fissured lips, strawberry tongue, unilateral cervical node enlargement, and swelling of the hands and feet with erythema of the palms and soles. Within five days of the onset of fever, a maculopapular erythematous rash can appear, often beginning in the perineal area. Commonly, two to three weeks after the onset of the fever, the patient develops periungual desquamation [1].
The diagnosis of complete Kawasaki disease, also called classic or typical Kawasaki disease, is based on the presence of ≥5 days of fever and the presence of ≥4 of the five main clinical features. Children who don’t present with sufficient clinical criteria but who have consistent laboratory or echocardiographic findings can be diagnosed with incomplete Kawasaki disease (also sometimes referred to as atypical Kawasaki disease), which accounts for approximately 20% of cases [2]. The treatment consists of high doses of IVIG given preferentially within the first 10 days of fever, associated with acetylsalicylic acid, which significantly reduces the development of coronary artery aneurysms and ectasia from 15-25% to about 5% [3].
Despite the treatment efficacy, Kawasaki disease is now the leading cause of acquired heart disease in children in developed countries, exceeding rheumatic heart disease. Long term follow-up of patients with persistent coronary artery aneurysms shows an increased incidence of acute coronary syndrome [4]. Despite complete regression on coronary angiography, patients with a history of coronary aneurysm have persistent intimal thickening and endothelial dysfunction, which could represent a long term risk factor for premature coronary atherosclerosis [5]. Even in patients who did not have coronary lesions, some data suggest endothelial dysfunction and increased arterial stiffness, without clear clinical implications [6].
The incidence of Kawasaki disease differs widely among ethnic groups and is highest in Japan, where 330.2/100,000 children aged 0 to 4 years are diagnosed every year [7], followed by other Asian countries. In Europe and North America, the reported incidence is between 4.9 (Denmark) and 19.0/100,000/year (USA) in children under 5 years of age [8]. The influence of genetic factors in the development of the disease is illustrated by the higher rates of Kawasaki disease in the Asian populations having migrated to countries with low incidence [9] and the high relative risk of Kawasaki disease among family members [10].
Since the epidemiology of Kawasaki disease in Switzerland is unknown, we conducted a prospective national data collection with the aim of determining the epidemiology, diagnostic modalities, treatment, and early outcomes of children diagnosed with Kawasaki disease.
Methods
All children under the age of 17 hospitalised in Switzerland and reported by the Swiss Paediatric Surveillance Unit (SPSU) between March 2013 and February 2017 with a diagnosis of Kawasaki disease were included.
We defined complete Kawasaki disease as per the 2004 American Heart Association (AHA) criteria [3]: the presence of ≥5 days of fever and ≥4 of the 5 main clinical features (cutaneous rash, cervical lymphadenopathy >1.5 cm diameter, conjunctivitis, changes of lips or oral/pharyngeal erythema, and extremity changes). The cases with less than 4 clinical signs were considered incomplete.
Coronary arteries were described as “normal”, dilated” or “aneurysm” and known sizes were expressed as z-scores using the Boston equation [11], calculating the body surface area with the Haycock formula [12].
Announcement was done through the SPSU (www.spsu.ch), which is a surveillance system for rare paediatric diseases in hospitalised children in each of the 33 Swiss paediatric hospitals set up by the Swiss Pediatric Association and the Federal Office of Public Health. It covers the 1.3 million Swiss paediatric hospital population. After receiving the initial anonymous report by the SPSU, we collected the following data for each patient with a data collection form addressed to the physician in charge of the patient during the hospital stay: demographics (birth date, gender, race, canton of residence), presentation of Kawasaki disease (symptoms, dates of first symptom and diagnosis), investigations needed to establish the diagnosis (laboratory values, cardiac investigations as well as radiological and microbiological studies), and treatment (doses and date of intra-venous immunoglobulins [IVIG] and acetylsalicylic acid administration, other treatment for Kawasaki disease or for alternative diagnosis).
The primary outcome of the study was to calculate the incidence of Kawasaki disease in Switzerland. The secondary outcome was the analysis of the demographic characteristics of the patients, the method and time needed to establish the diagnosis, the treatments administered, and the short term (end of hospital stay) clinical outcomes.
Comparative data analysis was done for patients with complete and incomplete forms of Kawasaki disease and for children below and above 5 years of age. Given that most of the epidemiological studies about Kawasaki disease include only children under 5 [8, 13], we decided to compare that age group with older children. This allows us to compare this subgroup with other studies and to explore if the older patients have different characteristics than the ones that are usually included in studies. Children at extreme ages (under 1 year and over 8 years) were also compared to children in the more “classical” age group. We chose these criteria for extreme ages based on an American epidemiological study which demonstrated more coronary artery lesions in children under 1 and over 8 years old [14].
Statistical analysis was performed with STATA 15.0 (STATA Corp., College Station, Texas, USA).
Continuous variables are expressed as mean and standard deviation, while categorical variables are presented as absolute numbers and percentage. The different groups were compared by the unpaired student t-test for continuous variables and Pearson’s chi squared test for categorical variables, respectively. A p-value <0.05 was considered significant.
The incidence of Kawasaki disease was calculated using the data of the Federal Statistical Office of Switzerland, in relation to the permanent residents of the country in the relevant calendar year.
The research protocol was approved by the Institutional Ethics Board. Because of the anonymous data collection, the Ethic Committee waived the informed consent. The study was performed in compliance with the 1964 Helsinki declaration and its later amendments.
Results
The SPSU reported 257 cases of Kawasaki disease with a return of 252 (98%) data collection forms. Of these, 77 patients were excluded: 64 (24.9%) because of missing data on duration of fever and 13 (5.1%) because they were diagnosed and treated before the 5th day of fever (figure 1).

Figure 1 Flow chart of the study showing the inclusion and exclusion process of the patients. 257 children were reported by the SPSU. 5 data collection forms were not returned and therefore were excluded of the study. 64 forms didn’t mention fever duration and 13 patients were diagnosed with Kawasaki disease before completing 5 days of fever. These patients were excluded of the study. 175 patients were included in the cohort.
Therefore, 175 patients were included in the analysis, with a mean age of 38.2 (+/–31.0) months, 105 (60%) of them were boys (table 1). The patients under 5 years of age represented 81% of the children diagnosed with Kawasaki disease, as illustrated in figure 2. The most frequent clinical signs reported were a rash in 146 patients (85.4%) and changes of the lips and oral/pharyngeal mucosa in 145 patients (83.4%). Complete Kawasaki disease was reported in 107 patients (61.1%).
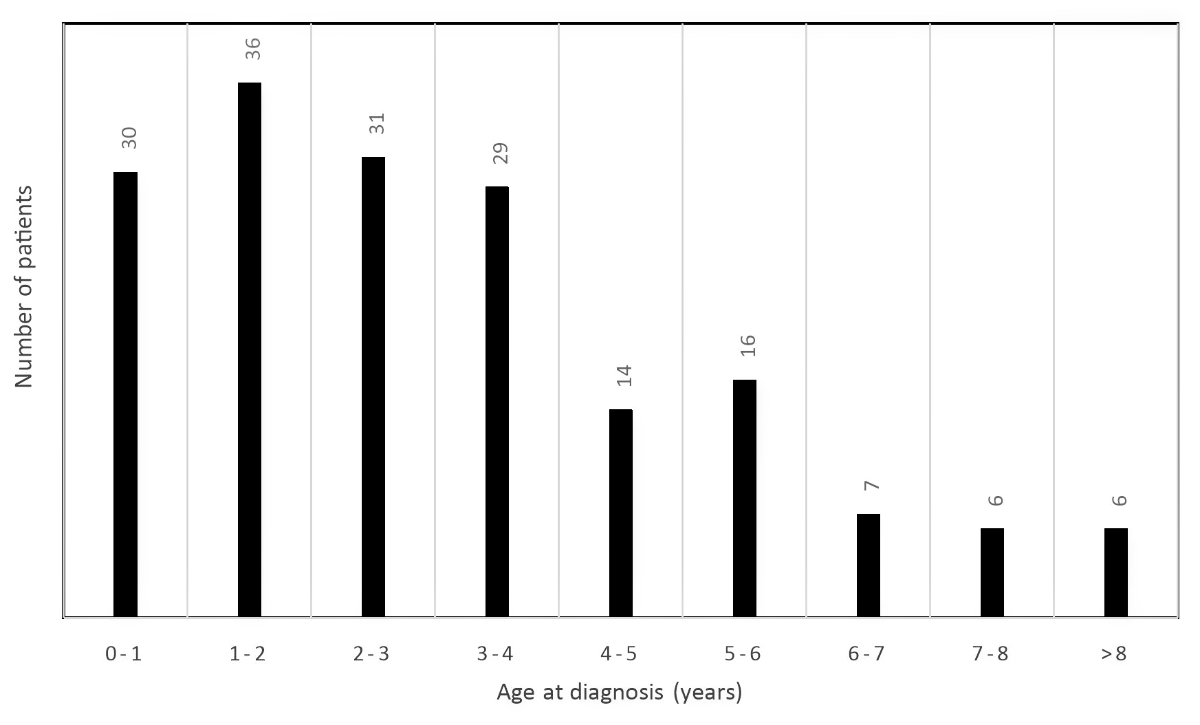
Figure 2 Bar chart showing the distribution of the children diagnosed with Kawasaki disease by age group. Most of the patients were between 0 and 4 years old.
Table 1Baseline characteristics. Data are provided as mean and standard deviation, respectively as absolute number and percentage, as appropriate. All patients: n = 175.
| Gender: male |
105 (60%) |
| Weight (kg) |
14.7 ±7.5 |
| Age at onset (months) |
38.2 ±31.0 |
| Clinical signs |
Total days of fever |
8.5 ±3.7 |
| Rash (n = 171) |
146 (85%) |
| Adenopathy (n = 168) |
89 (53%) |
| Conjunctivitis (n = 171) |
136 (80%) |
| Extremity changes (n = 168) |
106 (63%) |
| Lips & mouth changes (n = 172) |
145 (83%) |
| Diagnosis |
Complete Kawasaki Disease |
107 (61%) |
| Number of days between 1st symptom and diagnosis (n = 174) |
7.3 ±4.1 |
| Number of days between diagnosis and transthoracic echocardiography (n = 170) |
1.1 ± 2.5 |
| Lab results |
CRP (g/L) (n = 174) |
108 ±82 |
| White blood cells (G/L) (n = 172) |
17 ±8.2 |
| Platelets (G/L) (n = 170) |
375 ±182 |
| Haemoglobin (g/L) (n = 172) |
103 ±16 |
| Urinalysis performed |
134 (78%) |
| Other examinations |
Abdominal ultrasound performed |
84 (48%) |
| Throat swab performed |
52 (30%) |
| Bone marrow analysis performed |
1 (0.6%) |
| Cardiac exams |
Abnormal ECG (n = 120) |
17 (14%) |
| Abnormal transthoracic echocardiography (n = 174) |
91 (52%) |
| – Pericardial effusion (n = 154) |
23 (15%) |
| – Myocardial dysfunction (n = 165) |
2 (1.2%) |
| – Valvular regurgitation (n = 152) |
14 (9.2%) |
| – Perivascular brightness (n = 168) |
39 (23%) |
| – Perivascular brightness as single transthoracic echocardiography anomaly |
18 (10%) |
| – Coronary findings: right coronary artery (n = 173)
|
Normal |
149 (86%) |
| Dilated |
13 (7.5%) |
| Aneurysmal |
11 (6.4%) |
| z-score (data from n = 23 patients)
|
4.8 ±3.2 |
| – Coronary findings: left coronary artery (n = 173) |
Normal |
123 (71%) |
| Dilated |
37 (21%) |
| Aneurysmal |
13 (7.5%) |
| z-score (data from n = 35 patients) |
6.7 ±4.3 |
| – Patients with bilateral coronary arteries lesions |
17 (9.7%) |
In addition to the usual clinical signs of Kawasaki disease, the children included in the study presented with various other clinical features, most often digestive complaints (n = 25, 14.3%), osteo-articular (n = 8, 4.6%) and ear-nose-throat symptoms (n = 6, 3,4%). Differential diagnosis was actively sought: 134 (78.4%) children had urine analysis, an abdominal ultrasound was performed in 84 (48.3%) patients, throat cultures were performed in 52 (30.1%) cases.
The diagnosis of Kawasaki disease was made at a mean of 7.3 (+/–4.1) days after the first symptom. The first echocardiography was performed at a mean of 1.1 days of diagnosis (range: 6 days before to 24 days after diagnosis).
The seasonality of Kawasaki disease was ill-defined, with a non-homogenous pattern within the four years of the study. The only clear trend concerns the lowest diagnosis rate, which occurred in September and October (Figure 3). The geographical repartition of the patients, determined with their home location, is illustrated in figure 4.
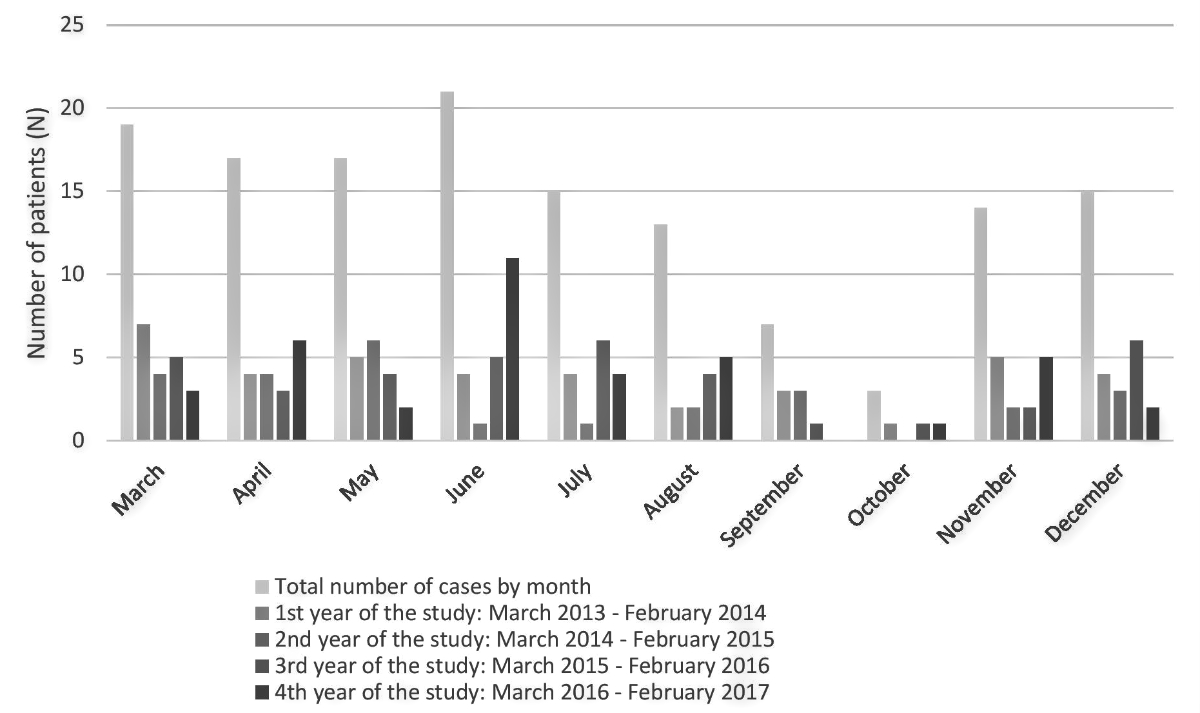
Figure 3 Bar chart showing the seasonality of Kawasaki disease in Switzerland, with a nadir in September and October.
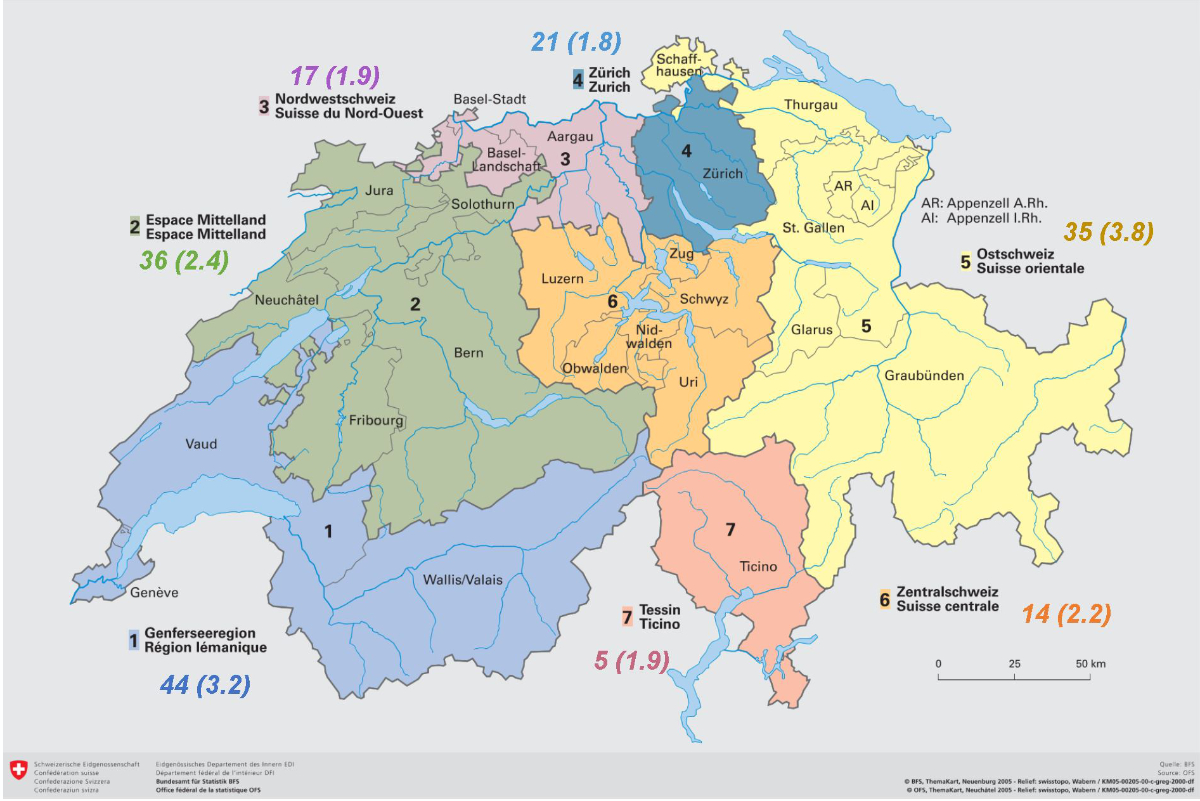
Figure 4 Geographical repartition of patients with Kawasaki disease in Switzerland, by residence. The major incidence was in “Suisse orientale” with 3.8/100,000 children under 18 years old per year and the lowest in Zurich with 1.8/100,000 children under 18 years old per year. (Comment: Three children were foreigners diagnosed during holidays. They don't appear on the map but are included in the study.). Copyright: Office fédéral de la statistique
All patients had an echocardiography, which was abnormal in 91 patients (52.3%). The detailed results are presented in table 2.
Table 2Treatment. Continuous variables are expressed as mean and standard deviation (SD). Categorical variables are expressed as absolute number and percentage. All patients: n = 175.
| IVIG administered (n = 174) |
174 (100%) |
| – Dose (g/kg) (n = 166) |
2.0 ±0.2 |
| – Number of days between diagnosis and 1st IVIG (n = 173) |
0.2 ±0.6 |
| – 2nd IVIG course (n = 164) |
39 (24%) |
| – Number of days between 1st and 2nd IVIG doses (n = 38) |
2.4 ±1.9 |
| Acetylsalicylic acid (n = 174) |
173 (99%) |
| – Initial acetylsalicylic acid dose (mg/kg) (n = 172) |
72 ±25 |
| – Duration initial acetylsalicylic acid (days) (n = 141) |
6.3 ±6.6 |
| – Chronic acetylsalicylic acid dose (mg/kg) (n = 129) |
7.0 ±12 |
| Further treatment |
31 (18%) |
| – 2nd line treatment |
16 (9.1%) |
| – 2nd line treatment: corticosteroids |
13 (7.4%) |
| – 2nd line treatment: infliximab |
2 (1.1%) |
| – 2nd line treatment: rituximab |
1 (0.6%) |
| – 3rd line treatment (infliximab) |
1 (0.6%) |
We calculated z-scores for the patients for whom we had a coronary artery diameter documented (table 3).
An ECG was performed in only 163 children (66.0%) during their hospital stay, of which 17 (14.2%) were described as abnormal, most of them showing non-specific repolarisation abnormalities.
Table 3Z-scores. Data are provided as mean and standard deviation.
|
z-score
|
| Right coronary artery (n = 23) |
4.8 ±3.2 |
| Left coronary artery (n = 35) |
6.7 ±4.3 |
All but 1 child (n = 174, 100%) received IVIG at a dose of 2.0 g/kg. A second dose of IVIG was necessary in 39 (23.8%) patients. Children who had a second IVIG infusion had significantly more coronary artery lesions than those who had only one (table 4).
Table 4Relation between the number of coronary artery lesions and the need for a second dose of intra-venous immunoglobulins. Data are provided as absolute numbers.
|
IVIG: 1 dose (n = 135)
|
IVIG: ≥2 doses (n = 39)
|
p-value
|
| Presence of coronary artery lesions |
36 |
19 |
|
| Absence of coronary artery lesions |
99 |
20 |
0.0115 |
A second line treatment was given to 16 (9.4%) children, after or instead of a 2nd IVIG infusion (table 2). Acetylsalicylic acid was prescribed to 173 (99.4%) patients, at anti-inflammatory doses for a mean of 6.3 (±6.6) days and thereafter at anti-thrombotic doses for a duration dependent on subsequent echocardiographic results.
One child (0.6%) died during the acute phase of the disease, despite an intensive treatment with Extra Corporeal Membrane Oxygenation.
We compared the children who were 5 years old or younger (n = 142) to the older children (n = 33): the latter group had a significantly higher rate of complete Kawasaki disease (78.8% vs 57.4%, p = 0.021). Despite the fewer clinical signs in younger children, there was no significant difference in the time needed to diagnose Kawasaki disease in the two groups and the cardiac outcomes were similar (Table 5). The least represented clinical sign, cervical adenopathy, was significantly more prevalent in children aged 5 or more (48.2% vs 74.2%, p = 0.009).
Table 5Presentation characteristics among children younger versus older than 5 years. Continuous variables are expressed as mean [range]. Categorical variables are expressed in absolute numbers (%).
|
≤5 years old (n = 142)
|
>5 years old (n = 33)
|
p-value
|
| Clinical signs |
Total days of fever |
8.1 [5-28] |
10 [6-30] |
0.006 |
| Rash |
118 (85%) |
28 (88%) |
0.707 |
| Adenopathy |
66 (48%) |
23 (74%) |
0.009 |
| Conjunctivitis |
108 (78%) |
28 (85%) |
0.339 |
| Extremity changes |
89 (65%) |
17 (55%) |
0.291 |
| Lips & mouth changes |
114 (81%) |
31 (97%) |
0.03 |
| Diagnosis |
Complete Kawasaki disease |
81 (57%) |
26 (79%) |
0.021 |
| Number of days between 1st symptom and diagnosis |
7.1 [1-23] |
8.2 [1-29] |
0.1897 |
| Number of days between diagnosis and transthoracic echocardiography |
1 [-6-13] |
1.5 [-2-24] |
0.3113 |
| Cardiac exams |
Abnormal ECG |
15 (16%) |
2 (8.0%) |
0.32 |
| Abnormal transthoracic echocardiography |
77 (55%) |
14 (42%) |
0.207 |
| – Pericardial effusion |
18 (15%) |
5 (16%) |
0.835 |
| – Myocardial dysfunction |
0 (0%) |
2 (6.5%) |
0.003 |
| – Valvular regurgitation |
10 (8.2%) |
4 (13%) |
0.383 |
| – Perivascular brightness |
33 (24%) |
6 (19%) |
0.573 |
| – Right coronary artery |
Normal |
124 (88%) |
25 (78%) |
| Dilated |
10 (7.1%) |
3 (9.4%) |
| Aneurysmal |
7 (5.0%) |
4 (13%) |
0.246 |
| – Left coronary artery |
Normal |
95 (68%) |
28 (85%) |
| Dilated |
34 (24%) |
3 (9.1%) |
| Aneurysmal |
11 (7.9%) |
2 (6.1%) |
0.13 |
We also compared extremes of ages (less than 1 year old and more than 8 years old) with ages in between. The diagnosis of Kawasaki disease was made significantly later in the “extreme age” group patients (8.6 (±0.9) vs 7.0 (±0.3) days, p = 0.0129), they had a significantly longer duration of fever (9.8 (±0.9) vs 8.1 (±0.3) days, p = 0.013) and had significantly more echocardiographic abnormalities (n = 26 (70.3%) vs n = 65 (47.5%), p = 0.014) at diagnosis (table 6).
Table 6Comparison between children at the extreme spectrum of age (defined as <1 year or >8 years) versus typical age (between 1 and 8 years). Continuous variables are expressed as mean [range]. Categorical variables are expressed in absolute numbers (%).
|
<1 year old &>8 years old (n = 37)
|
1-8 years old (n = 138)
|
p-value
|
| Clinical signs |
Total days of fever |
9.8 [5–30] |
8.1 [5–19] |
0.0129 |
| Rash |
32 (87%) |
114 (85%) |
0.83 |
| Adenopathy |
13 (36%) |
76 (58%) |
0.022 |
| Conjunctivitis |
28 (78%) |
108 (80%) |
0.769 |
| Extremity changes |
19 (54%) |
87 (65%) |
0.225 |
| Lips & mouth changes |
30 (81%) |
115 (85%) |
0.543 |
| Diagnosis |
Complete Kawasaki disease |
18 (49%) |
89 (65%) |
0.079 |
| Number of days between 1st symptom and diagnosis |
8.6 [2–29] |
7.0 [1–21] |
0.0309 |
| Number of days between diagnosis and transthoracic echocardiography |
0.7 [–3–10] |
1.2 [–6–24] |
0.2739 |
| Cardiac exams |
Abnormal ECG |
9 (35%) |
8 (8.5%) |
0.001 |
| Abnormal transthoracic echocardiography |
26 (70%) |
65 (48%) |
0.014 |
| – Pericardial effusion |
4 (13%) |
19 (15%) |
0.784 |
| – Myocardial dysfunction |
0 (0%) |
2 (1.6%) |
0.452 |
| – Valvular regurgitation |
4 (13%) |
10 (8.3%) |
0.469 |
| – Perivascular brightness |
10 (29%) |
29 (22%) |
0.339 |
| – Right coronary artery |
Normal |
26 (72%) |
123 (90%) |
|
| Dilated |
3 (8.3%) |
10 (7.3%) |
|
| Aneurysmal |
7 (19%) |
4 (2.9%) |
0.001 |
| Left coronary artery |
Normal |
19 (54%) |
104 (75%) |
|
| Dilated |
11 (31%) |
26 (19%) |
|
| Aneurysmal |
5 (14%) |
8 (5.8%) |
0.039 |
Discussion
This is the first national study describing the epidemiology of Kawasaki disease in Switzerland. It follows a large retrospective single-centre study performed in our Paediatric Cardiology Unit analysing patients under 18 years old with a diagnosis of Kawasaki disease between 1981 and 2014 [15]. Of note, while most epidemiological studies regarding Kawasaki disease only included children under the age of 5, we were able to include all children under 17 years old. This allowed us to obtain a more complete view of the local epidemiology. Furthermore, considering the collecting data system we used and its excellent coverage [16], we can reasonably assume to have included the vast majority of the children treated for Kawasaki disease in Switzerland.
New recommendations about Kawasaki disease diagnosis and treatment were published in 2017 [2]. The most important change in diagnosis is that experienced clinicians are allowed to diagnose Kawasaki disease after only 3 days of fever if the clinical presentation is typical. Given that our inclusion criteria were chosen before 2017, the definition of Kawasaki disease used in this study is more stringent regarding the number of days of fever than the current one.
The overall annual incidence of Kawasaki disease in children under 17 years of age in Switzerland was 3.1 /100,000 [CI 95% 2.6–3.7] per year. For children less than 5 years old, the incidence was 8.4/100,000 [95% CI 6.7–10.2] per year.
Had we included all 253 patients regardless of the number of days of fever before the diagnosis, the incidence of Kawasaki disease would have been 11.9 /100,000 [95% CI 10.9-13.0] per year for children <5 years old and 4.5/100,000 [95% CI 3.9–5.1] per year for children <17 years old.
In Europe, the incidence of Kawasaki disease for children <5 years old ranges between 4.9 (Denmark) and 15.2/100,000 per year (Ireland) [17]. Switzerland therefore lies in the medium range among European countries.
The demographic data of our cohort regarding age and gender is consistent with the international literature [18].
Seasonal variation was low and non-consistent between the four years of the study, except for September and October, during which the lowest incidence was reported. Japanese data over a 14-years period shows some similarities, with a nadir in October and two peaks in January and June, with the incidence in between being high but not peaking. The authors mention a viral trigger as a possible explanation [19].
The diagnosis was made in a timely fashion and concordant with other studies [20, 21] and the distribution of the clinical signs corresponds to the typical description reported the literature [22]. Some children had their first TTE some days before the diagnosis of Kawasaki disease was attributed, which can reflect the early evocation of Kawasaki disease as a differential diagnosis and the easy access to specialised care in Switzerland. It is possible that some of these patients developed further cardiac anomalies, which then wouldn’t have been described in the study unless they were still present at discharge (question forms asked for initial and discharge ETT). Even if this is a limitation of the study, we think that such transient anomalies in a small hypothetical subset of children is not extremely relevant.
Remarkably, the presence of a cervical lymphadenopathy seemed to be related to the age group, the patients aged 5 or more having a higher prevalence than the younger ones. A possible explanation is linked to lymphoid organ maturation, which takes place around 5–6 years of life. Therefore, clinical features linked to the lymphoreticular system may be less obvious in younger patients [23].
Nearly 40% of presentations were incomplete Kawasaki disease, in front of reported proportions between 15 and 36% [24]. The 2004 AHA guidelines define incomplete Kawasaki disease as unexplained fever ≥5 days associated with 2 or 3 clinical signs and compatible laboratory and/or echocardiography findings. In this study, we considered all children who did not meet the complete definition of Kawasaki disease but were diagnosed with Kawasaki disease by their paediatrician or cardiologist as incomplete cases of Kawasaki disease. It is possible that the higher rate of incomplete Kawasaki disease in Switzerland reflects overdiagnosis, some patients presenting with less than 2 clinical signs.
The incidence of cardiac lesions in our cohort is higher than 8%, which is the number reported in the last Japanese survey, covering the years 2015 and 2016 [7]. The 2004 AHA guidelines included perivascular brightness in the positive echocardiographic findings in Kawasaki disease but, due to low inter- and intra-observer reliability and unclear significance [25], it was removed from the 2017 recommendations [2]. In our cohort, 18 (10.3%) patients presented with perivascular brightness as a unique echocardiographic abnormality. It is possible that these children were overdiagnosed with Kawasaki disease. For the counting of the coronary artery lesions, we decided to inventory each coronary artery separately, each lesion being a separate risk factor for subsequent myocardial infarction. In our cohort, 17 (9.7%) children presented bilateral coronary artery lesions. Even by adjusting our definition to the Japanese one, which considers only coronary aneurysms/dilatations/stenosis, valvular lesions, and myocardial infarcts (our definition included perivascular brightness and pericardial effusion as well), the incidence of cardiac lesions would still be significantly higher in the present study. The results of a recent Spanish report are more consistent with ours [26]. According to data derived from both Japanese and Caucasian children [27, 28], early IVIG administration (defined as administration before day 5 of illness) is associated with a decreased incidence of coronary artery lesions. In the Japanese study, children received IVIG on day 5 of the illness. In both the currently presented Swiss cohort and the Spanish study, they are treated later. The promptness of diagnosis and treatment in Japan could explain that difference. The implementation of the new AHA 2017 guidelines, in which diagnosis can be made before 5 days of fever, might therefore help in reducing the incidence of coronary artery lesions.
In this study, the diagnosis was usually made timely so as to administer IVIGs within day 10 of fever. The international recommendations for the treatment were followed, and the vast majority of the patients received IVIG and acetylsalicylic acid. In our cohort, the number of patients who needed a second dose of IVIG is higher than the 15-20% described by Li et al. in their meta-analysis [29]. Unlike the AHA recommendations, which define IVIG resistance as persistence or recrudescence of fever after 36 hours after completion of the first IVIG infusion, most of our patients received the second IVIG infusion after only 24 hours. The higher incidence of IVIG refractory patients in our study could reflect hasty retreatment.
The management of the cases which did not respond to the first IVIG infusion was heterogeneous: as an alternative to a second dose of IVIG, resistant cases were treated with corticosteroids, infliximab and/or rituximab. The international recommendations from 2004 [3] suggest as the first option a second dose of IVIG, given that it has a dose-response effect (low level of evidence). Steroids are recommended if the second IVIG infusion fails to resolve the fever. Even though corticosteroids added to IVIG for the initial treatment in patients with severe Kawasaki disease based on prediction scores lower the rate of coronary arteries lesions [30], the efficacy of corticosteroids to treat IVIG-resistant children is not clearly established [2]. Infliximab is described as an option under investigation. The 2017 update [2] adds that, after a phase I study, Infliximab is considered a safe alternative to a second IVIG infusion. Successful use of Rituximab is described only in 1 case report [31].
Comparison between this Swiss national cohort and the one analysing 30 years of retrospective data in our centre in Lausanne [15] shows similar demographic data and clinical features at diagnosis. The increase in the proportion of incomplete Kawasaki disease over time described in the retrospective cohort seems to be confirmed by this study. The proportion of abnormal initial echocardiography was more than 10% higher in the “historical” cohort than in this study. The different way to describe TTE anomalies in these two studies doesn’t allow a good comparison but perivascular brightness was described in 53% of patients in the retrospective study but in only 23% in this one. Since the diagnosis was made around day 7 of symptoms for both groups, a significant delay in performing the exam doesn’t explain this difference. The appreciation of perivascular brightness is very subjective and poorly reproducible. For this reason perivascular brightness is not part of the 2017 recommendations (see above).
The subgroup analysis showed that in very young (< 1year old) and older children (>8 years old), the diagnosis of Kawasaki disease, and therefore the initiation of treatment, is often delayed. The same subgroup has more cardiac anomalies and coronary lesions. Given that this study was not designed to assess causality, we can only hypothesise about the link between treatment delay and coronary lesions. It is worth noting that retrospective American studies show an increase in coronary artery lesions in children under 1 and over 10 years old without precise description of timing of IVIG administration [14, 32, 33].
This study has limitations. The first is the relatively high number of missing data regarding the number of days of fever, leading to the exclusion of these patients from the study and thus potentially leading to a slight underestimation of the incidence of Kawasaki disease. This is even more true since these patients would probably have been included as Kawasaki disease under the new AHA guidelines.
The second important limitation is that coronary artery z-scores were not part of the initial data collection form. The questionnaire asked only for qualitative assessment and size of the coronary arteries, which was rarely documented if the qualitative assessment was normal.
The main strength of this study is its prospective, nationwide coverage. Furthermore, an outstanding response rate was achieved. This demonstrates the indispensable work of national organisations like the SPSU which make paediatric studies on infrequent diseases possible.
Finally, this is the first epidemiological study of Kawasaki disease in Switzerland.
Further follow-up of this cohort will allow researchers to determine the medium and long-term outcomes of these patients.
Acknowledgements
We thank the representatives of theSwiss Pediatric Surveillance Unit (SPSU): M. Albisetti, W. Bär, M. Bianchetti, L. Buetti, H. U. Bucher, F. Cachat, A. Castiglioni, C. Däster, P. Diebold, Z. Dovhunovà, S. Ferroni, S. Fluri, M. Gebauer, M. Gehri, E. Giannoni, S. Grupe, L. Hegi, K. Held-Egli, P. Imahorn, T. Karen, C. Kind, L. Kottanattu, B. Laubscher, U. Lips, H. Madlon, V. Maghaouri-Slim, A. Malzacher, J. McDougall, J.-C. Minet, M. Mönkhoff, A. Moser, A. Niederer, V. Pezzoli, N. Piol, K. Posfay Barbe, G. Ramos y Munoz, L. Reinhard, T. Riedel, H. Roten, C. Rudin, K. P. Rühs, M. Russo, V. Schlumbom, N. Schöbi, S. Stirnemann, E. Süess, G. Simonetti, C. Stüssi, R. Tabin, M. Tomaske, R. Villiger, S. Wellmann, J. Wildhaber, M. Wopmann, G. Zeilinger, S.-A. Zoubir, A. Zemmouri, the SPSU board: C. Rudin, V. Bernet, C. Posfay Barbe, I. Bolt, B. Laubscher, G. Simonetti, M. Mäusezahl, D. Beeli and all the dedicated physicians for taking care of the patients and helping to complete the questionnaires.
Prof. Nicole Sekarski
Pediatric Cardiology Unit
Mother-Child Department
University Hospital of Lausanne
CH-1011 Lausanne
nicole.sekarski[at]chuv.ch
References
1.
Son MB
,
Newburger JW
. Kawasaki disease. Pediatr Rev. 2013 Apr;34(4):151–62. https://doi.org/10.1542/pir.34-4-151 https://doi.org/10.1542/pir.34.4.151
2.
McCrindle BW
,
Rowley AH
,
Newburger JW
,
Burns JC
,
Bolger AF
,
Gewitz M
, et al.; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention
. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017 Apr;135(17):e927–99. https://doi.org/10.1161/CIR.0000000000000484
3.
Newburger JW
,
Takahashi M
,
Gerber MA
,
Gewitz MH
,
Tani LY
,
Burns JC
, et al.; Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease; Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics
. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004 Oct;110(17):2747–71. https://doi.org/10.1161/01.CIR.0000145143.19711.78
4.
Holve TJ
,
Patel A
,
Chau Q
,
Marks AR
,
Meadows A
,
Zaroff JG
. Long-term cardiovascular outcomes in survivors of Kawasaki disease. Pediatrics. 2014 Feb;133(2):e305–11. https://doi.org/10.1542/peds.2013-1638
5.
Iemura M
,
Ishii M
,
Sugimura T
,
Akagi T
,
Kato H
. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000 Mar;83(3):307–11. https://doi.org/10.1136/heart.83.3.307
6.
Chen KY
,
Curtis N
,
Dahdah N
, et al
(2016) Kawasaki Disease and Cardiovascular Risk: A Comprehensive Review of Subclinical Vascular Changes in the Longer Term. Acta Paediatr n/a-n/a. https://doi.org/https://doi.org/10.1111/apa.13367
7.
Makino N
,
Nakamura Y
,
Yashiro M
,
Kosami K
,
Matsubara Y
,
Ae R
, et al.
Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015-2016. Pediatr Int. 2019 Apr;61(4):397–403. https://doi.org/10.1111/ped.13809
8.
Uehara R
,
Belay ED
. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79–85. https://doi.org/10.2188/jea.JE20110131
9.
Holman RC
,
Christensen KY
,
Belay ED
, et al
(2010) Racial/Ethnic Differences in the Incidence of Kawasaki Syndrome among Children in Hawai‘i. 69:4
10.
Uehara R
,
Yashiro M
,
Nakamura Y
,
Yanagawa H
. Kawasaki disease in parents and children. Acta Paediatr. 2003 Jun;92(6):694–7. https://doi.org/10.1111/j.1651-2227.2003.tb00602.x
11.
McCrindle BW
,
Li JS
,
Minich LL
,
Colan SD
,
Atz AM
,
Takahashi M
, et al.; Pediatric Heart Network Investigators
. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007 Jul;116(2):174–9. https://doi.org/10.1161/CIRCULATIONAHA.107.690875
12.
Haycock GB
,
Schwartz GJ
,
Wisotsky DH
. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978 Jul;93(1):62–6. https://doi.org/10.1016/S0022-3476(78)80601-5
13.
Makino N
,
Nakamura Y
,
Yashiro M
,
Ae R
,
Tsuboi S
,
Aoyama Y
, et al.
Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239–45. https://doi.org/10.2188/jea.JE20140089
14.
Belay ED
,
Maddox RA
,
Holman RC
,
Curns AT
,
Ballah K
,
Schonberger LB
. Kawasaki syndrome and risk factors for coronary artery abnormalities: united States, 1994-2003. Pediatr Infect Dis J. 2006 Mar;25(3):245–9. https://doi.org/10.1097/01.inf.0000202068.30956.16
15.
de La Harpe M
,
di Bernardo S
,
Hofer M
,
Sekarski N
. Thirty Years of Kawasaki Disease: A Single-Center Study at the University Hospital of Lausanne. Front Pediatr. 2019 Jan;7:11–11. https://doi.org/10.3389/fped.2019.00011
16. SPSU_Annual Report 2017.pdf
17.
Singh S
,
Vignesh P
,
Burgner D
. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015 Nov;100(11):1084–8. https://doi.org/10.1136/archdischild-2014-307536
18.
Rowley AH
,
Shulman ST
. The Epidemiology and Pathogenesis of Kawasaki Disease. Front Pediatr. 2018 Dec;6:374. https://doi.org/10.3389/fped.2018.00374
19.
Burns JC
,
Cayan DR
,
Tong G
,
Bainto EV
,
Turner CL
,
Shike H
, et al.
Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005 Mar;16(2):220–5. https://doi.org/10.1097/01.ede.0000152901.06689.d4
20.
Heaton P
,
Wilson N
,
Nicholson R
,
Doran J
,
Parsons A
,
Aiken G
. Kawasaki disease in New Zealand. J Paediatr Child Health. 2006 Apr;42(4):184–90. https://doi.org/10.1111/j.1440-1754.2006.00827.x
21.
Tulloh RM
,
Mayon-White R
,
Harnden A
,
Ramanan AV
,
Tizard EJ
,
Shingadia D
, et al.
Kawasaki disease: a prospective population survey in the UK and Ireland from 2013 to 2015 [Erratum in: Arch Dis Child. 2020 Mar;105] [3] [:e5. PMID: 30104394]. Arch Dis Child. 2019 Jul;104(7):640–6. https://doi.org/10.1136/archdischild-2018-315087
22.
Saundankar J
,
Yim D
,
Itotoh B
,
Payne R
,
Maslin K
,
Jape G
, et al.
The epidemiology and clinical features of Kawasaki disease in Australia. Pediatrics. 2014 Apr;133(4):e1009–14. https://doi.org/10.1542/peds.2013-2936
23.
Oh JH
. Understanding Kawasaki Disease on the Ground of Pediatric Growth and Lymphoid Tissue Maturation. Korean Circ J. 2017 Jan;47(1):29–30. https://doi.org/10.4070/kcj.2016.0408
24.
Yu JJ
. Diagnosis of incomplete Kawasaki disease. Korean J Pediatr. 2012 Mar;55(3):83–7. https://doi.org/10.3345/kjp.2012.55.3.83
25.
Rabinowitz EJ
,
Rubin LG
,
Desai K
,
Hayes DA
,
Tugertimur A
,
Kwon EN
, et al.
Examining the Utility of Coronary Artery Lack of Tapering and Perivascular Brightness in Incomplete Kawasaki Disease. Pediatr Cardiol. 2019 Jan;40(1):147–53. https://doi.org/10.1007/s00246-018-1971-z
26.
Fernandez-Cooke E
,
Barrios Tascón A
,
Sánchez-Manubens J
,
Antón J
,
Grasa Lozano CD
,
Aracil Santos J
, et al.; KAWA-RACE study group
. Epidemiological and clinical features of Kawasaki disease in Spain over 5 years and risk factors for aneurysm development. (2011-2016): KAWA-RACE study group. PLoS One. 2019 May;14(5):e0215665. https://doi.org/10.1371/journal.pone.0215665
27.
Abrams JY
,
Belay ED
,
Uehara R
,
Maddox RA
,
Schonberger LB
,
Nakamura Y
. Cardiac Complications, Earlier Treatment, and Initial Disease Severity in Kawasaki Disease. J Pediatr. 2017 Sep;188:64–9. https://doi.org/10.1016/j.jpeds.2017.05.034
28.
Tse SM
,
Silverman ED
,
McCrindle BW
,
Yeung RS
. Early treatment with intravenous immunoglobulin in patients with Kawasaki disease. J Pediatr. 2002 Apr;140(4):450–5. https://doi.org/10.1067/mpd.2002.122469
29.
Li X
,
Chen Y
,
Tang Y
,
Ding Y
,
Xu Q
,
Sun L
, et al.
Predictors of intravenous immunoglobulin-resistant Kawasaki disease in children: a meta-analysis of 4442 cases. Eur J Pediatr. 2018 Aug;177(8):1279–92. https://doi.org/10.1007/s00431-018-3182-2
30.
Chen S
,
Dong Y
,
Yin Y
,
Krucoff MW
. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2013 Jan;99(2):76–82. https://doi.org/10.1136/heartjnl-2012-302126
31.
Sauvaget E
,
Bonello B
,
David M
,
Chabrol B
,
Dubus JC
,
Bosdure E
. Resistant Kawasaki disease treated with anti-CD20. J Pediatr. 2012 May;160(5):875–6. https://doi.org/10.1016/j.jpeds.2012.01.018
32.
Ghimire LV
,
Chou FS
,
Mahotra NB
,
Sharma SP
. An update on the epidemiology, length of stay, and cost of Kawasaki disease hospitalisation in the United States. Cardiol Young. 2019 Jun;29(6):828–32. https://doi.org/10.1017/S1047951119000982
33.
Callinan LS
,
Tabnak F
,
Holman RC
,
Maddox RA
,
Kim JJ
,
Schonberger LB
, et al.
Kawasaki syndrome and factors associated with coronary artery abnormalities in California. Pediatr Infect Dis J. 2012 Sep;31(9):894–8. https://doi.org/10.1097/INF.0b013e31825c4d7c



