Assessment of SARS-CoV-2 tests costs and reimbursement tariffs readjustments during the COVID-19 pandemic
DOI: https://doi.org/10.4414/SMW.2022.w30168
Giorgia
Caruanaa, René
Brouilleta, Onya
Opotaa, Gilbert
Greubab
aInstitute of Microbiology, Lausanne University Hospital and University of Lausanne, Switzerland
bService of Infectious Diseases, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Summary
INTRODUCTION: While laboratories have been facing limited supplies of reagents for diagnostic tests throughout the course of the COVID-19 pandemic, national and international health plans, as well as billing costs, have been constantly adjusted in order to optimize the use of resources. We aimed to assess the impact of SARS-CoV-2 test costs and reimbursement tariff adjustments on diagnostic strategies in Switzerland to determine the advantages and disadvantages of different costs and resource saving plans.
MATERIALS AND METHODS: We specifically assessed the cost of diagnostic SARS-COV-2 RT-PCR using five different approaches: i) in-house platform, ii) cobas 6800® (Roche, Basel, Switzerland), iii) GeneXpert® SARS-CoV-2 test (Cepheid, Sunnyvale, CA, USA), iv) VIASURE SARS-CoV-2 (N1 + N2) Real-Time PCR Detection Kit for BD MAX™ (Becton Dickinson, Franklin Lake, NJ, USA), v) cobas® Liat® SARS-CoV-2 & Influenza A/B (Roche, Basel, Switzerland). We compared these costs to the evolution of the reimbursement tariffs.
RESULTS: The cost of a single RT-PCR test varied greatly (as did the volume of tests performed), ranging from as high as 180 CHF per test at the beginning of the pandemic (February to April 2020) to as low as 82 CHF per test at the end of 2020. Depending on the time period within the pandemic, higher costs did not necessarily mean greater benefits for the laboratories. The costs of molecular reagents for rapid tests were higher than of those for classic RT-PCR platforms, but the rapid tests had reduced turnaround times (TATs), thus improving patient care and enabling more efficient implementation of isolation measures, as well as reducing the burden of possible nosocomial infections. At the same time, there were periods when the production or distribution of these reagents was insufficient, and only the use of several different molecular platforms allowed us to sustain the high number of tests requested.
CONCLUSIONS: Cost-saving plans need to be thoroughly assessed and constantly adjusted according to the epidemiological situation, the clinical context and the national resources in order to always guarantee that the highest performing diagnostic solutions are available. Not all cost-saving strategies guarantee good analytical performance.
Introduction
SARS-CoV-2 joined the already quite long list of (14 or more) pandemics since 1500 [1]. Lessons from the past meant it was clear from the beginning of the epidemic that measures for minimizing deaths were not compatible with the minimization of the economic impact of COVID-19 [2]. Economists hypothesized three main mechanisms for how this virus could affect the global economy: i) by directly influencing production (reduced supply), ii) by affecting supply chains and market disruption and iii) by its financial impact on markets (increased demand) [3]. These mechanisms are now affecting the supply and demand of COVID-19 vaccines and have been present throughout the past year at a laboratory level for molecular reagents, as well as for swabs and viral transport media.
Indeed, throughout the SARS-CoV-2 pandemic, all laboratories played a critical role but simultaneously faced limited supplies of reagents for diagnostic tests, with most of them struggling to meet growing demand [4]. At the same time, national and international health plans, as well as billing costs, were constantly adjusted throughout the course of the pandemic in order to optimize the use of resources. Figure 1 shows the example of variations in Swiss billing costs for the molecular diagnosis of SARS-CoV-2 infection during the past year. This not only induced a certain level of instability in the management of laboratories due to changes in the number of tests they were required to perform each day depending on the epidemiologic period, but also led to a series of problems related to health insurance billing costs for the population.
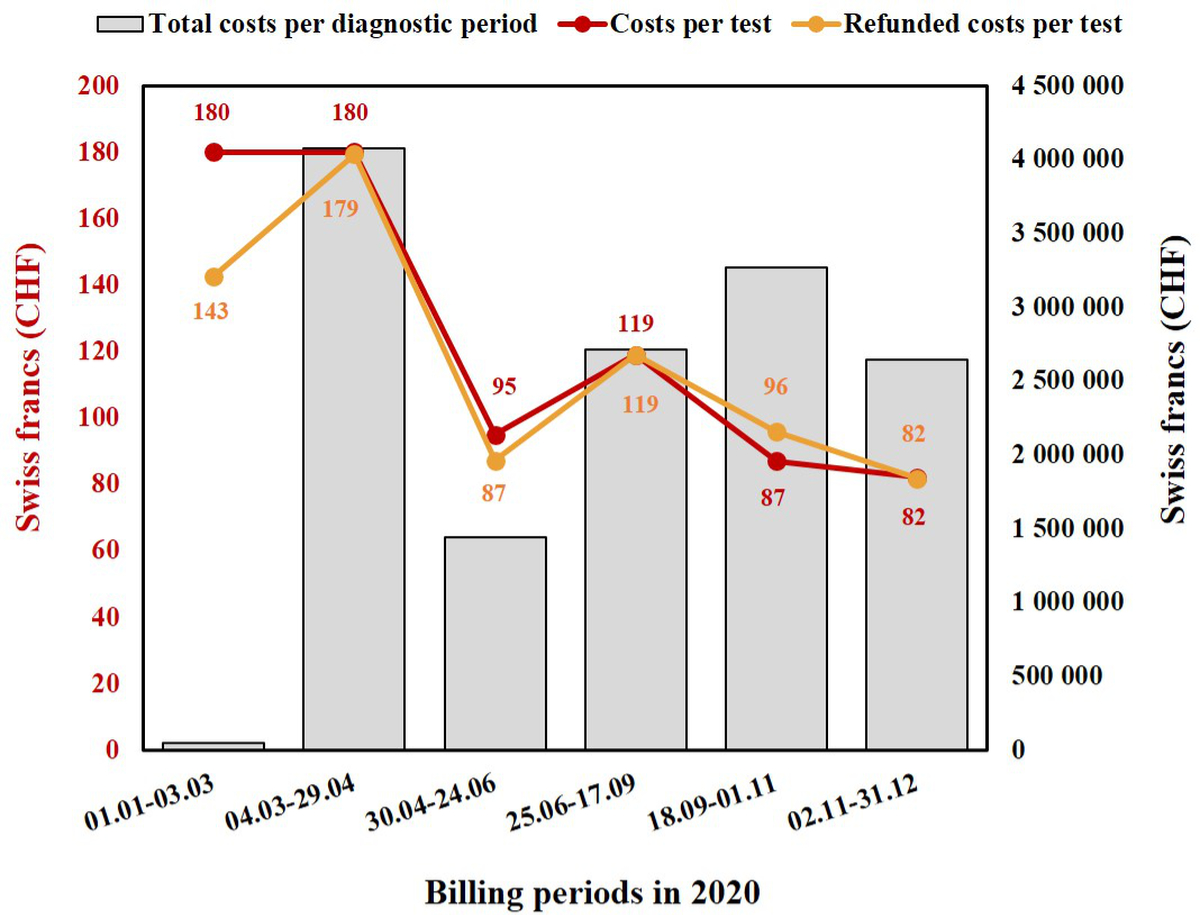
Figure 1 Changes in billing costs per RT-PCR (CHF) and total cumulative costs for all RT-PCRs per billing period throughout 2020 at Lausanne University Hospital (Switzerland).
Grey columns represent the total amount of money spent for each billing period, based on the total number of SARS-CoV-2 RT-PCR tests performed. Each red dot represents the cost per SARS-CoV-2 RT-PCR test for that precise billing period, while orange dots represent the refunded costs per test for each billing period.
In Switzerland, during a pandemic billing costs are determined based on the Epidemics Act, which is part of the communicable disease legislation [5]. Through this, the Swiss Confederation’s Federal Council creates the organizational, professional and financial frameworks required to detect, monitor, prevent and control communicable diseases. The costs are mainly based on the rates already used for similar analyses [6] and are subsequently adapted based on the number of tests required as well as the number of tests performed, while also taking into consideration the level of laboratory automation and the eventual purchase costs of new machinery.
At Lausanne University Hospital, we started testing patients from 14 February using our in-house molecular platform [7]. From February 25, following the detection of the first SARS-CoV-2 positive patient in Switzerland, the number of analyses required rapidly increased and in order to be able to respond to the constantly increasing demand, we had to recruit more laboratory technicians and also adapt our diagnostic workflow by using several molecular platforms [8, 9].
With this study, we aimed to assess the impact of test costs and adjustment scales on diagnostic strategies in Switzerland throughout the first year of the SARS-CoV-2 pandemic (2020) in order to determine the advantages and disadvantages of different costs and resource saving plans. Such findings would be applicable at an international level and would also provide an insight into laboratory management during SARS-CoV-2 pandemics.
Methods
Definitions
Reagent costs per test correspond to the effective costs paid to the manufacturers for the reagents needed to perform each SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) test.
Test reimbursement tariffs describes the amount, in Swiss francs (CHF), reimbursed to the diagnostic laboratories for each test performed.
The burden of reagent costs on reimbursements was defined as the ratio between the RT-PCR reagent costs per test and the total amount reimbursed to laboratories per test.
Technician-related costs were not included in our analysis.
The order tax, an administrative tax only applied to outpatient subjects, was not included in the tariff and costs analysis.
Molecular techniques
SARS-COV-2 real-time RT-PCR was performed on one of the following five molecular platforms: i) our automated, high-throughput molecular diagnostic (MDx) platform [7, 10], ii) cobas 6800® SARS-CoV-2 (Roche, Basel, Switzerland) [8], iii) GeneXpert® SARS-CoV-2 test (Cepheid, Sunnyvale, CA, USA) [11], iv) VIASURE SARS-CoV-2 (N1 + N2) Real-Time PCR Detection Kit for BD MAX™ (Becton Dickinson, Franklin Lake, NJ, USA), v) cobas® Liat® SARS-CoV-2 & Influenza A/B (Roche, Basel, Switzerland).
Laboratory activity
Laboratory costs and the number of tests performed for SARS-CoV-2 molecular diagnosis were obtained through our laboratory information system (LIS). In Switzerland, the costs are “rated” by the Federal Office of Social Insurances (abbreviated “OFAS” in French): one OFAS point roughly corresponds to 1 CHF (which currently – in February 2022 – corresponds to 0.91 Euro).
Ethical declaration
This article was prepared according to STANDARD guidelines for diagnostic accuracy studies reporting. The data on laboratory costs and performance were obtained partially through a quality project at our institution (CHUV, Lausanne, Switzerland), as well as from the routine budget evaluation at our institute. According to national law (Swiss Federal Act on Human Research), the performance and publishing of the results of such a project can be done without asking for the permission of the competent research ethics committee.
Results
The burden (performances and costs) of laboratory activity for the molecular diagnosis of SARS-CoV-2 infection was evaluated from 1 January 2020 to 31 December 2020. Six billing periods were identified for 2020, each one lasting about two months. During these periods, the cost of a single RT-PCR test varied greatly (as did the volume of tests performed), ranging from as high as 180 CHF per test at the beginning of the pandemic (February to April) to as low as 82 CHF per test at the end of the year (fig. 1). At the same time, reimbursement at the regional or national level varied as well, and not necessarily accordingly (fig. 1). The first two billing periods (up to the end of April, during the first wave of infections) had higher billing costs and proportionally lower reimbursements, and also corresponded to the time when test costs were covered by personal health insurance. Notably, during the initial period, the social insurance system did not systematically reimburse our laboratory, since officially there was no tariff until 3rd March 2020. Thus, the mean effective reimbursement was not 180 CHF (which, for comparison, is what we billed for influenza RT-PCR tests), but only 143 CHF. Then, starting from 4th March, nearly all the tests we billed were reimbursed, explaining why the reimbursement rate (179 CHF) was close to the official tariff of 180 CHF. The remaining small difference was likely due to the few subjects without health insurance.
Starting from May 2020, the test costs were covered by the government, first (May and June) at a regional and then (from the end of June 2020) at a national level. At the time of the second wave (September to December 2020), laboratories and facilities (including instruments and technical personnel) had already been adapted to process a large number of samples, explaining the reduction in the cost per test and the corresponding reimbursement (figures 1 and 2).
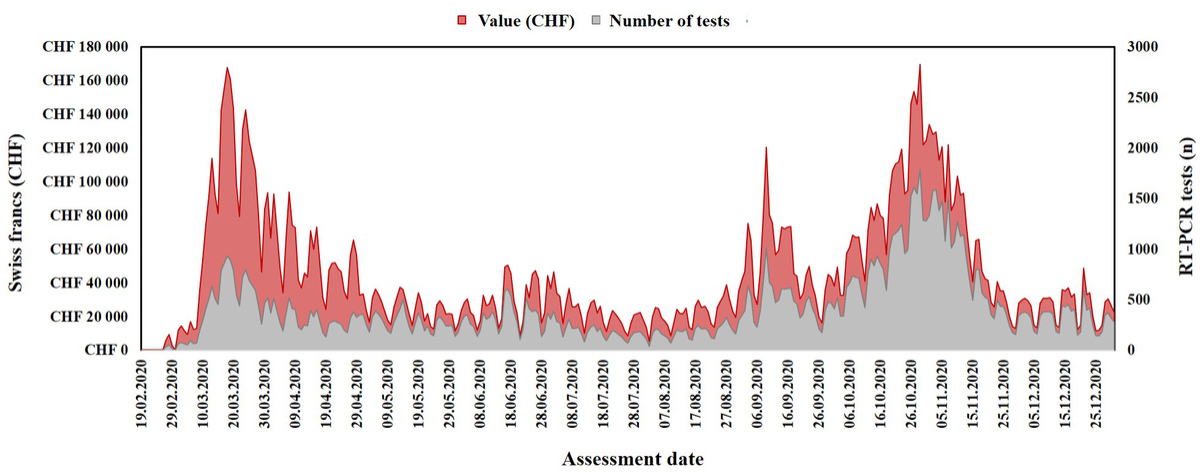
Figure 2 Laboratory reimbursements for SARS-CoV-2 RT-PCR tests compared to the number of tests performed throughout 2020 at Lausanne University Hospital (Switzerland).
The evaluation starts after 14 February 2020, when SARS-CoV-2 RT-PCR first became available at our institute. Data were collected per 10 days period.
The presence of a fully automated molecular diagnostic platform and our experience in the development of specific RT-PCRs allowed us to respond quickly to the crisis, which meant we were able to handle a huge number of samples per day during the initial period (February and March 2020). This was done despite the absence, at that time, of any SARS-CoV-2 test compatible with one of the industrial systems already present in our institute, such as cobas (Roche, Switzerland). Subsequently, the use of several molecular platforms allowed us to sustain the high number of tests requested and, at the same time, to cope with the lack of reagents, which was luckily limited to one rapid molecular platform (GeneXpert®). As shown in figure 3, from 24th March 2020 the most used molecular platform was the cobas 6800©. This instrument was already present in our laboratory and was chosen for SARS-CoV-2 RT-PCR because of its convenient bilateral connection to our LIS and its shorter turnaround time (TAT) due to its fully closed (from extraction to amplification) automated system [8]. The implementation of further rapid molecular systems (Cobas® Liat® and BD MAX™) was only possible during the last months of 2020 (fig. 3), and to date, cobas®, Liat® and GeneXpert® still provide the majority of rapid SARS-CoV-2 screening. The costs of rapid molecular reagents are naturally higher than those for conventional RT-PCR platforms such as cobas, and this needs to be taken into account when assessing the burden of these costs on the reimbursements (tab. 1). However, the higher reagent costs are offset by the reduced TAT, which improves patient care and the efficient implementation of isolation measures. Looking at the variation in costs (fig. 1), it is clear that depending on the time period within the pandemic (and hence the test demand) and the number/type of diagnostic platforms used, higher test costs do not necessarily correspond to a higher reimbursement for the laboratory, and this will naturally have an impact on the laboratory’s general expenditure budget. This impact is especially important in laboratories with low volumes of analyses and analyses which are not fully automated.
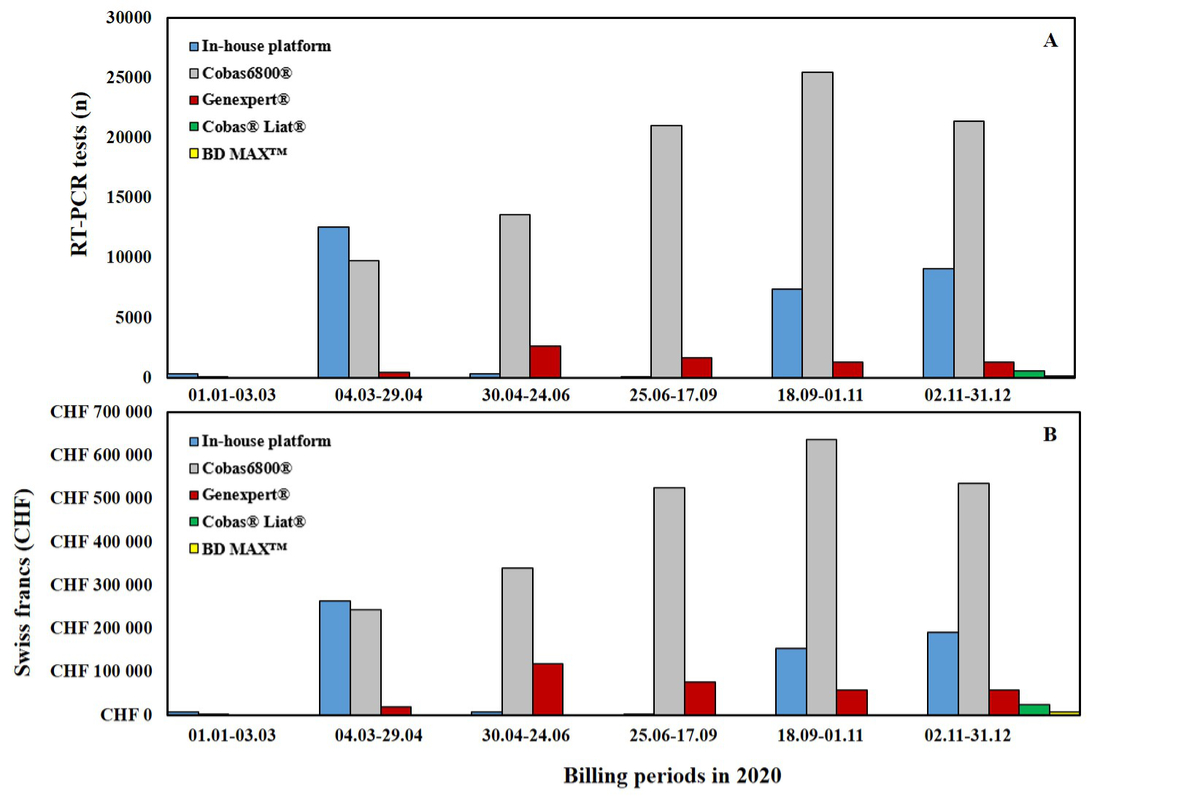
Figure 3 Variations in the number of RT-PCR tests performed and the cumulative costs per molecular platform per billing period throughout 2020 at Lausanne University Hospital (Switzerland).
A) Cumulative number of RT-PCR tests performed per molecular platform per billing period.
B) Cumulative costs (Swiss francs) of RT-PCR tests performed per molecular platform per billing period.
Table 1Reagent costs for different molecular platforms and the percentage these costs account for of the reimbursement per test according to the official tariff during the first wave (180 CHF) and during the last two months of 2020 (82 CHF).
|
Platform
|
Total number of tests
|
Time to result (hours)
|
Reagent costs per test (CHF)
|
Burden of reagent costs on reimbursement
|
|
Tarif per test: 180 CHF
|
Tarif per test: 82 CHF
|
| In-house platform |
29,738 |
~ 4.3 |
21 |
11.70% |
25.70% |
| Cobas6800®
|
91,168 |
~ 3.4 |
25 |
13.90% |
30.60% |
| Genexpert®
|
7,246 |
~ 0.75 |
45.25 |
25.20% |
55.40% |
| Cobas® Liat® |
570 |
~ 0.33 |
42 |
– |
51.50% |
| BD MAX™ |
120 |
~ 2 |
53 |
– |
65% |
Discussion
By closely assessing the variations in RT-PCR test costs and their reimbursements, we were able to highlight the economic impact of cost adjustment scales on our laboratory.
The test costs were naturally higher at the beginning of the pandemic, when (i) RT-PCR tests specific to the newly discovered pathogen were under development, (ii) these tests were the only diagnostic method and (iii) demand was still low due to the lower number of cases, making the hands-on time per test much larger. Despite the high reimbursement costs observed during this period, these were often not high enough to cover the laboratory’s testing expenses in the short term, when the number of RT-PCR tests requested was very low (less than two tests per day). Subsequently, as the virus spread in Switzerland, the diagnostic demand grew rapidly and the laboratory had to increase its number of lab technicians and technology, acquiring new machines. In this phase, the costs were still high because of the costs of new machine purchases, as well as initial adjustments in the laboratory workflow (digitalization of the process and increased lab technicians’ working hours). In Switzerland, the healthcare system relies on private insurance. As in all private healthcare-based systems, sicker people’s access to required investigations and treatments is economically facilitated through lower deductibles. This meant that during the early phases of the pandemic, when the test costs were billed to private healthcare insurance companies, people with comorbidities, and thus low deductibles, were more likely to be tested compared to their healthy counterparts. This determined and impaired SARS-CoV-2 screening and likely caused a general under-diagnosis in the otherwise healthy population [12]. Also, due to the relatively limited amount of the RT-PCR reagents available, only contacts of positive cases and symptomatic individuals at risk of unfavourable outcome were then invited to be tested, adding a further bias to the testing strategy.
As we have shown for Switzerland, once they had adapted their technology and personal to the new demands caused by the ongoing pandemic and with a very high demand for tests, the test costs decreased without a negative impact on laboratories. It is very important, however, that economic strategies have a minimal impact on test performance. In October 2021, almost one and a half years after the beginning of the pandemic and with a disease prevalence of around 3%, the Swiss national directives provided for total coverage of the costs of RT-PCR screening only for patients with COVID-19 symptoms. For COVID-19 asymptomatic patients, the costs of screening with RT-PCR tests were left to the tested individuals, unless the laboratory performed specimens pooling. At the same time, this asymptomatic population could be provided with five rapid antigenic self-tests per month free of charge. This strategy has been criticized for multiple reasons. First, specimen pooling can constitute a cost-effective solution with a minor decrease in sensitivity only if limited to low resource settings with very low (≤1%) disease prevalence [13, 14]. This is due to the fact that each positive pool needs to be dissolved (re-tested sample by sample), which is time consuming (drastically increasing the costs) and increases the risk of contamination. For this reason, the decision to implement this strategy must be considered very carefully, especially in light of the ongoing epidemiological situation which has proved, over the last year, to change very quickly (to date, February 2022, the prevalence is estimated at around 40%). Second, rapid antigen tests (RATs) have been shown to maintain good performance for population screening only within the first four days of symptoms and for viral loads above 1 million copies per ml [15]. They are not effective when assessing the COVID-19 asymptomatic population, with sensitivities for this group as low as 28–33% [16, 17]. Hence, RAT screening strategies are recommended mainly in symptomatic outpatient settings during the first few days of symptoms [18], especially where access to molecular methods is limited. Despite clear recommendations from the Swiss Federal Office of Public Health and from the Swiss Society of Microbiology to only use antigen tests in the symptomatic population within the first four days of symptoms [18, 19], RATs still represent the test of choice in some screening centres, including for asymptomatic subjects, based on the assumption that RT-PCR is too expensive. This likely had a major negative impact from December 2020 to March 2021 by reducing the overall efficacy of testing to identify chains of transmission. Indeed, a recent epidemiological study showed that over one month, as many as 24 clusters of more than three cases in a population of 880,000 would not have been detected by using only RATs [20].
In conclusion, cost-saving plans need to be thoroughly assessed and constantly adapted according to the epidemiological situation, the clinical context and the national resources in order to always guarantee that the highest performing diagnostic solutions are available, bearing in mind that higher single test costs do not necessarily mean higher costs overall.
Data sharing statement
The data for this study are not shared publicly but can be provided upon reasonable request and for peer review.
Acknowledgements
We would like to thank all the staff of the Institute of Microbiology of the Lausanne University Hospital, including all the biomedical technicians of the molecular diagnostic laboratory for routine RT-PCRs.
Contribution: GC conceived and designed the study, performed the literature search, and drafted and revised the article. RB performed the data analyses. OO provided essential reagents and materials for the study. GG conceived and designed the study, provided essential reagents and materials and supervised the study. All authors critically revised the article and approved the submitted version.
Prof. Gilbert Greub
Institut Universitaire de Microbiologie
Département de médecine de laboratoire et pathologie (DMLP)
Centre Hospitalier Universitaire Vaudois (CHUV)
Rue du Bugnon 48
CH-1011 Lausanne
gilbert.greub[at]chuv.ch
References
1.
Taubenberger JK
,
Morens DM
. Influenza: the once and future pandemic. Public Health Rep. 2010 Apr;125(3 Suppl 3):16–26.
2.
Anderson RM
,
Heesterbeek H
,
Klinkenberg D
,
Hollingsworth TD
. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar;395(10228):931–4. https://doi.org/10.1016/S0140-6736(20)30567-5
3.
Bachman D
. The economic impact of COVID-19 (novel coronavirus). https://www2.deloitte.com/us/en/insights/economy/covid-19/economic-impact-covid-19.html, 2020 2021).
4.
Lippi G
,
Plebani M
. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks [CCLM]. Clin Chem Lab Med. 2020 Jun;58(7):1063–9. https://doi.org/10.1515/cclm-2020-0240
5. Bundesgesetz über die Bekämpfung übertragbarer Krankheiten des Menschen. Loi fédérale sur la lutte contre les maladies transmissibles de l’homme. Legge federale sulla lotta contro le malattie trasmissibili dell'essere umano., in: D.B.d.S.E.L.A.f.d.l.C.s.L.A.f.d.C. Svizzera. (Ed.) 2012, p. 28.
[6] Verordnung des EDI über Leistungen in der obligatorischen Krankenpflegeversicherung. Ordonnance du DFI sur les prestations dans l’assurance obligatoire des soins en cas de maladie. Ordinanza del DFI sulle prestazioni dell’assicurazione obbligatoria delle cure medico-sanitarie., in: D.E.D.d.I.L.D.f.d.l.i.I.D.f. dell’interno. (Ed.) 1995, p. 70.
7.
Greub G
,
Sahli R
,
Brouillet R
,
Jaton K
. Ten years of R&D and full automation in molecular diagnosis. Future Microbiol. 2016;11(3):403–25. https://doi.org/10.2217/fmb.15.152
8.
Opota O
,
Brouillet R
,
Greub G
,
Jaton K
. Comparison of SARS-CoV-2 RT-PCR on a high-throughput molecular diagnostic platform and the cobas SARS-CoV-2 test for the diagnostic of COVID-19 on various clinical samples. Pathog Dis. 2020 Nov;78(8):ftaa061. https://doi.org/10.1093/femspd/ftaa061
9.
Marquis B
,
Opota O
,
Jaton K
,
Greub G
. Impact of different SARS-CoV-2 assays on laboratory turnaround time. J Med Microbiol. 2021 May;70(5). https://doi.org/10.1099/jmm.0.001280
10.
Pillonel T
,
Scherz V
,
Jaton K
,
Greub G
,
Bertelli C
. Letter to the editor: SARS-CoV-2 detection by real-time RT-PCR, Euro Surveill 25(21) (2020).
11.
Moraz M
,
Jacot D
,
Papadimitriou-Olivgeris M
,
Senn L
,
Greub G
,
Jaton K
, et al.
Universal admission screening strategy for COVID-19 highlighted the clinical importance of reporting SARS-CoV-2 viral loads. New Microbes New Infect. 2020 Nov;38:100820. https://doi.org/10.1016/j.nmni.2020.100820
12.
Reddy SR
,
Ross-Degnan D
,
Zaslavsky AM
,
Soumerai SB
,
Wharam JF
. Impact of a high-deductible health plan on outpatient visits and associated diagnostic tests. Med Care. 2014 Jan;52(1):86–92. https://doi.org/10.1097/MLR.0000000000000008
13.
Wacharapluesadee S
,
Kaewpom T
,
Ampoot W
,
Ghai S
,
Khamhang W
,
Worachotsueptrakun K
, et al.
Evaluating the efficiency of specimen pooling for PCR-based detection of COVID-19. J Med Virol. 2020 Oct;92(10):2193–9. https://doi.org/10.1002/jmv.26005
14.
Ben-Ami R
,
Klochendler A
,
Seidel M
,
Sido T
,
Gurel-Gurevich O
,
Yassour M
, et al.; Hebrew University-Hadassah COVID-19 Diagnosis Team
. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin Microbiol Infect. 2020 Sep;26(9):1248–53. https://doi.org/10.1016/j.cmi.2020.06.009
15.
Caruana G
,
Croxatto A
,
Kampouri E
,
Kritikos A
,
Opota O
,
Foerster M
, et al.
Implementing SARS-CoV-2 Rapid Antigen Testing in the Emergency Ward of a Swiss University Hospital: the INCREASE Study. Microorganisms. 2021 Apr;9(4):798. https://doi.org/10.3390/microorganisms9040798
16.
Caruana G
,
Lebrun LL
,
Aebischer O
,
Opota O
,
Urbano L
,
de Rham M
, et al.
The dark side of SARS-CoV-2 rapid antigen testing: screening asymptomatic patients. New Microbes New Infect. 2021 Jul;42:100899. https://doi.org/10.1016/j.nmni.2021.100899
17.
Torres I
,
Poujois S
,
Albert E
,
Colomina J
,
Navarro D
. Evaluation of a rapid antigen test (Panbio™ COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin Microbiol Infect. 2021;27(4):636.e1–4. https://doi.org/10.1016/j.cmi.2020.12.022
18.
Adrian Egli RL
. Katia Jaton, Gilbert Greub, Recommendation of the Swiss Society of Microbiology for usage of SARS-CoV-2 specific antigen tests, Pipette - Swiss. Lab Med. 2020;6:18–20.
19.
FOPH
. Coronavirus : Tests. https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/testen.html, (accessed January 25.2021).
20.
Ladoy A
,
Opota O
,
Carron PN
,
Guessous I
,
Vuilleumier S
,
Joost S
, et al.
Size and duration of COVID-19 clusters go along with a high SARS-CoV-2 viral load : a spatio-temporal investigation in Vaud state, Switzerland, (2021) 2021.02.16.21251641.


