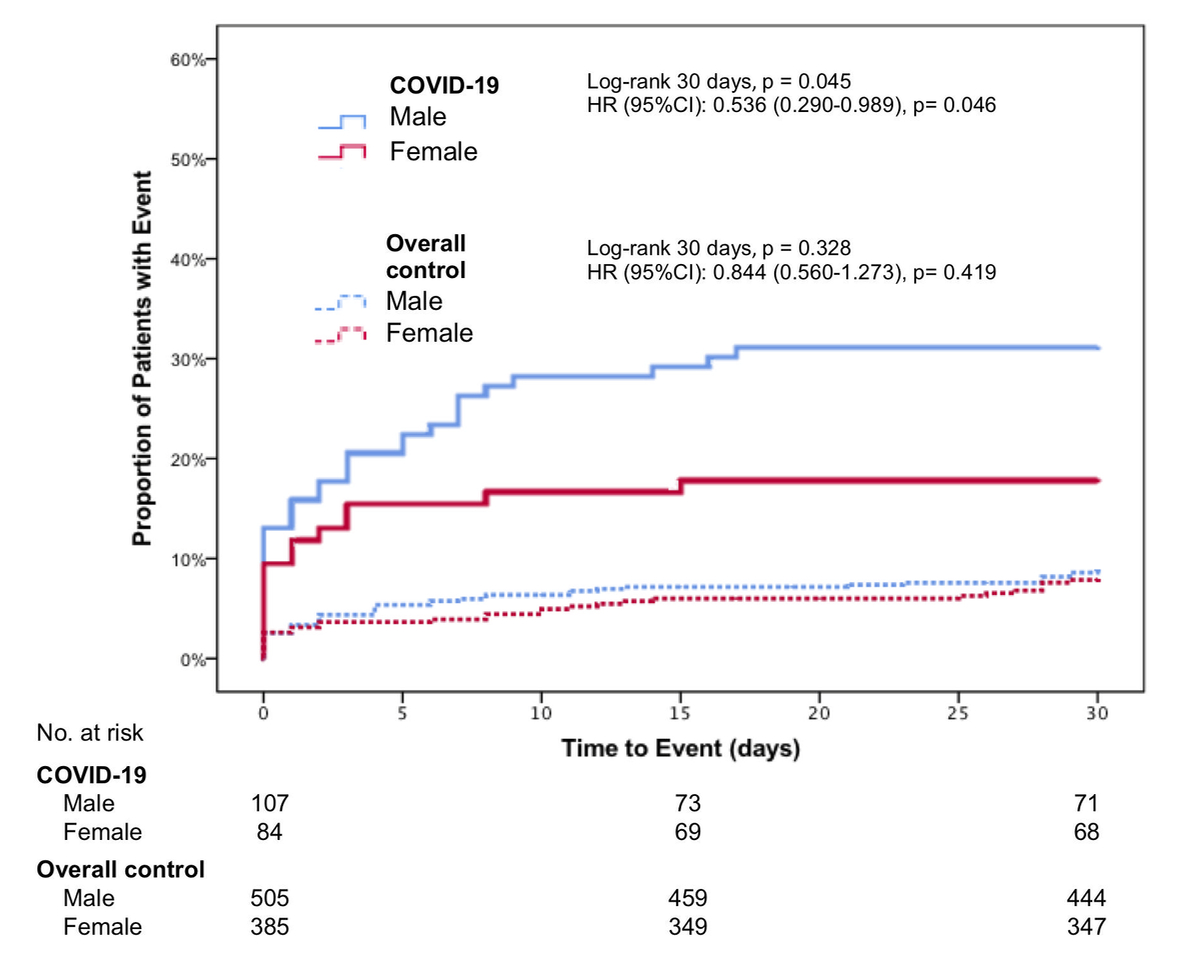
Figure 1 Composite outcome of admission to ICU, rehospitalization for respiratory complication and death within 30 days in COVID-19 cases and overall control; depicted hazard ratios (HR) are age-corrected.
DOI: https://doi.org/10.4414/SMW.2022.w30167
A novel pneumonia-like illness outbreak that started in China in December 2019 resulted in a worldwide pandemic, as declared by the World Health Organization on March 11, 2020. The cause was identified as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a positive-sense, single-stranded RNA virus [1]. Several studies have reported an association between male sex and severe outcome, including intensive care unit (ICU) admission and death, in SARS-CoV-2 infected patients [2–5]. On the other hand, there is little data providing information about sex differences in coronavirus disease 2019 (COVID-19) incidence. Recent global data reported by the Global Health 50/50 research initiative show that the infection rates seem to be comparable for both sexes [2, 6]. Regardless, most of these studies only included patients with confirmed SARS-CoV-2 infections and lacked adequate controls of patients with similar symptoms and disease severity but without SARS-CoV-2 infection. Thus, we aimed to investigate whether sex disparities in outcomes are present in patients with COVID-19, and whether these are specific to COVID-19 or generalizable to all patients with symptoms suggestive of COVID-19 infection upon ER presentation.
The prospective cohort COronaVIrus surviVAl (COVIVA, ClinicalTrials.gov NCT04366765) study included unselected patients aged 18 years or older presenting to the emergency room (ER) of the University Hospital Basel, Switzerland, with clinically suspected or confirmed SARS-CoV-2 infection during the first wave of the COVID-19 pandemic between March and June, 2020. All patients with clinical suspicion of COVID-19 underwent nasopharyngeal SARS-CoV-2 swab tests. Patients were considered SARS-CoV-2 positive (cases) if, in addition to clinical signs and symptoms of COVID-19, one or more SARS-CoV-2 PCR swab tests performed on the day of ER presentation or within 14 days prior to or after the ER presentation were positive. The remaining patients with only negative SARS-CoV-2 swab test results were considered as controls. All participating patients or their legally authorized representatives consented by signing a local general consent form. This study was conducted according to the principles of the Declaration of Helsinki and was approved by the local ethics committee (EKNZ identifier 2020–00566).
The authors designed the study, gathered and analysed the data according to the STROBE guidelines for reporting observational studies [7] (table S1 in the appendix), vouched for the data and analysis, wrote the paper, and decided to submit it for publication.
All patients underwent a thorough clinical assessment by the treating ER physician according to local standard operating procedures (SOPs). Vital parameters including heart rate, blood pressure, oxygen saturation and respiratory rate were assessed in every patient. The patients’ management was left to the discretion of the attending physicians in accordance with local SOPs, which did not contain any sex-specific recommendations.
Blood samples were routinely taken at the time of ER presentation in every patient (both cases and controls). Besides routine laboratory parameters, high-sensitivity troponin T (hs-cTn), natriuretic peptides, D-dimers, procalcitonin and ferritin were measured for every patient as part of the local SOP for suspected COVID-19 patients. The timing and type of subsequent laboratory measurements during hospital stay were left to the discretion of the treating physicians and were not part of this study’s protocol.
Thirty days after discharge, patients were contacted by telephone or in writing by research physicians or study nurses and information about current health, hospitalizations and adverse events were obtained using a predefined set of questions and item checklists. The records of other hospitals/ICUs and primary care physicians, as well as national death registries, were screened for additional information, if applicable.
The primary outcome measure was a composite of ICU admission, rehospitalization due to respiratory complications and all-cause death at 30 days. Secondary outcomes included the components of the composite primary endpoint, case management and length of hospital stay, as well as incidence of intubation, haemodynamic support and acute respiratory distress syndrome during the course of the index hospitalization.
To determine the final diagnosis that led to the index ER presentation and the clinical suspicion of COVID-19, trained physicians reviewed all medical data available, including 30 days post-discharge follow-up information, and chose from a predefined list the diagnosis which best fitted each patient. The predefined main categories included, but were not limited to, COVID-19, non-SARS-CoV-2 infections (e.g. other respiratory, gastrointestinal or urogenital infections), cardiovascular disease (acute coronary syndrome, rhythm disorder, congestive heart failure, pulmonary embolism), other non-infectious pulmonary disease (e.g. lung tumour, asthma, chronic obstructive pulmonary disease) and neurologic disease (e.g. stroke, seizure).
Data are expressed as medians and interquartile ranges (IQR) for continuous variables and as numbers and percentages (%) for categorical variables. All variables were compared using Mann–Whitney U tests for continuous variables and Pearson’s chi-squared or Fisher’s exact test for categorical variables, as appropriate. In this analysis, COVID-19 patients (cases) were compared to the unselected SARS-CoV-2 negative patients (overall control group, table S2 in the appendix), as well to the subgroup of patients with acute respiratory infections but no COVID-19 (respiratory control group, e.g. viral infection of the upper airways, bronchitis, pneumonia). The composite outcome was plotted in Kaplan–Meier curves and the log-rank test was used to assess differences between groups. The association between sex and the composite outcome was assessed using age-adjusted Cox proportional hazards models. In the case of multiple events, the time to the first event within 30 days was considered. Predefined subgroup analyses were performed for patients under and over 55 years of age and with or without cardiac disease, hypertension, diabetes, obesity, smoking and pneumopathy. The cut-off age of 55 years was chosen to assess sex disparities with respect to hormonal changes before and after menopause [8]. In a second multivariable Cox proportional hazards model, we aimed to further investigate the predictive value of sex if adjusted for age and numerous comorbidities using a stepwise-backward approach. The small sample size and number of events meant that this was not possible. Prespecified subgroup comparisons were performed using multivariable Cox models, including treatment with a covariable interaction term, and were summarized using a forest plot.
All hypothesis testing was two-tailed and p-values of less than 0.05 were considered significant. No correction for multiple testing was applied. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY).
From March 2020 to June 2020, 1,086 patients presented at the ER with symptoms suggesting SARS-CoV-2 infection (e.g. dyspnoea, coughing, fever). Follow-up at 30 days was complete in 1,081 patients, meaning 5 patients were excluded from the analysis (none of them with COVID-19, see figure S1 in the appendix). The baseline characteristics of the unselected study population are listed in table S3 in the appendix. Overall, 43.4% were female and 56.6% male (p <0.001). 191 patients (18%) tested positive for SARS-CoV-2, with a median age of 57 years (IQR 44–69 years; table 1). The prevalence of COVID-19 was similar in women (n = 84, 17.9%) and men (n = 107, 17.5%, p = 0.855).
Table 1Clinical characteristics.
| SARS-CoV-2 positive (Cases) | SARS-CoV-2 negative (Overall control group) | SARS-CoV-2 negative with respiratory infection (Respiratory control group) | |||||||
| All ( n = 191) | Female ( n = 84) | Male ( n = 107) | All ( n = 890) | Female ( n = 385) | Male ( n = 505) | All (n = 323) | Female (n = 142) | Male (n = 181) | |
| Age – years [IQR] | 57 [44,69] | 57 [41,67] | 58 [46,69] | 57 [38,74] | 61 [43,74] | 57 [44,69] | 59 [41,74] | 57 [38,74] | 61 [43,74] |
| Age ≥55 years | 103 (54%) | 45 (54%) | 58 (54%) | 499 (56%) | 201 (52%) | 298 (59%) | 174 (54%) | 70 (49%) | 104 (58%) |
| Risk factors and history | |||||||||
| Comorbidity burden – median [IQR] | 1 [0,3] | 1 [0,3] | 2 [0,3] | 2 [0,3] | 1 [0,3] | 2 [1,4] | 2 [0,3] | 1 [0,3] | 2 [1,4] |
| Cardiac disease | 38 (20%) | 11 (13%) | 27 (25%) | 261 (29%) | 85 (22%) | 176 (35%) | 92 (29%) | 30 (21%) | 62 (34%) |
| CAD | 21 (11%) | 2 (2%) | 19 (18%) | 131 (15%) | 26 (7%) | 105 (21%) | 43 (13%) | 11 (8%) | 32 (18%) |
| Hypertension | 81 (42%) | 33 (39%) | 48 (45%) | 367 (41%) | 146 (37.9%) | 221 (44%) | 142 (44%) | 58 (41%) | 84 (46%) |
| Smoking | 58 (30%) | 19 (23%) | 39 (36%) | 361 (41%) | 107 (28%) | 254 (50%) | 159 (49%) | 50 (35%) | 109 (60%) |
| COPD | 9 (5%) | 2 (2%) | 7 (7%) | 111 (12%) | 36 (9%) | 75 (15%) | 58 (18%) | 20 (14%) | 38 (21%) |
| Diabetes | 34 (18%) | 12 (14%) | 22 (21%) | 137 (15%) | 41 (11%) | 96 (19%) | 51 (16%) | 18 (13%) | 33 (18%) |
| Obesity | 74 (39%) | 30 (36%) | 44 (41%) | 278 (31%) | 100 (26%) | 178 (35%) | 91 (28%) | 33 (23%) | 58 (32%) |
| Renal insufficiency | 26 (14%) | 10 (12%) | 16 (15%) | 145 (16.3%) | 52 (14%) | 93 (18%) | 39 (12%) | 16 (11%) | 23 (13%) |
| Stroke/TIA | 10 (5%) | 3 (4%) | 7 (7%) | 70 (8%) | 25 (7%) | 45 (9%) | 19 (6%) | 5 (4%) | 14 (8%) |
| Symptoms | |||||||||
| Beginning of symptoms in days – median [IQR] | 7 [3,11] | 7 [3,11] | 7 [3,11] | 3 [2,8] | 3 [2,8] | 3 [2,7] | 3 [2,8] | 3 [2,8] | 3 [2,7] |
| Fever | 104 (55%) | 42 (50%) | 62 (58%) | 353 (40%) | 155 (40%) | 198 (39%) | 147 (46%) | 67 (47%) | 80 (44%) |
| Chills | 31 (16%) | 11 (13%) | 20 (19%) | 165 (18.5%) | 84 (22%) | 81 (16%) | 68 (21%) | 38 (27%) | 30 (17%) |
| Cough | 126 (66%) | 52 (62%) | 74 (69%) | 465 (52%) | 207 (54%) | 258 (51%) | 242 (75%) | 108 (76%) | 134 (74%) |
| Dyspnoea | 81 (42%) | 38 (45%) | 43 (40%) | 438 (49%) | 192 (50%) | 246 (49%) | 185 (57%) | 82 (58%) | 103 (57%) |
| Clinical parameters – median [IQR] | |||||||||
| Temperature* in °C | 37.1 [36.8,38] | 37.1 [36.8,38] | 37.2 [36.8,38] | 37 [36.5,37.7] | 37 [36.6,37.5] | 37 [36.5,37.7] | 37 [36.5,37.7] | 37 [36.6,37.5] | 37 [36.5,37.7] |
| Respiratory rate* | 20 [16,24] | 20 [16,23] | 20 [16,25] | 18 [16,23] | 19 [16,23] | 18 [16,22] | 18 [16,23] | 19 [16,23] | 18 [16,22] |
| SaO2* in % | 97 [95,98] | 97 [96,98] | 96 [94,98] | 97 [95,98] | 97 [96,99] | 97 [95,98] | 97 [95,98] | 97 [96,99] | 97 [95,98] |
| Heart rate* | 89 [80,103] | 89 [76,101] | 90 [82,103] | 88 [75,103] | 89 [75,102] | 87 [74,103] | 88 [75,103] | 89 [75,102] | 87 [74,103] |
| Blood pressure – systolic* in mmHg | 135 [122,149] | 128 [116,153] | 136 [124,148] | 137 [121,156] | 135 [120,156] | 138 [123,155] | 137 [121,156] | 135 [120,156] | 138 [123,155] |
| Blood pressure – diastolic* in mmHg | 82 [71,90] | 80 [71,86] | 83 [72,90] | 81 [72,90] | 80 [70,86] | 82 [75,91] | 81 [72,90] | 80 [70,86] | 82 [75,91] |
| BMI in kg/m² | 29 [25,32] | 29 [25,33] | 29 [25,32] | 26 [23,30] | 25 [22,31] | 25 [23,29] | 26 [23,30] | 25 [22,31] | 26 [23,29] |
| Laboratory parameters – median [IQR] | |||||||||
| Leucocytes in *109/l | 6.27 [4.95,8.34] | 5.96 [4.53,7.92] | 6.71 [5.12,8.74] | 8.48 [6.6,11.1] | 9.1 [6.91,12.01] | 8.82 [6.82,11.7] | 8.82 [6.82,11.7] | 8.48 [6.6,11.1] | 9.1 [6.91,12.01] |
| Lymphocytes – absolute in *109/l | 1.07 [0.72,1.57] | 1.3 [0.82,1.77] | 0.98 [0.69,1.35] | 1.58 [0.92,2.14] | 1.42 [0.9,2.02] | 1.47 [0.9,2.08] | 1.47 [0.9,2.08] | 1.58 [0.92,2.14] | 1.42 [0.9,2.02] |
| Lymphocytes in % | 19.2 [11.9,26.9] | 23.5 [14.8,30.6] | 17.1 [11.4,22.6] | 18.9 [10,28.7] | 16.2 [9.6,25.5] | 17.2 [9.8,26.6] | 17.2 [9.8,26.6] | 18.9 [10,28.7] | 16.2 [9.6,25.5] |
| CRP in mg/l | 28.9 [2.6,73.4] | 12.1 [1.5,49.1] | 36.2 [7.8,106.6] | 5.9 [1,40.6] | 10.4 [1.3,53.7] | 7.6 [1.2,47.6] | 7.6 [1.2,47.6] | 5.9 [1,40.6] | 10.4 [1.3,53.7] |
| Procalcitonin in µg/l | 0.09 [0.059,0.182] | 0.065 [0.059,0.146] | 0.102 [0.059,0.237] | 0.072 [0.059,0.287] | 0.103 [0.059,0.332] | 0.093 [0.059,0.332] | 0.093 [0.059,0.332] | 0.072 [0.059,0.287] | 0.103 [0.059,0.332] |
| D-dimer in µg/l | 0.58 [0.35,1.19] | 0.6 [0.37,1.22] | 0.57 [0.34,1.18] | 0.56 [0.29,1.12] | 0.59 [0.29,1.27] | 0.58 [0.29,1.19] | 0.58 [0.29,1.19] | 0.56 [0.29,1.12] | 0.59 [0.29,1.27] |
| Ferritin in ng/ml | 387 [164,823] | 193 [84,395] | 578 [308,1194] | 121 [62,224] | 206.5 [116,410] | 163 [85,329] | 163 [85,329] | 121 [62,224] | 206.5 [116,410] |
| Creatinine in µmol/l | 76 [62,95] | 62 [54,75] | 85 [74,100] | 62 [55,77] | 83 [71,99] | 75 [61,93] | 75 [61,93] | 62 [55,77] | 83 [71,99] |
| hs-troponin T in ng/l | 7 [4,14] | 5 [3,13] | 8.5 [5,15] | 5 [3,16.5] | 12 [6,26] | 9 [4,22] | 9 [4,22] | 5 [3,16.5] | 12 [6,26] |
| NT-proBNP in pg/ml | 77 [49,242] | 66 [49,208] | 82.5 [49,250] | 110 [49,370] | 117 [49,569] | 114.5 [49,462] | 114.5 [49,461.5] | 110 [49,370] | 117 [49,569] |
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; IQR: interquartile range; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; TIA: transient ischaemic attack; SaO2: arterial oxygen saturation; BMI: body mass index; CRP: C-reactive protein; hs-troponin T: high-sensitivity troponin T; NT-proBNP: N-terminal pro brain natriuretic peptide
In the total study population, as well as in those with COVID-19, men had a significantly higher burden of comorbidity, more often had cardiac disease or coronary artery disease (CAD), were more often smokers, and had higher infection parameters (CRP, leucocytes), ferritin and hs-cTn levels compared to women. In contrast, procalcitonin levels were higher only in male SARS-CoV-2 positive patients, which was not the case for controls. Comparable findings were observed in the subgroups of SARS-CoV-2 positive patients <55 and ≥55 years (table S4 in the appendix).
SARS-CoV-2 positive female patients were less frequently hospitalized (51% vs. 66%, p = 0.034), intubated (10% vs. 21%, p = 0.037) or received haemodynamic support (8% vs. 20%, p = 0.029) than men. In contrast, no sex differences for the above-mentioned endpoints could be observed in the overall control group or the respiratory control group (table 2).
Table 2Outcomes.
| SARS-CoV-2 positive (Cases) | SARS-CoV-2 negative (Overall control group) | SARS-CoV-2 negative with respiratory infection (Respiratory control group) | ||||||||||
| All ( n = 191) | Female ( n = 84) | Male ( n = 107) | p-value | All ( n = 890) | Female ( n = 385) | Male ( n = 505) | p-value | All (n = 323) | Female (n = 142) | Male (n = 181) | p-value | |
| Case management | ||||||||||||
| – Outpatient | 77 (40%) | 41 (49%) | 36 (34%) | 0.034* | 451 (50%) | 209 (54%) | 242 (48%) | 0.069* | 186 (57%) | 87 (61%) | 99 (54%) | 0.215* |
| – Inpatient | 114 (60%) | 43 (51%) | 71 (66%) | 444 (50%) | 179 (46%) | 265 (52%) | 138 (43%) | 55 (39%) | 83 (46%) | |||
| Length of stay in days – median [IQR] | 3 [0,8] | 0 [0,6] | 5 [0,11] | 0.003* | 0 [0,6] | 0 [0,5] | 0 [0,6] | 0.022* | 0 [0,6] | 0 [0,5] | 0 [0,6] | 0.045* |
| Composite endpoint** | 48 (25%) | 15 (18%) | 33 (31%) | 0.045 | 96 (11%) | 37 (10%) | 59 (12%) | 0.323 | 33 (10%) | 13 (9%) | 20 (11%) | 0.328 |
| – ICU admission | 40 (21%) | 13 (16%) | 27 (25%) | 63 (7%) | 25 (6%) | 38 (8%) | 23 (7%) | 10 (7%) | 13 (7%) | |||
| – All-cause death within 30 days | 16 (8%) | 5 (6%) | 11 (10%) | 61 (7%) | 21 (6%) | 40 (8%) | 23 (7%) | 8 (6%) | 15 (8%) | |||
| – Rehospitalization for respiratory complications | 4 (2%) | 1 (1%) | 3 (3%) | 24 (3%) | 8 (2%) | 16 (3%) | 10 (3%) | 4 (3%) | 6 (3%) | |||
| Intubation | 30 (16%) | 8 (10%) | 22 (21%) | 23 (3%) | 11 (3%) | 12 (2%) | 15 (5%) | 7 (5%) | 8 (4%) | |||
| Haemodynamic support | 28 (15%) | 7 (8%) | 21 (20%) | 26 (3%) | 10 (3%) | 16 (3%) | 14 (4%) | 6 (4%) | 8 (4%) | |||
| ARDS | 26 (14%) | 7 (8%) | 19 (18%) | 6 (1%) | 2 (1%) | 4 (1%) | 4 (1%) | 2 (1%) | 2 (1%) | |||
* Analyses and p-values are exploratory
** Admission to ICU, rehospitalization for respiratory complication and all-cause death within 30 days
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; IQR: interquartile range; ICU: intensive care unit; ARDS: acute respiratory distress syndrome
The composite primary outcome occurred in 144 of 1,081 (13%) patients, with a significantly higher incidence of 25% (48/191 patients) in COVID-19 cases compared to 11% (96/890 patients) in the overall control group (p<0.001) and 10% (33/323) in the respiratory control group.
Importantly, within the SARS-CoV-2 positive population the incidence of the composite outcome was significantly lower in women (18%, 15/84 patients) than in men (31%, 33/107 patients, log-rank p = 0.045; age-adjusted HR for female sex 0.536, 95%CI 0.290–0.989, p = 0.046). Both age and sex were independent predictors of the composite outcome in COVID-19 positive patients. In contrast, no sex-related differences were observed among the SARS-CoV-2 negative patients, neither in the overall control group (age-adjusted HR 0.844, 95%CI 0.560–1.273, p = 0.419) nor in the respiratory control group (age-adjusted HR 0.840, 95%CI 0.418–1.688, p = 0.624). Figure 1 shows the Kaplan–Meier curves for the occurrence of the composite outcome within 30 days for men and women in the SARS-CoV-2 positive and negative groups.

Figure 1 Composite outcome of admission to ICU, rehospitalization for respiratory complication and death within 30 days in COVID-19 cases and overall control; depicted hazard ratios (HR) are age-corrected.
Figure S2 in the appendix shows the same for the respiratory control group.
When stratified by age, in COVID-19 patients under the age of 55 years, no sex differences in the primary outcome could be observed (HR 1.275, 95%CI 0.369–4.405, p = 0.701, figure 2).
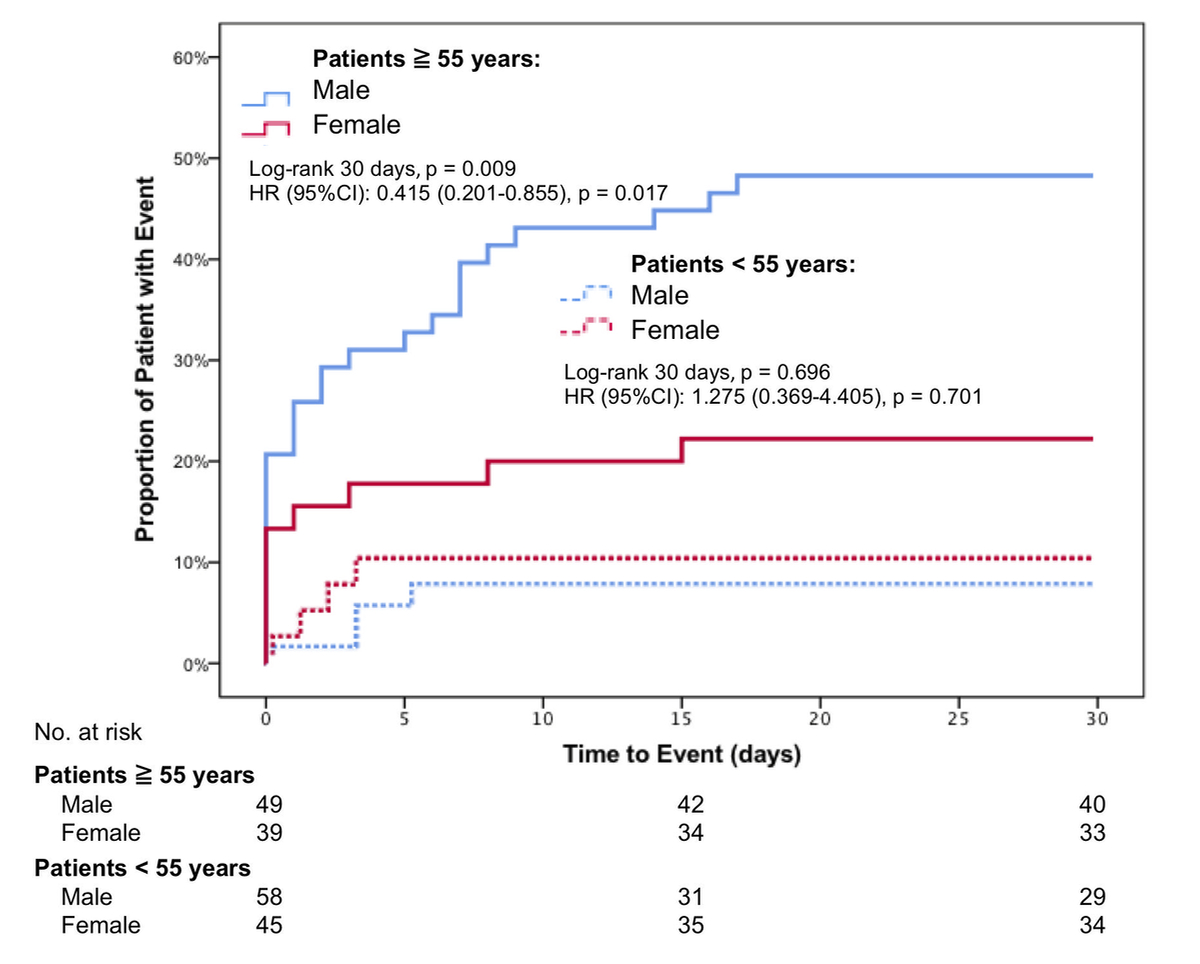
Figure 2 Composite outcome of admission to ICU, rehospitalization for respiratory complication and death within 30 days in SARS-CoV-2 positive patients under and over the age of 55 years.
In contrast, in COVID-19 patients ≥55 years, female sex was associated with a significantly superior event-free survival (HR 0.415, 95%CI 0.201–0.855, p = 0.017).
In additional subgroup analyses, a consistent trend towards better outcomes in women could be observed in the majority of subgroups without reaching the level of significance (figure 3).
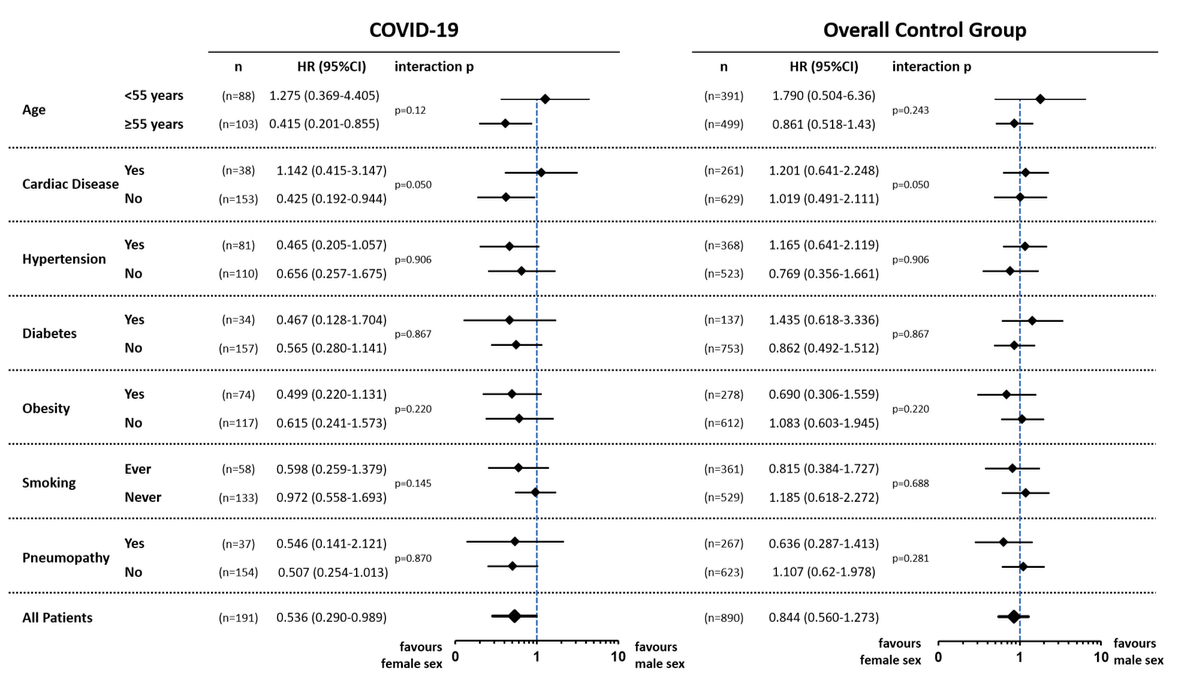
Figure 3 Forest plot showing age-adjusted hazard ratios (HR) and their corresponding 95% confidence intervals to quantify the impact of sex on the composite primary outcome in predefined subgroups within the COVID-19 cases (first column) and the controls (second column).
In this prospective cohort study of consecutive patients presenting with suspected SARS-CoV-2 infection to the ER of the University Hospital in Basel, Switzerland, we explored the impact of sex on clinical outcome in COVID-19 patients and in comparable controls.
First, the prevalence of confirmed COVID-19 infections in patients presenting to the ER was comparable between women and men. Second, the incidence of adverse clinical outcomes, defined as the composite of ICU admission, rehospitalization for respiratory distress or death within 30 days, was almost halved in women compared to in men with COVID-19, regardless of age. In addition, female patients with SARS-CoV-2 infection were less often hospitalized, intubated or in need of haemodynamic support. Third, sex-related differences in outcome were most prominent in patients aged 55 years and older and were seen across a range of additional subgroup analyses. Fourth, the observed sex disparities were consistently associated with COVID-19 infections and were not found in SARS-CoV-2 negative patients, nor in patients with other upper respiratory infections or pneumonia.
Among patients presenting to the ER with suspected COVID-19, male sex was more prevalent, as had already been observed in patients with similar symptoms before the pandemic [9]. However, in line with the most recent data, our results showed that the prevalence of SARS-CoV-2 infection was similar in men and women [2, 6, 10, 11].
Male sex and older age have been described as predictors of adverse outcome [4, 12–14]. However, whether this is specific to COVID-19 has not yet been clearly established. To the best of our knowledge, this is one of the few studies recruiting consecutive patients presenting to the ER with symptoms suggesting SARS-CoV-2 infection and therefore with parallel enrolment of an adequate control group. Importantly, we could demonstrate that the highest risk was observed in male COVID-19 patients over the age of 55, whose risk was substantially higher compared to their age-matched female counterparts.
Thus, a postulated protective effect of female sex hormones, which are presumably present in patients <55 years, seems to be neglectable regarding disease severity and outcome in symptomatic COVID-19 patients [15, 16]. In contrast, some data in mouse models even suggest a stronger immunological response driven by oestrogen. The sex disparities observed in this analysis could be explained by a plethora of possible reasons, such as differences in cardiovascular comorbidities, genetic factors and immune functions [15–17]. Some influencing factors could also possibly be attributed to gender, which is defined by social and cultural norms, roles and behaviours. As an example, women tend to take over the role of the primary caregiver not only at home, but also in the health care system. Based on our exploratory subgroup analyses, cardiovascular comorbidities and risk factors do not themselves seem to the primary factors accounting for the sex differences observed in COVID-19, as a numerical advantage for women was observed across most risk categories. The innate and adaptive immune response to infections is generally stronger in women than in men [18, 19]. Moreover, some sex-related immunological aspects change according to age, which leads to men being potentially more susceptible to infections and therefore having worse outcomes [15]. Thus, sex differences in immune composition and response may be another reason for the observed sex differences in SARS-CoV-2 infection outcome. The key finding of this study is that the observed sex disparities in COVID-19 could not be seen in either the control group of unselected patients presenting with symptoms suggestive of COVID-19 but testing negative for SARS-CoV-2 or in a subgroup of patients with other upper respiratory infections and pneumonia. Accordingly, the sex disparities seem not to be generalizable to all patients presenting to the ER with symptoms suggestive of COVID-19, but may be related to disease-specific mechanisms involved in COVID-19.
The main limitation of our study is its single centre design, with all data coming from one Swiss tertiary centre. However, during the current COVID-19 pandemic the number of affected patients, disease severity and the overloading of limited health care resources have differed widely between regions and health care systems. While most of the data reported are from severely affected regions, our data represent a typical Central European setting with a health care system that was not overloaded. Accordingly, event rates observed in this analysis are lower than the ones reported from North America or China [20, 21]. Second, the study may be insufficiently powered for multivariable analysis due to the limited number of SARS-CoV-2 positive patients, and also, only adjusting for age could have led to substantial residual confounding. Accordingly, no direct causalities can be extrapolated from our findings, as our data only allow us to explore associations. Nevertheless, given the design of this study (i.e. consecutive inclusion of unselected control patients presenting with comparable symptoms in the same time period), the observed results still add valuable information to the literature. In particular, they allow us to put findings observed in COVID-19 patients into perspective when compared with other acute conditions, including respiratory infections from other causes. Our findings need to be validated in larger future studies, as residual confounding could be substantial. Third, the patients for this analysis were recruited if they had symptoms suggesting COVID-19, leading to their triage to the ER during the first wave of the pandemic. Therefore, our findings cannot be extrapolated to the general population or to asymptomatic patients with SARS-CoV-2 infection.
Despite similar prevalences of SARS-CoV-2 infection in women and men, female sex is associated with better outcome in symptomatic COVID-19 patients presenting to the ER, particularly in patients aged years. Sex disparities seem to be specific to COVID-19, as they were not observed in comparable controls. Further studies are needed in order to explain the underlying mechanisms.
We thank the clinical staff for their valuable help during the study period and all the patients for their participation in the study.
The COVIVA study was supported by the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel and an unrestricted grant by Roche Diagnostics.
Dr. Arslani has received a research grant from the Swiss Academy of Medical Sciences and the Bangerter Foundation (YTCR 09/19) and the Swiss National Science Foundation (P500PM_202963).
Dr. Twerenbold reports research support from the Swiss National Science Foundation (Grant No P300PB_167803), the Swiss Heart Foundation, the Swiss Society of Cardiology, the Cardiovascular Research Foundation Basel, the University of Basel and the University Hospital Basel, as well as speaker honoraria/consulting honoraria from Abbott, Amgen, Astra Zeneca, Roche, Siemens, Singulex and Thermo Scientific BRAHMS.
Dr. Kuster reports research support from the Swiss National Science Foundation (Grant No IZCOZ0_189877) and the Cardiovascular Research Foundation Basel, Switzerland, that are unrelated to this work, and speaker honoraria/consulting honoraria from Janssen-Cilag.
Dr. Gebhard reports funding from the Swiss National Science Foundation (Grant No 31CA30196140), the University of Basel and the University Hospital of Basel.
Authors not named here have disclosed no conflicts of interest.
1. Guan WJ , Ni ZY , Hu Y , Liang WH , Ou CQ , He JX , et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. Epub 2020/02/29. doi: https://doi.org/10.1056/NEJMoa2002032. PubMed PMID: 32109013; PubMed Central PMCID: PMCPMC7092819.
2. Gebhard C , Regitz-Zagrosek V , Neuhauser HK , Morgan R , Klein SL . Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. Epub 2020/05/27. doi: https://doi.org/10.1186/s13293-020-00304-9. PubMed PMID: 32450906; PubMed Central PMCID: PMCPMC7247289.
3. Abate BB , Kassie AM , Kassaw MW , Aragie TG , Masresha SA . Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020;10(10):e040129. Epub 2020/10/09. doi: https://doi.org/10.1136/bmjopen-2020-040129. PubMed PMID: 33028563; PubMed Central PMCID: PMCPMC7539579.
4. Jin JM , Bai P , He W , Wu F , Liu XF , Han DM , et al. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front Public Health. 2020;8:152. Epub 2020/05/16. doi: https://doi.org/10.3389/fpubh.2020.00152. PubMed PMID: 32411652; PubMed Central PMCID: PMCPMC7201103.
5. Iaccarino G , Grassi G , Borghi C , Carugo S , Fallo F , Ferri C , et al. Gender differences in predictors of intensive care units admission among COVID-19 patients: The results of the SARS-RAS study of the Italian Society of Hypertension. PLoS One. 2020;15(10):e0237297. Epub 2020/10/07. doi: https://doi.org/10.1371/journal.pone.0237297. PubMed PMID: 33022004; PubMed Central PMCID: PMCPMC7537902.
6. Sex gaC-GH. [13 March, 2021]. Available from: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/
7. von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014 Dec;12(12):1495–9. https://doi.org/10.1016/j.ijsu.2014.07.013
8. Zhu D , Chung HF , Dobson AJ , Pandeya N , Giles GG , Bruinsma F , et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4(11):e553-e64. Epub 2019/10/08. doi: https://doi.org/10.1016/s2468-2667(19)30155-0. PubMed PMID: 31588031; PubMed Central PMCID: PMCPMC7118366. https://doi.org/10.1016/S2468-2667(19)30155-0
9. Muller B , Harbarth S , Stolz D , Bingisser R , Mueller C , Leuppi J , et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. Epub 2007/03/06. doi: https://doi.org/10.1186/1471-2334-7-10. PubMed PMID: 17335562; PubMed Central PMCID: PMCPMC1821031.
10. Suleyman G , Fadel RA , Malette KM , Hammond C , Abdulla H , Entz A , et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270. Epub 2020/06/17. doi: https://doi.org/10.1001/jamanetworkopen.2020.12270. PubMed PMID: 32543702; PubMed Central PMCID: PMCPMC7298606.
11. Tolia VM , Chan TC , Castillo EM . Preliminary Results of Initial Testing for Coronavirus (COVID-19) in the Emergency Department. West J Emerg Med. 2020;21(3):503-6. Epub 2020/04/01. doi: https://doi.org/10.5811/westjem.2020.3.47348. PubMed PMID: 32223871; PubMed Central PMCID: PMCPMC7234708.
12. Grasselli G , Greco M , Zanella A , Albano G , Antonelli M , Bellani G , et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345-55. Epub 2020/07/16. doi: https://doi.org/10.1001/jamainternmed.2020.3539. PubMed PMID: 32667669; PubMed Central PMCID: PMCPMC7364371.
13. Zheng Z , Peng F , Xu B , Zhao J , Liu H , Peng J , et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. Epub 2020/04/27. doi: https://doi.org/10.1016/j.jinf.2020.04.021. PubMed PMID: 32335169; PubMed Central PMCID: PMCPMC7177098.
14. Lakbar I , Luque-Paz D , Mege JL , Einav S , Leone M . COVID-19 gender susceptibility and outcomes: A systematic review. PLoS One. 2020;15(11):e0241827. Epub 2020/11/04. doi: https://doi.org/10.1371/journal.pone.0241827. PubMed PMID: 33141872; PubMed Central PMCID: PMCPMC7608911 speaker for MSD, Pfizer and as consultant for Amomed, Aguettant and Gilead. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
15. Klein SL , Flanagan KL . Sex differences in immune responses. Nat Rev Immunol. 2016 Oct;16(10):626–38. https://doi.org/10.1038/nri.2016.90
16. Scully EP , Haverfield J , Ursin RL , Tannenbaum C , Klein SL . Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442-7. Epub 2020/06/13. doi: https://doi.org/10.1038/s41577-020-0348-8. PubMed PMID: 32528136; PubMed Central PMCID: PMCPMC7288618.
17. Maleki Dana P , Sadoughi F , Hallajzadeh J , Asemi Z , Mansournia MA , Yousefi B , et al. An Insight into the Sex Differences in COVID-19 Patients: What are the Possible Causes? Prehosp Disaster Med. 2020;35(4):438-41. Epub 2020/07/01. doi: https://doi.org/10.1017/s1049023x20000837. PubMed PMID: 32600476; PubMed Central PMCID: PMCPMC7327162. https://doi.org/10.1017/S1049023X20000837
18. Potluri T , Fink AL , Sylvia KE , Dhakal S , Vermillion MS , Vom Steeg L , et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4:29. Epub 2019/07/18. doi: https://doi.org/10.1038/s41541-019-0124-6. PubMed PMID: 31312529; PubMed Central PMCID: PMCPMC6626024.
19. Gemmati D , Bramanti B , Serino ML , Secchiero P , Zauli G , Tisato V . COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int J Mol Sci. 2020;21(10). Epub 2020/05/20. doi: https://doi.org/10.3390/ijms21103474. PubMed PMID: 32423094; PubMed Central PMCID: PMCPMC7278991.
20. Palaiodimos L , Kokkinidis DG , Li W , Karamanis D , Ognibene J , Arora S , et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. Epub 2020/05/19. doi: https://doi.org/10.1016/j.metabol.2020.154262. PubMed PMID: 32422233; PubMed Central PMCID: PMCPMC7228874.
21. Zhou F , Yu T , Du R , Fan G , Liu Y , Liu Z , et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-62. Epub 2020/03/15. doi: https://doi.org/10.1016/S0140-6736(20)30566-3. PubMed PMID: 32171076; PubMed Central PMCID: PMCPMC7270627.
The appendix is available in the pdf version of the article.