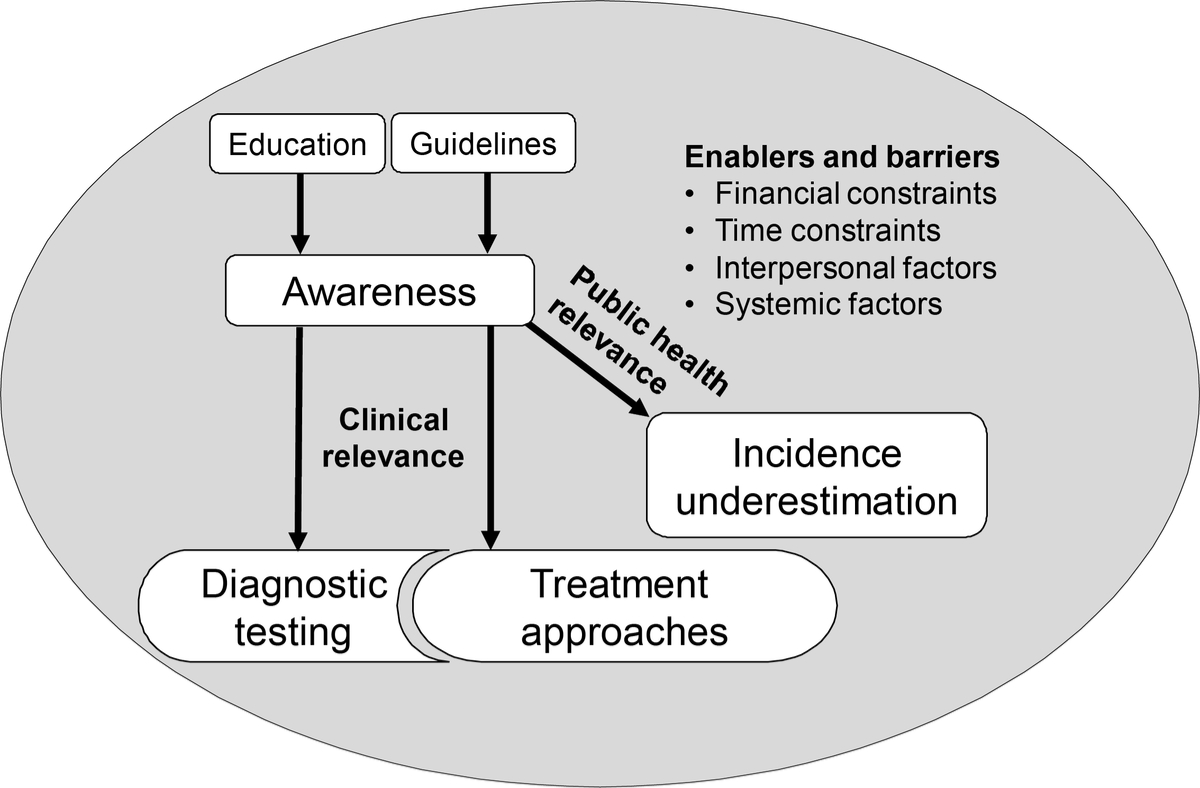
Figure 1 Overview of the themes and their relationships with each other which emerged from the in-depth interviews with 46 Swiss physicians on pneumonia and Legionnaires’ disease.
DOI: https://doi.org/10.4414/SMW.2022.w30157
Legionnaires’ disease is characterised by severe pneumonia and caused by the gram-negative Legionella spp. bacteria. In Switzerland, reporting cases of Legionnaires' disease to the National Notification System for Infectious diseases (NNSID) managed by the Federal Office of Public Health (FOPH) is mandatory [1]. Between 2008 and 2019, the number of reported cases has more than doubled, reaching an annual incidence of 6.5 cases per 100,000 population in 2019 [2]. At the same time, the burden of disease for Legionnaires' disease might be underestimated [3–5], due to under-ascertainment or underdiagnosis through lack of testing or limitations of the various diagnostic methods [6].
We previously investigated the trend of diagnostic test frequency for Legionnaires' disease and showed that the number of tests performed increased between 2007 and 2016, along with the number of reported cases [7]. During this period, the Legionella urinary antigen test (UAT) was most widely applied throughout all study years. Based on these data alone, the increase in testing and notified cases could not be explained. Particularly, there was no information available in the notification database to describe and contextualise these findings with regard to physician-related factors. The underdiagnosis of Legionnaires' disease has been partially attributed to a lack of awareness about the disease among physicians, and growing case numbers likely represent increased awareness (and hence, testing and case detection). For example, Ticino, a canton in the south of Switzerland, has a notification rate four times higher than the rest of Switzerland, which is often attributed to a heightened awareness of local physicians leading to a cycle of confirmation biases. In other words, high case numbers lead to increased awareness and intensive testing, which in turn results in more cases identified [8]. Indeed, we have found that most diagnostic tests were performed in Ticino, yet there the positivity rate was also found to be the highest [7]. Hence, to explain the growing legionellosis test numbers, we need to understand the processes leading to diagnoses and diagnostics.
The diagnosis of Legionnaires' disease is dependent on pneumonia case management. Pneumonia is often classified as nosocomial pneumonia or community-acquired pneumonia (CAP). For the purpose of this study, we focused on CAP. Current case management is rooted in numerous available guidelines for CAP. The Swiss guidelines on the management of CAP in use at the time of the study (2019–2020) were published in 2006 by the Swiss Society of Infectious Diseases (SSI) [9]. This guideline is based on the European Respiratory Society (ERS) / European Society for Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines from 2005 [10] and was endorsed in the Swiss Government’s latest document on legionellosis [11]. In 2016, an update of the German CAP and lower respiratory tract infection management guideline was published, which widened its scope to the Austrian and Swiss contexts [12, 13]. In 2021, these guidelines received another update, with the SSI among its authors [14]. The SSI updated the CAP guideline in their online collection as well [15].
Most guidelines agree that microbiological investigation is generally only recommended for hospitalised patients or patients with severe CAP. CAP severity is frequently based on the CURB-65 or Pneumonia Severity Index (PSI) score, but should be individually assessed. There are minor differences in this recommendation, for example, the SSI guideline from 2006 associating severe CAP, warranting a Legionella UAT, with ICU admission [9]. Legionnaires' disease testing is also recommended when clinically and epidemiologically suspected. Since the clinical presentation of Legionnaires' disease does not differ from other atypical pneumonias, both indications for testing (disease severity and suspicion) are subject to interpretation. Therefore, efforts were made to develop a scoring system to identify Legionella infections based on clinical parameters, some of which have proven useful in recent validation studies [16–20]. The causative pathogen guides the pneumonia treatment. For Legionella infections, treatment with an antibiotic that can reach high intracellular concentrations (e.g., fluoroquinolones or macrolides) significantly decreases mortality from 60–70% to 10–20% [21, 22].
Physicians' acceptance and uptake of recommendations for the management of Legionnaires' disease and their use of subjective testing guidelines has been largely unexplored. It is unclear what factors influence decision-making from the assessment of a patient with symptoms of lower respiratory tract infection to the initiation of detailed clinical investigations for Legionnaires' disease. The aim of this study was to explore physicians’ awareness of Legionnaires' disease and their decision-making processes regarding the clinical management of CAP in Switzerland. Investigating the decision pathway for diagnosis (or diagnostics in particular) of Legionnaires' disease and the role of corresponding guidelines and other influencing factors provides essential insights into explanations for the increase in testing and determinants of underestimation of Legionnaires' disease to further contextualise increasing reported case numbers.
We conducted qualitative face-to-face in-depth interviews with Swiss physicians using a semi-structured interview guide (table 1). We asked physicians about (1) their diagnosis and management of CAP, (2) awareness and knowledge about Legionnaires' disease in Switzerland, and (3) their approaches to diagnosing and managing Legionnaires' disease. The interview guide was designed following a review of national and international guidelines for the management of CAP. It was purposively designed to allow for a general discussion of pneumonia before prompting participants to discuss Legionnaires' disease specifically. We collected feedback from the FOPH and tested the interview guide with selected physicians. The interview guide was further refined with the data collection team and during the interviews, for example to prioritise questions in case the interview was running over time.
Table 1Overview of the semi-structured interview guide for in-depth interviews on pneumonia and Legionnaires' disease with physicians in Switzerland.
| Topics | Sample questions |
| Information sources and guidelines | Do you consult guidelines to diagnose and treat pneumonia? If so, which ones? |
| Assuming that you have enough time and that all costs are covered, would you like additional training? What should this training cover? | |
| Pneumonia diagnosis | Could you walk me through a typical approach for diagnosing and treating a patient presenting with an acute respiratory infection/pneumonia? |
| Do you initiate aetiological testing? When? Why? How often? | |
| Experience with Legionnaires' disease | Legionnaires' disease may not be so common in daily practice – What do you know about Legionnaires' disease ? |
| Have you ever treated a patient with Legionnaires' disease ? Could you describe this instance(s)? | |
| Opinions on Legionnaires' disease | Could you describe the main challenges in obtaining adequate information on Legionnaires' disease ? |
| Do you think we miss Legionnaires' disease cases in Switzerland? Why? |
Additionally, we collected participants’ demographic data including location, years of clinical practice and current position.
Physicians who encounter pneumonia patients in daily practice were eligible for participation. We purposively sampled a wide variety of physicians in all care levels in order to examine an array of possible diagnostic pathways. We aimed to enrol six physicians per language region and healthcare level. To recruit hospital physicians, we compiled a list of primary, secondary and tertiary hospitals and aimed to cover most cantons. First contact was made with the hospital secretariat, which distributed the recruitment letter and informed consent forms in their departments. We started with the interviews at the largest institutions, typically the university hospitals. We randomly selected GPs at family medicine practices from a publicly available registry and contacted them directly. The selection of GPs was based on the same regional stratification criteria as for hospital-based clinicians. After the initial interviews, we recruited more physicians using the snowball system in accordance to the strata defined above. We stopped data collection upon reaching data saturation.
Data were collected between October 2019 and February 2020. We obtained written informed consent from all participants before conducting the interviews. The interviews were audio recorded. Six female interviewers, from backgrounds in medicine, biomedicine, sociology and ethnology were trained in qualitative data collection. They conducted interviews in German, French and Italian. The interviews lasted between 30 and 60 minutes and took place in the physicians’ offices and workplaces (n = 41) or online/ by telephone (n = 5).
We transcribed interviews verbatim and translated them into German or English (depending on the data collectors’ language skill). For quality control reasons, four interviews were translated independently by two researchers. The interviews were organised and processed using the framework method [23]. We used MAXQDA to analyse the transcripts. We used the consolidated criteria for reporting qualitative research (COREQ) to organise and report our results [24].
We analysed the interview transcripts using thematic analysis, which involves coding excerpts of transcripts and identifying common themes in the data [25]. A coding tree was prepared a priori based on the interview guide and the research objectives, such as level of awareness of physicians or the different steps taken in the case management of pneumonia (such as anamnesis, aetiological testing and mandatory reporting). This tree was used to code three interviews independently by two researchers: FBF (doctoral researcher in epidemiology) and JF (MSc student in epidemiology). The coding of these interviews was discussed and the code tree adapted iteratively. One researcher (JF) then coded all interviews with the adapted coding tree. FBF and JF repeatedly met during this process to clarify any uncertainties or disagreements.
During this process, we identified several themes, which were not directly linked to the interview guide or our pre-existing knowledge and assumptions, but which nonetheless came up repeatedly. We reiterated the coding process using the newly generated and developed, data-driven themes and codes (e.g., diagnostic uncertainty). This inductive analysis of the data allowed us to further consider the (unexpected) processes behind the interplay between diagnostics and treatment. In a final step, we held a workshop with the data collectors to validate the themes and conclusions of this study. MJD (postdoctoral medical sociologist) and DM (senior epidemiologist) supervised the conduct of the study and the analysis and interpretation of the results.
Ethical approval was obtained from the “Ethikkommission Nordwest- und Zentralschweiz” (ID 2019-01708). We do not identify any study participants by name to ensure participant confidentiality and anonymity.
We interviewed 46 physicians. The sample of physicians was well-balanced based on language-region and employment at different healthcare levels (see table 2 for details). More than 70% of interviewed physicians were male. At the time of the interview, participants’ average years of medical practice was 23 years. On average, the physicians practising at a university hospital had about 10 years less medical practice than physicians working elsewhere did. Six physicians practised at multiple healthcare levels, such as working part-time in a cantonal hospital and in their own family medicine practice. Two of the interviewed GPs were part of a Sentinella study on Legionnaires' disease [26].
Table 2Characteristics of physicians included in the study on pneumonia and Legionnaires' disease case management in Switzerland.
| n | % | ||
| Language region | German | 12 | 26.1 |
| French | 20 | 43.5 | |
| Italian | 14 | 30.4 | |
| Sex | Female | 13 | 28.3 |
| Male | 33 | 71.7 | |
| Healthcare level | GP | 19a | 41.3 |
| (Median years of medical practice: 23) | |||
| Regional hospital | 13 | 28.3 | |
| (Median years of medical practice: 29) | |||
| Cantonal hospital | 12 | 26.1 | |
| (Median years of medical practice: 28) | |||
| University hospital | 9 | 19.6 | |
| (Median years of medical practice: 13) | |||
| Speciality | Pulmonology | 7 | 15.2 |
| Infectiology | 11 | 23.9 | |
| Emergency medicine | 7 | 15.2 | |
| General medicine | 17 | 37 | |
| Other | 8 | 17.4 | |
| Years of medical practice | 0–5 years | 1 | 2.2 |
| 6–10 years | 3 | 6.7 | |
| 11–20 years | 12 | 26.7 | |
| 21–30 years | 16 | 35.6 | |
| Over 30 years | 13 | 28.9 | |
| Total | 46 | 100 | |
a Two GPs participated in the Legionnaires' disease Sentinella study [26]
In the following sections, we discuss five inter-related themes we identified during data analysis. These include (1) awareness of Legionnaires' disease, (2) underestimation of Legionnaires' disease, (3) treatment approaches, (4) the interdependency of diagnostics and treatment approaches and (5) the enablers and barriers affecting all other identified themes. In figure 1, we visualise the relationship between the themes. For example, physicians’ education and awareness of the guidelines strongly influenced their awareness about Legionnaires' disease. Awareness around Legionnaires' disease and/or existing pneumonia guidelines impacted both clinical and public health aspects. On the clinical side, awareness informed the diagnostic testing and treatment approaches, which are mutually dependent on each other.

Figure 1 Overview of the themes and their relationships with each other which emerged from the in-depth interviews with 46 Swiss physicians on pneumonia and Legionnaires’ disease.
Overall, physicians demonstrated a high level of awareness of Legionnaires' disease by including it in their considerations for pneumonia diagnosis, before we specifically prompted them about it during the interviews. Additionally, many physicians were able to provide details on host and exposure risks, the clinical presentation, treatment and the transmission routes and prevention efforts. Physicians from Ticino were also well aware of the high Legionnaires' disease incidence in their region. Physicians working in hospitals demonstrated the highest level of awareness and in-depth knowledge. They reported a large emphasis on Legionnaires' disease during their education, both initial medical school training and in continuing education. One hospital physician described:
“I feel that there is enough knowledge about Legionnaires' disease. Somehow, as a medical student, pneumonia is drummed into you. If you have something less typical, then it is always Legionella - not Coxiella or tularaemia. Tuberculosis or Legionella are the big two to think about when you do not have a normal bacterial pneumonia. I have the feeling that it is also somewhat a mystified disease. You know [that it is Legionnaires' disease], I think, even if you haven't had anything to do with it for a very long time.” (University hospital in the German-speaking part of Switzerland, female, 13 years medical practice)
The few physicians who self-reported being unaware were all GPs. Most GPs stated that they had never encountered a Legionnaires' disease case in their practice, or would likely not be aware if they had. After referral of patients with severe pneumonia to the hospital, GPs explained that thereafter they often do not learn about patients’ outcomes in detail. Because of this lack of feedback and the rarity of Legionnaires' disease, after several years of practice, some physicians might fail to recall it. One physician explained:
“There are things that I learned in medical school that I just never saw again. In addition, to be honest, I don't even know what [Legionnaires' disease] is and I don't really have the intellectual concepts available any more either. (…) If I am no longer exposed to these things, then they disappear from my professional life. However, I am aware that I don't know some things. And if [the patient] doesn't really make progress, then I'm pretty quick with referrals.” (GP in the French-speaking part of Switzerland, male, 23 years of medical practice)
However, GPs did not perceive this lack of in-depth knowledge as problematic. They saw their responsibility in triaging patients (referring them to the hospital or specialists when necessary) and initiating timely empirical antibiotic treatment in pneumonia patients, which did not necessarily entail identifying the causative pathogen.
Despite the high awareness for Legionnaires' disease, most physicians believed that the incidence is underestimated in Switzerland. Three main reasons for underestimation were named. First, many physicians agreed that Legionnaires' disease cases presenting with mild pneumonia on an outpatient basis would be missed owing to omission of the aetiological investigation. Second, few cases might be missed owing to oversights in ordering the diagnostic tests or limitations of the tests themselves. And third, for a large proportion (estimated 30–50%) of pneumonia cases no causative agent can be found even if aetiological investigation is attempted. One physician explained:
“Unfortunately, for probably the vast majority of our pneumonia patients, even if we do good diagnostics or standard good diagnostics – not scientifically good diagnostics – but routine diagnostics, we find no pathogen. That's more common [among pneumonia patients] than pneumococcus [laughs].” (Cantonal hospital in the German-speaking part of Switzerland, male, 37 years of medical practice)
Even considering the underestimation, most physicians did not consider Legionella spp. as an important pathogen for pneumonia. In this regard, some physicians mentioned a limited clinical relevance of Legionella, as it can be treated appropriately and a minor public health relevance due to the low case numbers.
Appropriate antibiotic prescription for pneumonia in general and Legionnaires' disease in particular was perceived as a highly relevant topic. Many physicians steered the discussion toward the issue of antibiotic treatment. Depending on their treatment approach, interviewed physicians could be divided into two groups: (1) those who encourage testing and targeted antibiotic treatment and (2) those who promote empirical treatment with less testing.
The physicians in the first group, primarily hospital physicians, perceived the results of diagnostic tests as an opportunity to initiate targeted antibiotic treatment to reduce antibiotic resistance. Although most physicians were aware of this need, these physicians working in hospitals exhibited more ownership of that responsibility as part of their professional role. Apart from impeding antimicrobial resistance, they also reported clinical considerations with fewer antibiotics prescribed resulting in fewer side effects and less negative effects on the microbiome. Especially macrolides, which are often used to treat Legionnaires' disease, were noted to have adverse effects. One physician summarised the importance of diagnostic testing:
“Unfortunately, too little emphasis is placed on carrying out regular diagnostics. In my opinion, doctors too often use combination therapy with a macrolide or a quinolone and also use it for a relatively long period (…). [With good diagnostics] we can de-escalate the treatment, and we can use a narrow-spectrum antibiotic with a clear conscience. This means less side effects, less negative effects on antimicrobial resistance and less negative effects on the microbiome. That's why it's important to me personally, that we put microbial diagnostics in the foreground again. (…). In many cases, it is only a viral pneumonia (…). Then you don't have to treat with antibiotics. Viral pneumonia has a more severe progression if treated with antibiotics. Patients and especially the treating physicians need to be made aware of this, so nobody says, ‘I gave an antibiotic to be on the safe side. It won't do any harm.' We know that it does harm.” (Cantonal hospital in the German-speaking part of Switzerland, male, 37 years of medical practice)
In the second group of physicians, there was one primary through-line in favour of empirical treatment, whereby physicians favoured a pragmatic and maximalist approach to improve patients’ health quickly. Most GPs belonged to this group, stating that improving the patient health was their priority. One physician commented:
“You have to ask yourself the question: Will I provide better care if I do [diagnostic testing] than if I don't? And other doctors will answer you differently, but as an infectiologist with my experience, I tell you I don't need that most of the time. I do a good job with an empirical approach.” (Cantonal hospital in the French-speaking part of Switzerland, male, 34 years of medical practice)
In our interviews, questions about appropriate treatment could not be explored without discussing diagnostics. The Legionella UAT belongs to the standard tests for inpatient diagnostics. According to several physicians, their hospitals made adapted guidelines available, which supported the use of UAT for patients admitted to or presenting at the emergency ward with pneumonia symptoms. In contrast, GPs reported generally not testing for Legionnaires' disease. If patients need to be referred to the hospital, GPs assumed that testing is initiated there. Regardless of the setting, the Legionella UAT was well known and the initial test of choice to diagnose Legionnaires' disease to all physicians. Whereas the test was primarily appreciated for its ease of use and rapid results, some hospital physicians and GPs expressed that the UAT was too costly and time-consuming to receive results, when it should and could be a point-of-care test. There were also concerns about the test’s sensitivity, which closely tied together with the physicians’ awareness of the limitations of the UAT to reliably detect only Legionella pneumophila serogroup 1 and their trust in the diagnostic tests. To address the UAT’s limitations, hospital physicians in particular mentioned the usage of polymerase chain reaction (PCR) diagnostics, but considered PCR mostly as a second line test if patients were severely ill or the UAT returned negative but the suspicion for Legionnaires' disease was strong. Many physicians recognised problems in determining the aetiology, but they felt that this could only be solved by innovation in diagnostics rather than through adjustments on their case management side.
Physicians expressed two contrasting perspectives regarding the influence of a Legionnaires' disease test result on treatment. These perspectives were contingent upon physicians’ trust in diagnostics. On the one hand, some physicians reported that the result of a diagnostic test does not affect treatment. These physicians were particularly conscious of diagnostic uncertainty and would not de-escalate the antibiotic treatment even if the UAT were negative. One physicians described his decision-making process:
“Given a [pneumonia] patient has a good disease progression, what we don't know is when to stop a macrolide therapy? If I haven't confirmed [the diagnosis] with the UAT, that doesn't mean that I have ruled out an atypical pathogen. Because the test is too unspecific. Or it could be that I didn't look for [the pathogen] before starting the antibiotics, then it's possible that it can't be detected. I can't do a PCR on every patient due to cost reasons. So one treats perhaps a bit broader – empirically. (…) I think there is a small need for improvement; after all it depends on the diagnostics. And when microbiological diagnostics become more accessible, perhaps also cheaper and more precise, then this problem will be solved. (…) With Legionella if you have a negative UAT, other serotypes are not excluded. We have enough guidelines. I think it's the precision of the diagnostics [that need improvement].” (Regional hospital in Italian-speaking part of Switzerland, male, 13 years medical practice)
They also highlighted that a high degree of suspicion of Legionnaires' disease should overrule the test result. On the other hand, some physicians reported de-escalating the antibiotic treatment if the UAT were negative, as one physician explained:
“We rely on these Legionella UATs and I think that if the UAT is negative, you [should] stop the Legionella therapy. I personally want to prescribe as few antibiotics as possible. If you already have a negative test result, then I rely on that. And I think – I checked – about 90% of the strains are covered [by the UAT]. It's not 100%, but it's very high.” (Cantonal hospital in the German-speaking part of Switzerland, female, 19 years medical practice)
Physicians reported only a few instances where they would add antibiotic prescriptions after a positive test result, since treatment would most often be initiated with an antibiotic active against Legionnaires' disease. We also observed some uncertainty concerning the correct approach, which the comment below illustrates:
“Recently, due to one or two pneumonia cases that were presented and discussed [in the internal hospital seminars], I became a little unsure. (…) We often say ‘OK, if the UAT is negative, then you can stop [the therapy] again’. I think that's not quite right.” (University hospital in the German-speaking part of Switzerland, male, 6 years of medical practice)
Apart from adhering to guidelines and the clinical considerations physicians made when met with patients presenting with pneumonia / Legionnaires' disease, we noted during interviews that other more distal factors shaped the decision-making process in clinical practice. The factors most often mentioned were cost concerns (for both patients and the health systems), time constraints, lack of resources/equipment, and patients’ expectations.
Most physicians were highly cost aware toward diagnostic testing. They believed that currently testing is not sufficiently targeted to at-risk patients, resulting in a perceived unnecessary burden on the health system. As one interviewee said:
“The aim is to focus more on [targeted] diagnostic tests. [We should be] really focusing on severe patients, as testing is worthwhile there, but for all others it is not needed. I think there is an overuse of diagnostic tests; we could save money if we would do it in a certain way... targeted to patients where the pre-test probability [of a positive finding] is higher.” (University hospital in the French-speaking part of Switzerland, female, 17 years medical practice)
Yet at hospitals, even though the physicians were cost conscious, they estimated testing accounts for a small portion of total hospitalisation costs only. Mostly GPs felt the need to save resources, which discouraged testing. They voiced concerns about being sanctioned or seen as a "bad doctor", if they increased the diagnostic test volume. One GP noted:
“I'm sure I missed legionellosis cases, but the problem is that there is so much pressure from the health insurance companies not to do examinations [i.e., aetiological tests], that I prefer saving my laboratory resources for follow-ups or other diagnoses [than Legionnaires' disease]. Since the treatment is not going to be changed, there is just no point in making a specific diagnosis. [...] They should pay us for these diagnostic tests, and stop bothering us with cost issues. We can't be asked to work more and do more examinations and at the same time be punished for doing so. It just does not make any sense.” (GP in the French-speaking part of Switzerland, male, 35 years of medical practice)
Overall, physicians saw antibiotics as too cheap and diagnostic testing as too expensive, which encouraged treatment with broad-spectrum antibiotics. Many physicians, in particular GPs, saw conflicting interests in promoting microbiological investigations for public health benefits (such as improved surveillance activities of pathogens and antimicrobial resistance), and the need for cost-effective and resource-saving treatment of patients.
Congruously, a lack of time was the next major consideration affecting testing and treatment decisions. Some physicians, particularly GPs, noted a lack of time to pursue continuing education and stay on top of current medical and public health advancements. Further, GPs noted that the prescription of broad-spectrum antibiotics was time-efficient, as it would lessen the need for follow-up with the patients. Overall, they saw a conflict in devoting time to continued education, the in-depth investigation of cases, the efficient care of many of their patients and timely referral once a case becomes complicated. As one interviewee put it:
“I don't think [most doctors have enough knowledge on pneumonia], me included. We don't have enough time [to know everything]. As primary care providers, if we have patients with pneumonia, we just give Augmentin and wait and see what happens. And if it doesn't work, maybe we add a Klacid and that's how we do medicine. However, I'm convinced that's wrong and I'm convinced we don't have the time [to do better]. If I want to take good care of my patients, as a primary care provider, I have to draw a line somewhere and say ‘I can treat uncomplicated pneumonia here in my clinic. If it gets difficult, I know exactly where to turn to. However, I really think the body of knowledge has grown so much (…) I think we have a conflict there. We don't have that time any more.” (Regional hospital in Italian-speaking part of Switzerland, male, 31 years medical practice)
The lack of resources was exemplified by GPs generally not being able to perform the Legionella UAT in-house. In addition, hospital physicians questioned why the UAT was slow in delivering a test result and not more accessible, which they would see as an improvement in the diagnosis of Legionnaires' disease:
“[The UAT] is a standard test, it's not particularly difficult. It can be carried out in almost every laboratory, even in small laboratories or in private laboratories. It is like a pregnancy test. I think it can be done as a point-of-care test by a clinician in the emergency ward. But it has to be done in an accredited laboratory and so the barrier to do it is actually large. If we could make it possible for the test to be carried out by GPs or in the emergency wards, then we would certainly improve diagnostics. In our laboratory, for Legionella, the ‘tolerance time’, the time until we get the result, is much too long. The test takes 15 minutes and if you send it to the laboratory, the result should be there in an hour. Now we only receive the result after four hours or even the next day. Then it's no longer a point-of-care test, that's our frustration.” (Cantonal hospital in the German-speaking part of Switzerland, male, 37 years of medical practice)
Lastly, GPs reported prescribing antibiotics based on patients’ wishes. They did note, however, that the frequency of patient requests for antibiotic treatment seemed to have declined over time. They also explained the need to justify expensive diagnostics for patients, which prevented them from costly testing. One GP gave an example:
“When you are in a practice you are much more cost-conscious not only for yourself but also for the patients, because in the hospital everything is included in the flat rate but in the practice everything is charged separately. And you don't want to scare the patient [with the laboratory costs]; or you have to explain it to them because then patients come back to you and say "I have received an 850 francs invoice from the laboratory!" Then you have to be able to justify it.” (GP in Italian-speaking part of Switzerland, female, 22 years medical practice)
Overall, many physicians reported taking a variety of considerations and factors into account when deciding on a diagnosis and treatment pathway. However, GPs seemed to be most affected by constraints beyond clinical considerations.
Through analysis of 46 in-depths interviews with physicians, we provide an in-depth, qualitative understanding of physicians’ awareness on Legionnaires’ disease and practices for diagnosis and treatment in Switzerland. We did not observe major regional differences, but we found physicians working at hospital level regardless of whether regional, cantonal or university hospitals were comparable in their views and opinions, whereas GPs differed in comparison to hospital physicians in most aspects.
Previous research on the "true" burden and the trend of Legionnaires' disease suggested a lack of awareness or changing awareness as causes [3–5]. However, we found no evidence supporting this hypothesis in our study; awareness about Legionnaires' disease was generally high. Swiss physicians in our sample see it neither as a major public health threat nor as an emerging disease. This assessment seems to be based on the low number of contacts with Legionnaires' disease patients and the fact that appropriate treatment is available, regardless of whether the pathogen has been identified. Indeed, annually "only" 6 to 7 cases per 100,000 inhabitants are notified. However, due to the ongoing increase in case numbers, Legionnaires' disease is now among the 10 most notified disease among the 52 infectious diseases under surveillance in Switzerland [27]. Additionally, Switzerland has the second highest Legionnaires' disease notification rate in Europe, behind Slovenia (9.4 in 2019) [5]. Nevertheless, almost all physicians in this sample agreed that Legionnaires' disease is likely underestimated, primarily owing to a small proportion of pneumonia cases for whom microbiological investigation are initiated and an even smaller proportion where it is successful [28, 29].
Additionally, diagnostic work-up of pneumonia and, therefore, Legionella seems to be limited to the hospital setting. Many physicians stated following internal hospital guidelines, where, in line with current Swiss guidelines, UATs were listed as standard test procedures for patients presenting at the emergency ward with pneumonia symptoms or for patients admitted to the hospital [9].
In contrast, GPs were well aware that aetiological testing is not recommended for outpatients presenting with mild pneumonia symptoms. As GPs often do not know the causative agent of a pneumonia they treat and are, therefore, not consciously confronted with Legionnaires' disease cases, they tend to be less sensitised to the disease than physicians working in hospitals. It is likely that most notified Legionnaires' disease cases in Switzerland are treated in the hospitals. Yet this also implies that patients with mild symptoms being treated in an outpatient setting will not be diagnosed and Legionnaires' disease cases could potentially be missed. Hence, disease severity (and its assessment) and health-seeking behaviour likely influence testing and, therefore, case numbers.
Several assessment scores exist to assist the physicians in determining the disease severity of CAP, such as the PSI and the CURB-65 or CRB-65 score, all of which are listed in the 2006 SSI guideline [9, 30, 31]. In the last decade, several additional scores were developed and validated to differentiate Legionnaires' disease from pneumonias of other origins based on clinical parameters [16, 17, 20]. Yet none of these scores were specifically mentioned in the interviews and were not at the forefront of the physicians’ considerations. Consistent with the low emphasis on these scores, a 2012 study showed that the CRB-65 is not routinely assessed in Switzerland [31]. Without objective parameters to assess disease severity, the decision for testing is subject to the physicians’ empirical intuition. Although most of the hospital physicians in our study demonstrated high awareness of the clinical signs and risk factors for Legionnaires' disease, and reported that their current level of education and training was adequate, there was considerable uncertainty about narrowing down the population-at-risk which warrants testing.
In our interviews, we found two contrasting attitudes toward treatment and diagnosis: (1) low confidence in diagnostics and a preference for empirical treatment; and (2) a preference for diagnosis in favour of narrow-spectrum antibiotics. The UAT is the most commonly used diagnostic test for Legionella infections in Switzerland [7]. The physicians from this study highlighted several features that they appreciated about these tests. Nonetheless, confidence in the accuracy of the UAT is interlinked with its use and the approach to antibiotic treatment. Some physicians would continue treatment even with a negative test result, as they were cautious regarding the sensitivity of the UAT and its limitation of only detecting Legionella pneumophila serotype 1.
Indeed, a systematic review found a sensitivity of 0.74 for the UAT and reported a rate of 26% false negatives [32]. A more recent study found an even higher false negative rate of 44.4% [33]. Several publications suggested that the UAT should be used solely to confirm (the presence of) Legionella, but not for ruling it out [32, 34]. Therefore, from a clinical perspective and in cases where antibiotics with Legionella spp. coverage have already been administered, it could be argued that an UAT need not be performed at all if it does not further influence the treatment. However, a single-centre study from Switzerland highlighted that in 90% of hospitalised cases, macrolide therapy was discontinued once an UAT was negative [35]. Another Swiss study in 2004 showed that a negative UAT would lead to a shorter treatment duration than recommended, but not to withdrawal of macrolides or quinolones [36]. After a positive UAT, non-Legionella targeting antibiotics were discontinued. The addition of antibiotics was of less importance, because Legionella was mostly covered already in the empiric treatment regimen. The 2006 SSI guideline does not specifically mention the de-escalation of therapy, but the 2021 S3 update devotes a chapter to this topic and antibiotic stewardship in general [9, 14].
Ideally, physicians could base their choice of appropriate treatment for a patient on scientific evidence, but the literature seems to be inconclusive. Some studies conclude that respiratory fluoroquinolones or a combination of a β-lactam with a macrolide is a superior empirical treatment strategy compared with β-lactam monotherapy [37]. Similarly, it was found that the initial therapy with an antibiotic active against Legionella (quinolones or macrolides) reduces the likelihood of transfer to the intensive care unit (ICU) [38] and treatment failure in severe CAP cases [39]. The latter study, however, cautions against the excessive use of fluoroquinolones. This is supported by Dutch researchers, who recently advised against the excessive use of quinolones in view of the low incidence of Legionnaires' disease cases and recommended a diagnostic workup for Legionella based on the CURB-65 score [40]. Other studies found that the increased use of narrow-spectrum antibiotics following an antibiotic stewardship intervention did not compromise patients’ health outcomes [41]. There was also the recommendation to use narrow-spectrum antibiotics for all patients except for those with severe pneumonia or a high risk for an adverse outcome [35]. Harmonisation of the clinical implications of Legionella UATs could facilitate the decision-making for physicians and lead to more consistent testing and treatment approaches.
Previous research demonstrated a wide range of intrinsic (such as fear of negative health outcomes) and extrinsic (such as time pressure) factors that influence antibiotic prescribing behaviour [42]. In our study, we found similar effects influencing not only treatment but also diagnostic approaches. In previous sections, we discussed the influence of extrinsic (patient-related) clinical considerations and intrinsic diagnostic uncertainty on physicians' decision-making. Other reported factors that affected diagnostic and treatment approaches were often system-related, such as cost and time constraints.
For most physicians, financial considerations were paramount. Antibiotics are less costly than diagnostic tests, which account for the majority of hospital costs [43, 44]. A Dutch study reported potential healthcare cost reductions from de-escalation of antibiotic treatment after a negative Legionnaires' disease test result. However, such antibiotic de-escalation based on a negative UAT result is not always recommended according to Dutch guidelines [44–46]. In our study, hospital physicians, while cost-conscious, appeared less constrained by financial considerations than GPs.
The GPs’ empirical approach to care (in line with current guidelines) likely accounts partially for the underestimation of Legionnaires' disease cases, yet it is noteworthy that GPs felt the need to reconcile various demands. They reported lacking time for continuing education and in-depth investigation of individual patients, lacking the resources to perform diagnostic testing, and being under pressure to be cost-effective for patients and the healthcare system. The concern of being reprimanded or considered a “bad doctor” for overuse of diagnostic tests could originate from the revised (and lowered) tariffs/reimbursement GPs can claim following tariff point revisions in the 2000s. Additionally, in Switzerland, the UAT may only be performed in accredited laboratories, which does not allow their use in in-house diagnostics of family medicine practices. Lastly, GPs seemed to be more affected by interpersonal factors and communication with their patients than hospitalists. GPs placed more emphasis on a diagnosis and treatment approach that was in accordance with the patient’s wishes and expectations.
Diagnosing Legionnaires' disease, i.e., using a diagnostic test, should serve two purposes: to improve patient outcomes and to promote public health by gaining knowledge on the spread of pathogens and their contribution to the burden of disease. The impact on individual and public health of not diagnosing Legionnaires' disease is not easy to assess. There is a lack of knowledge about the spectrum of disease severity in Legionnaires' disease, since primarily severe, i.e. hospitalised cases, are detected. It can only be speculated how large the pool of mild cases is. Results from the German CAPNETZ study suggest that the numbers of hospitalised and ambulatory cases with Legionella infection are similar [47]. Furthermore, we do not know how many of the severe cases could have been prevented through earlier detection at the GP level. From a public health perspective, diagnosing Legionnaires' disease at the primary care level, which would reduce underestimation, supports accurate monitoring. It further guides future case management and potentially facilitates the identification of infectious sources, which allows the implementation of accurate prevention and control measures. However, rolling out the UAT to primary healthcare levels would be hampered by the lower test sensitivity in patients with mild symptoms [48]. In hospital settings, improving diagnostic methods is the most obvious approach to reduce the underestimation of cases, for example by heavily relying on PCR diagnostics as has been done in New Zealand [49]. The utility of Legionella clinical scores should also be further investigated. For individual health, empirical treatment might be sufficient, if there is little transition of undetected mild cases to severe hospitalised cases. If the burden of mild cases is found to be high, CAP guidelines should be adjusted.
To minimise bias, we aimed to limit Legionnaires' disease prompts before and during the first part of the interview. Due to the purposive sampling, physicians who were either aware of Legionnaires' disease or had a special interest in the topic might be overrepresented. Hence, the generally high level of awareness found might not be representative. However, this qualitative study aimed at uncovering realities and opinions that shape the awareness and testing (and case detection) approaches for legionellosis. The study was not designed to quantify our findings. We note however, that several GPs reported having limited knowledge which shows that, at least for them, knowledge and previous information was not a barrier to participation, but rather that these GPs saw the study as a platform to express their experiences and constraints.
Physicians in Switzerland showed high awareness of Legionella spp. and the “Legionnaires' disease” disease system, suggesting that we should broaden the discussion of Legionnaires' disease underestimation beyond a lack of awareness. A majority of Legionnaires' disease notifications originate in hospital settings since GPs rarely perform aetiological testing, which is as currently recommended. This implies that mild cases may not be detected. Physicians uniformly agreed that Legionnaires' disease is underdiagnosed, largely due to a general difficulty in identifying the causative agent of pneumonia. Most study participants were aware of and reported testing and treatment decisions in adherence to the current guidelines.
There are challenges in balancing multiple interests and constraints that affect physician practices. Specifically, this relates to clinical benefit to the patient, antibiotic stewardship, and time and cost efficiency for both the patient and the healthcare system. Physicians reported uncertainties about the reliability of the UAT for Legionnaires' disease and the correct approaches to antibiotic stewardship and de-escalation of therapy. There is a need for better diagnostics to help physicians reduce underestimation of Legionnaires' disease and improve antibiotic stewardship without compromising patients’ health outcomes. Additionally, questions about the extent of missed mild cases and cases transitioning from mild to severe owing to non-diagnosis and ineffective treatment need to be answered to assess the public and individual health impact of non-testing at GP level and, therefore, the appropriateness of current guidelines.
We gratefully acknowledge the contributions of the following persons: Audrey Lanyan, Amélie Viret, Anja Orschulko, Deniz Dogan and Linda Eggs for supporting the data collection and the validation of the findings. Jean-Baptiste Puginier supported the translation of the interviews. We thank Julia Fanderl for supporting the coding and analysis of the data and Melina Bigler for reviewing the manuscript.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
This study was funded by the Swiss Federal Office of Public Health.
1. Communicable Diseases Legislation – Epidemics Act , (EpidA), (2016).
2. Bulletin BAG . Week 1+2/2021: Federal Office of Public Health; 2021.
3. Fastl C , Devleesschauwer B , van Cauteren D , Lajot A , Leroy M , Laisnez V , et al. The burden of legionnaires’ disease in Belgium, 2013 to 2017. Arch Public Health. 2020 Oct;78(1):92. https://doi.org/10.1186/s13690-020-00470-7
4. Cassell K , Gacek P , Rabatsky-Ehr T , Petit S , Cartter M , Weinberger DM . Estimating the true burden of Legionnaires’ disease. Am J Epidemiol. 2019 Sep;188(9):1686–94. https://doi.org/10.1093/aje/kwz142
5. Legionnaires’ disease . Annual epidemiological report for 2019. Stockholm: ECDC: European Centre for Disease Prevention and Control, 2021.
6. Beauté J , Robesyn E , de Jong B ; European Legionnaires’ Disease Surveillance Network . Legionnaires’ disease in Europe: all quiet on the eastern front? Eur Respir J. 2013 Dec;42(6):1454–8. https://doi.org/10.1183/09031936.00089113
7. Fischer FB , Schmutz C , Gaia V , Mäusezahl D . Legionnaires’ disease on the rise in Switzerland: A denominator-based analysis of national diagnostic data, 2007–2016. Int J Environ Res Public Health. 2020;17(19):7343. PubMed PMID: doi:https://doi.org/10.3390/ijerph17197343
8. Gysin N . Legionnaires' disease in Switzerland: analysis of Swiss surveillance data, 2000 to 2016 - spatial and seasonal determinants [MPH thesis]; 2018.
9. Laifer G , Flückiger U , Scheidegger C . Management of community acquired pneumonia (CAP) in adults (ERS/ESCMID guidelines adapted for Switzerland). Swiss Society for Infectious Diseases; 2006.
10. Woodhead M , Blasi F , Ewig S , Huchon G , Ieven M , Ortqvist A , et al.; European Respiratory Society; European Society of Clinical Microbiology and Infectious Diseases . Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005 Dec;26(6):1138–80. https://doi.org/10.1183/09031936.05.00055705
11. Legionellen und Legionellose BAG-/BLV-Empfehlungen. Federal Office of Public Health; Federal Food Safety and Veterinary Office, 2018.
12. Höffken G , Lorenz J , Kern W , Welte T , Bauer T , Dalhoff K , et al.; Paul-Ehrlich-Society of Chemotherapy; German Respiratory Diseases Society; German Infectious Diseases Society; Competence Network CAPNETZ for the Management of Lower Respiratory Tract Infections and Community-acquired Pneumonia . Guidelines of the Paul-Ehrlich-Society of Chemotherapy, the German Respiratory Diseases Society, the German Infectious Diseases Society and of the Competence Network CAPNETZ for the Management of Lower Respiratory Tract Infections and Community-acquired Pneumonia. Pneumologie. 2010 Mar;64(3):149–54. https://doi.org/10.1055/s-0029-1243910
13. Ewig S , Höffken G , Kern WV , Rohde G , Flick H , Krause R , et al. Behandlung von erwachsenen Patienten mit ambulant erworbener Pneumonie und Prävention - Update 2016. Pneumologie. 2016 Mar;70(3):151–200. https://doi.org/10.1055/s-0042-101873
14. Ewig S , Kolditz M , Pletz M , Altiner A , Albrich W , Drömann D , et al. Behandlung von erwachsenen Patienten mit ambulant erworbener Pneumonie – Update 2021. Pneumologie. 2021 Sep;75(9):665–729. https://doi.org/10.1055/a-1497-0693
15. Albrich W , Boillat-Blance N , Kahlert C , Hauser C , Ott S , Pedrazzini B . Pneumonie / Ambulant-erworbene Pneumonie CAP (D). 2021 [cited 2022 Feb 7]. Available from: https://ssi.guidelines.ch/guideline/3007
16. Miyashita N , Horita N , Higa F , Aoki Y , Kikuchi T , Seki M , et al. Validation of a diagnostic score model for the prediction of Legionella pneumophila pneumonia. J Infect Chemother. 2019 Jun;25(6):407–12. https://doi.org/10.1016/j.jiac.2019.03.009
17. Bolliger R , Neeser O , Merker M , Vukajlovic T , Felder L , Fiumefreddo R , et al. Validation of a prediction rule for Legionella pneumonia in emergency department patients. Open Forum Infect Dis. 2019 Jun;6(7):ofz268. https://doi.org/10.1093/ofid/ofz268
18. Ito A , Ishida T , Washio Y , Yamazaki A , Tachibana H . Legionella pneumonia due to non-Legionella pneumophila serogroup 1: usefulness of the six-point scoring system. BMC Pulm Med. 2017;17(1):211-. doi: https://doi.org/10.1186/s12890-017-0559-3. PubMed PMID: 29246145.
19. Cunha BA . Severe Legionella pneumonia: rapid presumptive clinical diagnosis with Winthrop-University Hospital’s weighted point score system (modified). Heart Lung. 2008 Jul-Aug;37(4):311–20. https://doi.org/10.1016/j.hrtlng.2007.12.003
20. Fiumefreddo R , Zaborsky R , Haeuptle J , Christ-Crain M , Trampuz A , Steffen I , et al. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med. 2009 Jan;9(1):4. https://doi.org/10.1186/1471-2466-9-4
21. Phin N , Parry-Ford F , Harrison T , Stagg HR , Zhang N , Kumar K , et al. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect Dis. 2014 Oct;14(10):1011–21. https://doi.org/10.1016/S1473-3099(14)70713-3
22. Velazco JF . Legionnaires’ disease treatment. In: Surani S, editor. Hospital acquired infection and Legionnaires' disease: IntechOpen; 2020.
23. Gale NK , Heath G , Cameron E , Rashid S , Redwood S . Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13(1):117. doi: https://doi.org/10.1186/1471-2288-13-117. PubMed PMID: 24047204; PubMed Central PMCID: PMCPMC3848812.
24. Tong A , Sainsbury P , Craig J . Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007 Dec;19(6):349–57. https://doi.org/10.1093/intqhc/mzm042
25. Braun V , Clarke V . Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. https://doi.org/10.1191/1478088706qp063oa
26. Sentinella: Themen 2019. Federal Office of Public Health; 2019.
27. Federal Office of Public Health . Meldepflichtige übertragbare Krankheiten und Erreger: Leitfaden zur Meldepflicht. 2020.
28. Carugati M , Aliberti S , Reyes LF , Franco Sadud R , Irfan M , Prat C , et al. Microbiological testing of adults hospitalised with community-acquired pneumonia: an international study. ERJ Open Res. 2018 Oct;4(4):00096–02018. https://doi.org/10.1183/23120541.00096-2018
29. Shoar S , Musher DM . Etiology of community-acquired pneumonia in adults: a systematic review. Pneumonia. 2020 Oct;12(1):11. https://doi.org/10.1186/s41479-020-00074-3
30. Lim WS , van der Eerden MM , Laing R , Boersma WG , Karalus N , Town GI , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003 May;58(5):377–82. https://doi.org/10.1136/thorax.58.5.377
31. Widmer CC , Bachli EB . Quality of care in patients with community acquired pneumonia and sepsis in a Swiss hospital. Swiss Med Wkly. 2012 Feb;142(0506):w13510. https://doi.org/10.4414/smw.2012.13510
32. Shimada T , Noguchi Y , Jackson JL , Miyashita J , Hayashino Y , Kamiya T , et al. Systematic review and metaanalysis: urinary antigen tests for Legionellosis. Chest. 2009 Dec;136(6):1576–85. https://doi.org/10.1378/chest.08-2602
33. Muyldermans A , Descheemaeker P , Boel A , Desmet S , Van Gasse N , Reynders M ; National Expert Committee on Infectious Serology . What is the risk of missing legionellosis relying on urinary antigen testing solely? A retrospective Belgian multicenter study. Eur J Clin Microbiol Infect Dis. 2020 Apr;39(4):729–34. https://doi.org/10.1007/s10096-019-03785-8
34. Rojas E , Naqvi S , Balakrishnan B . 985: Urine antigen testing for Legionnaires’ disease: Use it to rule in, not rule out! Crit Care Med. 2021;49(1):490. https://doi.org/10.1097/01.ccm.0000729828.56129.4d
35. Piso RJ , Arnold C , Bassetti S . Coverage of atypical pathogens for hospitalised patients with community-acquired pneumonia is not guided by clinical parameters. Swiss Med Wkly. 2013 Sep;143(3738):w13870. https://doi.org/10.4414/smw.2013.13870
36. Garbino J , Bornand JE , Uçkay I , Fonseca S , Sax H . Impact of positive legionella urinary antigen test on patient management and improvement of antibiotic use. J Clin Pathol. 2004;57(12):1302-5. doi: https://doi.org/10.1136/jcp.2004.018861. PubMed PMID: PMC1770495.
37. Garin N , Marti C . Community-acquired pneumonia: the elusive quest for the best treatment strategy. J Thorac Dis. 2016 Jul;8(7):E571–4. https://doi.org/10.21037/jtd.2016.05.13
38. Falcone M , Russo A , Tiseo G , Cesaretti M , Guarracino F , Menichetti F . Predictors of intensive care unit admission in patients with Legionella pneumonia: role of the time to appropriate antibiotic therapy. Infection. 2021 Apr;49(2):321–5. https://doi.org/10.1007/s15010-020-01565-7
39. Ott SR , Hauptmeier BM , Ernen C , Lepper PM , Nüesch E , Pletz MW , et al. Treatment failure in pneumonia: impact of antibiotic treatment and cost analysis. Eur Respir J. 2012 Mar;39(3):611–8. https://doi.org/10.1183/09031936.00098411
40. Van Berge Henegouwen JM , Groeneveld GH , de Boer MG , Visser LG . A more restrictive use of quinolones in patients with community acquired pneumonia is urgently needed. Neth J Med. 2017 Dec;75(10):462–3.
41. Schweitzer VA , van Heijl I , Boersma WG , Rozemeijer W , Verduin K , Grootenboers MJ , et al.; CAP-PACT Study Group . Narrow-spectrum antibiotics for community-acquired pneumonia in Dutch adults (CAP-PACT): a cross-sectional, stepped-wedge, cluster-randomised, non-inferiority, antimicrobial stewardship intervention trial. Lancet Infect Dis. 2022 Feb;22(2):274–83. https://doi.org/10.1016/S1473-3099(21)00255-3
42. Teixeira Rodrigues A , Roque F , Falcão A , Figueiras A , Herdeiro MT . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013 Mar;41(3):203–12. https://doi.org/10.1016/j.ijantimicag.2012.09.003
43. Spoorenberg SM , Bos WJ , Heijligenberg R , Voorn PG , Grutters JC , Rijkers GT , et al. Microbial aetiology, outcomes, and costs of hospitalisation for community-acquired pneumonia; an observational analysis. BMC Infect Dis. 2014 Jun;14(1):335. https://doi.org/10.1186/1471-2334-14-335
44. Vestjens SM , Wittermans E , Spoorenberg SM , Grutters JC , van Ruitenbeek CA , Voorn GP , et al. Inter-hospital variation in the utilization of diagnostics and their proportionality in the management of adult community-acquired pneumonia. Pneumonia. 2018 Dec;10(1):15. https://doi.org/10.1186/s41479-018-0059-0
45. Wiersinga WJ , Bonten MJ , Boersma WG , Jonkers RE , Aleva RM , Kullberg BJ , et al.; Dutch Working Party on Antibiotic Policy; Dutch Association of Chest Physicians . SWAB/NVALT (Dutch Working Party on Antibiotic Policy and Dutch Association of Chest Physicians) guidelines on the management of community-acquired pneumonia in adults. Neth J Med. 2012 Mar;70(2):90–101.
46. Wiersinga WJ , Bonten MJ , Boersma WG , Jonkers RE , Aleva RM , Kullberg BJ , et al. Management of community-acquired pneumonia in adults: 2016 guideline update from the Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT). Neth J Med. 2018 Jan;76(1):4–13.
47. von Baum H , Ewig S , Marre R , Suttorp N , Gonschior S , Welte T , et al.; Competence Network for Community Acquired Pneumonia Study Group . Community-acquired Legionella pneumonia: new insights from the German competence network for community acquired pneumonia. Clin Infect Dis. 2008 May;46(9):1356–64. https://doi.org/10.1086/586741
48. Blázquez RM , Espinosa FJ , Martínez-Toldos CM , Alemany L , García-Orenes MC , Segovia M . Sensitivity of urinary antigen test in relation to clinical severity in a large outbreak of Legionella pneumonia in Spain. Eur J Clin Microbiol Infect Dis. 2005 Jul;24(7):488–91. https://doi.org/10.1007/s10096-005-1361-3
49. Priest PC , Slow S , Chambers ST , Cameron CM , Balm MN , Beale MW , et al. The burden of Legionnaires’ disease in New Zealand (LegiNZ): a national surveillance study. Lancet Infect Dis. 2019 Jul;19(7):770–7. https://doi.org/10.1016/S1473-3099(19)30113-6
50. Woodhead M , Blasi F , Ewig S , Garau J , Huchon G , Ieven M , et al.; Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases . Guidelines for the management of adult lower respiratory tract infections—full version. Clin Microbiol Infect. 2011 Nov;17 Suppl 6:E1–59. https://doi.org/10.1111/j.1469-0691.2011.03672.x
Recommendations for Community-acquired pneumonia (CAP) in Switzerland at the time of the study were to treat mild outpatient cases with Amoxiciline/Clavulanate or Doxycycline [51]. Moderately ill, hospitalised patientsshould be treatedwith Amoxiciline/Clavulanate +/– Clarithromycine. Severecases, admitted to the ICU shouldreceive Ceftriaxone + Clarithromycine. If Legionella spp. has been identified the patients should be treated with a macrolide or quinolone. Treatment is recommended to last at least 14 days for Legionella spp., but shorter duration are possible if the patient is afebrile. The Swiss guideline published by the Swiss Society of Infectious Diseases in 2006 is based on the European Respiratory Society (ERS) / European Society for Clinical Microbiology and Infectious Diseases (ESCMID) guidelines from 2005 [9, 10]. The ERS/ESCMID guidelines were updated in 2011 and recommendations slightly changed: Newer broad-spectrum antibiotics (such as Amoxiciline/Clavulanate) are reserved for thirdline treatment when the traditional well-known agents cannot be used [50]. For Legionella spp. treatment respiratory quinolones should be preferred over macrolides. The newest update of the S3 guideline, which was conceived together with the SSI, recommends a macrolide for all cases of severe pneumonia, which can be discontinued afterthree days and clinical stabilisation of the patient if no atypical pathogen could be identified [14]. Confirmed Legionnaires' disease cases should be treated with a quinolone. Treatment duration as short as five days might be possible.
Most of the physicians in our study mentioned antibiotic prescriptions in line with these recommendations. Nine different antibiotics belonging to four different antibiotic classes were named by the physicians for treating pneumonia: β-lactams, quinolones, macrolides and tetracycline. Macrolides were most often mentioned for Legionnaires' disease treatment – few physicians also mentioned their adverse effects. As GPs stated to hardly perform diagnostic tests, they were more in favour for empirical treatment with broad-spectrum antibiotics for patients presenting with pneumonia. GPs most frequently reported initiating treatment with β-lactams such as amoxicillin and penicillin. If the patient's clinical condition would not improve, clarithromycin is added. Yet, also a considerable number of GPs mentioned macrolide and quinolone treatment. In a hospital setting, macrolide and quinolone treatment prevailed.