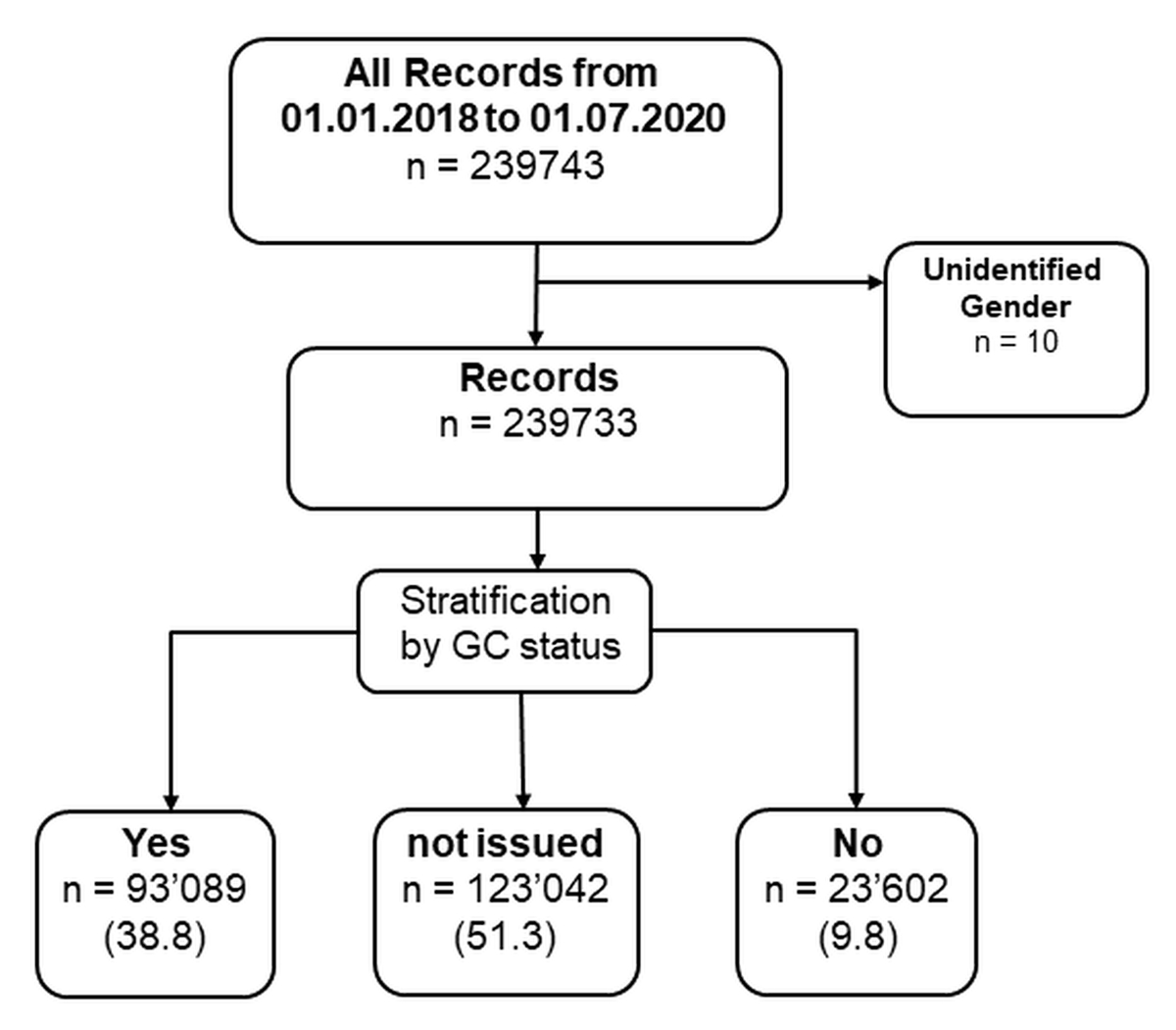
Figure 1 General Consent data processing workflow.
DOI: https://doi.org/10.4414/SMW.2022.w30159
Informed consent (IC) is one of the core elements of clinical research which is reflected in national legislations around the world, in addition to international guidelines such as the International Conference on Harmonization - Good Clinical Practice (ICH-GCP) [1]. A patient’s participation in human research projects requires sufficient or full information on all relevant aspects of the specific project followed by the voluntary, verbal and written confirmation of the subject’s willingness [2]. As the gold standard of consent, IC is used in the majority of human research projects around the world [3].
The concept of General Consent (GC) was developed as a consequence of technological advancement and the growth of large data/sample repositories [4]. With databases and biobanks at the disposal of researchers, the collection of specific Informed Consent, for every patient and every project, is extremely resource intensive and time consuming [5, 6]. In Switzerland, General Consent is considered a comprehensive “one-time” consent which allows the use of a patient’s routinely collected health-related data/samples for future unspecified research projects and for its storage in databases and biobanks [7–9].
At the University Hospital Zurich (USZ), General Consent was strenuously implemented as a consequence of the Federal Act on Research involving Human Beings (HRA, Human Research Act) which came into force in 2014 [7]. It has become a standard element of the admissions process for in- and outpatients in every clinic. All patients at the USZ are informed about General Consent by their clinic and can autonomously and without consequence to medical care, agree (GC = yes), or disagree (GC = no) to the further use of their data/samples in human research projects. Both Informed Consent and General Consent include documentation by means of written, signed, and dated consent forms followed by approval of the specific research project by the local cantonal ethics committee (EC).
The advantages of General Consent include uncomplicated collection and use of routinely collected data and samples. This promotes development of new approaches and technologies, the expansion of new information sources, and the establishment of data and sample networks – a big step forward for cooperative research [6]. Nevertheless, the implementation of such liberal consent process has always raised the important question of adequate patient protection and the ethical conformity of these projects [4, 10, 11].
Various research groups such as Brown et al. or Cassidy et al. have thoroughly investigated the different factors (age, gender, and ethnicity) influencing Informed Consent choice and patient recruitment into clinical trials [12, 13]. However, none have investigated the factors influencing a patients General Consent choice concerning routinely collected data in unspecified research projects. Therefore, the aim of this paper will be to investigate the demographic and medical factors influencing General Consent choice in USZ patient population.
This cross-sectional study was conducted at the University Hospital Zurich, Switzerland. We used completely anonymous data which does not require informed consent by participants or approval by the ethics committee. This conforms to both local law and research policies of the University Hospital Zurich [14]. Our study adhered to STROBE guidelines (STrengthening the Reporting of OBservational studies in Epidemiology) [15].
Our dataset was derived from the clinical record system, KISIM, in which all records from 01/2018 to 07/2019 were considered (figure 1). We included all inpatients and outpatients, from any clinical unit.

Figure 1 General Consent data processing workflow.
Routinely collected health data (age, gender, diagnosis, number of visits, and General Consent choice) were anonymously extracted from the clinical data platform, exported to raw data files, which in turn were imported into a separate database management system. Number of visits is defined as in- and outpatient visits to the USZ after the first consultation. We used structured query language (SQL) statements for database management and processing so that only minor data restructuring steps were necessary during statistical analysis.
Depending on the patient’s choice, they were assigned to one of the three General Consent status groups: Yes, No, or Not issued. Not issued General Consents (GC status = not issued) included forms which were not handed out to the patients or were not returned to hospital administration. General Consent status was used to stratify the population and perform comparative statistical analysis to describe the sample characteristics according to their category (figure 1).
Clinical departments at the University Hospital Zürich have similar processes for collection of General Consent. Generally, data derived from the clinical record system (KISIM) showed that clinical departments with high patient turnover had lower General Consent collection rates (GC not issued) due to increased workload. Collection rates were also highly influenced by consultation time (time spent with patient at first contact) as well as the effort and organisation of the administrative teams. Furthermore, departments with vulnerable patient populations showed low General Consent collection rates. This included the geriatric department, maternity ward, and the psychiatric department.
Monthly internal General Consent quality assessment reports derived from the clinical record system (KISIM) only showed slight differences in General Consent behaviour between in- and outpatients. Since January 2021, the monthly General Consent “yes” proportion was stable at 83% for outpatients and 83–86% for inpatients, respectively. The process for General Consent collection is re-evaluated in all departments on a regular basis and corrective measures are implemented. Internal quality documents provided by the General Consent project management team of the USZ suggest that the General Consent collection rate is increasing in most departments of the USZ.
We investigated the potential associations between demographic and medical factors and General Consent status, using multinomial and logistic regression models (tables 1, 2 and 3). In the first model, the “GC =not issued” group was used as the reference group whereas in the second model the “GC = no” group was used as the reference. The models analysed potential associations between the outcome, General Consent status, and the exposures which included number of diagnoses (sum of ICD-10 codes and free text diagnoses using name mapping tables), visits (included both inpatient and outpatient stays), gender, and age, in which the latter was calculated in decades (age/10). At the USZ, General Consent collection is known to be dependent on the “clinic of admission”. We controlled (confounder) for the 43 clinics in our model. We conducted all tests as 2-sided and determined p-values of ≤0.001 to be indicative for statistical significance. Statistical analyses were performed using the software R, version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria) and the stargaze”” package [16] was used to build our regression model.
Table 1Multinomial logistic regression – General Consent status: consented vs. not issued.
| Variable | OR | CI | p value |
| Age (in 10 year intervals) | 1.07 | 1.06; 1.08 | <0.001 |
| Sex (female) | 1.02 | 1.00; 1.04 | 0.018 |
| Number of diagnoses | 1.55 | 1.54; 1.57 | <0.001 |
| Visits | 1.11 | 1.10; 1.11 | <0.001 |
Reference group: “not issued”
CI = confidence interval; OR = odds ratio of positive General Consent choice (consented)
Tabel 2Multinomial logistic regression – General Consent status: declined vs. not issued.
| Variable | OR | CI | p value |
| Age(in 10 year intervals) | 1.01 | 1.00; 1.02 | <0.001 |
| Sex (female) | 1.23 | 1.25; 1.33 | <0.001 |
| Number of diagnoses | 1.52 | 1.51; 1.53 | <0.001 |
| Visits | 1.10 | 1.09; 1.12 | <0.001 |
Reference group: “not issued”
CI = confidence interval; OR = odds ratio of negative General Consent choice (declined)
Table 3Logistic regression – General Consent status: declined vs. consented.
| Variable | OR | CI | p value |
| Age (in 10 year intervals) | 1.05 | 1.0; 1.07 | <0.001 |
| Sex (female) | 0.79 | 0.76; 0.81 | <0.001 |
| Number of diagnoses | 1.02 | 1.02; 1.03 | <0.001 |
| Visits | 1.00 | 1.00; 1.01 | 0.013 |
Reference group: “declined”
CI = confidence interval; OR = odds ratio of positive General Consent choice (consented)
Additionally, observations were made about the distribution of General Consent status over the different age groups of the USZ patient population through descriptive statics (figures 2 and 3).

Figure 2 Age in General Consent status groups.
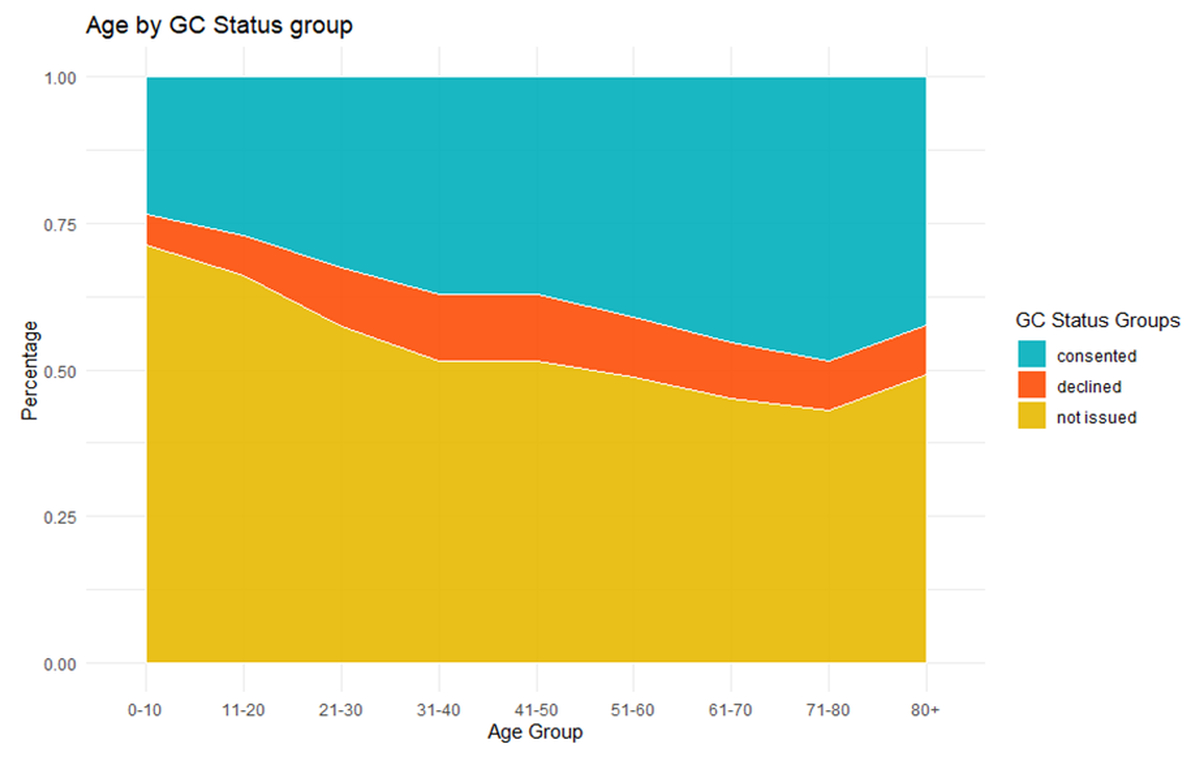
Figure 3 General Consent status distribution in age categories.
This study used anonymous data and does not require approval of an ethics committee [14].
Overall, a total of 239,733 patients were included in our analysis (figure 1) of which the majority had not been issued a General Consent (GC = not issued, 51.3%). 116,691 (48.7%) patients signed a General Consent of which 79.7% had agreed (GC = yes) and the minority, 20.2%, had declined (GC = no) to the use of their data/samples.
The total population consisted of 125,668 female (52.4%) and 114,065 male (47.6%) individuals in which the mean age, number of diagnoses and number of visits were 48.34 years (SD: 21), 2.33 diagnoses (SD: 2.43) and 1.69 readmission (SD: 2.39), respectively (table 4).
Table 4Baseline characteristics for General Consent status groups consented, declined, and not issued (total n = 239,733).
| Variable | Consented | Declined | Not issued | |
| n (%) | 93,089 (38.8) | 23,602 (9.8) | 123,042 (51.3) | |
| Age (median, IQR) | 52.00 [36.00, 68.00] | 47.00 [34.00, 62.00] | 45.00 [30.00, 62.00] | |
| Sex (n, %) | Male | 44,732 (18.7) | 9,643 (4.0) | 59,690 (24.9) |
| Female | 48,357 (20.1) | 13,959 (5.8) | 63,352 (26.4) | |
| n diagnoses | 2.00 [1.00, 4.00] | 2.00 [1.00, 3.00] | 1.00 [1.00, 2.00] | |
| Visits | 1.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | |
n = number, *IQR = Interquartile range
Respondents in our study population visited one of the 43 different USZ clinics, who were responsible for the collection of General Consent from their patients. Patients were treated predominantly in the ophthalmology department (23,987, 10%), the dermatology department (24,017, 10%) and the emergency department (22,724, 9.5%).
The regression models (tables 1, 2 and 3) controlled for the 43 different inter-clinic collection rates.
Higher number of diagnoses and visits lead to a statistically significant increase in the collection of General Consent (GC = no and GC = yes) (tables 1 and 2). The female gender was found to associate with decreased odds in positive General Consent choice (more GC = no) (table 3) whereas age and number of diagnoses was found to associated with increased odds in positive General Consent choice (more GC = yes) (table 3).
Multinomial logistic regression predicts that higher age associates with increased odds in positive General Consent choice (tables 1 and 3, figure 2).
Further investigation shows, there are differences in General Consent choice and collection depending on the age group (figures 3 and 4). Overall, more General Consents were not issued in younger patients (<18 years) and geriatric patients (80+) (figures 3 and 4).
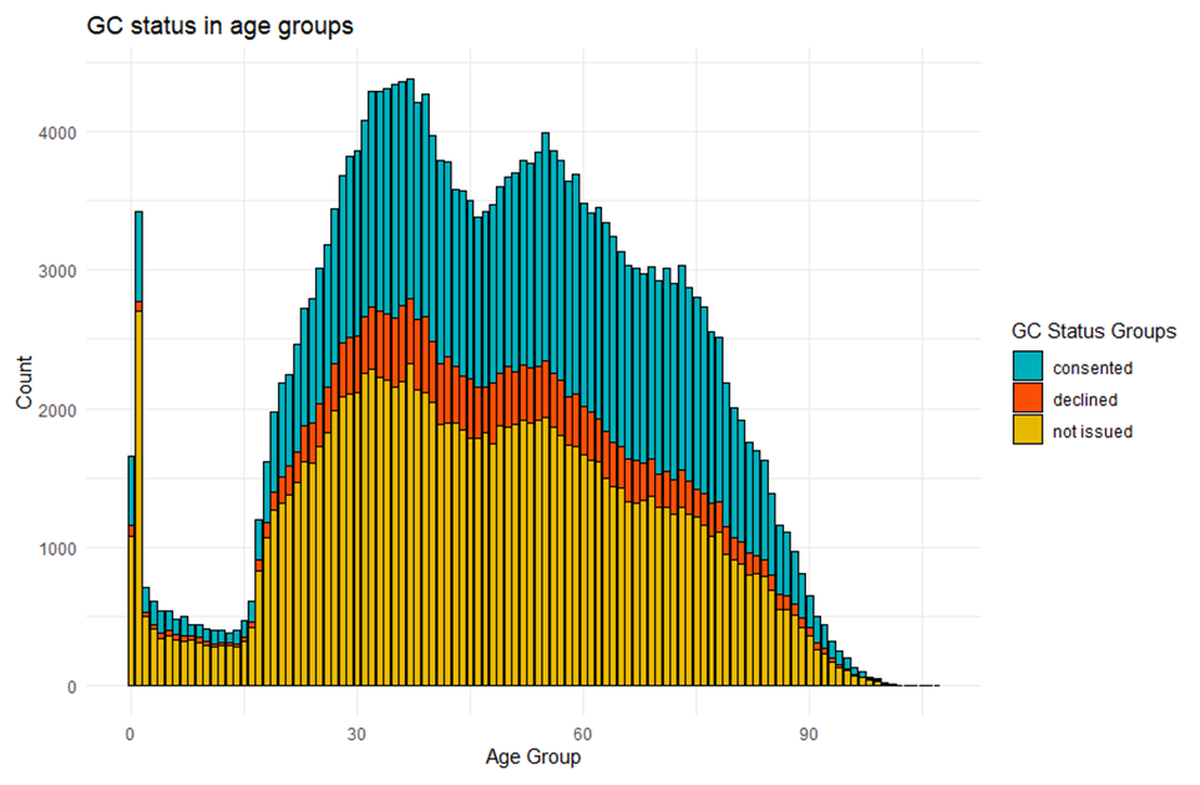
Figure 4 General Consent status in all ages.
In this cross-sectional study, we used patient data stored in the clinical information system of the University Hospital Zurich from 01/2018 to 07/2019 to investigate the association of demographic and medical factors on General Consent choice. The female gender associated with increased negative General Consent choice whereas age was associated with positive General Consent choice. Additionally, number of diagnoses and visits were predictive for both positive as well as negative General Consent status.
The use of routinely collected data/samples in research is becoming increasingly more important and will continue to do so in the future. General Consent is a simplified consent process, in which the patients agree to the further use of their data/samples for multiple different research projects, which are deemed low-risk [7]. General Consent simplifies the conduct of research projects with data/samples and ultimately leads to the promotion of cooperative research, as patients no longer need to consent to each project specifically. The difficulty lies in improved access to health-related data/samples and simultaneously maintaining patient privacy, rights, and ethical conformity [11]. Investigation of the factors influencing a patient’s General Consent choice may help improve the information of these target groups in future.
Previous literature has found that women are historically under-represented in clinical research [17], which is corroborated by our results. In clinical trials the concerns of these women included lack of information, strenuous study procedures, interference with personal life and a discomfort with the clinical environment [12]. Since collection of General Consent does not require additional visits from patients, previous research may have underestimated the other effects connected to patient consent such as lack of information (on data privacy, regulations, and general science), incomprehension, and distrust in clinical institutions.
Additionally, our investigation showed that older patients were more willing to say yes to General Consent, contrary to previous findings [18]. Studies have shown that there are challenges recruiting elderly patients with the most frequent concerns found to be travelling to the investigative institution [13]. Since General Consent does not require additional travel, it may explain the increased willingness of these patients to participate in General Consent studies. Clinical trials in older patients are often difficult to conduct due to comorbidities, co-medications and differences in pharmacokinetics and pharmacodynamics [13]. The use of these data/samples in research may prove critical in overcoming these challenges in future. An additional factor to consider may be that younger patients are more aware of the data/sample privacy risks, which may lead to the decline in positive General Consent choice in comparison to the older population [19,20]. General Consent choice may also vary in different age groups. Our results show there are higher rates of “not issued” General Consents in very young (1-10 years) and old patients (+85) whose consent forms are often signed by a parent or surrogate. The signature of surrogates raises not only a practical issue but also an ethical concern which we wish to investigate in future.
Furthermore, our results suggest that multi-morbid patients seem more likely to agree to General Consent which may be explained through the increased medical severity in this patient population. This is a positive step forward as patients with multiple diagnoses are preferably not recruited into clinical trials [21]. Number of visits was found to associate with both negative as well as positive General Consent choice. This is in line with USZ General Consent processes. General Consent is usually signed by the patients at their first visitation and if this does not occur the form will be handed out at the next visitation.
In the context of our investigation, we would also like to discuss the contemporary ethical and legal concerns regarding General Consent. Current discussions about the conformity of the General Consent have raised the question if patients are being adequately informed about the use of their data and samples in research [10, 11]. However, increasing the length, complexity, legal and medical jargon of consent forms is known to decrease understanding amongst patient populations [22]. We therefore suggest a targeted and individual nationwide communication concept, such as online information platforms and “expert chats” for specific and/or general questions regarding the patient’s interest. A national effort will increase the trust in the scientific community, identify patient concerns as well as find shortcomings in the General Consent collection processes. All communication tools and platforms should explicitly and coherently inform patients about the general concepts of research and science; the benefits of this research; give an overview on current projects working with General Consent data/samples; have detailed explanations on the risks of data privacy and security; and include up to date information on ethical, legal, and regulatory requirements. Furthermore, the collection of General Consent requires a large administrative effort which has been established through hospital processes. This study may serve as a basis to approach these target groups and increase interest and participation of the general population in research projects.
The study we conducted has several strengths: To our knowledge, this is one of the first large and systematic investigations looking into the factors influencing General Consent choice in a population. We considered in- and outpatients from all clinics of the USZ over a period longer than a year, thus limiting selection bias. The exposures were investigated in a very large sample size, which decreased the margin of error.
There are however limitations: due to the large sample size of our data, we may have found small effects, which are not medically or practically relevant. We did not investigate if these patient populations are also underrepresented in actual human research projects. Furthermore, our data did not include other important confounders such as nationality, religion, and profession.
In a subsequent study we wish to investigate additional exposures such as nationality and socio-economic factors and the impact of the COVID-19 pandemic on General Consent behaviour. If feasible, we will also include a second university hospital into the analysis to increase the generalisability of our findings. Further areas of interest include if this effect is seen in other countries, investigation of the patients’ comprehension of General Consent, and important efforts to increase comprehension through adaptive consent designs such as dynamic and eConsent [23].
In conclusion, General Consent is a necessary development towards a future in which large repositories of data and samples will be accessible for research. It will be a fine line navigating patient protection and privacy regulations in addition to promoting research with a simplified consent process. Investigation into the factors influencing General Consent may improve the targeted information these patient populations may receive as well as guarantee ethical conformity.
The datasets generated and/or analysed during the current study are not publicly available due to the protection of patient data but are available from the corresponding author on reasonable request.
Authors’ contributions: AG structured and acquired the data, completed the statistical analysis and designed the graphs/figures. AB supported AG and collected additional information. RG supervised the planning, conduct and completion of the project. FJ verified legal and regulatory statements made in manuscript. AG and AB wrote the first draft of manuscript and FJ and RG thoroughly reviewed and edited it. AB made all changes for the review process.
We thank Cyril Simmen for the initial review of the manuscript and we thank Prof. Gabriela Senti for the final review.
Alexandra Griessbach, MSc
Clinical Trials Center
University Hospital Zurich
Moussonstrasse 2
CH-8044 Zurich
alexandragriessbach[at]gmail.com
The project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1. Vijayananthan A , Nawawi O . The importance of Good Clinical Practice guidelines and its role in clinical trials. Biij. 2008 Jan;4(1):e5. https://doi.org/10.2349/biij.4.1.e5
2. European Medicines Agency . Guideline for good clinical practice E6(R2) [Internet]. European Medicines Agency; 2026 Dez. Verfügbar unter: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf
3. Cocanour CS . Informed consent-It’s more than a signature on a piece of paper. Am J Surg. 2017 Dec;214(6):993–7. https://doi.org/10.1016/j.amjsurg.2017.09.015
4. Hansson MG , Dillner J , Bartram CR , Carlson JA , Helgesson G . Should donors be allowed to give broad consent to future biobank research? Lancet Oncol. 2006 Mar;7(3):266–9. https://doi.org/10.1016/S1470-2045(06)70618-0
5. Chandler R , Brady KT , Jerome RN , Eder M , Rothwell E , Brownley KA , et al. Broad-scale informed consent: A survey of the CTSA landscape. J Clin Transl Sci. 2019 Sep;3(5):253–60. https://doi.org/10.1017/cts.2019.397
6. Mikkelsen RB , Gjerris M , Waldemar G , Sandøe P . Broad consent for biobanks is best - provided it is also deep. BMC Med Ethics. 2019 Oct;20(1):71. https://doi.org/10.1186/s12910-019-0414-6
7. Die Bundesversammlung der Schweizerischen Eidgenossenschaft . Bundesgesetz über die Forschung am Menschen,Humanforschungsgesetz, HFG [Internet]. Jan 1, 2014. Verfügbar unter: https://www.admin.ch/opc/de/classified-compilation/20061313/index.html#
8. Die Bundesversammlung der Schweizerischen Eidgenossenschaft . Bundesgesetz über den Datenschutz (DSG) [Internet]. Juli 1, 1993. Verfügbar unter: https://www.admin.ch/opc/de/classified-compilation/19920153/index.html
9. Swissethics. Template Generalkonsent 2019 [Internet]. [zitiert 28.06.2021. Verfügbar unter: <span style="background-color: rgb(179, 157, 219); color: rgb(0, 0, 0); font-size: 15px;">https://swissethics.ch/documents/generalkonsent</span>
10. Fey M . Der Generalkonsent aus Sicht der klinischen Forschung. Bull Med Suisses [Internet]. 20. Oktober 2020 [zitiert 7. November 2020]; Verfügbar unter: https://doi.emh.ch/bms.2020.19285
11. Sprecher F , Talanova V. Verbesserungspotenzial des Generalkonsents. Schweizerische Ärztezeitung. 16. September 2020;101(38):1197–200.
12. Brown DR , Fouad MN , Basen-Engquist K , Tortolero-Luna G . Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Ann Epidemiol. 2000 Nov;10(8 Suppl):S13–21. https://doi.org/10.1016/S1047-2797(00)00197-6
13. Cassidy EL , Baird E , Sheikh JI . Recruitment and retention of elderly patients in clinical trials: issues and strategies. Am J Geriatr Psychiatry. 2001;9(2):136–40. https://doi.org/10.1097/00019442-200105000-00005
14. Kantonale Ethikkommission Zürich [Internet]. Kanton Zürich. [zitiert 30. Dezember 2020]. Verfügbar unter: https://www.zh.ch/de/gesundheitsdirektion/ethikkommission.html
15. Vandenbroucke JP , von Elm E , Altman DG , Gøtzsche PC , Mulrow CD , Pocock SJ , u. a. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 16. Oktober 2007;4(10):e297.
16. Hlavac M . stargazer: Well-Formatted Regression and Summary StatisticsTables. R package version 5.2.2. https://CRAN.R-project.org/package=stargazer. 2018.
17. McCarty CA , Nair A , Austin DM , Giampietro PF . Informed consent and subject motivation to participate in a large, population-based genomics study: the Marshfield Clinic Personalized Medicine Research Project. Community Genet. 2007;10(1):2–9. https://doi.org/10.1159/000096274
18. Shenoy P , Harugeri A . Elderly patients’ participation in clinical trials. Perspect Clin Res. 2015 Oct-Dec;6(4):184–9. https://doi.org/10.4103/2229-3485.167099
19. Olson KE , O’Brien MA , Rogers WA , Charness N . Diffusion of Technology: Frequency of Use for Younger and Older Adults. Ageing Int. 2011 Mar;36(1):123–45. https://doi.org/10.1007/s12126-010-9077-9
20. Tell. Am digitalen Puls der Bevölkerung [Internet]. Schweiz: Tell; 2019. Verfügbar unter: https://cdn2.hubspot.net/hubfs/4264594/Tell%20report%202019/tell_Bericht_DE.pdf
21. Hanlon P , Hannigan L , Rodriguez-Perez J , Fischbacher C , Welton NJ , Dias S , et al. Representation of people with comorbidity and multimorbidity in clinical trials of novel drug therapies: an individual-level participant data analysis. BMC Med. 2019 Nov;17(1):201. https://doi.org/10.1186/s12916-019-1427-1
22. Beskow LM , Friedman JY , Hardy NC , Lin L , Weinfurt KP . Developing a Simplified Consent Form for Biobanking. Goodyear M, Herausgeber. PLoS ONE. 8. Oktober 2010;5(10):e13302.
23. Weber M , Griessbach A , Grossmann R , Blaser J. A FHIR-Based eConsent App for the Digital Hospital. Stud Health Technol Inform. 16. Juni 2020;270:3–7.